Abstract
Objectives:
Standardized criteria for disease activity and end of treatment of systemic treatment of atopic dermatitis have not been established. The objective of this study is to explore the experience using upadacitinib retrospectively, to find clues to weaning from systemic treatment, and to determine the algorithm for setting treatment goals and terminating systemic treatment.
Methods:
Fourteen patients treated with upadacitinib between 1 November 2021 and 31 December 2023 were enrolled in the study. Topical anti-inflammatory treatments were combined. Treatment outcomes were established according to the European Task Force of Atopic Dermatitis guidelines. To evaluate disease status and control, we adopted the Treat to target strategy. Changes in serum biomarkers (TARC and IgE) were also observed.
Results:
All patients achieved EASI 50 after 52 weeks. At 76 weeks, 80% and 30% of patients achieved EASI 75 and EASI 90, respectively. Four patients completed upadacitinib, five patients discontinued treatment, and five patients remained on treatment. Two patients achieved complete remission without topical anti-inflammatory treatment. Two patients discontinued due to adverse events. Patients with better treatment adherence, mainly topical treatment, tended to be able to withdraw from UPA. IgE increased from baseline in 11 patients (78.6%) and TARC increased in 14 patients (100%). These biomarkers decreased from the peak 24–48 weeks after initiating treatment, after the improvement of eczema.
Conclusion:
Combining systemic and topical treatments effectively induces remission in AD patients. Transitioning off systemic treatment begins by achieving remission maintained solely with topical therapy, emphasizing the importance of adherence.
Introduction
Recently, systemic treatment of atopic dermatitis (AD) by molecularly targeted therapeutics has become popular. Systemic treatment is recommended for patients, including adolescents, with moderate-to-severe AD who do not adequately respond to topical anti-inflammatory drugs [1]. Upadacitinib (UPA), a systemic treatment for moderate-to-severe AD, is a potent inhibitor of Janus kinase 1 (JAK1). UPA suppresses the transduction of AD-related cytokines, including IL-4, IL-13, IL-31, and TSLP. The efficacy and safety of UPA have been demonstrated in phase III trials, and the success rate for inducing remission is increasing [2–4]. The criteria for UPA induction are clearly stated, and remission is possible if the criteria are met. However, no standardized algorithm for disease activity or drug termination during systemic treatment has been established, and clinicians are forced to respond on an individual basis at their discretion in clinical practice. The objective of this study is to explore the retrospectively explore UPA experiences to identify clues to weaning from systemic treatment and to determine an algorithm for setting treatment goals and terminating systemic treatment.
Materials and methods
Patients
Patients with AD, who were treated with UPA between 1 November 2021 and 31 December 2023, were enrolled in the study. The UPA implementation criteria were as follows: Investigator’s Global Assessment (IGA) Score of 3 (moderate) or higher, Eczema Area and Severity Index (EASI) score of 16 or higher, or a severely inflamed skin rash over a large area of the face [5–7]. The diagnosis and severity classification of AD were determined based on the Japanese guidelines. UPA (15 mg) was administered orally once a day, and topical anti-inflammatory treatment and skincare were continued. Dupilumab (DUP) treatment was switched to UPA. Topical anti-inflammatory treatments were temporarily intensified during flare-ups, and the UPA dose was increased to 30 mg, depending on the severity of the disease.
Data collection
The following data were extracted from medical records: patient characteristics; clinical indices, including EASI, body surface area (BSA) [8], IGA, the self-reported Patient Global Assessment of (PtGA) of disease severity, Pruritus Numerical Rating Scale (Pruritus NRS) [9], the Patient-Oriented Eczema Measure (POEM) [10] and the Children’s Dermatology Life Quality Index (CDLQI) [11]; and serum biomarkers, including total serum IgE levels (IgE), thymus and activation-regulated chemokine (TARC), eosinophil count, and lactate dehydrogenase (LDH).
Patient follow-up
Patients were followed up every 4 weeks and clinical indices were measured. Serum biomarkers (TARC and IgE) were measured before starting treatment and at 4, 12, 24, 48, 72, and 96 weeks after starting treatment. To evaluate disease status and control, we adopted the treat-to-target (T2T) strategy [12]. T2T strategy consisted of PtGA (Patient global assessment), the EASI (Disease domain), Pruritus NRS, POEM, and CDLQI. If the global assessment goal was reached and at least one of the disease domains was achieved, the treatment was continued (Continuation: C). If one of the disease domains was not achieved, treatment was optimized through discussion between the healthcare provider and patient (Discuss and Optimize: D/O). If at least one of the patient’s global assessment goals and disease domains was achieved, the treatment was modified (M) (Figure 1).
