Abstract
Hereditary angioedema (HAE) adversely affects patients’ social and daily life significantly, and the disease burden is high. We recruited study participants from a patient-participatory registry, Rare and Undiagnosed Diseases Study (RUDY) Japan, to better understand the broader effect of HAE on patients’ lives. Thirteen patients with HAE who registered between January 2019 and March 2021 completed an online questionnaire to record details of episodes and the angioedema quality of life (AE-QoL) questionnaire, in which they described the degree to which QoL was impaired. In all, 58 episodes were recorded, and self-reported data were accumulated from 24 returned AE-QoL questionnaires. The episodes peaked between approximately 06:00 and 07:00 h and between approximately 16:00 and 20:00 h. Of the 58 episodes, 38 (65.5%) were treated; 24 (63.2%) were treated at patients’ homes. The drugs used in treatment were hospital-administered human C1-inhibitor preparations for 14 episodes and home-administered icatibant for 26 episodes. Time between episode onset and treatment initiation and between episode onset and full recovery tended to be shorter with icatibant treatment at home. The total AE-QoL score was 37.1 ± 22.9, indicating great impairment of QoL, with particularly high scores in the fatigue/mood and fears/shame domains. Most previous studies of real-world HAE episodes have been retrospective, based on patients’ memories. Collaboration between patients and researchers revealed the location of episodes, the time of day they appeared, treatments for episodes, and the degree to which patients’ QoL was impaired by HAE in this prospective study.
Introduction
Hereditary angioedema (HAE) is a rare genetic disease in which vascular permeability is increased in the deep skin, mucous membranes, or both, with recurrent episodes of localized edema. The incidence of HAE is approximately 1 in 50,000 people [1], but differences between races are unclear. One study reported HAE in 133 cases in 40 families from China [2], but another study reported HAE in only a dozen families [3]. No reports describe the incidence of HAE in Japan. Fewer HAE patients may be diagnosed at medical institutions in Asia compared with the number of cases reported in Europe and the United States, but whether differences in reported cases are due to racial differences or low awareness of the disease is unclear. HAE is classified into three types: type 1 is due to lack of the C1-inhibitor (C1-INH), type 2 is due to dysfunction of C1-INH, and the C1-inhibitor functions normally in the third type (HAE-nC1INH). The edema may be intestinal, which causes abdominal pain, and it may occur in the airway, causing asphyxia. Once formed, the edema lasts for several days, which significantly hampers patients’ social and daily life, and the disease burden is high. However, because episodes occur suddenly in daily life, it is difficult to assess disease activity and impairment of quality of life (QoL) accurately. Questionnaire surveys are typically used to record information retrospectively; therefore, accuracy is dependent on patients’ recall. To reduce potential recall bias and to more accurately describe the timing of episodes, symptom development, and time to recovery, we used an established online platform codeveloped by researchers, medical practitioners, and patients to study HAE.
Methods
Participants
Patients with HAE were recruited to participate in the Rare and Undiagnosed Diseases Study (RUDY) Japan project, in which patients, researchers, and medical practitioners work together to promote medical research and collect information from patients living with rare diseases [4]. Patients were diagnosed with HAE after being examined by a physician at a medical institution in their place of residence.
Our study included patients with HAE who were enrolled in RUDY Japan between January 2019 and March 2021. The treatment options available in Japan during this period are described below. Patients interested in participating in the study were invited to create an account on a dedicated website1 containing information on the RUDY Japan project. Participating physicians shared relevant information on the project with their patients having HAE, who were then encouraged to contribute to the study by providing information on their diagnosis and assisting with questionnaire development and improvement. Additionally, patients with HAE actively involved in relevant patient organizations were also invited to share information on the project with others to aid recruitment.
