- Department of Dermatology, Graduate School of Biomedical and Health Sciences, Hiroshima University, Hiroshima, Japan
In the treatment process of atopic dermatitis, pompholyx may occurs on palms and soles. However, there is limited knowledge about which patients are more likely to develop pompholyx and when it is expected to occur. Therefore, this study investigated the occurrence of pompholyx after the start of induction remission treatment. We collected medical information from 48 AD patients who visited Hiroshima University Hospital for the first time between January 2019 and December 2020, from the time of their first visit to 12 months later. In the initial group of 48 patients, 10 individuals developed pompholyx within 1 year after their first visit. There was no clear connection between the occurrence of pompholyx and treatment received. Most of the patients who developed pompholyx were above the age of 30. Serum thymus and activation-regulated chemokine (TARC) levels tended to be higher at the first visit in the group that later developed pompholyx compared to the group that did not. In conclusion, patients over the age of 30 with higher serum TARC levels may develop pompholyx while their AD is improving. It is crucial to provide these patients with information about the possibility of developing pompholyx during the course of their AD treatment and that it would be cured in the course of treatment.
Introduction
The treatment strategy for atopic dermatitis (AD) is rapid induction of remission with anti-inflammatory treatments, including topical agents, and maintenance of remission [1]. Rapid improvement of the dermatitis may be followed shortly afterward by the appearance of pompholyx (also known as dyshidrotic eczema) to appear on palms and soles (Supplementary Figure S1) [2, 3]. This phenomenon has been reported in studies of patients with severe AD who are admitted for treatment [3]. However, there is limited knowledge regarding the characteristics of these patients and the timing of the development of pompholyx. If pompholyx occurs early in the course of AD treatment, then patients might perceive it as a recurrence of AD or a side effect of topical treatment despite the fact that treatment has long been underway. Such misconceptions can lead to poor treatment adherence. In clinical practice, explaining possible adverse events in advance during the course of treatment can help to reduce patients’ anxiety about treatment. Therefore, this study investigated the relationships of the timing of treatment and patients’ backgrounds with the occurrence of pompholyx after the start of induction remission treatment in patients with no previous history of pompholyx.
Methods
We collected medical information from 48 AD patients who visited Hiroshima University Hospital for the first time between January 2019 and December 2020. The data were collected from the initial visit up to 12 months afterward, and these patients had no previous history of developing pompholyx at the time of their first visit. It was clinically determined that “skin manifestations on palms and soles with vesicles not with erythema” were pompholyx. Ethical approval was obtained from the Ethics Review Committee of Hiroshima University (E2082).
Results
In the initial group of 48 patients, 10 individuals developed pompholyx within 1 year after their first visit (Supplementary Table S1). There was no clear connection found between the occurrence of pompholyx and the specific type of treatment received. None of the patients had a medical history of metal allergy, any obvious changes in their dietary intake, nor exercise habits before or after the onset of pompholyx. The 48 patients were male:female 33:15, and among them, those who developed pompholyx were more likely to be female compared to the ratio of inclusion, male:female 3:7 in the pompholyx-incident group and male:female 30:8 in the pompholyx-non-incident group (Supplementary Table S1). Notably, most of the patients who developed pompholyx were above the age of 30 (Supplementary Figure S2). The onset of pompholyx was variable, with two cases experiencing it from 11 to 30 days after treatment, three cases from 31 to 100 days, and five cases after more than 100 days. The duration of treatment in this study was defined as that from the time of the first visit to our hospital. The duration from onset to healing of pompholyx ranged from less than 1 month in four cases, 1–3 months in four cases, and approximately 6 months in two cases. All cases achieved a full recovery within the observation period.
We investigated what differences there were in severity of AD and biomarker values at the first visit between the pompholyx group and the nonpompholyx group. Serum thymus and activation-regulated chemokine (TARC) levels tended to be higher at the first visit in the group that later developed pompholyx compared to the group that did not (Figure 1A). On the other hand, there were no obvious differences in LDH, eosinophil, or IgE levels between the two groups (Figures 2A–C). Although the Eczema Area and Severity Index (EASI) score at the first visit were not clearly different between the pompholyx group and the nonpompholyx group, all cases were above 7 in the pompholyx group (Figure 1B). The onset of pompholyx coincided with an improvement in the EASI score by more than 10 points (Figure 3). Furthermore, serum TARC levels were lower at the onset of pompholyx blisters compared to the levels recorded at the first visit (Figure 1C).