FIGURE 1
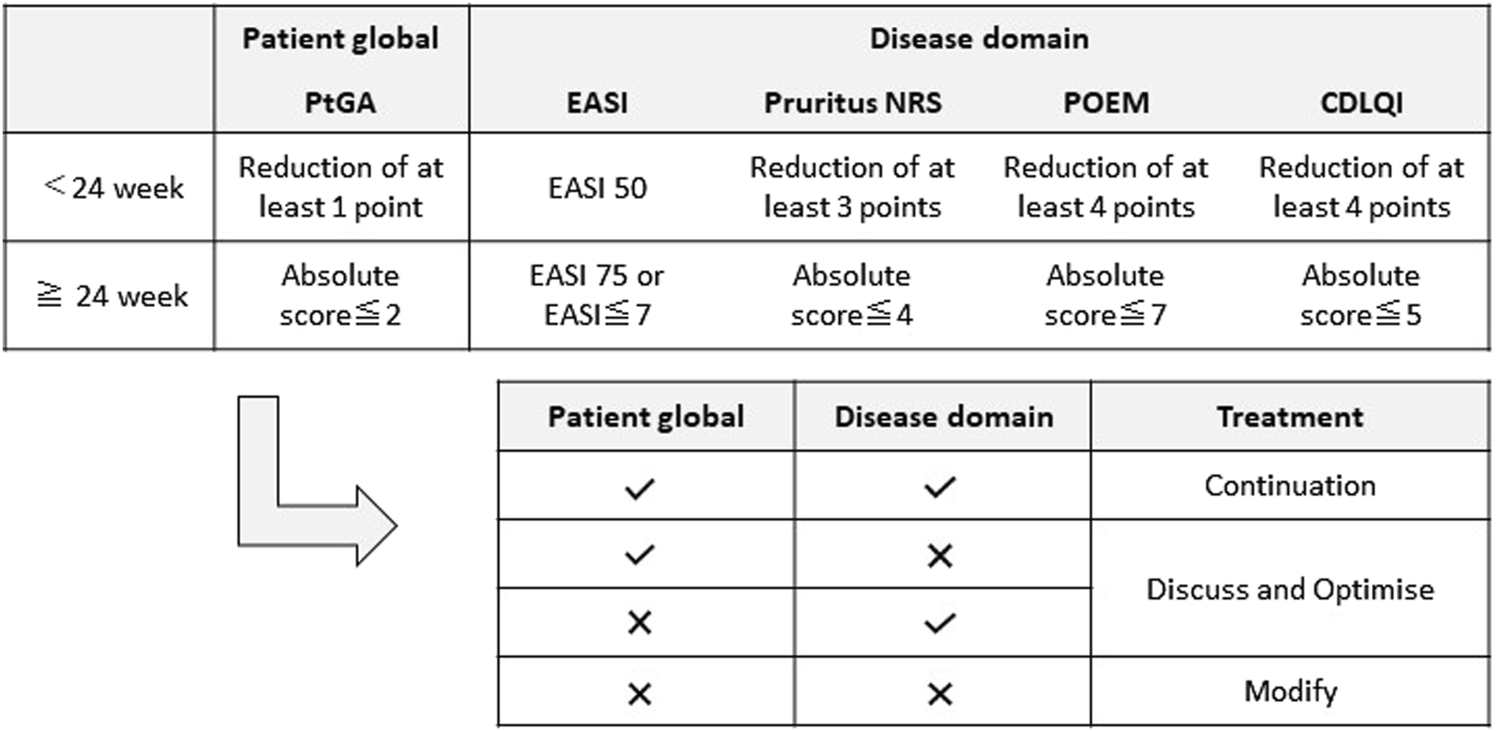
Treat-to-target strategy. PtGA, Patient self-reported Global Assessment of disease severity; EASI, Eczema Area and Severity Index; Pruritus NRS, Pruritus Numerical Rating Scale; POEM, Patient-oriented eczema measure; CDLQI, Children’s Dermatology Life Quality Index.
Outcomes
Endpoints were reaching the treatment goals. The treatment goals were defined according to the European Task Force of Atopic Dermatitis (ETFAD) [13] as follows: 1) complete remission (CR), no flare-up for 8 weeks without topical treatment (topical corticosteroids, calcineurin inhibitors, delgocitinib and difamilast); 2) incomplete remission (IR): no flare-up for 8 weeks and using less than 30 g/month topical treatment in children and less than 60 g/month topical treatment after patients age 15 years or older; 3) control (Co), using systemic treatment and/or topical treatment of more than 30 g/month in children and less than 60 g/month age 15 years or older. Patients were divided into three groups based on outcomes at the end of observation: terminating UPA, discontinuing UPA, and ongoing UPA. The outcomes and the reasons for the outcomes were examined retrospectively. The rate of achieving EASI 50/EASI 75/EASI 90, the rate of EASI transition, the rate of achieving IGA 0/1 (the percentage of patients with an IGA of 0 or 1), and the percentage of patients with improved pruritus NRS by at least 4 points from baseline were evaluated.
Statistical analysis
Data normality was checked using the Shapiro–Wilk test. Groups were compared using Mann–Whitney U tests. EZR (Saitama Medical Center, Jichi Medical School) was used for statistical treatment. p < 0.05 was considered statistically significant.
Results
Patient backgrounds
Fourteen patients, aged 12–19 (median, 15 years) years when beginning UPA treatment, were included in the study. The average EASI scores were 37.8 (28.4–40.3) and 80% (71.3%–80%) BSA was affected in all patients with more than severe disease [median (interquartile range: IQR)]. All patients had AD for more than 10 years, with repeated exacerbations and remissions and a mixture of chronic findings, including lichenified lesions, pruritic nodules, serous papules, and crusts [median (IQR)]. Facial and pruritic types were more common, but the erythroderma type was present in two patients. Three patients used DUP for 90–100 weeks before starting UPA treatment; these patients only achieved EASI 50 before switching to UPA. The preintroduction treatment details, pre-treatment IGA, pruritus NRS, POEM, CDLQI, IgE, TARC, LDH, and peripheral blood eosinophil counts are shown in Table 1.
TABLE 1
| Sex (n, %) | |
| Male | 12 (86.7) |
| Female | 2 (14.3) |
| Age (years) | 15 (14–16) |
| BMI (kg/m2) | 20.4 (18.7–22.4) |
| Diagnostic age of AD (year) | 1 (0.8–1.15) |
| Duration since AD diagnosis (year) | 14 (12.3–15.8) |
| Comorbidities (n, %) | |
| Food allergy | 2 (14.3) |
| Allergic rhinitis | 4 (28.6) |
| Asthma | 2 (14.3) |
| Urticaria | 0 (0) |
| Pre-treatment (n, %) | |
| Topical corticosteroid | |
| Medium | 13 (92.8) |
| Strong | 14 (100) |
| Very strong | 5 (35.7) |
| Delgocitinib | 4 (28.6) |
| Topical tacrolimus | 2 (14.3) |
| Dupilumab | 3 (21.4) |
| Moisturizers | 14 (100) |
| Clinical Index | |
| EASI | 37.8 (28.4–40.3) |
| BSA (%) | 80 (71.3–80.0) |
| IGA | |
| Moderate (IGA = 3) | 7 (50) |
| Severe (IGA = 4) | 7 (50) |
| Pruritus NRS | 7.3 (3.3–8.0) |
| POEM | 15 (10–17) |
| CDLQI | 14 (2–20) |
| Serum biomarkers | |
| Total serum IgE level (IU/mL) | 5,043 (753–9,518) |
| TARC (pg/mL) | 1,140 (811–2,115) |
| Eosinophils (×109/L) | 386 (156–660) |
| LDH (IU/mL) | 219 (192–281) |
Patient characteristics at baseline.