RUDY Japan
The URL of the website for participants to access and complete the survey is provided below.2
Questionnaire
The web-based survey for this study consisted of two questionnaires. The first questionnaire, which was developed in collaboration with medical professionals, researchers, and three patients, was for recording details of episodes [5]. Questions concerned the time of day of the episode, the anatomical location of swelling, whether the episode was treated, the medications used, where treatment was administered, and how long it took for symptoms to resolve. The platform allowed the same patient to enter information about different episodes over time. The second questionnaire was the Japanese version of the angioedema quality of life questionnaire (AE-QoL) [6]. In this study, the AE-QoL questionnaire was administered twice a year, in March and September. A description of the AE-QoL domains and scoring is provided in Supporting Information.
Description of AE-QoL domains and scoring
The patients’ QoL in relation to episodes of edema occurring in the preceding 4 weeks was evaluated using the AE-QoL questionnaire [6, 7] that contains 17 questions in four domains, including function (questions 1–4); fatigue/mood (questions 6–10); fear/shame (questions 12–17); and nutrition (questions 5 and 11). The responses to the questions were scored using a scale ranging from 0 (never) to 4 (frequently). The score percentages were calculated for each domain (0–100), and the total score was defined as the mean score percentage for the 17 questions (0–100). Higher scores indicated greater QoL impairment.
HAE treatments available in Japan between 2019 and 2021
The HAE treatment measures available in Japan during the study period included a dry concentrated human C1 inhibitor (C1-INH) preparation (Berinert® P; 1000–1500 IU intravenous injection or drip infusion, CSL Behring, King of Prussia, PA, United States) that was administered in a hospital; icatibant (FIRAZYR®, 30 mg subcutaneous injection; Takeda Pharmaceutical Company, Tokyo, Japan), which was used for acute episodes and could be administered at home or in a hospital; and tranexamic acid and danazol, which were used for long-term prophylaxis.
Statistical analysis
Scores for each domain and the total score of AE-QoL were analyzed with Student’s t-test. A p-value of <0.05 was considered significant.
Ethics statement
Approval of the research protocol: The study was approved by the Clinical Research Ethics Review Committee of Hiroshima University (E-1341) and Osaka University (16234(T1)-12).
Informed Consent: Informed consent (written) was obtained from the participants.
Results
Thirteen patients (four men, nine women) were recruited between January 2019 and March 2021. The participants’ average age was 47.4 13.8 years. Nine patients (69.2%) had type 1 or type 2 HAE, three patients (23.1%) had HAE-nC1INH, and in one patient (7.7%), the type was unknown (Table 1). A total of 58 episode records and 24 AE-QoL questionnaires were collected. HAE episodes occurred across all time periods, with peaks between approximately 06:00 and 07:00 h and between approximately 16:00 and 20:00 h (Figure 1A). Facial edema occurred in 14 episodes (24.1%); edema occurred in the trunk in 42 episodes (72.4%) and in the extremities in 18 episodes (31.0%); and laryngeal edema occurred in five episodes (8.6%; Figure 1B). Gastrointestinal symptoms were present in 35 episodes (60.3%), and all those episodes were accompanied by abdominal edema. No association between the time period and site of attacks was observed.
TABLE 1
| Age (years) | Sex | HAE subtype | Number of attacks | AE-QoL number |
|---|---|---|---|---|
| 25 | M | 1 or 2 | 2 | 1 |
| 32 | F | HAE-nC1INH | 7 | 4 |
| 35 | F | 1 or 2 | 1 | 2 |
| 37 | F | 1 or 2 | 6 | 1 |
| 38 | F | 1 or 2 | 4 | 3 |
| 43 | F | HAE-nC1INH | 11 | 2 |
| 47 | F | HAE-nC1INH | 16 | 2 |
| 51 | F | 1 or 2 | 2 | 0 |
| 55 | M | Unknown | 1 | 1 |
| 58 | F | 1 or 2 | 4 | 4 |
| 59 | F | 1 or 2 | 0 | 1 |
| 65 | M | 1 or 2 | 1 | 0 |
| 71 | M | 1 or 2 | 3 | 3 |
Patient demographics.
