- 1Department of Dermatology and Skin Oncology, Chutoen General Medical Center, Kakegawa, Japan
- 2Allergic Disease Research Center, Chutoen General Medical Center, Kakegawa, Japan
An identical group of disorders has been called “interstitial granulomatous dermatitis” and “palisaded neutrophilic and granulomatous dermatitis.” In addition, a drug-induced subset of this condition has been named “interstitial granulomatous drug reaction (IGDR).” More recently, “reactive granulomatous dermatitis (RGD)” has been proposed as an umbrella term encompassing these three disorders. A considerable number of RGD cases are associated with systemic conditions, including autoimmune diseases, such as rheumatoid arthritis and systemic lupus erythematosus, inflammatory bowel disease, and malignancies. IGDR has also been shown to be caused by certain medications. We present a case of a 74-year-old Japanese man showing an asymptomatic, well-demarcated erythema eruption on the bilateral inguinal region extending to the lower abdomen and proximal thigh area. He had hyperlipidemia and had been taking pravastatin for 4 years. A biopsy specimen taken from the erythematous lesion revealed infiltration of lymphocytes and histiocytes in the upper and lower dermis. Interstitial infiltrates of histiocytes and lymphocytes were found as they were dispersed between collagen bundles. Immunostaining showed CD68+ macrophages intermingled with CD4+ and CD8+ T cells. Based on the clinical and histologic findings, the eruption was diagnosed as RGD. Because of the possibility of IGDR, pravastatin was stopped. His eruption completely disappeared within 6 months after the discontinuation. The list of drugs that cause RGD includes angiotensin-converting enzyme inhibitors, antihistamines, β-blockers, antidepressants, anticonvulsants, tocilizumab and ustekinumab. In addition, various statins have been reported to induce drug eruptions. The eruption types are mainly eczematous or lichenoid reactions, with psoriasiform or ichthyosiform reactions occurring rarely. There was only one report documenting that RGD occurred in patients administered rosuvastatin, with annular, violaceous plaques distributed on the extremities, proximal trunk and intertriginous areas. Pravastatin induces lichenoid eruption, but RGD has not been documented. Our case suggests that RGD is underestimated as an adverse effect of statins possibly because of its unusual cutaneous manifestations.
Introduction
An identical group of disorders has been called “interstitial granulomatous dermatitis” and “palisaded neutrophilic and granulomatous dermatitis” [1]. In addition, a drug-induced subset of this condition has been named “interstitial granulomatous drug reaction (IGDR)”. More recently, “reactive granulomatous dermatitis (RGD)” has been proposed as an umbrella term encompassing these three disorders [2, 3]. A considerable number of RGD cases are associated with systemic conditions, including autoimmune diseases, such as rheumatoid arthritis and systemic lupus erythematosus, inflammatory bowel disease, and malignancies [1, 2]. Drugs are another cause, as represented by IGDR [1–3]. Here, we report a case of RGD showing a unique skin eruption induced by pravastatin.
Case description
A 74-year-old Japanese man was referred to us because of a 6-month history of an asymptomatic tinea-like erythematous eruption on the right inguinal region. It had been growing centrifugally at a rate of approximately 2 cm per month and had gradually extended to the left inguinal area and lower abdomen. He had hyperlipidemia and had been taking pravastatin sodium, an inhibitor of HMG-CoA reductase, 5 mg daily, for 4 years. His family history was unremarkable. On examination, the patient had a well-demarcated erythema on the inguinal and proximal thigh areas (Figure 1A), extending to the lower abdomen (Figure 1B). The surface was smooth with no scaling or papules. No fungi were observed in the potassium hydroxide preparation. Sensory perception was not disturbed. Blood chemistry, including liver enzymes and hemoglobin A1c, were all within normal limits. T-cell interferon-γ production tests for tuberculosis, antinuclear antibodies, and anti-SS-A or -SS-B antibodies were negative. The lymphocyte transformation test with pravastatin was negative.