Data are presented as median (interquartile range) or n (%).
BMI, body mass index; AD, atopic dermatitis; EASI, eczema area and severity index; BSA, body surface area; IGA, Investigator’s Global Assessment; Pruritus NRS, pruritus numerical rating scale; PtGA, Patient self-reported Global Assessment of disease severity; POEM, Patient-Oriented Eczema Measure; CDLQI, Children’s Dermatology Life Quality Index; TARC, thymus and activated-regulated chemokine; LDH, lactate dehydrogenase.
Efficacy
The patients were observed for a maximum of 96 (30–64) weeks [median (IQR)]. EASI score changes are shown in Figure 2A. In most cases, the symptoms rapidly improved after 4 weeks of treatment with UPA. EASI 50, EASI 75, and EASI 90 scores were evaluated up to 96 weeks after beginning treatment; 78.6% of patients achieved EASI 50 at 4 weeks and maintained EASI 100 after 52 weeks. EASI 75 was achieved by four patients at 4 weeks, 69.2% at 52 weeks, and 80% at 76 weeks. EASI 90 was achieved by 30% of patients at 76 weeks (Figure 2B). The percentage of patients achieving IGA 0/1 and pruritus NRS improvements of 4 or more points from baseline increased significantly at 4 weeks of treatment, followed by a flat to gradual improvement trend after 4 weeks (Supplementary Figure S1). At the end of the observation period, four patients ended UPA treatment, five patients discontinued UPA treatment, and five patients were continuing UPA treatment.
FIGURE 2
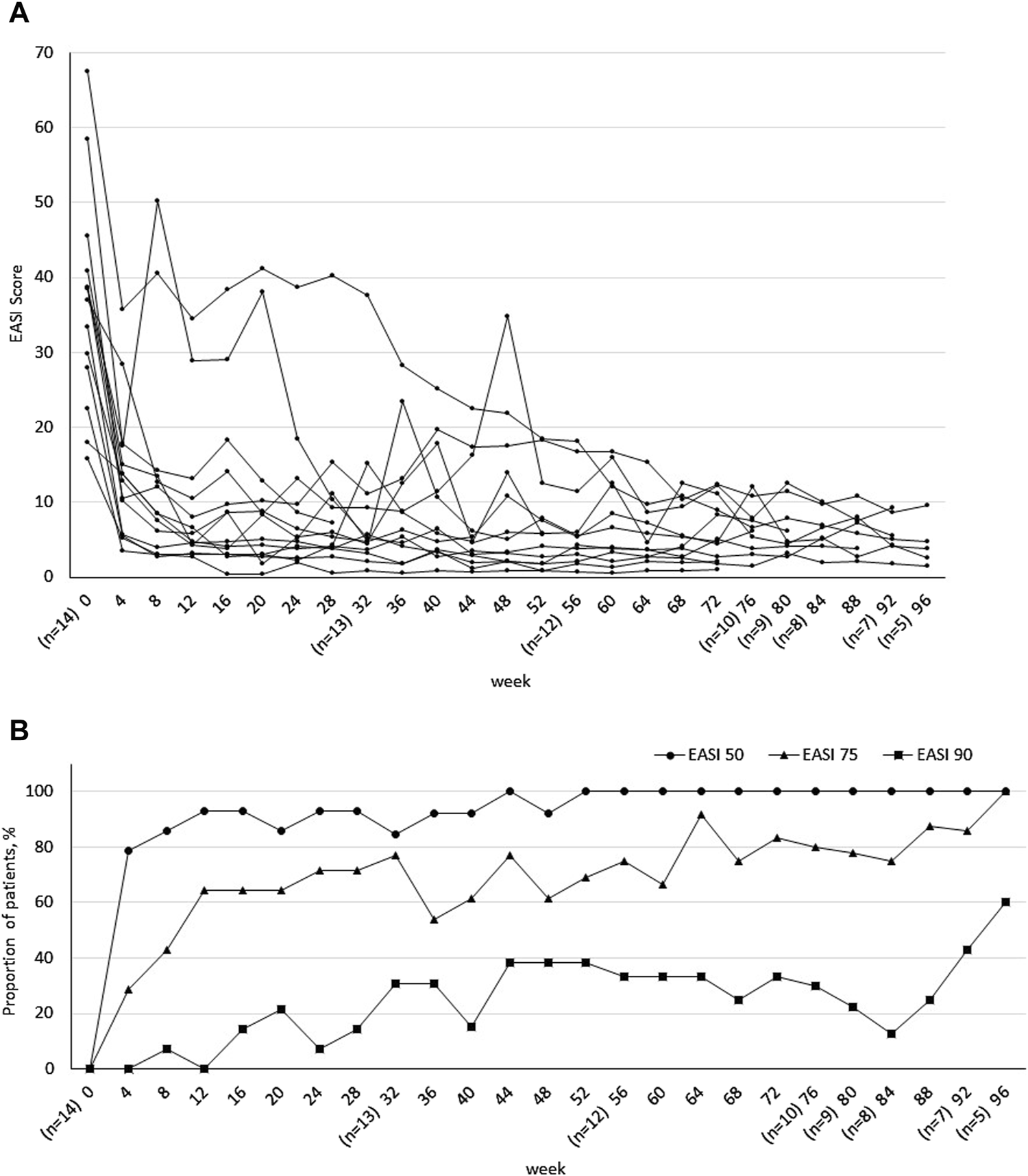
(A) Progress in Eczema Area and Severity Index (EASI). (B) Achievement rates of EASI 50, EASI 75, and EASI 90 over 96-week treatment period. Number of patients included varied depending on treatment progress at the end of the observation period.
