Dear Editors,
Ulcerative colitis (UC) is an inflammatory bowel disease (IBD) characterized by relapsing and remitting inflammation affecting the colon and rectum. Although erythema nodosum and pyoderma gangrenosum are well-known cutaneous manifestations of UC, vasculitis is a rare symptom associated with this disease [1]. Vedolizumab is an antibody against the gut-selective α4β7 integrin that is approved for use for UC and Crohn’s disease. Vedolizumab is rarely associated with cutaneous side effects including vasculitis. We report here a case of IgAV that developed during vedolizumab therapy for UC.
A 71-year-old Japanese woman visited our hospital complaining of purpura on her limbs for a few days. She had been suffering from UC refractory to corticosteroids and mesalazine for more than 11 years, but it was well-controlled by vedolizumab treatment for the past 28 months. She also had recurrent episodes of hemoptysis 2 months before the appearance of palpable purpura, which occurred 1 month after the 16th administration of vedolizumab. She had no history of smoking or respiratory disease. However, she had been coughing up small amounts of yellow phlegm for a few months before the onset of hemoptysis. Computed tomography (CT) of the chest showed ground glass opacities and consolidation with thickening of the tracheal walls in the left lower lobe compared to the image before the start of vedolizumab (Figures 1A, B). Sputum cytopathology was class 2, and bacterial culture detected Haemophilus parainfluenzae and Pseudomonas aeruginosa. One month of oral erythrosine therapy resulted in only a slight improvement in lung consolidation (Figure 1C). One month after the 17th dose of vedolizumab, palpable purpura of 1–3 cm in size appeared on the lower limbs (Figure 1D), followed by hemoptysis a few days later. She did not complain of joint pain, numbness, or dyspnea. A skin biopsy specimen showed leukocytoclastic vasculitis (Figures 