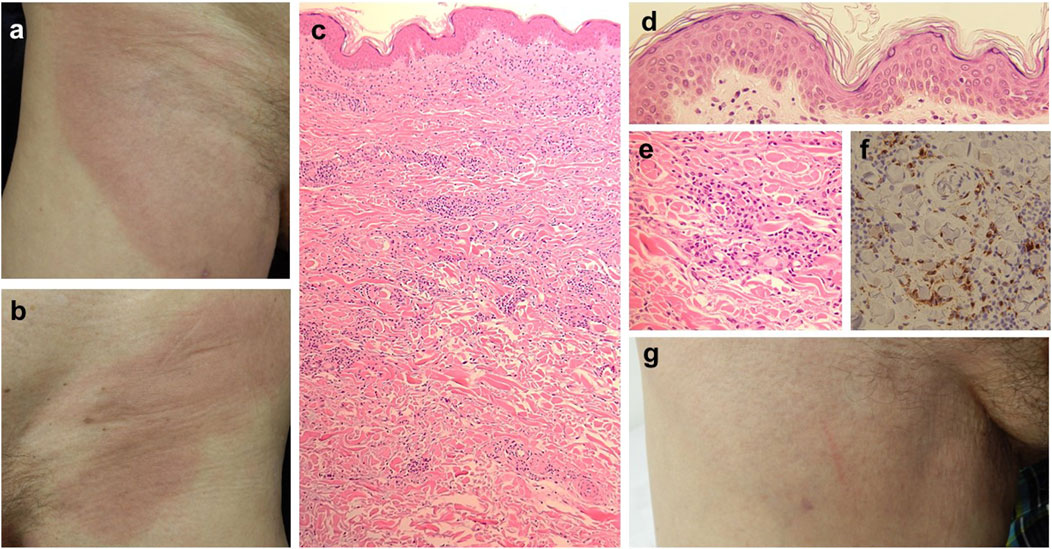
Figure 1. Clinical presentation and histopathological findings. On the initial examination, the patient had a well-demarcated erythema on the right (A) and left (B) inguinal region, extending to the thighs. Histopathology (H&E stain), showed an infiltration of lymphocytes and histiocytes in the upper and lower dermis [(C), x50)], mainly between collagen bundles. Interfacial dermatitis with lymphocytes is noted [(D), x200]. Histiocytes and lymphocytes are dispersed between collagen fibers ((E), x200), Immunostaining for CD68 shows positive histiocytes in the infiltrate [(F), x200]. The clinical picture shows the disappearance of the eruption on the right inguinal region 6 months after discontinuation of pravastatin (G).
A biopsy specimen taken from the erythematous lesion revealed infiltration of lymphocytes and histiocytes in the upper to lower dermis (Figure 1C). Interface dermatitis was present (Figure 1D), but neither tissue eosinophilia nor atypia of lymphocytes were observed, suggesting that our case was more likely a granulomatous reaction. Interstitial infiltrates of histiocytes and lymphocytes were found scattered between collagen bundles (Figure 1E). No mucin deposition or frank necrobiosis of collagen was noted. Ziehl-Neelsen staining did not reveal any positive microorganisms within the histiocytes, excluding the possibility of tuberculosis or Hansen’s disease. Immunostaining revealed CD68+ macrophages (Figure 1F) intermingled with CD4+ and CD8+ T cells. Based on the clinical and histologic findings, the eruption was diagnosed as RGD.
Because of the possibility of IGDR, pravastatin was stopped. The patient’s eruption gradually improved, and 6 months after the discontinuation, it completely disappeared (Figure 1G).
Discussion
The list of drugs that cause RGD includes angiotensin-converting enzyme inhibitors, antihistamines, β-blockers, antidepressants, anticonvulsants, tocilizumab, and ustekinumab [4, 5]. In addition, various statins have been reported to induce drug eruption, such as simvastatin, pitavastatin, atorvastatin, and fluvastatin, and, with equal frequency, lovastatin, pravastatin and rosuvastatin [6]. The eruption types are mainly eczematous or lichenoid reactions [6], with psoriasiform or ichthyosiform reactions occurring rarely.