HAE, hereditary angioedema; HAE-nC1INH, HAE with normal C1 inhibitor; AE-QoL, angioedema quality of life questionnaire.
FIGURE 1
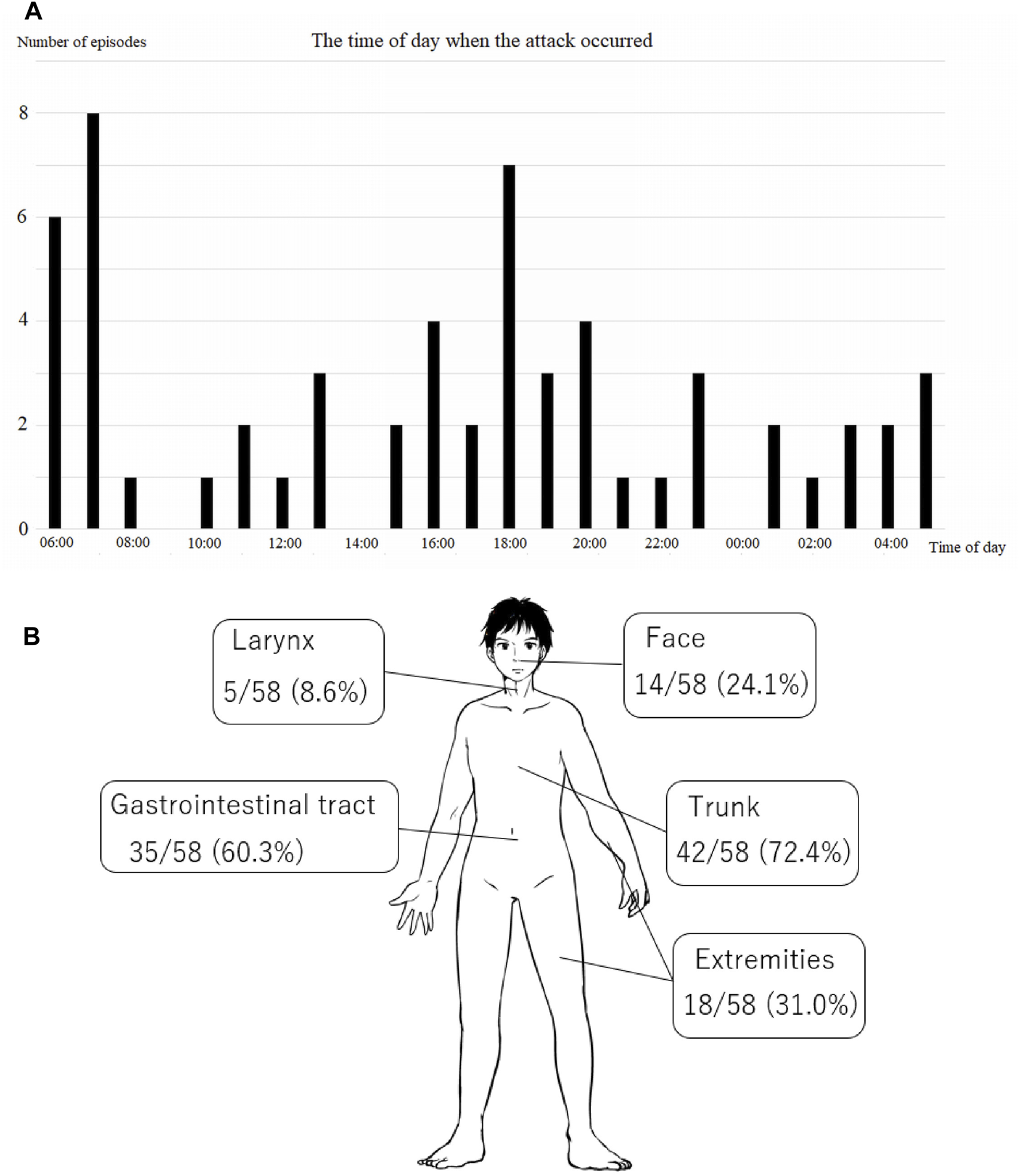
Episodes of edema. (A) The time periods when the episodes occurred. The episodes peaked at approximately 07:00 and 18:00 h. (B) The sites of the episodes. Swelling of the abdomen or gastrointestinal tract was relatively common.
