Dear Editors,
Eosinophilic granulomatosis with polyangiitis (EGPA) is a multisystem inflammatory disorder characterized by blood/tissue eosinophilia, asthma, and necrotizing vasculitis with granulomatosis. Among diverse symptoms, neuropathy often persists regardless of other symptomatic activity and poses a treatment resistance, contributing significantly to the morbidity of the disease. We present a favorable clinical efficacy of mepolizumab, a humanized monoclonal antibody against interleukin-5 (IL-5), in a woman with refractory peripheral neuropathy (PN)-associated with EGPA.
A 60-year-old Japanese woman presented with a 2-week history of low-grade fever and itchy rash on the trunk. Later, she developed uncomfortable pain and numbness on the bilateral lower extremities that immediately spread upwards. Numbness was felt throughout the whole legs, like an electric current running, and was also present from the palms to the fingertips in both hands. Her past history included childhood-onset asthma. Physical examination revealed multiple purpura and erythema, varying in size up to the fingertips, distributed diffusely over the lower extremities and trunk (Figures 1A–C). Some of the purpuric lesions were palpable.
FIGURE 1
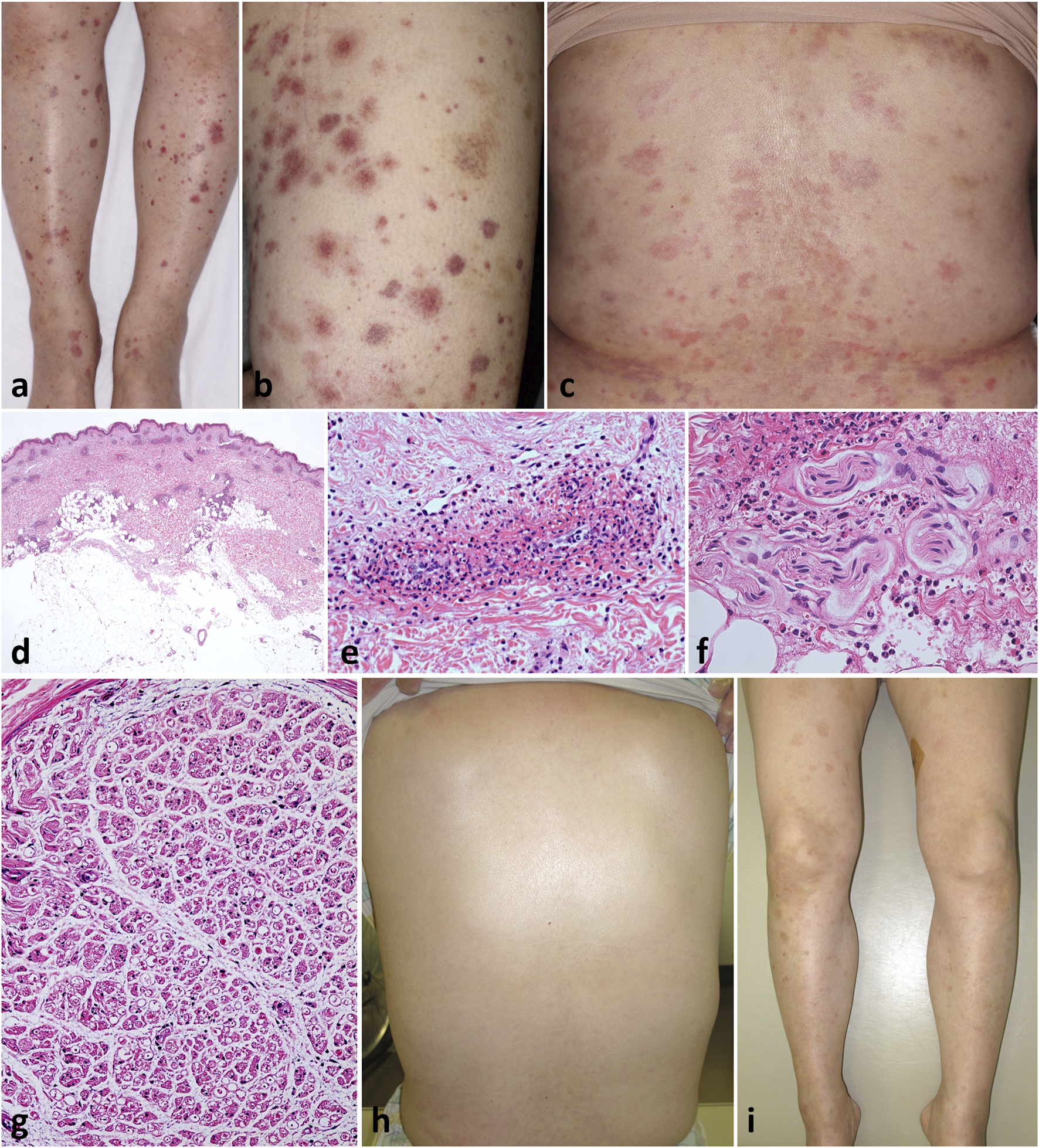
Clinicopathological findings Varying-sized, multiple, non-coalescent purpura were diffusely scattered on the legs (A) and thighs (B). Multiple erythema and pigmentations were present on the trunk (C). Patchy perivascular and periappendage infiltration of inflammatory cells throughout the dermis but not in the subcutaneous fat [(D), H&E, loupe image]; the former consisted of numerous leukocytes and eosinophils with fibrinoid necrosis of the vessel walls [(E), H&E, ×100], and the latter of perineural infiltration with damaged vessels [(F), H&E, ×200]. Histology of sural nerve biopsy showing decreased number and unequal distribution of nerve fibers with perineural edema [(G), H&E, ×2]. Clinical picture showing prompt resolution of eruption 1 week after receiving oral prednisolone (H,I).
