Abstract
Acquired idiopathic generalized anhidrosis is a rare disease characterized by systemic anhidrosis or hypohidrosis without other systemic diseases. However, its etiology remains unclear. Autoimmune mechanisms seem to be involved in the development of acquired idiopathic generalized anhidrosis. Although steroid pulse therapy is the most commonly used therapy, it lacks a high level of evidence. On the other hand, pilocarpine, a muscarinic receptor agonist that stimulates exocrine glands, increases saliva and tear secretion as well as sweating. Here, we report treatment progresses of steroid pulse therapy and oral pilocarpine in our department. Between 2012 and 2021, we treated 10 patients of acquired idiopathic generalized anhidrosis. All patients were administered oral pilocarpine as the first therapy, three (30%) of whom had increased sweating. Minor side effects were observed, however, no serious side effects were observed. Five patients who did not respond to oral pilocarpine were subsequently treated with steroid pulse therapy to which four (80%) showed significant response. Two patients were subsequently administered oral pilocarpine as post-therapy, and remission was maintained for up to 81 months. Oral pilocarpine could be used to relieve symptoms in patients with acquired idiopathic generalized anhidrosis prior to steroid pulse therapy and as maintenance therapy after steroid pulse therapy.
Introduction
Acquired idiopathic generalized anhidrosis (AIGA) is a rare disease characterized by systemic anhidrosis or hypohidrosis without autonomic and neurological abnormalities [1, 2]. Before diagnosing AIGA, it is crucial to rule out systemic diseases such as diabetes mellitus, Sjögren’s syndrome, Fabry’s disease, and congenital ectodermal dysplasia [1]. Symptoms of AIGA include heat retention and/or heat stroke during exercise and outdoor work. It has also been associated with the development of cholinergic urticaria and elevated serum immunoglobulin (Ig) E levels [1]. These symptoms impair the patient’s quality of life. AIGA is classified into idiopathic pure sudomotor failure (IPSF), sudomotor neuropathy, and/or sweat gland failure [2]. However, its pathogenesis is unknown, and some autoimmune mechanisms are thought to be involved, given the response to steroid pulse therapy [1].
While steroid pulse therapy has been adapted to treat AIGA, the evidence supporting its efficacy is not strong [2–7], leading to moderate recommendations grade according to the Japanese guideline [1]. Pilocarpine is a muscarinic receptor agonist that stimulates the exocrine glands and increases saliva secretion, tear secretion, and sweating. We for the first time reported seven AIGA cases treated with oral pilocarpine elsewhere [8]. In the current report, we added more AIGA cases treated with oral pilocarpine and presented two AIGA cases, successfully treated with steroid pulse therapy, were administered oral pilocarpine as post-therapy. The cases used pilocarpine as post-therapy have not been previously reported.
Case description
Between 2012 and 2021, among patients who visited our department, 13 patients were diagnosed as having AIGA. All patients underwent laboratory tests to confirm that they were negative for thyroid-stimulating hormone, free thyroxine, free triiodothyronine, and anti-SS-A/SS-B antibodies and had no underlying diseases. Iodine-starch test (Minor test) in a hot room (35°C; humidity: 65%–75%) for 15 min was performed to confirm the range of anhidrotic areas. Patients were diagnosed with AIGA based on the diagnostic criteria [1]. All procedures used in this research were approved by the Medical Ethics Review Committee of the University of Tsukuba Hospital (approval number: R04-234).
The patients were asked about the duration of AIGA and the presence of heat retention and cholinergic urticaria. Serum IgE levels were measured during the initial visit. Skin biopsies were performed from the anhidrotic and hidrotic areas, and histopathological examinations were performed. Before treatment, informed consent about the therapy, including oral pilocarpine and steroid pulse therapy were obtained. Three patients did not expect treatment because of mild symptoms. Pilocarpine (15 mg/day) was orally administered daily. Steroid pulse therapy was administered using a 3-consecutive-day intravenous injection of methylprednisolone (1,000 mg/day). The efficacy of oral pilocarpine and steroid pulse therapy was determined using patients’ self-assessment of sweating, heat retention, and cholinergic urticaria.