Patients terminating UPA treatment
The median duration of UPA treatment was 60 (52–61) weeks [median (IQR)]. The four patients who achieved EASI 80 could be managed with topical anti-inflammatory drugs or moisturizers alone after completion of treatment. These four patients were managed with only anti-inflammatory topical drugs or moisturizers since the end of the study and are progressing to CR or IR without flare-ups (Table 2).
TABLE 2
| Case ID | 2 | 3 | 8 | 10 |
|---|---|---|---|---|
| Duration of UPA administration (weeks) | 64 | 28 | 60 | 60 |
| Reasons for ending UPA | IR | IR | IR | IR |
| Clinical Index at the end of UPA | ||||
| EASI | 3.6 | 3.8 | 0.6 | 4 |
| Improvement rate of EASI (%) | 84.0 | 88.6 | 96.2 | 94.7 |
| IGA | 1 | 1 | 0 | 1 |
| BSA (%) | 5 | 1 | 0 | 3 |
| Outcomes | IR | CR | CR | IR |
| Treatment after UPA | TCS Difamilast |
Moisturizer | Moisturizer | TCS Difamilast |
| Treat-to-target strategy | C | C | C | C |
| Flare-up within 8 weeks | - | - | - | - |
Patients terminating upadacitinib treatment.
CR, complete remission; IR, incomplete remission; TCS, topical corticosteroid; C, continuation; EASI, eczema area and severity index; BSA, body surface area; IGA, Investigator’s Global Assessment; UPA, upadacitinib.
Patients discontinuing UPA treatment
Five patients discontinued UPA treatment (Table 3). The median duration of UPA treatment was 36 (28–56) weeks [median (IQR)]. The reasons for discontinuation included financial burden in three patients and adverse events, including acne and blood creatinine kinase (CPK) elevation in one patient each. Case 5 was originally on DUP for 92 weeks but could not achieve EASI 50 and was switched to UPA. Four weeks after switching to UPA, the patient achieved EASI 75, but had an acne exacerbation and switched back to DUP at 16 weeks. The DUP was completed at 60 weeks, EASI 90 was achieved at 72 weeks, and the patient is now in CR. Case 12 had an elevated CPK of 772 U/L, so the UPA was terminated, and the patient was transitioned to management with topical corticosteroid (TCS) and difamilast.
TABLE 3
| Case ID | 5 | 6 | 7 | 9 | 12 |
|---|---|---|---|---|---|
| Duration of UPA administration (weeks) | 12 | 36 | 28 | 56 | 64 |
| Reasons for discontinuing UPA | Acne | Financial burden | Financial burden | Financial burden | CPK elevation |
| Clinical Index at the end of UPA | |||||
| EASI | 4.8 | 13.2 | 9.3 | 5.6 | 7.2 |
| Improvement rate of EASI (%) | 76.7 | 65.8 | 74.9 | 86.3 | 75.8 |
| IGA | 2 | 3 | 3 | 1 | 2 |
| BSA (%) | 2 | 8 | 10 | 8 | 9 |
| Outcomes | CR | Co | Co | Co | Co |
| Treatment after UPA | Dupilumab | TCS Delgocitinib |
TCS Delgocitinib |
TCS | TCS Difamilast |
| Treat-to-target strategy | C | D/O | D/O | C | C |
| Flares within last 8 weeks | - | + | + | + | + |
Patients discontinuing upadacitinib therapy.
CR, complete remission; Co, Control; TCS, topical corticosteroid; C, continuation; D/O, discuss and optimize; EASI, eczema area and severity index; BSA, body surface area; IGA, Investigator’s Global Assessment; UPA, upadacitinib.
Patients with ongoing UPA
At the end of the observation period, five patients were still undergoing UPA treatment (Table 4). Three patients (cases 1, 4, and 14) exhibited poor treatment adherence, including neglected UPA medication or inadequate topical treatment or skin care, such as not being able to take a bath. After consulting with the patients and their guardians, treatment was continued because further improvement could be expected with appropriate topical treatment. Eczema status and adherence improved in one patient (Case 1). Cases 1 and 13 were given a C on T2T strategy but continued UPA treatment because they had not achieved 8 weeks of flare-free status.
TABLE 4
| Case ID | 1 | 4 | 11 | 13 | 14 |
|---|---|---|---|---|---|
| Duration of UPA administration (weeks) | 96 | 96 | 80 | 52 | 28 |
| Reasons for ongoing UPA | Poor adherence | Poor adherence | Considering ending | Unstable symptoms | Poor adherence |
| Latest clinical Index | |||||
| EASI | 3.8 | 8.9 | 5.7 | 5.7 | 7.2 |
| Improvement rate of EASI (%) | 91.7 | 92.9 | 85.3 | 79.6 | 81.3 |
| IGA | 1 | 2 | 1 | 1 | 1 |
| BSA (%) | 10 | 16 | 4 | 5 | 7 |
| Outcome | Co | Co | IR | Co | Co |
| Ongoing basic treatment | TCS Difamilast |
TCS | TCS | TCS Difamilast |
TCS |
| Treat-to-target strategy | C | D/O | C | C | D/O |
| Flare-up within 8 weeks | + | + | - | + | + |
Patients with ongoing upadacitinib therapy.
Co, Control; IR, incomplete remission; TCS, topical corticosteroid; C, continuation; D/O, discuss and optimize; EASI, eczema area and severity index; BSA, body surface area; IGA, Investigator’s Global Assessment; UPA, upadacitinib.
