Dear Editors,
Acquired reactive perforating collagenosis (ARPC) is a perforating dermatosis that is characterized by the transepidermal elimination of necrotic collagen. Its characteristic clinical features are umbilicated hyperkeratotic papules or nodules. Epidemiologically, most ARPC cases accompany diabetes mellitus [1]. Eosinophilic granulomatosis with polyangiitis (EGPA) is multisystemic, necrotizing, small- and medium-vessel vasculitis with eosinophilic inflammation [2]. We report a case of ARPC and EGPA that developed simultaneously in a patient with diabetes.
A male in his mid-70s developed pruritic disseminated erythema on the trunk. He had had type 2 diabetes for 20 years. The eruptions changed to extremity-dominant erythematous papules and necrotizing nodules. Subsequently, necrotizing nodules on a palm deepened and ulcerated (Figure 1A), and multiple keratotic papules emerged on the lower extremities (Figure 1B) despite the oral administration of prednisolone (10–15 mg/day). Initial investigations revealed significant peripheral blood eosinophilia (white blood cell count, 10.4×103/μL; neutrophils, 5.6×103/μL; lymphocytes, 1.7×103/μL; eosinophils, 2.6×103/μL). Serum myeloperoxidase-anti-neutrophil cytoplasmic antibody (ANCA) and proteinase3-ANCA were negative. His serum immunoglobulin E level was elevated (918.9 IU/mL). Skin biopsies from the nodules on his palm showed extensive eosinophilic infiltration in the epidermis and dermis, in addition to eosinophilic septal panniculitis. Small-vessel vasculitis with fibrinoid necrosis was observed in the deep dermis (Figures 1C–E). Keratotic papules on his leg showed degenerated necrotic foci with epidermal cupping. Degenerated collagen