Of the 58 recorded episodes, 38 (65.5%) were treated. Of the 20 untreated episodes, 1 (5%) included throat symptoms. However, the attack that caused swelling in the throat was minor. The most common reason for not treating symptoms was that they were mild (14 episodes [70%]); other reasons were that the episodes occurred nocturnally, during holidays, or outside hospital opening hours (5 episodes [25%]) and to avoid missing school or employment (1 episode [5%]). Of the 38 episodes that were treated, 20 (52.6%) had some prodromal symptoms or triggers. Reported triggers for episodes included changes in temperature, mental tension and stress, lack of sleep, and menstruation. We could not identify factors specific to the time of day when most episodes occurred. In all, 24 episodes (63.2%) were treated at home; of these, two were also treated in a healthcare setting. The plasma-derived C1-INH preparation (pdC1-INH) was used alone in 12 episodes (31.6%), and icatibant was used alone in 24 (63.1%) and both were used in 2 (5.3%). For 22 episodes (57.9%), treatment was completed at home with icatibant self-injection alone.
Whereas icatibant can be self-administered at home, pdC1-INH must be administered at a healthcare facility in Japan. Therefore, the time between the onset of symptoms and the start of treatment tended to be shorter with icatibant than with pdC1-INH (Figure 2A). The time from the onset of an episode to the start of recovery was less than 30 min for half the episodes treated with icatibant; in contrast, for most episodes treated with pdC1-INH, this interval was >30 min (Figure 2B). The time from episode onset to full recovery was also longer with pdC1-INH than with icatibant (Figure 2C).
FIGURE 2
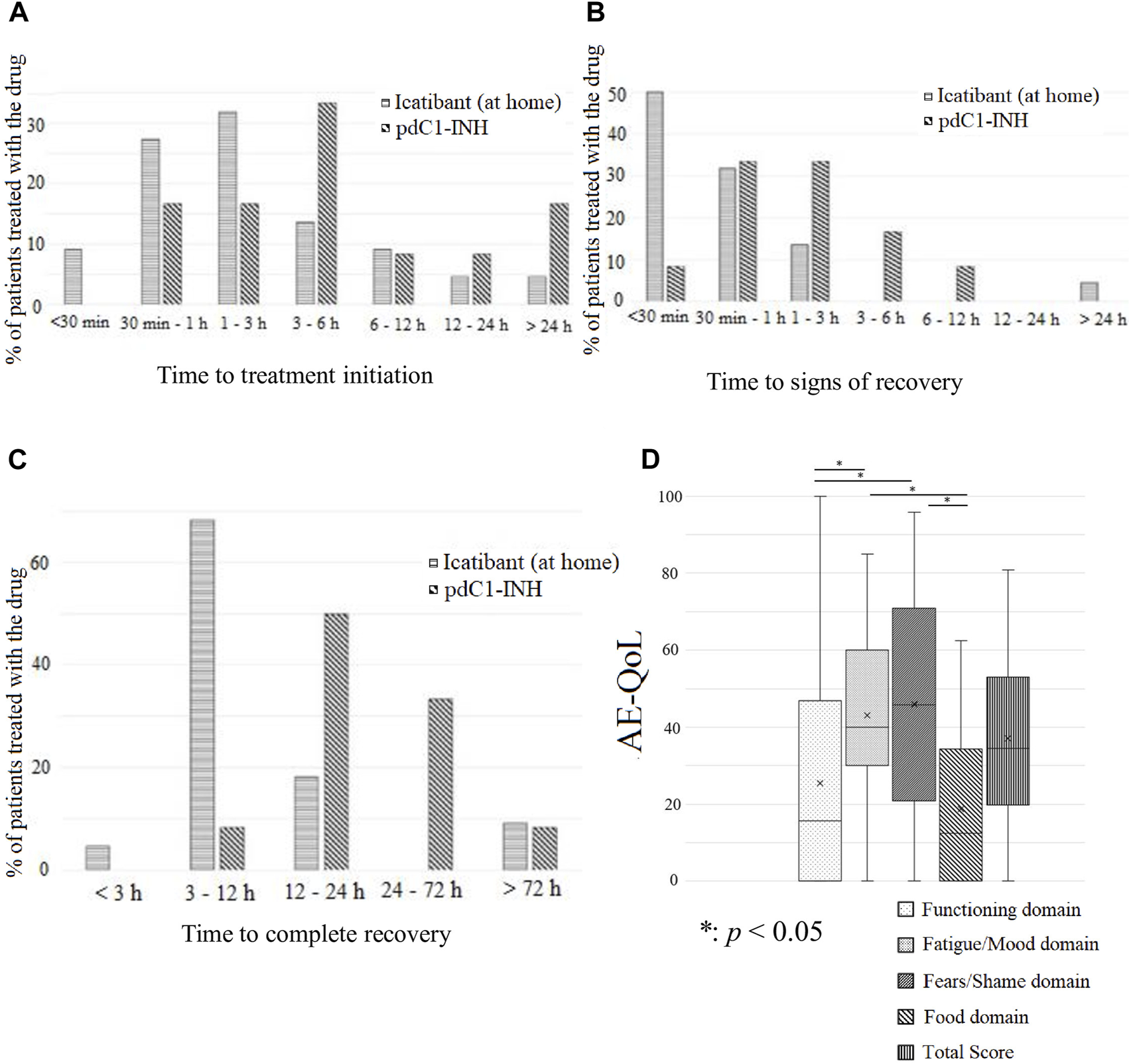