Biomarkers
After starting UPA treatment, IgE increased from baseline in 11 patients (78.6%) and TARC increased in 14 patients (100%). IgE peaked in 5 (35.7%) patients at 24 (24–42) weeks and TARC peaked in 11 (78.6%) patients at 24 (24–36) weeks (Figures 3A, B). No significant changes were observed in eosinophil counts before and after treatment (Figure 3C).
FIGURE 3
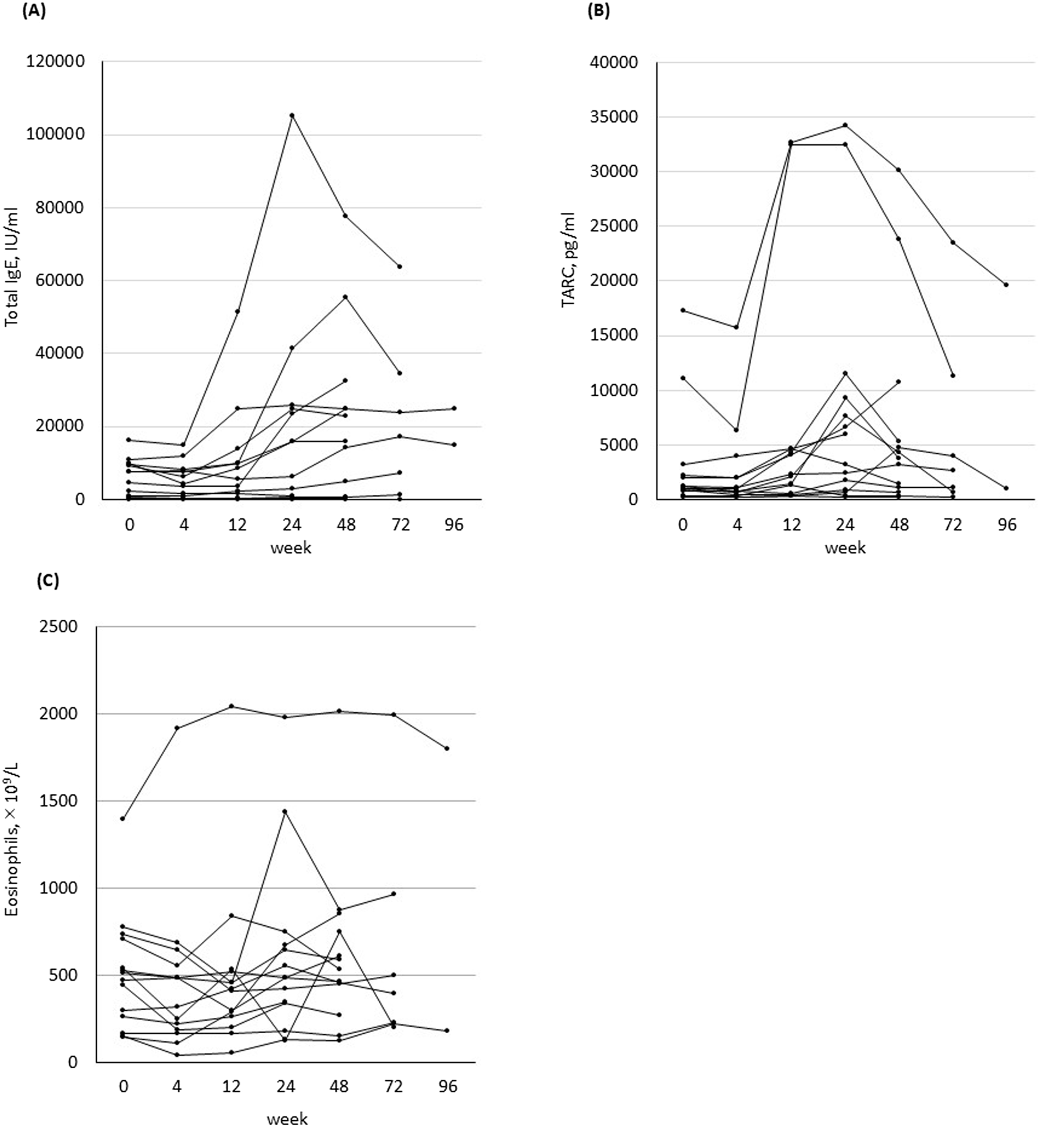
Change in biomarkers before and 4, 12, 24, 48, 72, and 96 weeks after treatment in all 14 patients who started treatment with upadacitinib. (A) Total serum IgE level. (B) Thymus and activation-regulated chemokine (TARC) level. (C) Blood eosinophil counts.
Safety
Adverse events included acne in three patients, elevated blood CPK in two patients (highest 772 IU/L and 434 IU/L), stye in one patient, COVID-19 infection in one patient, and influenza infection in two patients. In all patients, acne improved after using topical nadifloxacin and benzoyl peroxide. No oral antibiotics were necessary. UPA was discontinued in the patient with a CPK of 772 IU/L. Both patients with elevated CPK levels were untreated and CPK levels spontaneously decreased. One patient with acne was switched from UPA to DUP and exhibited improvement. No liver dysfunction, renal dysfunction, hemopenia, or adverse events led to the discontinuation of the drug.
Discussion
In this study, 4 of 14 patients who were treated with UPA were able to withdraw from UPA, with a median treatment duration of 60 weeks (52–61 weeks). Patients with better treatment adherence, mainly topical treatment, tended to be able to withdraw from UPA. Two of the four patients who weaned from UPA were able to achieve CR without the need for topical anti-inflammatory drugs. To the best of our knowledge, this is the first report of a combination of T2T strategy and goal setting that incorporates the frequency of flare-ups and treatment leading to weaning from UPA.