The clinical characteristics of the ten patients treated for AIGA are summarized in Table 1. Case 1 and 6 overlapped with our cases reported previously [8]. There were six male and four female patients with an age range of 14–70 years (median, 31 years). The median disease duration was 10 months. Most patients experienced heat retention. Eight patients had cholinergic urticaria (patients 1–3, 5–9). Serum IgE levels were elevated in four cases (patients 1, 6, 8, 10). The total area of anhidrosis ranged between 65% and 98% (median: 89%) of the body. Histopathological examination revealed lymphocytic infiltration around sweat glands in six cases (60%; patients 1, 3, 7–10). Degeneration of sweat ducts in three cases (30%; patients 1, 2, 4). Two patients showed normal findings (20%; patients 5, 6). Ten patients chose oral pilocarpine as the first treatment, because the treatment will be more safe and convenient than steroid pulse therapy (Table 2). Adverse effects were observed in three patients: dizziness (patient 2), increased saliva secretion (patient 5), and pruritus (patient 7). Patient 2 continued oral pilocarpine at a decreased dose (10 mg/day), and promptly improved. The other two patients discontinued oral pilocarpine. Three of ten patients (30%) experienced increased sweating within several days (patients 1–3). Patients 1 and 3 improved both heat retention and cholinergic urticaria. Patient1-3 were then continued oral pilocarpine for 8–18 months. Improvement in symptoms was not observed in the other seven patients after treatment for between 1 and 3 months. Out of five of them who were subsequently treated with 2 or 3 cycles of steroid pulse therapy, four showed remission (80%). Four patients (patients 6–9) felt increased sweating, three (patients 7–9) had improvement in heat retention, and patients 7 and 8 had improvement in cholinergic urticaria. Patient 8 experienced liver injury after steroid pulse therapy. He underwent further examinations at the gastroenterology. Iodine-starch test, which was performed in patient 6 after successful steroid pulse therapy, revealed remarkable decrease in anhidrotic areas from 65% to 20% and increase in sweating in both anhidrotic and normal hidrotic areas (Figure 1).
TABLE 1
| Case | Age/sex | Disease duration (months) | Heat retention | Cholinergic urticaria | Serum IgE (IU/mL) | Anhidrotic area (%) | Pathological findings |
|---|---|---|---|---|---|---|---|
| 1 | 14/Ma | 3 | N/Ab | + | 535 | 80 | Degeneration of sweat ducts, lymphocytic infiltration |
| 2 | 43/Fc | N/A | + | + | 146 | 60 | Degeneration of sweat ducts |
| 3 | 43/M | 108 | + | + | 65 | 90 | Lymphocytic infiltration |
| 4 | 70/F | N/A | + | - | N/A | 90 | Degeneration of sweat ducts |
| 5 | 22/M | 17 | + | + | 71 | N/A | Normal |
| 6 | 34/M | 3 | + | + | 1,131 | 65 | Normal |
| 7 | 24/M | 1 | N/A | + | 37 | N/A | Lymphocytic infiltration |
| 8 | 28/M | 132 | N/A | + | 265 | 88 | Lymphocytic infiltration |
| 9 | 62/F | 2 | N/A | + | <5 | 95 | Lymphocytic infiltration |
| 10 | 20/F | 60 | + | - | 281 | 98 | Lymphocytic infiltration |
Clinical characteristics of patients with AIGA.
Abbreviation:
M, male.
F, female.
N/A, not applicable.
TABLE 2
| Case | Age/sex | Oral pilocarpine therapy | Steroid pulse therapy | Prognosis | ||||
|---|---|---|---|---|---|---|---|---|
| Duration of treatment (moa) | Adverse effects | Effects | No. of courses | Adverse effects | Effects | |||
| 1 | 14/M | 18 | - | Increased sweating, no heat retention and Chol Ub | - | No relapse in 90 months | ||
| 2 | 43/F | 8 | dizziness | Increased sweating | - | Continuation | ||
| 3 | 43/M | 9 | - | Increased sweating, no heat retention and Chol U | - | Continuation | ||
| 4 | 70/F | 1 | - | - | - | |||
| 5 | 22/M | 0.5 | Increased in saliva secretion | - | - | |||
| 6 | 34/M | 2 | - | - | 3 | - | Increased sweating | No relapse in 80 months |
| 7 | 24/M | 0.5 | pururitus | - | 3 | - | Increased sweating, no heat retention | Taking bilastine, monterlukast sodium, famotidine and Kampo |
| 8 | 28/M | 2 | - | - | 3 | Liver injury | Increased sweating, no heat retention and Chol U | After steroid pulse therapy, taking pilocarpine for 19 months. Relapse 81 months after cessation of pilocarpine |
| 9 | 62/F | 2 | - | - | 2 | - | Increased sweating, no heat retention and Chol U | After steroid pulse therapy, taking pilocarpine for 10 months and no relapse |
| 10 | 20/F | 3 | - | - | 2 | - | - | ongoing |
Treatments and prognosis of patients with AIGA.