Duration of attacks and quality of life (QoL) of patients with HAE. (A) The time from attack onset to treatment initiation was shorter with icatibant treatment at home than with plasma-derived C1-INH preparation pdC1-INH treatment alone. (B) The time until signs of recovery was shorter with icatibant at home than with pdC1-INH treatment alone. (C) The time until complete recovery from the onset of episodes was shorter with icatibant treatment at home than with pdC1-INH treatment alone. (D) In the angioedema QoL, the scores for fatigue/mood and fears/shame domains were high, i.e., severely impaired in QoL of the patients.
With regard to QoL impairment, analysis of AE-QoL data collected between January 2019 and March 2021 revealed a total score of 37.1 ± 22.9, with scores of 25.5 ± 29.4, 43.1 ± 22.8, 46.0 ± 28.4, and 18.8 ± 19.8 for the function, fatigue/mood, fears/shame, and food domains (Figure 2D).
Discussion
A previous study of 121 Japanese HAE patients from 2016 to 2017 demonstrated that patients experienced an average of 15.7 attacks per year [8]. At that time, only pdC1-INH preparations were available among first line modern medications recommended by the guideline [9] for acute attacks in Japan. Of the 82% of patients who received pdC1-INH, 69% experienced a therapeutic response. However, only 53.1% of attacks were treated. In our survey, 65.5% of registered attacks were treated. During patient recruitment, written confirmation of their diagnosis (i.e., HAE subtype) and attendance at the hospital was obtained from the attending doctors. The current study included a relatively large proportion of patients with HAE-nC1INH and HAE-unknown, preventing direct comparison with previous evidence. However, the availability of self-injectable icatibant in addition to hospital-administered pdC1-INH may have contributed to the increase in opportunities for therapeutic intervention for attacks.