The efficacy of systemic treatment using molecularly targeted therapeutics in AD has been demonstrated [2–4]. In this study, the results of remission induction were comparable to those in clinical trials. However, the potential recurrence after discontinuation of systemic treatment, prolongation of systemic treatment, and difficulties in weaning off systemic treatment are concerning. In this study, more patients in the CR/IR group who weaned off from UPA exhibited better adherence, including topical treatment adherence, compared with patients in the Co group, and patients in the Co group had better adherence than patients in the CR/IR group. Maintaining treatment adherence is the key to successful treatment. Adolescent patients tend to have lower adherence compared to other age groups due to misinformation, the establishment of their own management methods, and decreased contact with healthcare providers [14]. Systemic treatment is fast-acting, improves eczema and itching, and reduces BSA [2–4]. Reduced BSA is expected to decrease the amount of time spent on topical treatment and skin care and the amount of ointment use, thus reducing the burden of daily management and improving treatment adherence. Once symptoms improve in response to systemic treatment, topical treatment and skin care are often neglected. Topical treatment is important for maintaining long-term remission and weaning from systemic therapy. It is important to fully educate patients about the necessity of topical treatment as adherence can be expected to increase with improvements in BSA.
In clinical practice, even if UPA is effective, reduced dosage or discontinuation is sometimes required for various reasons, including financial burden, adverse events, and patient anxiety due to long-term administration. UPA treatment was discontinued in this study due to financial burden and adverse events. In Japan, the subsidy system for medical expenses for children covers different ages in different municipalities. In the patient’s area, the eligibility for medical expenses was limited to 15 years of age, and the patient was no longer able to continue UPA. In the phase III study (AD Up study), discontinuation was considered when the CPK elevation was >4 times the normal value [3]. In this study, the upper limit of normal (ULN) was approximately 2.8 times higher than the ULN, but UPA was discontinued due to patient and family concerns. Acne is the most frequent adverse event in patients treated with UPA, especially in adolescents compared to adults [2–4]. Acne can be improved with standard treatment [15], but in this study, UPA was discontinued due to the child’s strong aversion to acne, prioritizing adherence and motivation.
The T2T strategy has been proposed as a management method for the systemic treatment of moderate-to-severe AD, and this strategy may be effective in understanding the pathophysiology of AD, maintaining treatment adherence, and preventing treatment rampancy [16, 17]. The T2T strategy has also been employed for treating rheumatoid arthritis [18] and inflammatory bowel disease [19], providing good control and preventing random dosing. This study confirmed that its efficacy in maintaining eczema control, motivation, and adherence.
In this study, total IgE levels and TARC were increased compared with baseline and deviated from EASI and clinical symptoms, suggesting the involvement of IL-21 and IL-31. IL-21 is a negative regulator of IgE production that inhibits IgE class switching via JAK1.3/STAT3. UPA-induced inhibition of this negative regulator may promote IgE class switching [20]. IL-31 has been recognized as having a dual role in Th2-type inflammation: it activates Th2-type inflammation in the early phase of diseases, but it also suppresses Th2-type inflammation through negative feedback in the chronic phase [21]. In an asthma model using IL-31RA knockout mice, it was demonstrated that IL-31R signaling plays a negative regulatory role in inflammation by inhibiting the proliferation of CD4+ T cells, leading to a reduction in Th2-type cytokine production [22]. The JAK/STAT activation of IL-31 involves STAT1/3/5. In patients with chronic AD, IL-31 might be in a state of suppressing Th2-type inflammation. Then, the inhibition of JAK/STAT activation by UPA might lead to the suppression of IL-31 and the activation of IL-4 and IL-13, resulting in the expression of TARC. These biomarkers decreased from their peak 24–48 weeks after initiating treatment, following the improvement of eczema. Future research is needed to determine remission and identify the time lag between improved clinical indices and biomarkers during UPA treatment.
Based on this study, we proposed an algorithm for systemic treatment (Figure 4). “Continuation of T2T strategy” and “flare-free status for at least 8 weeks” were used as good control criteria to determine whether to terminate systemic treatment or to step down treatment. The duration of flare-free status was based on the ETFAD definition. In this study, it was difficult to determine this because the treatment response and control status of individual patients had to be considered. Therefore, “at least 8 weeks” was used. Setting clear goals is important while using the ETFAD definition [13], sharing decision-making, tightly controlling the treatment with T2T strategy [12], and evaluating treatment according to the frequency of flare-ups. One goal of systemic treatment is to reduce the disease severity to a level that allows the maintenance of long-term remission with topical treatment and skin care alone. Thus, repeating the same evaluation after stepping down to topical treatment is important.