fibers were eliminated through the necrotic foci. Numerous neutrophils were seen in the adjacent dermis, and moderate infiltration of eosinophils was seen in the mid-dermis around the papules (Figures 1F–H). Immunohistochemically, matrix metalloproteinase (MMP)-2 and MMP-9 were highly expressed in the adjacent dermis (Figures 1I, J). Combination therapy of prednisolone (20 mg/day), minocycline (200 mg/day) and diaphenylsulfone (50 mg/day) was successful. However, during the tapering of the prednisolone, the erythematous nodules recurred and he developed bronchial asthma. The addition of mepolizumab (300 mg/month) was effective.
FIGURE 1
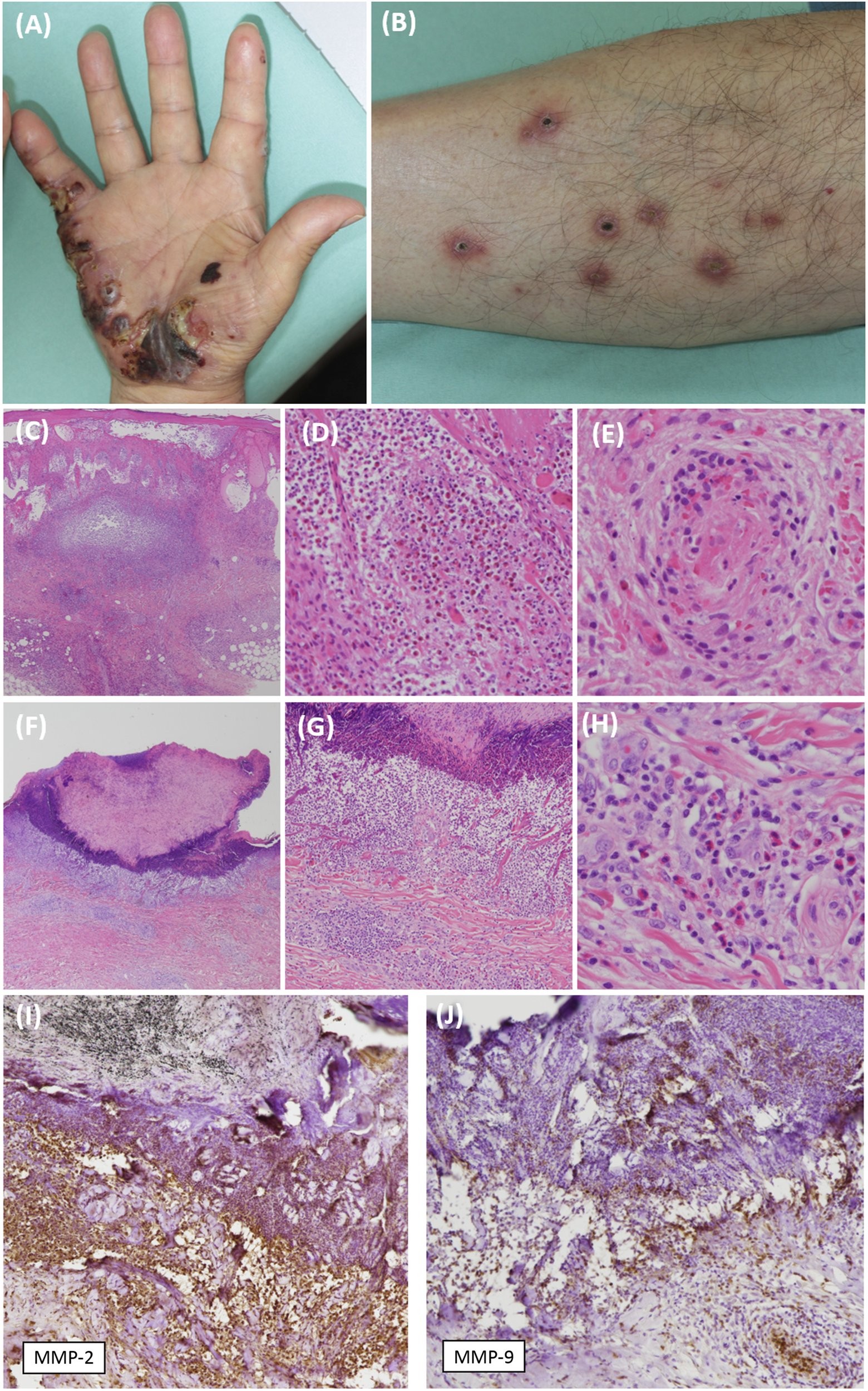
Clinical and pathological features of the skin lesions. (A) A tender necrotizing nodule on the patient’s palm. (B) A firm, keratotic papule on the patient’s lower extremity. (C,D) A skin biopsy sample from a papule on the palm demonstrates massive eosinophilic infiltration [hematoxylin-eosin (HE), original magnification ×20 (C), ×100 (D)]. (E) The specimen also shows inflammatory cell infiltration and fibrinoid necrosis in a small vessel in the deep dermis (HE, original magnification ×200). (F) A skin biopsy sample from the papule on the thigh demonstrates degenerated collagen bundles that have been eliminated to form a cup-shaped epidermal depression (HE, original magnification ×20). (G) Numerous neutrophils are seen directly under the depression (HE, original magnification ×100). (H) Eosinophilic infiltration is seen further down in the mid-dermis (HE, original magnification ×200). (I,J) Intense immunolabeling for MMP-2 and MMP-9 is observed in the dermis adjacent to the cup-shaped lesion (original magnification ×100).