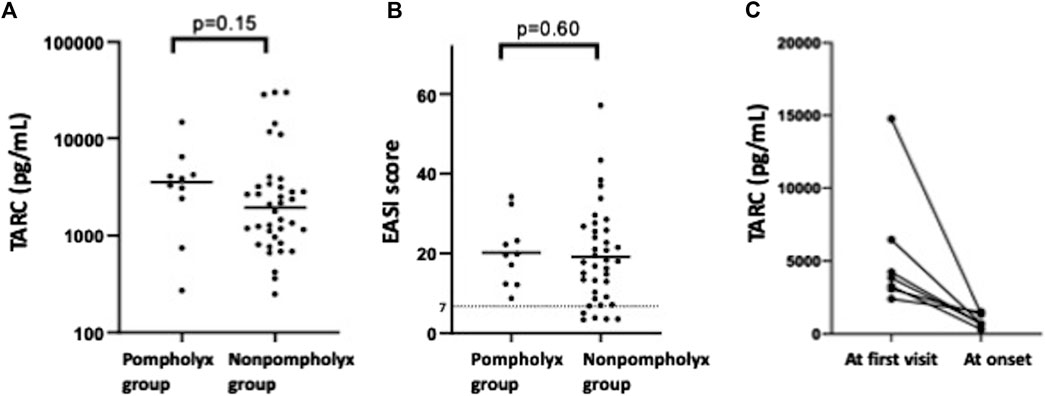
Figure 1. Variations in disease severity and TARC levels associated with pompholyx. (A) Comparison of TARC levels at the first visit between patients with and without pompholyx. (B) EASI score at the first visit in patients with and without pompholyx. (C) Changes in TARC levels between the first visit and onset of pompholyx. Note: EASI score: 0 = clear, 0.1–1.0 = almost clear, 1.1–7.0 = mild, 7.1–21.0 = moderate, 21.1–50.0 = severe, 50.1–72.0 = very severe.
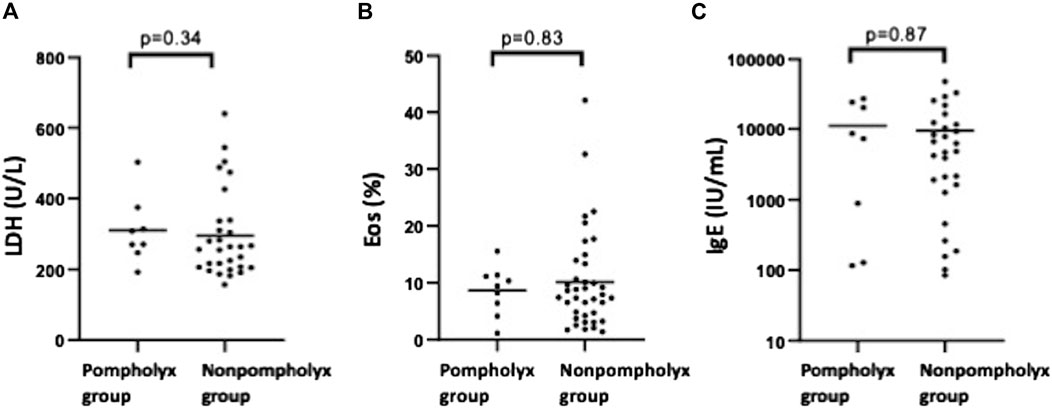
Figure 2. Comparison of LDH, eosinophil, and IgE levels between pompholyx and nonpompholyx groups. Statistical analysis using Mann Whitney U-test revealed no significant differences in LDH, eosinophil, or IgE levels at the first visit between patients who developed pompholyx during treatment (pompholyx group) and those who did not (nonpompholyx group).
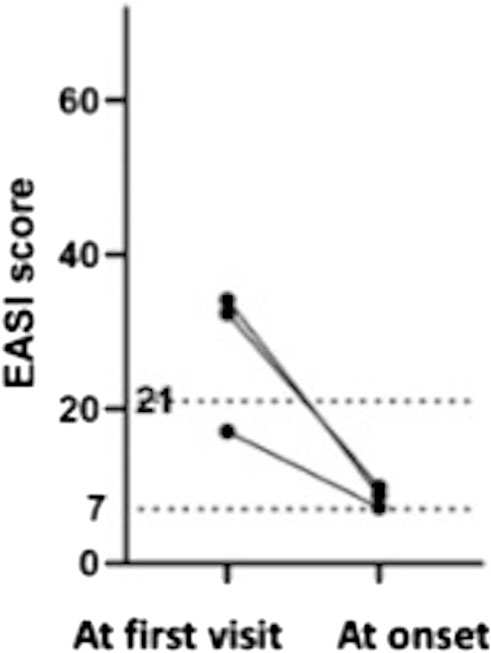
Figure 3. Changes in EASI scores in patients with pompholyx during treatment. Among the cases with available EASI scores at the first visit and at the onset of pompholyx, the EASI score at the onset of pompholyx showed an improvement of more than 10 compared to the first visit.