FIGURE 4
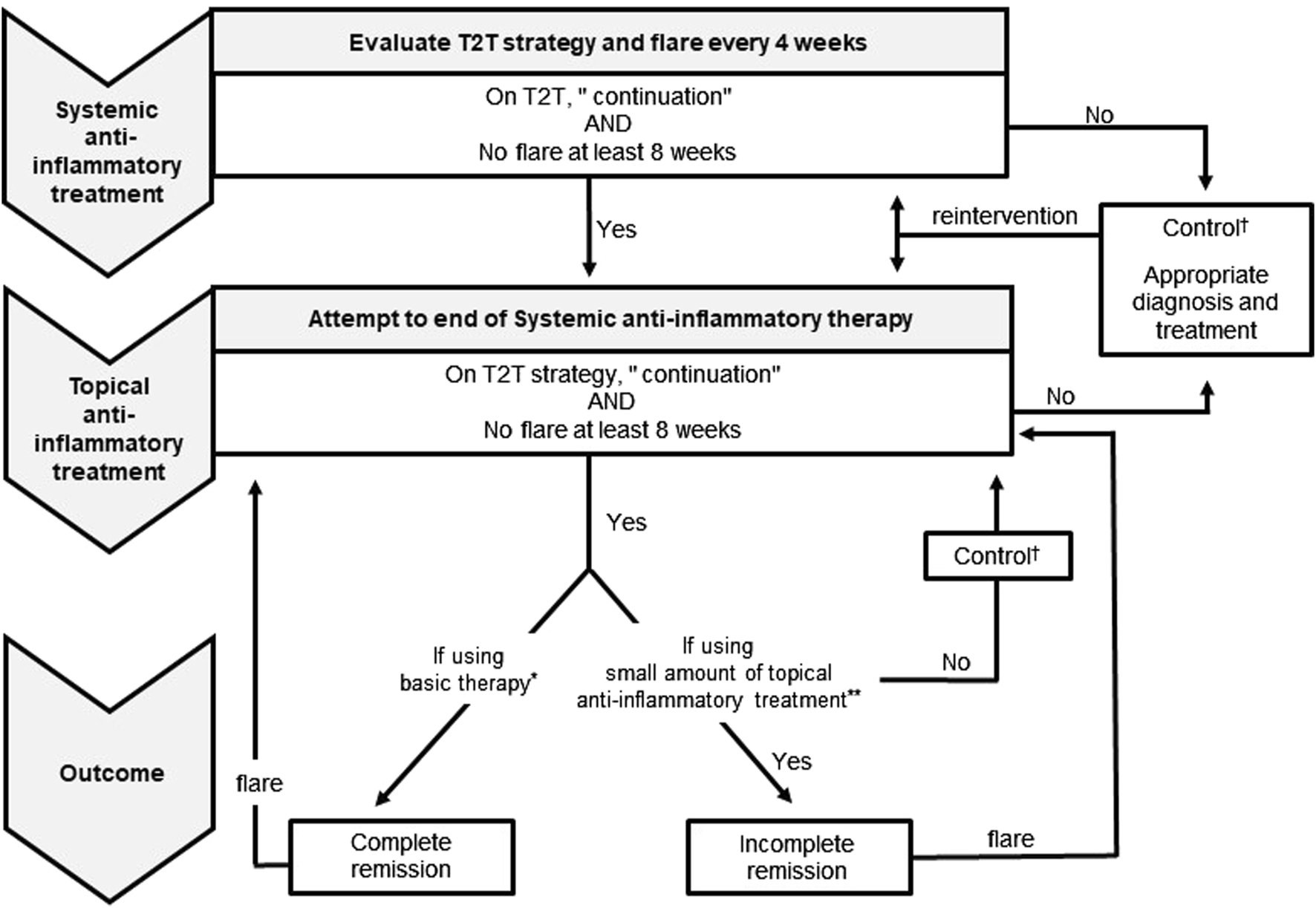
Proposed atopic dermatitis systemic treatment algorithm and definitions. * Irritant/allergen avoidance and emollient use are included. ** Topical anti-inflammatory treatment use is <30 g/month in children and <60 g/month after 15 years of age. † Under control using systemic and/or topical anti-inflammatory treatment. T2T, Treat-to-target strategy.
There were several limitations to this study. First, this was a single-center noncontrolled, nonrandomized, retrospective, observational study with a small number of subjects. Second, this study was conducted at a single institution. Thus, the scale was limited. In the future, a prospective study using this algorithm with a large number of cases should be implemented after establishing clear goals and useful evaluation criteria. Furthermore, measuring the T2T strategy in clinical practice is a time-consuming and laborious task, and a more convenient control tool should be considered in the future.
The synergistic effects of systemic and topical treatments can induce remission in patients with AD. Topical treatment is the basis of AD treatment and should be continued after the start of systemic treatment. The first step in weaning patients from systemic treatment is to achieve remission that can be maintained with topical treatment alone. Thus, maintaining treatment adherence is particularly important.
Statements
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
The studies involving humans were approved by the Institutional Review Board (IRB) of Saitama Medical University Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
TK designed the work, contributed to the acquisition, analysis, and interpretation of the data, and drafted the manuscript. KO, TS, and EM contributed to the acquisition and interpretation of the data. TI contributed to designing the work and revised the manuscript critically for important intellectual content. All authors contributed to the article and approved the submitted version.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We thank the family for their support and consent. We gratefully acknowledge the assistance provided by Enago, the editorial brand of Crimson Interactive Pvt. Ltd., for English language, grammar, punctuation, and spelling under Advance Editing.
Conflict of interest
TK received lecture fees from AbbVie GK, Sanofi, Torii Pharmaceutical and Otsuka Pharmaceutical Co., Ltd. EM received research support from Maruho Co., Ltd. TI received research support from Grants-in-Aid for Scientific Research (JSPS KAKENHI Grant Number JP 19K08879) and Seimens Healthcare Diagnostics. TI received lecture fees from KYORIN Pharmaceutical Co., Ltd., Pfizer Inc., AMCO INC., Otsuka Pharmaceutical Co., Ltd., Saitama Prefectural Office, the Japanese Society of Occupational and Environmental Allergy, and the Japanese Society of Allergology.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/jcia.2024.13404/full#supplementary-material
SUPPLEMENTARY FIGURE S1(A) Percentage of patients achieving pruritus NRS 4-point improve menta from baseline. (B) Percentage of Patients Achieving IGA 0/1. The number of patients included varied depending on treatment progress at the end of the observation period. Pruritus NRS, Pruritus Numerical Rating Scale; IGA, Investigator’s Global Assessment.
References
1.
Saeki H Ohya Y Furuta J Arakawa H Ichiyama S Katsunuma T et al Executive summary: Japanese guidelines for atopic dermatitis (ADGL) 2021. Allergol Int (2022) 71:448–58. 10.1016/j.alit.2022.06.009
2.
Guttman-Yassky E Teixeira HD Simpson EL Papp KA Pangan AL Blauvelt A et al Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (measure up 1 and measure up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet (2021) 397:2151–68. 10.1016/S0140-6736(21)00588-2
3.
Silverberg JI Bruin-Weller M Bieber T Soong W Kabashima K Costanzo A et al Upadacitinib plus topical corticosteroids in atopic dermatitis: week 52 AD up study results. J Allergy Clin Immunol (2022) 149:977–87.e14. 10.1016/j.jaci.2021.07.036
4.