The advantages of patient-participatory registries are two-way communication and data accumulation. As a result, the design of the study was more suitable for collecting data that reflected patients’ needs. The time of day when HAE episodes are most prevalent had not been reported previously; this study revealed that most episodes coincided with times of day when people are most active, such as commuting to and from work (05:00–09:00 h and 16:00–20:00 h), and of the 38 episodes that were treated, 20 (52.6%) had some prodromal symptoms or triggers. However, attacks frequently reported between 6:00–7:00 may have occurred during sleep and were noticed upon awakening.
Laryngeal edema occurred for few episodes, but gastrointestinal symptoms occurred in the majority. Almost one-third of episodes were not treated, mostly because patients felt symptoms were mild and were reluctant to go to the hospital. However, early intervention is recommended by the guideline for managing attacks of HAE [9]. The widespread use of self-treatment will obviate such reluctance to seek treatment. In a clinical trial with icatibant, symptoms begin to abate in 1.75 h and resolve completely in 5.0 h [10], whereas that with pdC1-INH reported to take 0.5 h before symptoms began to abate after pdC1-INH injection [11]. Our findings, however, show that with icatibant, symptoms started to improve earlier and completely resolved faster than with C1-INH. This result may reflect that icatibant was administered earlier than pdC1-INH.
The total AE-QoL score was 37.1 ± 22.9, close to 39, the threshold of moderate-to-severe impairment of QoL of patients with HAE [6]. Scores in the fatigue/mood and fears/shame domains were particularly high, suggesting that patients with HAE had not only daily physical functions, but also substantial anxiety and fear even without apparent symptoms. Figure 2D shows data from the 24 completed AE-QoL questionnaires. Although ascertainment of whether treatment significantly changed during the study period was not possible, modern and sufficiently effective medications for long-term prophylaxis (e.g., berotralstat and lanadelumab) were unavailable in Japan during this time, suggesting that any changes in HAE treatment protocols were unlikely to substantially affect the AE-QoL scores. This study has some limitations. The sample size of this study is small. We did not collect background information on each patient beyond the information shown in Table 1. Moreover, we did not link each patient to each record or associate attack records or QoL disturbance records.
This study in a patient-participatory registry revealed the basis of QoL in patients with HAE attack episodes, diurnal peaks, and the effectiveness of drugs in treating episodes. The expansion of participants and their time course of clinical observation is warranted for better understanding and management of HAE.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Clinical Research Ethics Review Committee of Hiroshima University (E-1341) and Osaka University (16234(T1)-12). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SaM, TA, and AT: study design, manuscript preparation; DM, RS, and KI: data collection; SaM and AT: statistical analysis of data; CY, AK, NH, MI, BY, YM, TT, ShM, MJ, JB, NG, JK, KK, and MH: interpretation of findings. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the Japan Society for the Promotion of Science Grant-in-Aid (Grant Numbers: JP15K15167, JP17K19812); JST RISTEX (Grant Number: JPMJRX18B3); and the Osaka University International Joint Research Promotion Program (awarded to KK). YM was supported by the Japan Foundation for Promoting Welfare of Small and Medium-Sized Enterprises (Nihon Full Happ), and MJ and NG were supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre.
Acknowledgments
We would like to express our sincere gratitude to Yukie Imamura for actively participating in the project since the launch of RUDY Japan, as well as all patients who participated in the questionnaire development discussions.
Conflict of interest
AT received honoraria (lecture fees) from Takeda Pharmaceutical Company Ltd., CSL Behring K.K., and Torii Pharmaceutical Company Ltd. MH received honoraria from CSL Behring, Takeda Pharmaceutical Company Ltd, and Torii Pharmaceutical Company Ltd. and consulting fees from Takeda, KalVista, and Pharvaris.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author disclaimer
The opinions expressed in this article belong to the authors and do not necessarily reflect those of the National Health Service, NIHR, UK Department of Health, and other funding bodies.
References
1.
Gompels MM Lock RJ Abinun M Bethune CA Davies G Grattan C et al C1 inhibitor deficiency: consensus document. Clin Exp Immunol (2005) 139(3):379–94. 10.1111/j.1365-2249.2005.02726.x
2.