There was only one report documenting that RGD occurred in patients administered rosuvastatin, with annular, violaceous plaques distributed on the extremities, proximal trunk, and intertriginous areas [7]. Pravastatin induces lichenoid eruptions [8], but RGD due to pravastatin has not been documented so far. The mechanism underlying pravastatin-induced RGD remains unclear. Since RGD is caused by different categories of drugs, the pathogenesis appears to be unrelated to the drug action. Instead, we speculate that drugs, such as pravastatin, immunologically activate histiocytes to proliferate in the skin or monocytes to infiltrate into the skin. Our case suggests that RGD is underestimated as an adverse effect of statins possibly because of its unusual cutaneous manifestations.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YT supervised writing the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Yang, C, Tang, S, Li, S, Ying, S, Zhu, D, Liu, T, et al. Underlying systemic diseases in interstitial granulomatous dermatitis and palisaded neutrophilic granulomatous dermatitis: a systematic review. Dermatology (2023) 239:287–98. doi:10.1159/000527461
2. Rosenbach, M, and English, 3rd JC. Reactive granulomatous dermatitis: a review of palisaded neutrophilic and granulomatous dermatitis, interstitial granulomatous dermatitis, interstitial granulomatous drug reaction, and a proposed reclassification. Dermatol Clin (2015) 33:373–87. doi:10.1016/j.det.2015.03.005
3. Bangalore Kumar, A, Lehman, JS, Johnson, EF, Cantwell, HM, Sartori Valinotti, JC, Sokumbi, O, et al. Reactive granulomatous dermatitis as a clinically relevant and unifying term: a retrospective review of clinical features, associated systemic diseases, histopathology and treatment for a series of 65 patients at mayo clinic. J Eur Acad Dermatol Venereol (2022) 36:2443–50. doi:10.1111/jdv.18203
4. Altemir, A, Iglesias-Sancho, M, Sola-Casas, MLÁ, Novoa-Lamazares, L, Fernández-Figueras, M, and Salleras-Redonnet, M. Interstitial granulomatous dermatitis following tocilizumab, a paradoxical reaction? Dermatol Ther (2020) 33(6):e14207. doi:10.1111/dth.14207
5. Walker, A, Westerdahl, JS, Zussman, J, and Mathis, J. Interstitial granulomatous drug reaction to ustekinumab. Case Rep Dermatol Med (2022) 2022:1461145. doi:10.1155/2022/1461145
6. Oda, T, Sawada, Y, Yamaguchi, T, Ohmori, S, Haruyama, S, Yoshioka, M, et al. Drug eruption caused by rosuvastatin. J Investig Allergol Clin Immunol (2017) 27:140–1. doi:10.18176/jiaci.0136
7. Hernández, N, Peñate, Y, and Borrego, L. Generalized erythematous-violaceous plaques in a patient with a history of dyslipidemia. Interstitial granulomatous drug reaction (IGDR). Int J Dermatol (2013) 52:393–4. doi:10.1111/j.1365-4632.2012.05670.x
Keywords: drug reaction, interstitial granulomatous dermatitis, pravastatin, reactive granulomatous dermatitis, interstitial granulomatous drug reaction
Citation: Kondo S, Yunoki M, Otsuka M and Tokura Y (2024) Case report: Reactive granulomatous dermatitis presenting with inguinal erythematous plaques in a patient administered with pravastatin. J. Cutan. Immunol. Allergy 7:13640. doi: 10.3389/jcia.2024.13640
Received: 09 August 2024; Accepted: 21 August 2024;
Published: 02 September 2024.
Copyright © 2024 Kondo, Yunoki, Otsuka and Tokura. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shumpei Kondo, a25kLnNobnBAZ21haWwuY29t
 Shumpei Kondo
Shumpei Kondo Marina Yunoki1
Marina Yunoki1 Yoshiki Tokura
Yoshiki Tokura