Abbreviation:
mo, months.
Chol U, cholinergic urticaria.
FIGURE 1
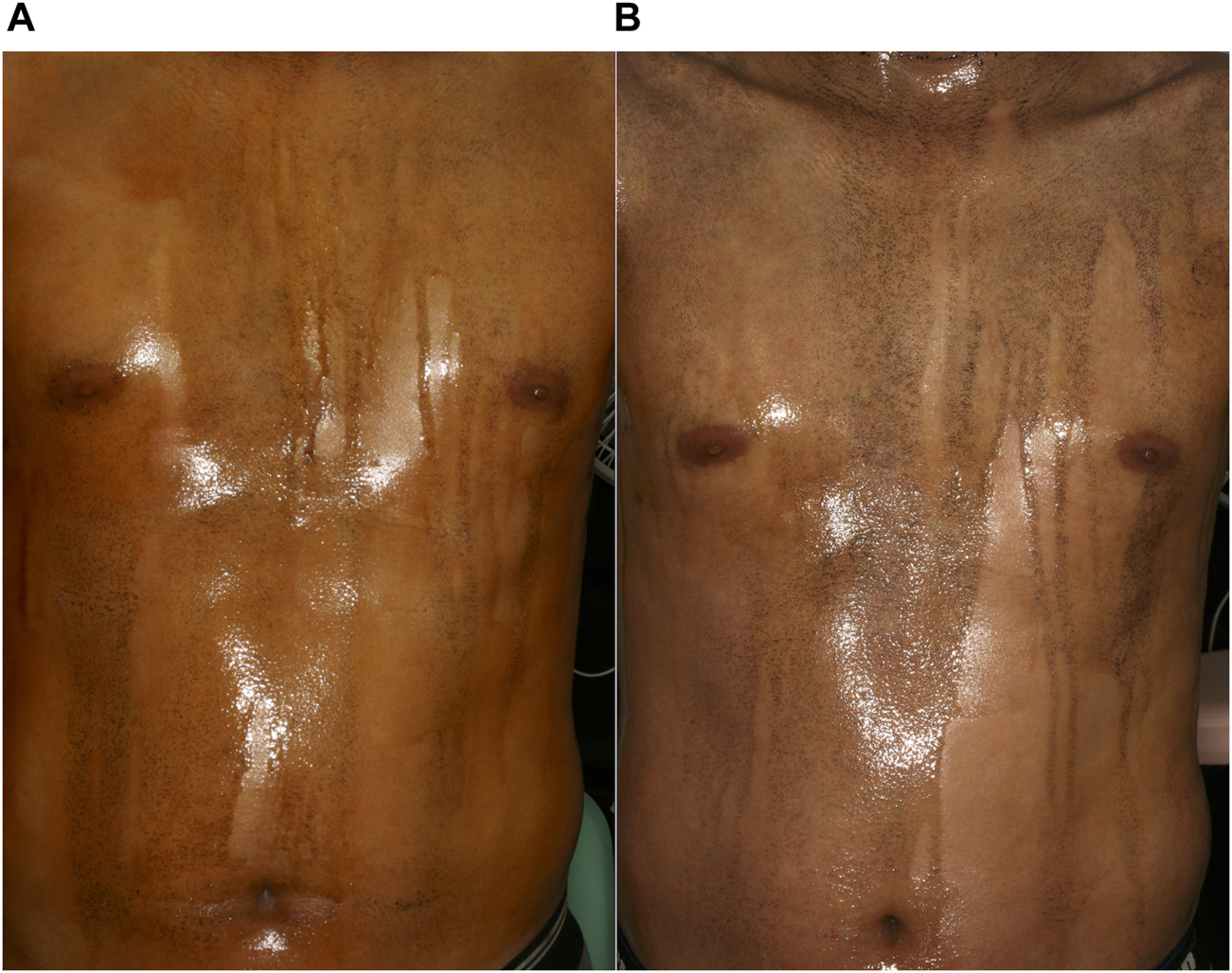
Iodine-starch test images of a patient with AIGA (Patient 6). (A) Large anhidrotic areas before steroid pulse therapy. (B) Increase in hidrotic areas and amount of sweating 3 months after the third steroid pulse therapy.