Ren HL Zhang HY . Clinical features of hereditary angioedema: analysis of 133 cases. Zhonghua Yi Xue Za Zhi (2007) 87(39):2772–6. 10.3760/j.issn:0376-2491.2007.39.010
3.
Yu TC Shyur SD Huang LH Wen DC Li JS . Paternal mosaicism and hereditary angioedema in a Taiwanese family. Ann Allergy Asthma Immunol (2007) 99(4):375–9. 10.1016/S1081-1206(10)60557-1
4.
Javaid MK Forestier-Zhang L Watts L Turner A Ponte C Teare H et al The RUDY study platform–a novel approach to patient driven research in rare musculoskeletal diseases. Orphanet J Rare Dis (2016) 11(1):150. 10.1186/s13023-016-0528-6
5.
Hamakawa N Kogetsu A Isono M Yamasaki C Manabe S Takeda T et al The practice of active patient involvement in rare disease research using ICT: experiences and lessons from the RUDY Japan project. Res Involv Engagem (2021) 7(1):9. 10.1186/s40900-021-00253-6
6.
Morioke S Takahagi S Kawano R Fukunaga A Harada S Ohsawa I et al A validation study of the Japanese version of the angioedema activity score (AAS) and the angioedema quality of life questionnaire (AE-QoL). Allergol Int (2021) 70(4):471–9. 10.1016/j.alit.2021.04.006
7.
Weller K Groffik A Magerl M Tohme N Martus P Krause K et al Development and construct validation of the angioedema quality of life questionnaire. Allergy (2012) 67:1289–98. 10.1111/all.12007
8.
Iwamoto K Yamamoto B Ohsawa I Honda D Horiuchi T Tanaka A et al The diagnosis and treatment of hereditary angioedema patients in Japan: a patient reported outcome survey. survey (2021) 70(2):235–43. 10.1016/j.alit.2020.09.008
9.
Maurer M Magerl M Betschel S Aberer W Ansotegui IJ Aygören-Pürsün E et al The international WAO/EAACI guideline for the management of hereditary angioedema–the 2021 revision and update. Allergy (2022) 77(7):1961–90. 10.1111/all.15214
10.
Hide M Fukunaga A Maehara J Eto K Hao J Vardi M et al Efficacy, pharmacokinetics, and safety of icatibant for the treatment of Japanese patients with an acute attack of hereditary angioedema: a phase 3 open-label study. Allergol Int (2020) 69(2):268–73. 10.1016/j.alit.2019.08.012
11.
Keating GM . Human C1-esterase inhibitor concentrate (Berinert). BioDrugs (2009) 23(6):399–406. 10.2165/11201100-000000000-00000
Summary
Keywords
angioedema, fatigue, patient participation, quality of life, registries
Citation
Morioke S, Aikyo T, Tanaka A, Matsubara D, Saito R, Iwamoto K, Yamasaki C, Kogetsu A, Hamakawa N, Isono M, Yamamoto BA, Matsumura Y, Takeda T, Manabe S, Javaid MK, Barrett J, Gray N, Kaye J, Kato K and Hide M (2024) Survey of hereditary angioedema episodes and quality of life impairment through a patient-participatory registry. J. Cutan. Immunol. Allergy 7:12626. doi: 10.3389/jcia.2024.12626
Received
28 December 2023
Accepted
18 March 2024
Published
28 March 2024
Volume
7 - 2024
Updates
Copyright
© 2024 Morioke, Aikyo, Tanaka, Matsubara, Saito, Iwamoto, Yamasaki, Kogetsu, Hamakawa, Isono, Yamamoto, Matsumura, Takeda, Manabe, Javaid, Barrett, Gray, Kaye, Kato and Hide.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Akio Tanaka, tantanakiotan@yahoo.co.jp; Michihiro Hide, ed1h-w1de-road@hiroshima-u.ac.jp
Deceased
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.