Dear Editors,
Lichen planus (LP) is a chronic inflammatory disease affecting the skin and/or mucous membranes. Although LP is primarily a T-cell-mediated disease, various autoantibodies, including those against BP180/type XVII collagen and desmogleins (DSGs), have been detected in some patients, especially those with the erosive form of oral LP (OLP) [1–3]. We report a case of OLP with low-titer autoantibodies to BP180 and DSG3.
A 29-year-old Japanese man presented with painful oral mucosal lesions that had persisted for several months. He was a non-smoker with no history of medication use or recent dental treatment. Physical examination revealed erosions and white reticular lesions on the bilateral buccal mucosa and tongue (Figure 1A). A biopsy specimen from the right buccal mucosa showed thickened epithelium, serrated rete ridges, intraepithelial and subepithelial band-like lymphocytic infiltrate, vacuolization of the basal layer, and Civatte bodies (Figure 1B). Acantholysis or subepithelial blistering was not observed. The results of routine laboratory tests were unremarkable, and serum anti-hepatitis C virus antibodies were negative. Low titers of serum IgG antibodies to BP180 NC16a (7.7 U/mL; cutoff value, <9.0 U/mL) and DSG3 (4.3 U/mL; cutoff value, <20.0 U/mL), but not DSG1 (<3 U/mL), were detected by chemiluminescent enzyme immunoassay. Direct immunofluorescence showed no deposition of IgG, IgA, or C3. Similarly, indirect immunofluorescence did not detect IgG or IgA deposition. Computed tomography did not reveal any signs of malignancy. The diagnosis of OLP was made, and topical tacrolimus therapy was started. The oral mucosal lesions improved within 4 months of treatment initiation. The autoantibody titers did not change significantly 8 months after diagnosis (Figure 1C).
FIGURE 1
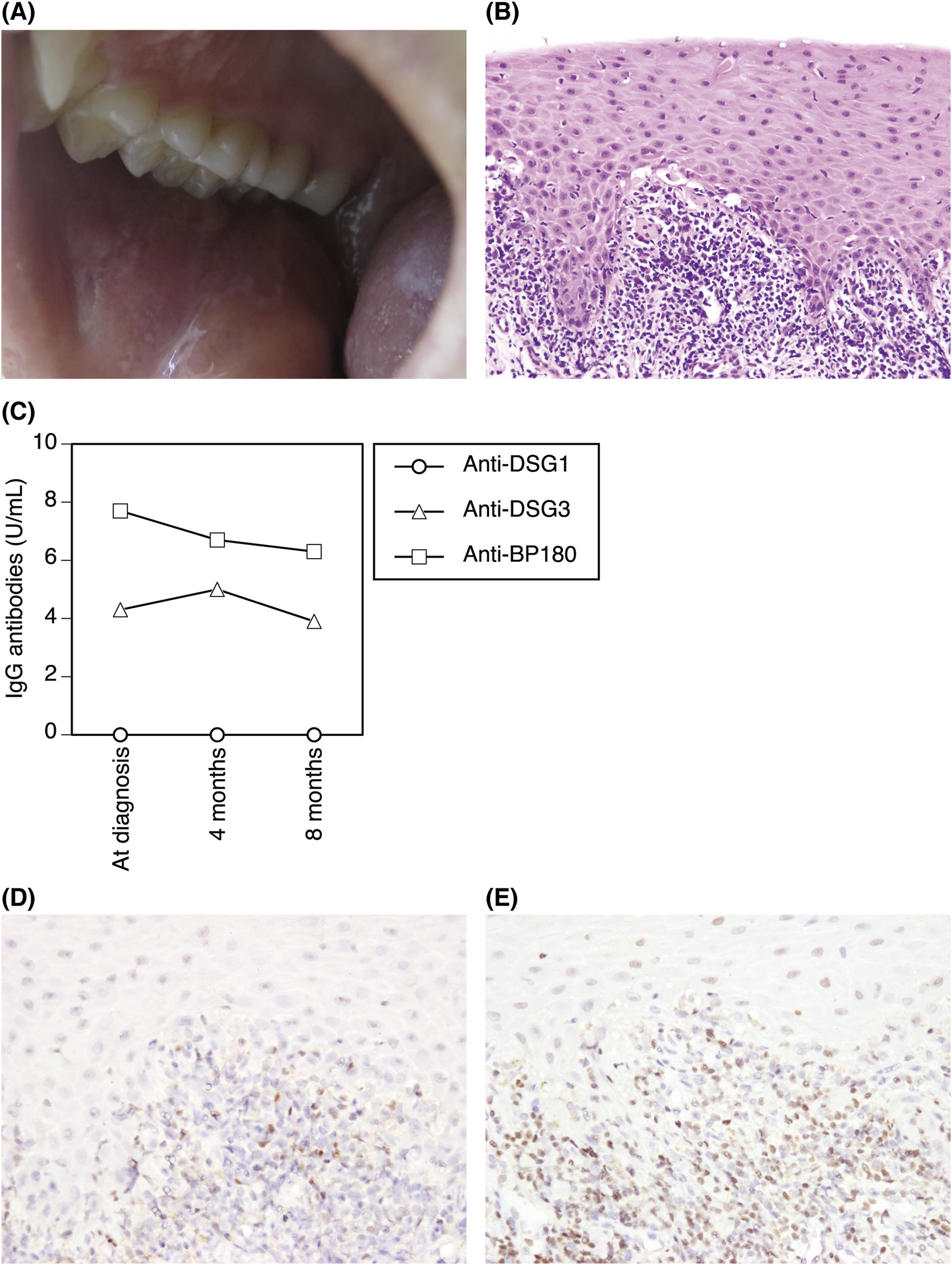
(A) Clinical presentation. Erosions and white reticular lesions on the buccal mucosa and tongue. (B) Histopathological findings of the buccal mucosal lesion. Thickened epithelium, serrated rete ridges, intraepithelial and band-like subepithelial lymphocytic infiltrate, vacuolization of the basal layer, and Civatte bodies (hematoxylin-eosin, original magnification ×200). (C) The autoantibody titers at diagnosis and 4 and 8 months after diagnosis. (D, E) Immunostaining of the infiltrates with anti-T-bet (D) and anti-GATA3 (E) antibodies (original magnification ×400).