Norito K Yukihiro O Hiroyuki M Masanori I Xiaofei H Kimitoshi I et al A phase 3 randomized, multicenter, double-blind study to evaluate the safety of upadacitinib in combination with topical corticosteroids in adolescent and adult patients with moderate-to-severe atopic dermatitis in Japan (rising up): an interim 24-week analysis. JAAD Int (2022) 6:27–36. 10.1016/j.jdin.2021.11.001
5.
Guidelines for the Promotion of Optimal Use. Pharmaceuticals and medical devices agency (PMDA). Available at: https://www.pmda.go.jp/files/000243653.pdf (Accessed April 15, 2024).
6.
Simpson E Bissonnette R Eichenfield LF Guttman-Yassky E King B Silverberg JI et al The validated investigator global assessment for atopic dermatitis (vIGA-AD): the development and reliability testing of a novel clinical outcome measurement instrument for the severity of atopic dermatitis. J Am Acad Dermatol (2020) 83:839–46. 10.1016/j.jaad.2020.04.104
7.
Hanifin JM Thurston M Omoto M Cherill R Tofte SJ Graeber M et al The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group, Exp Dermatol (2001) 10:11–8. 10.1034/j.1600-0625.2001.100102.x
8.
Amirsheybani HR Crecelius GM Timothy NH Pfeiffer M Saggers GC Manders EK . The natural history of the growth of the hand: I. Hand area as a percentage of body surface area. Plast Reconstr Surg (2001) 107:726–33. 10.1097/00006534-200103000-00012
9.
Phan NQ Blome C Fritz F Gerss J Reich A Ebata T et al Assessment of pruritus intensity: prospective study on validity and reliability of the visual analogue scale, numerical rating scale and verbal rating scale in 471 patients with chronic pruritus. Acta Derm Venereol (2012) 92:502–7. 10.2340/00015555-1246
10.
Charman CR Venn AJ Ravenscroft JC Williams HC . Translating patient-oriented eczema measure (POEM) scores into clinical practice by suggesting severity strata derived using anchor-based methods. Br J Dermatol (2013) 169:1326–32. 10.1111/bjd.12590
11.
Lewis-Jones MS Finlay AY . The children’s dermatology life quality index (CDLQI): initial validation and practical use. Br J Dermatol (1995) 132:942–9. 10.1111/j.1365-2133.1995.tb16953.x
12.
De Bruin-Weller M Biedermann T Bissonnette R Deleuran M Foley P Girolomoni G et al Treat-to-target in atopic dermatitis: an international consensus on a set of core decision points for systemic therapies. Acta Derm Venereol (2021) 101:adv00402. 10.2340/00015555-3751
13.
Wollenberg A Christen-Zäch S Taieb A Paul C Thyssen JP de Bruin-Weller M et al ETFAD/EADV eczema task force 2020 position paper on diagnosis and treatment of atopic dermatitis in adults and children. J Eur Acad Dermatol Venereol (2020) 34:2717–44. 10.1111/jdv.16892
14.
Kosse RC Bouvy ML Daanen M de Vries TW Koster ES . Adolescents’ perspectives on atopic dermatitis treatment-experiences, preferences, and beliefs. JAMA Dermatol (2018) 154:824–7. 10.1001/jamadermatol.2018.1096
15.
Mendes-Bastos P Ladizinski B Guttman-Yassky E Jiang P Liu J Prajapati VH et al Characterization of acne associated with upadacitinib treatment in patients with moderate-to-severe atopic dermatitis: a post hoc integrated analysis of 3 phase 3 randomized, double-blind, placebo-controlled trials. J Am Acad Dermatol (2022) 87:784–91. 10.1016/j.jaad.2022.06.012
16.
LeBovidge J Borok J Udkoff J Yosipovitch G Eichenfield LF . Atopic dermatitis: therapeutic care delivery: therapeutic education, shared decision-making, and access to care. Semin Cutan Med Surg (2017) 36:131–6. 10.12788/j.sder.2017.029
17.
Stalder JF Bernier C Ball A De Raeve L Gieler U Deleuran M et al Therapeutic patient education in atopic dermatitis: worldwide experiences. Pediatr Dermatol (2013) 30:329–34. 10.1111/pde.12024
18.
Rosina S Rebollo-Giménez AI Consolaro A Ravelli A . Treat-to-target in pediatric rheumatic diseases. Curr Rheumatol Rep (2023) 25:226–35. 10.1007/s11926-023-01112-x
19.
Bouguen G Levesque BG Pola S Evans E Sandborn WJ . Feasibility of endoscopic assessment and treating to target to achieve mucosal healing in ulcerative colitis. Inflamm Bowel Dis (2014) 20:231–9. 10.1097/01.MIB.0000437985.00190.aa
20.
Yang Z Wu CM Targ S Allen CDC . IL-21 is a broad negative regulator of IgE class switch recombination in mouse and human B cells. J Exp Med (2020) 217:e20190472. 10.1084/jem.20190472
21.
Huang J Yue H Jiang T Gao J Shi Y Shi B et al IL-31 plays dual roles in lung inflammation in an OVA-induced murine asthma model. Biol Open (2019) 8:bio036244. 10.1242/bio.036244
22.
Perrigoue JG Li J Zaph C Goldschmidt M Scott P de Sauvage FJ et al 31-IL-31R interactions negativelyity regulate type 2 inflammation in the lung. J Exp Med (2007) 204:481–7.
Summary
Keywords
adolescent, atopic dermatitis, Janus kinase, treat-to-target strategy, upadacitinib
Citation
Koga T, Okada K, Shimizu T, Morita E and Itazawa T (2024) Consideration of treatment goals and termination algorithm for adolescent atopic dermatitis using upadacitinib. J. Cutan. Immunol. Allergy 7:13404. doi: 10.3389/jcia.2024.13404
Received
18 June 2024
Accepted
08 August 2024
Published
22 August 2024
Volume
7 - 2024
Updates
Copyright
© 2024 Koga, Okada, Shimizu, Morita and Itazawa.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Takeshi Koga, takekoga@saitama-med.ac.jp
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.