As far as we know, this is the first reported case of ARPC and EGPA to develop simultaneously in a diabetic patient. The trigger for ARPC remains unclear, although mild superficial trauma or inflammatory cell infiltration have been hypothesized as causes [1]. Diabetic skin shows increased MMP-1, MMP-2 and lysyl oxidase expression and the fragmented and disorganized nanoscale morphology of collagen fibrils by atomic force microscopy [3]. The inhibition of MMP-2 and MMP-9 ameliorates collagen deposition disorders in diabetes model mice [4]. These findings indicate that MMPs are deeply involved in the degeneration and deposition of collagen fibers in the skin of patients with diabetes. In EGPA, the increased expression of both MMP-2 and MMP-9 were revealed in respiratory mucosa by immunohistochemistry and quantitative polymerase chain reaction [2]. There are reports that eosinophils themselves, as well as neutrophils, can release MMP-9 by tumor necrosis factor-α stimulation [5]. It is possible that the increased secretion of MMP-2 and MMP-9 in EGPA might be associated with the degeneration of collagen in the skin in this case. MMPs are pivotal enzymes for the degradation of the extracellular matrix. MMP-2 and MMP-9 degrade collagen type IV in the basement membrane [4]. As ARPC in diabetes is thought to be induced by minor injuries, such as from scratching [1], basement membrane fragility may contribute to induction. This case suggests that the increased secretion of MMPs in EGPA patients may affect the degeneration and deposition of collagen in diabetes and that it may be associated with the transepidermal elimination of altered collagen by making the basement membrane fragile.
Statements
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Nagoya University Hospital and Toyohashi Municipal Hospital (Approved numbers: 2022-0422 and 736, respectively). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
AM, SM, RF, YA, and MY contributed to acquiring the data, AM, TT, and MA wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by JSPS KAKENHI Grant Numbers. JP20K17315 and JP21H02941. This work was also supported by a grant from the Ministry of Health, Labor and Welfare of Japan (Health and Labor Sciences Research Grant for Research on Intractable Diseases: 23FC1039).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
Karpouzis A Giatromanolaki A Sivridis E Kouskoukis C . Acquired reactive perforating collagenosis: current status. J Dermatol (2010) 37(7):585–92. 10.1111/j.1346-8138.2010.00918.x
2.
Leone A Uzzo ML Gerbino A Tortorici S Tralongo P Cappello F et al Modulation of MMP-2 and MMP-9 in churg-strauss syndrome respiratory mucosa: potential monitoring parameters. Int J Immunopathol Pharmacol (2014) 27(2):299–304. 10.1177/039463201402700219
3.
Argyropoulos AJ Robichaud P Balimunkwe RM Fisher GJ Hammerberg C Yan Y et al Alterations of dermal connective tissue collagen in diabetes: molecular basis of aged-appearing skin. PLOS ONE (2016) 11(4):e0153806. 10.1371/journal.pone.0153806
4.
Zhou P Yang C Zhang S Ke ZX Chen DX Li YQ et al The imbalance of MMP-2/TIMP-2 and MMP-9/TIMP-1 contributes to collagen deposition disorder in diabetic non-injured skin. Front Endocrinol (Lausanne) (2021) 12:734485. 10.3389/fendo.2021.734485
5.
Schwingshackl A Duszyk M Brown N Moqbel R . Human eosinophils release matrix metalloproteinase-9 on stimulation with TNF-alpha. J Allergy Clin Immunol (1999) 104(5):983–9. 10.1016/s0091-6749(99)70079-5
Summary
Keywords
ARPC, perforating dermatosis, EGPA, eosinophils, vasculitis, MMP-2, MMP-9, diabetes
Citation
Miyazaki A, Taki T, Mori S, Fujishiro R, Arai Y, Yamada M and Akiyama M (2024) Acquired reactive perforating collagenosis and eosinophilic granulomatosis with polyangiitis developing simultaneously in a diabetic patient. J. Cutan. Immunol. Allergy 7:12594. doi: 10.3389/jcia.2024.12594
Received
21 December 2023
Accepted
25 January 2024
Published
05 February 2024
Volume
7 - 2024
Updates
Copyright
© 2024 Miyazaki, Taki, Mori, Fujishiro, Arai, Yamada and Akiyama.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tomoki Taki, white7petrolatum@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.