1E, F). Immunohistochemistry for immunoglobulin A (IgA) revealed IgA deposition on the vascular walls of the superficial dermis (Figure 1G). Laboratory findings were unremarkable, except for elevated serum IgA (533 mg/dL). Serum anti-neutrophil cytoplasmic antibodies were negative. A diagnosis of IgA vasculitis (IgAV) was made. The purpura disappeared promptly with 20 mg/day of oral prednisolone. Chest CT scans at 2 weeks after the initiation of prednisolone treatment showed substantial improvement in consolidation (Figure 1H).
FIGURE 1
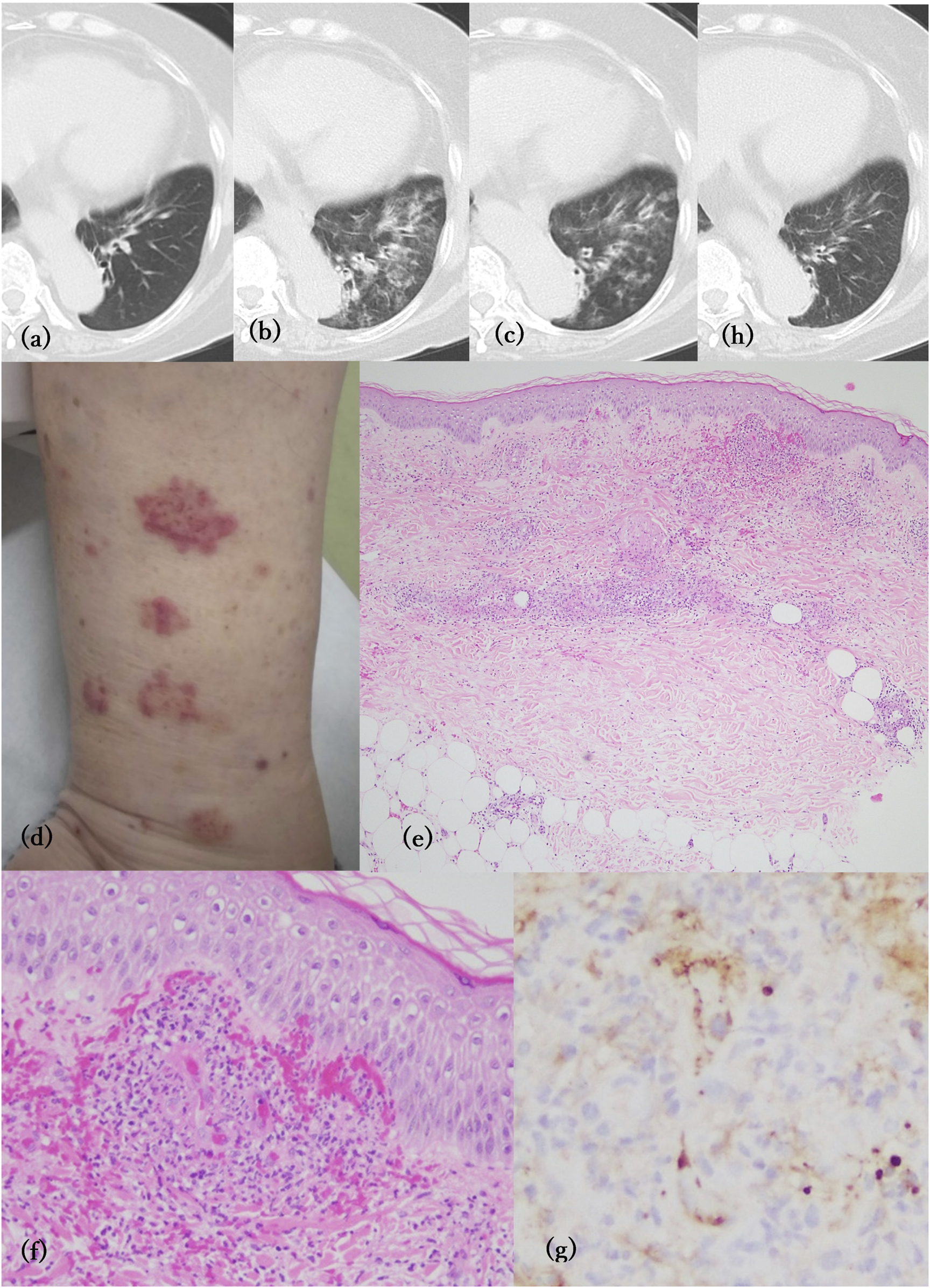
(A–C, H) Computed tomography of the chest No abnormal changes were observed before vedolizumab treatment (A). Consolidation with thickening of the tracheal wall in the left lower lobe at the first occurrence of pulmonary hemorrhage (B) and after 1 month of treatment with erythrosine (C). Two weeks after prednisolone treatment, the consolidation was almost gone (H). (D) Clinically palpable purpura appeared on the lower limbs. (E) Perivascular infiltration of inflammatory cells with erythrocyte extravasation in the dermis (H&E, X40). (F) Leukocytoclastic vasculitis observed in the superficial dermis (H&E, ×200). (G) Immunohistochemistry of IgA showing deposition on the vascular wall of the superficial dermis (×400).
Although our patient was suspected to have a lung infection causing pulmonary hemorrhage, the improvement of consolidation in the lung field was limited even after antibiotic therapy. However, it was completely improved with prednisolone treatment. Despite the absence of bronchoscopy in this instance, we attributed the diffuse alveolar hemorrhage to IgAV in consideration of the multifocal abrasive shadows and combined airspace consolidations with increased lung field density on CT scan in addition to the exacerbation of hemoptysis after the appearance of purpura of IgAV. We thus thought that pulmonary hemorrhage was the initial symptom of IgAV.