Abnormal laboratory tests included an increase of AST (73 U/L), ALT (75 U/L), CK (178 U/L), IgG (2,065 mg/dL), sIL-2R (3,008 U/mL), ESR (60 mm/h), CRP (0.93 mg/dL), and total IgE (256 U/L), with normal counts of eosinophils (6.2%, 271/μL). Anti-neutrophil cytoplasmic antibodies to myeloperoxidase (MPO-ANCA) and protein kinase 3 (PR3-ANCA), rheumatoid factor, and anti-CCP antibody were all negative.
Skin biopsy pathology from the right leg showed patchy infiltration of eosinophils and neutrophils around dermal blood vessels throughout the dermis, which displays fibrinoid necrosis of vessel walls (Figures 1D, E). Of note, there was perineural infiltration of numerous eosinophils and neutrophils surrounded by damaged perineural vessels (Figure 1F). Direct immunofluorescence was negative. Echocardiogram and whole-body CT scan did not show evidence for myocardial damage and other organ involvement, respectively, albeit with mild fatty liver. Nerve conduction studies demonstrated slight extension of sensory latencies (palm 2.12 and finger 3.3 ms; ref. <1.9 ms), and reduced sensory amplitude (palm 18.6 uV; ref. >20 uV and finger 4.6 uV; ref. >7 uV) and velocity in the right median nerve (palm 33.0 m/s and finger 42.4 m/s; ref. 47 m/s). The right sural nerve sensory conduction was blocked probably due to severe local edema. The amplitude and velocity of motor conduction in the right median, ulnar, and sural nerves were normal. These data indicated polyneuropathy as signs of peripheral neurological involvement. Sural nerve biopsy revealed decreased number and unequal distribution of myelinated nerve fibers with marked edema at perineural interstitials (Figure 1G). The overall clinicopathology with a preceding history of asthma suggest a diagnosis of EGPA with PN. At this stage, the disease activity relevant to the predictive 5-year mortality rate was assessed by the revised Five Factor Scoring, [1] which was 0.
Oral prednisolone (60 mg/day) was initiated, resulting in a prompt disappearance of the rash with resolved fever by week 3 (Figures 1H, I), but her PN became worse, especially from the entire fingers to the hands, extending to the bilateral wrists. High-dose intravenous immunoglobulin (IVIG) was introduced from week 4 and maintained monthly for a total of 12 courses, with gradual tapering of prednisolone to 5 mg/day.
While her disease activity was stable with continuous prednisolone doses for 6 years, the PN remained persistent and impaired her daily walking ability. Mepolizumab was then administered 300 mg monthly. After 1-month of mepolizumab therapy, all the neurological symptoms in the lower extremities significantly subsided, then followed by symptomatic relief of the fingers and palms, allowing a decrease in the IVIG frequency from monthly to nearly every 4 months thereafter. By 6-month, her PN became more tolerable daily, enabling a tapering of the prednisolone dose to 2 mg/day.