Discussion
Pompholyx is a disease typified by scattered or multiple vesicles of 2–5 mm in diameter localized on the palms and soles. The mechanism by which pompholyx develops is unknown, but some reports suggested that it is associated with sweat ducts or that it is an eczematous lesion. Pompholyx usually occurs and recurs during the summer season [4, 5]. The presence of AD is known to be a risk factor of pompholyx, but pompholyx as a complication of AD can occur at a time when dermatitis improves. In our study, the timing of the onset of pompholyx varied (Supplementary Figure S3). And we found no particular differences in the incidence of pompholyx depending on the season in which treatment was started. Previous reports have suggested that pompholyx tends to develop in patients with AD severe enough to require hospitalization after the disease has subsided [2, 3]. In our study, however, pompholyx developed in patients with moderate or severe AD, irrespective of their hospitalization status. Conversely, cases with an initial EASI scores below 7 did not develop pompholyx, indicating a potentially lower risk of pompholyx development in milder cases. Serum TARC levels tended to be higher at the first visit in the group that later developed pompholyx than in the group that did not develop pompholyx. TARC is a chemokine that attracts leukocytes and Th2 cells, and it enhances allergic reactions by promoting IgE production and eosinophil infiltration and activation, indicating the disease activity of AD. Conversely, because pompholyx develops during the inflammation recovery stage of AD, the pathogenesis of pompholyx might not necessarily be the same as that of AD. Our findings suggest that the severe inflammation and high disease activity of AD might have the potential to induce pompholyx via different mechanisms.
The limitations of this study included its retrospective, single-center nature and the use of data extracted from the medical information of a small number of cases. It is unclear whether pompholyx that occurs with the improvement of dermatitis in AD is the same skin rash as typical pompholyx. For this reason, some reports termed vesicles on the palms and soles, such as those observed in this study, as “pompholyx-like eruption.” [3].
In conclusion, patients over the age of 30 with moderate or severe AD may develop pompholyx while their AD is improving. It is crucial to provide these patients with information about the possibility of developing pompholyx as part of their AD treatment and that it would be cured in the course of treatment.
Data availability statement
Requests to access the datasets should be directed to EM, ZW1pbW05MjRAZ21haWwuY29t.
Ethics statement
The studies involving humans were approved by the Ethical Committee for Clinical Research of Hiroshima University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because the Ethics Review Committee has decided that there will be no invasive medical procedures in this study, only an opt-out presentation is required.
Author contributions
EM, SM, and AT participated in the design, interpretation of the studies and analysis of the data of the manuscript, and wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/jcia.2024.13559/full#supplementary-material
SUPPLEMENTARY FIGURE S1 | Clinical appearance of pompholyx. Vesicles are observed on palms, soles and edges of the feet.
SUPPLEMENTARY FIGURE S2 | Age distribution of patients with and without pompholyx during treatment. Comparison of the age distribution between patients who developed pompholyx during treatment (pompholyx group) and those who did not (nonpompholyx group). Pompholyx occurred in the age groups of 0–10 years and 31–60 years, while no cases were observed in the 11–30 years age group. Blue bars represent the pompholyx group, while gray bars represent the nonpompholyx groups.
SUPPLEMENTARY FIGURE S3 | Number of patients who developed pompholyx by month. Pompholyx cases were observed in all months except January, August, September, and November. There was no apparent seasonality in the onset of pompholyx.
References
1. Saeki, H. Clinical practice guidelines for the management of atopic dermatitis 2021. J Dermatol (2021) 131:2691. doi:10.14924/dermatol.131.2691
2. Norris, PG, and Levene, GM. Pompholyx occurring during hospital admission for treatment of atopic dermatitis. Clin Exp Dermatol (1987) 12:189–90. doi:10.1111/j.1365-2230.1987.tb01892.x
3. Horimukai, K, Tsumura, Y, Yamamoto, K, Shoda, T, Futamura, M, Nomura, I, et al. Pompholyx-like reactions occurring early in treatment of severe atopic dermatitis in children and adolescence. Arerugi (2011) 60:1543–9. doi:10.15036/arerugi.60.1543
4. Wollina, U. Pompholyx: a review of clinical features, differential diagnosis, and management. Am J Clin Dermatol (2010) 11:305–14. doi:10.2165/11533250-000000000-00000
Keywords: atopic dermatitis, pompholyx, dyshidrotic eczema, adverse event, severity
Citation: Murakami E, Morioke S and Tanaka A (2024) Improved atopic dermatitis accompanied by pompholyx. J. Cutan. Immunol. Allergy 7:13559. doi: 10.3389/jcia.2024.13559
Received: 19 July 2024; Accepted: 25 September 2024;
Published: 08 October 2024.
Copyright © 2024 Murakami, Morioke and Tanaka. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Akio Tanaka, dGFudGFuYWtpb3RhbkB5YWhvby5jby5qcA==
†ORCID: Emi Murakami, orcid.org/0000-0002-0211-9967; Satoshi Morioke, orcid.org/0000-0002-5822-4408; Akio Tanaka, orcid.org/0000-0002-5354-7064
 Emi Murakami
Emi Murakami Satoshi Morioke
Satoshi Morioke Akio Tanaka
Akio Tanaka