Anti-BP180, anti-DSG1, and anti-DSG3 autoantibodies have been detected in some OLP patients [1–3]. In most studies, these autoantibodies were present at low titers or were non-pathogenic [1–3]. This suggests that these autoantibodies are generated secondary to T-cell-mediated damage of keratinocytes, leading to the release of autoantigens.
The target antigens recognized by pathogenic cytotoxic T cells remain unidentified in the majority of LP cases. However, several studies have identified BP180- and/or DSG3-reactive T cells in the peripheral blood of LP patients [4]. These autoreactive T cells were IFN-γ-producing type 1 cells and/or IL-17A-producing type 17 cells [4]. The T-cell infiltrates in LP lesions predominantly consist of T-bet+ type 1 cells, whereas those in autoimmune blistering diseases (bullous pemphigoid and pemphigus vulgaris) are mainly GATA3+ type 2 cells [4, 5]. Interestingly, patients with LP pemphigoides, which is characterized by LP-like lesions associated with subepidermal blistering, linear IgG and C3 deposition along the basement membrane zone, and IgG autoantibodies to BP180 NC16a, had both type 1 and type 2 BP180-reactive T cells in the peripheral blood and GATA3+-cell-dominant T-cell infiltrates [4]. In our case, the infiltrates were also dominated by GATA3+ type 2 cells, which may explain the generation of autoantibodies (Figures 1D, E).
As autoantibodies against DSG1, DSG3, and BP180 can be detected in some OLP patients, the diagnosis should be based on a comprehensive assessment, including histopathology and immunofluorescence. Although it remains unclear whether these molecules are the primary target antigens for cytotoxic T cells and whether these patients have a potential risk for developing autoimmune blistering diseases due to epitope spreading over time, careful follow-up is essential.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the studies involving humans because our institution does not require ethical approval for reporting individual cases or case series. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We thank K. Gunshin for her technical assistance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
Herrero-González JE Parera Amer E Segura S Mas Bosch V Pujol RM Martínez Escala ME . Epithelial antigenic specificities of circulating autoantibodies in mucosal lichen planus. Int J Dermatol (2016) 55(6):634–9. 10.1111/ijd.12990
2.
Didona D Hertl M . Detection of anti-desmoglein antibodies in oral lichen planus: what do we know so far. Front Immunol (2022) 13:1001970. 10.3389/fimmu.2022.1001970
3.
Muramatsu K Nishie W Natsuga K Fujita Y Iwata H Yamada T et al Two cases of erosive oral lichen planus with autoantibodies to desmoglein 3. J Dermatol (2016) 43(11):1350–3. 10.1111/1346-8138.13493
4.
Schmidt T Solimani F Pollmann R Stein R Schmidt A Stulberg I et al TH1/TH17 cell recognition of desmoglein 3 and bullous pemphigoid antigen 180 in patients with lichen planus. J Allergy Clin Immunol (2018) 142(2):669–72.e7. 10.1016/j.jaci.2018.02.044
5.
Schinner J Cunha T Mayer JU Hörster S Kind P Didona D et al Skin-infiltrating T cells display distinct inflammatory signatures in lichen planus, bullous pemphigoid and pemphigus vulgaris. Front Immunol (2023) 14:1203776. 10.3389/fimmu.2023.1203776
Summary
Keywords
DSG3, GATA3, T-bet, T cells, type XVII collagen
Citation
Sato M, Kawai K, Hoshina Y, Terao K and Ibusuki A (2024) Oral lichen planus with low-titer autoantibodies to BP180 and desmoglein 3. J. Cutan. Immunol. Allergy 7:13676. doi: 10.3389/jcia.2024.13676
Received
20 August 2024
Accepted
27 September 2024
Published
03 October 2024
Volume
7 - 2024
Updates
Copyright
© 2024 Sato, Kawai, Hoshina, Terao and Ibusuki.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kazuhiro Kawai, kazkawai@m2.kufm.kagoshima-u.ac.jp
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.