IgAV can be triggered by infection, medications, or malignancy. IgAV rarely causes pulmonary hemorrhage. In our case, pulmonary hemorrhage without renal, gastrointestinal or joint symptoms preceded cutaneous purpura by 2 months and recurred intermittently after the appearance of the purpura. Pulmonary hemorrhage can be triggered by infection. Recently, a relationship between IBD, particularly UC, and airway lesions such as bronchiectasis has gained attention [2]. Since vedolizumab inhibits gut-selective lymphocyte migration, it is unlikely that it directly affects the immune system of the skin and lungs. However, it was possible that vedolizumab treatment altered the intestinal microbiota and affected the immune environment in the lungs as a consequence [2]. Therefore, previously unrecognized subclinical bronchiectasis combined with lower respiratory tract infection by Haemophilus parainfluenzae and Pseudomonas aeruginosa may have caused abnormal immune responses leading to the development of IgAV.
While a recent study focusing on IgAV in patients with IBD suggests that tumor necrosis factor-α blockers may promote the onset of IgAV [3], vedolizumab is rarely associated with IgAV. Although Gold et al. reported a case of IgAV as an infusion reaction to vedolizumab in a patient with Crohn’s disease [4], our case cannot be explained by the same mechanism. Vedolizumab was not discontinued in our patient for the following reasons. First, UC was well controlled with vedolizumab, and there was no exacerbation of UC even after the onset of IgAV. Second, the mechanism of action of vedolizumab is specific for T cells homing to the intestinal tract. Third, compared to TNF inhibitors, vedolizumab has much fewer reports of vasculitis side effects. In fact, Desai et al reported a case of IgAV in a patient with UC treated with vedolizumab who was successfully treated with systemic steroids without discontinuing vedolizumab [5]. Despite continued treatment with vedolizumab, our patient did not report any recurrence of purpura or hemoptysis even after the discontinuation of prednisolone. There was no exacerbation of UC in the meantime. However, since vedolizumab is a relatively new drug, it is necessary to investigate whether vedolizumab increases the risk of IgAV in patients with IBD.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
NI, TI, and SM was engaged in the diagnosis and treatment of the patient. NI and HF wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
He R Zhao S Cui M Chen Y Ma J Li J et al Cutaneous manifestations of inflammatory bowel disease: basic characteristics, therapy, and potential pathophysiological associations. Front Immunol (2023) 14:1234535. 10.3389/fimmu.2023.1234535
2.
Samuelson DR Welsh DA Shellito JE . Regulation of lung immunity and host defense by the intestinal microbiota. Front Microbiol (2015) 6:1085. 10.3389/fmicb.2015.01085
3.
Rasmussen C Abitbol V El Karoui K Bourrier A Paule R Vuitton L et al IgA vasculitis in patients with inflammatory bowel disease: new insights into the role of TNF-α blockers. Rheumatology (Oxford) (2022) 61:1957–65. 10.1093/rheumatology/keab662
4.
Gold SL Magro C Scherl E . A unique infusion reaction to vedolizumab in a patient with Crohn’s disease. Gastroenterology (2018) 155:981–2. 10.1053/j.gastro.2018.03.048
5.
Desai T Jurencak R Nair A Carman N Atypical immunoglobulin A vasculitis in a pediatric patient with ulcerative colitis. ACG Case Rep J (2023) 10:e01145. 10.14309/crj.0000000000001145
Summary
Keywords
IgA vasculitits, pulmonary hemorrhage, vedolizumab, ulcerative colitis, inflammatory bowel disease
Citation
Ikumi N, Ishikawa T, Minezaki S and Fujita H (2024) IgA vasculitis preceded by pulmonary hemorrhage in a patient with ulcerative colitis during treatment with vedolizumab. J. Cutan. Immunol. Allergy 7:13307. doi: 10.3389/jcia.2024.13307
Received
27 May 2024
Accepted
19 August 2024
Published
29 August 2024
Volume
7 - 2024
Updates
Copyright
© 2024 Ikumi, Ishikawa, Minezaki and Fujita.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hideki Fujita, fujita.hideki@nihon-u.ac.jp
ORCID: Natsumi Ikumi, orcid.org/0000-0002-3193-5779; Hideki Fujita, orcid.org/0000-0001-8657-0618
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.