One of the three patients who achieved remission with oral pilocarpine had no relapse for 90 months after discontinuation of oral pilocarpine (patient 1). Two other patients (patients 2 and 3) maintained remission without stopping treatment. After successful steroid pulse therapy, patient 6 had no relapse for 80 months. Patient 7 was treated with antihistamine therapy for persistent cholinergic urticarial. Patients 8 and 9 continued oral pilocarpine as post-therapy, because we think pilocarpine will work like sweat training [8]. Patient 8 experienced the first relapse 81 months after discontinuation of oral pilocarpine. Remission was maintained for 10 months in patient 9.
Discussion
Pilocarpine has been used to treat oral dryness associated with Sjögren’s syndrome and after radiotherapy for head and neck cancers [9]. Common side effects of it include nausea, abdominal pain, and dizziness, none of which are serious [10]. In our study, three patients experienced adverse effects: dizziness, pruritus, and increased salivation. All three patients experienced relief after reduction or discontinuation of oral pilocarpine. All patients successfully treated with oral pilocarpine showed a rapid increase in sweating and improvement in heat retention without recurrence of symptoms. In our previous report, we showed that anhidrotic areas were not remarkably reduced, but sweating increased in normal hidrotic areas in the iodine-starch test following oral pilocarpine therapy [8]. In this study, patients with self-assessed improvement in sweating following oral pilocarpine therapy also experienced increased sweating in the axillae and other originally normal hidrotic areas. Ogino et al. reported that pilocarpine iontophoresis induced sweating in control subjects but not in patients with AIGA [11]. These results suggest that increasing sweating in normal hidrotic areas by oral pilocarpine therapy leads to an improvement in heat retention. This is consistent with the dysfunction of acetylcholine receptors in IPSF, which is a major cause of AIGA. Repeated heat exposure (sweat training) can promote sweat gland activation, leading to increased sweating, particularly in normal skin [12]. Pilocarpine therapy may increase sweating in the normal hidrotic areas through these mechanisms. We believe that treatment with oral pilocarpine requires several months to induce remission.
Steroid pulse therapy is the most common treatment option for AIGA. It is reported that the initial response rate to steroid pulse therapy is 73% [3]. It is reported that infiltration of mast cells and CD3-positive cells around the sweat glands and ducts decreased after successful steroid therapy [13]. In our study, 80% of patients with AIGA who underwent steroid pulse therapy achieved increased sweating, improvement in heat retention, or decrease in cholinergic urticaria within 1 month. In a patient with successful steroid pulse therapy showed a remarkable decrease in anhidrotic areas in iodine -starch test. 91% of patients with AIGA who improved subjective symptoms reduced anhidrotic areas after steroid pulse therapy [3]. These results suggest that steroid pulse therapy and oral pilocarpine have different mechanisms underlying the improvement of AIGA; the former increases the areas and amount of sweating, and the latter increases the amount of sweating only in normal hidrotic areas. We administered oral pilocarpine as post-therapy to patients who were successfully treated with steroid pulse therapy. The cases used pilocarpine as post-therapy have not been previously reported. It was reported that the recurrence rate of steroid pulse therapy was 48%, and the recurrence mostly occurred within 1 year [3]. Therefore, we considered that prevention of recurrence after steroid pulse therapy is important. Some cases were administered oral steroid as post-therapy in the past [3]. But long-term use of systemic steroids has a risk of many adverse effects. We thought that pilocarpine as post-therapy may be effective for maintaining sweating because of functions such as sweat training, mainly in the restored sweating areas. In this study, post-therapy of oral pilocarpine resulted in long-term maintenance of remission. However, the number of cases was limited and it was a retrospective study, future studies are expected.
This study showed that oral pilocarpine was effective in some AIGA cases. Oral pilocarpine has less severe adverse effects than steroid pulse therapy. Oral pilocarpine could be used to relieve symptoms in patients with AIGA prior to steroid pulse therapy, and as maintenance therapy after steroid pulse therapy.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
All procedures used in this research were approved by the Medical Ethics Review Committee of the University of Tsukuba Hospital (approval number: R04-234). Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
HM contributed to the conception and design of the study, acquisition and analysis of data, and drafting of the manuscript. NK contributed to the conception and design of the study, data analysis, and critical manuscript revision. MO, YI, and NO contributed to investigation. TN critically revised the manuscript for important intellectual content. TN and JF contributed to the study conception and design and critically revised the manuscript for important intellectual content. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
Munetsugu T Fujimoto T Oshima Y Sano K Murota H Satoh T et al Revised guideline for the diagnosis and treatment of acquired idiopathic generalized anhidrosis in Japan. J Dermatol (2017) 44:394–400. 10.1111/1346-8138.13649
2.