The latest Japanese nationwide survey has explored an extremely high incidence of neuropathy, irrespective of the severity, in more than 90% of all EGPA cases (n = 965) [2]. EGPA-associated PN, a manifestation reflecting the vasculopathy, occurs more frequently in ANCA-positive cases (50%–70%) with a higher incidence of multiple mononeuritis pattern in sensory but also sometimes motor deficit. [3] PN is not a life threatening, but exclusively tends to persist and often gives the risk of disease morbidity due to prolonged muscle atrophy, neuropathic pain, and numbness, like our case. It remains a treatment challenge, with a relapse rate of 40% during tapering of preceding systemic corticosteroids [4]. Therefore, an early intervention with appropriate treatment intensity, including molecular-targeting drugs like mepolizumab, remains an issue that needs to be considered in individual cases and different clinical settings [3, 5]. Of interest, it has been reported that the mechanism of neuropathy may be different between MPO-ANCA-negative and MPO-ANCA-positive EGPA. Neuropathy in MPO-ANCA-negative EGPA is mainly due to occlusion of epineural vessels by excessive eosinophil infiltration. In our case, serum IgE and blood eosinophil counts were not elevated throughout the clinical course, but skin and nerve biopsies showed direct eosinophil infiltration into the skin and perineural blood vessels. Therefore, pathological confirmation of direct infiltration of eosinophils into target organ(s) would emphasize the therapeutic importance of targeted recruitment and activation of eosinophils. Biopsy of the affected organ is very helpful in planning the treatment strategy and motivating the early introduction of mepolizumab.
Mepolizumab directly binds to free IL-5, preventing interaction with its cell surface receptor. This blockade exerts a therapeutic effect by arresting the increased maturation of eosinophils in the bone marrow, while reducing eosinophil precursors, and also inhibiting their migratory activity to skin appendages, such as nerves and vasculature, and subsequent establishment of granulomatosis. In a recent retrospective study in refractory/relapsing EGPA (n = 147), mepolizumab exhibited a higher treatment efficacy with 78% of remission rate and a better safety profile compared to omalizumab and rituximab. Notably, the favorable effect of mepolizumab was a dose-independent (100 vs. 300 mg).
Further studies are warranted to determine whether mepolizumab in combination with preceding immunosuppressive drugs is superior or equivalent to monotherapy, as well as the timing and dose of administration [5], in EGPA-associated PN.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was obtained with written informed consent from the Institutional Ethics Committee of Fukui University Hospital. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
Guillevin L Pagnoux C Seror R Mahr A Mouthon L Toumelin PL et al The five-factor score revisited: assessment of prognoses of systemic necrotizing vasculitides based on the French Vasculitis Study Group (FVSG) cohort. Medicine (Baltimore) (2011) 90(1):19–27. 10.1097/MD.0b013e318205a4c6
2.
Makino H Sada KE . Clinical studies of the research committee on intractable vasculitides, the ministry of health, labour and welfare of Japan. Clin Exp Nephrol (2013) 17(5):697–9. 10.1007/s10157-013-0845-1
3.
Emmi G Bettiol A Gelain E Bajema IM Berti A Burns S et al Evidence-based guideline for the diagnosis and management of eosinophilic granulomatosis with polyangiitis. Nat Rev Rheumatol (2023) 19(6):378–93. 10.1038/s41584-023-00958-w
4.
Sada KE Amano K Uehara R Yamamura M Arimura Y Nakamura Y et al A nationwide survey on the epidemiology and clinical features of eosinophilic granulomatosis with polyangiitis (Churg-Strauss) in Japan. Mod Rheumatol (2014) 24(4):640–4. 10.3109/14397595.2013.857582
5.
Canzian A Venhoff N Urban ML Sartorelli S Ruppert AM Groh M et al Use of biologics to treat relapsing and/or refractory eosinophilic granulomatosis with polyangiitis: data from a European collaborative study. Arthritis Rheumatol (2021) 73(3):498–503. 10.1002/art.41534
Summary
Keywords
peripheral neuropathy, eosinophilic granulomatosis with polyangiitis, mepolizumab, intravenous immunoglobulin, corticosteroid therapy
Citation
Kawate K, Kasamatsu H, Nishimura K, Muneishi Y, Takashima W, Koizumi H, Iino S, Hayashi K, Oyama N and Hasegawa M (2024) A case of refractory peripheral neuropathy associated with eosinophilic granulomatosis with polyangiitis with successful tapering of systemic corticosteroid and reduced dosing frequency of high-dose intravenous immunoglobulin after combined use of mepolizumab. J. Cutan. Immunol. Allergy 7:12776. doi: 10.3389/jcia.2024.12776
Received
31 January 2024
Accepted
08 April 2024
Published
19 April 2024
Volume
7 - 2024
Updates
Copyright
© 2024 Kawate, Kasamatsu, Nishimura, Muneishi, Takashima, Koizumi, Iino, Hayashi, Oyama and Hasegawa.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Noritaka Oyama, norider@u-fukui.ac.jp
ORCID: Noritaka Oyama, orcid.org/0000-0003-4934-5205
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.