Nakazato Y Tamura N Ohkuma A Yoshimaru K Shimazu K . Idiopathic pure sudomotor failure: anhidrosis due to deficits in cholinergic transmission. Neurology (2004) 63:1476–80. 10.1212/01.wnl.0000142036.54112.57
3.
Iida T Nakamura M Inazawa M Munetsugu T Nishida M Fujimoto T et al Prognosis after steroid pulse therapy and seasonal effect in acquired idiopathic generalized anhidrosis. J Dermatol (2021) 48:271–8. 10.1111/1346-8138.15666
4.
Ohsima Y Yanagishita T Ito K Tamada Y Nishimura N Inukai Y et al Treatment of patients with acquired idiopathic generalized anhidrosis. Br J Dermatol (2013) 168:430–2. 10.1111/j.1365-2133.2012.11112.x
5.
Kageyama R Honda T Tokura Y . Acquired idiopathic generalized anhidrosis (AIGA) and its complications: implications for AIGA as an autoimmune disease. Int J Mol Sci (2021) 22:8389. 10.3390/ijms22168389
6.
Yoritaka A Hishima T Akagi K Kishida S . Successful steroid treatment of acquired idiopathic partial hypohidrosis. J Dermatol (2006) 33:265–7. 10.1111/j.1346-8138.2006.00064.x
7.
Kobayashi T Ito T Kobayashi Y Mitsuhashi Y Tsuboi R . Two cases of acquired idiopathic generalized anhidrosis successfully treated by steroid pulse therapy. J Dermatol (2014) 41:444–5. 10.1111/1346-8138.12480
8.
Kubota N Furuta J Onizawa S Saito A Nakamura Y Ishitsuka Y et al Oral pilocarpine alleviates the symptoms of acquired idiopathic generalised anhidrosis. Eur J Dermatol (2019) 29:422–3. 10.1684/ejd.2019.3573
9.
Tsifetaki N Kitsos G Paschides CA Alamanos Y Eftaxias V Voulgari PV et al Oral pilocarpine for the treatment of ocular symptoms in patients with Sjögren's syndrome: a randomised 12 week controlled study. Ann Rheum Dis (2003) 62:1204–7. 10.1136/ard.2002.003889
10.
Felberg S Dantas PEC Sato EH . Oral pilocarpine for the treatment of dry eye in patients with Sjögren’s syndrome. Arq Brasa Oftalmol (2022) 85:269–76. 10.5935/0004-2749.20220069
11.
Ogino J Saga K Kagaya M Kamada A Kaneko R Jimbow K . Idiopathic acquired generalized anhidrosis due to occlusion of proximal coiled ducts. Br J Dermatol (2004) 150:589–93. 10.1111/j.1365-2133.2004.05872.x
12.
Ogawa T Asayama M Miyagawa T . Effects of sweat gland training by repeated local heating. Jpn J Physiol (1982) 32:971–81. 10.2170/jjphysiol.32.971
13.
Fukunaga A Horikawa T Sato M Nishigori C . Acquired idiopathic generalized anhidrosis: possible pathogenic role of mast cells. Br J Dermatol (2009) 160:1337–40. 10.1111/j.1365-2133.2009.09113.x
Summary
Keywords
acquired idiopathic generalized anhidrosis, cholinergic urticaria, pilocarpine, steroid pulse therapy, sweat grand
Citation
Miyahara H, Kubota N, Okune M, Ishii Y, Okiyama N, Nomura T and Furuta J (2024) Case report: Ten cases of acquired idiopathic generalized anhidrosis treated with oral pilocarpine. J. Cutan. Immunol. Allergy 7:12902. doi: 10.3389/jcia.2024.12902
Received
26 February 2024
Accepted
23 May 2024
Published
04 June 2024
Volume
7 - 2024
Updates
Copyright
© 2024 Miyahara, Kubota, Okune, Ishii, Okiyama, Nomura and Furuta.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Noriko Kubota, kubota.noriko@md.tsukuba.ac.jp
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.