Dear Editors,
Psoriasis is a chronic inflammatory skin disease presenting scaly indurated erythema. Interleukin (IL)-17A plays a crucial role in the pathogenesis of psoriasis [1]. Anti-IL-17A antibodies have shown high therapeutic effectiveness for psoriasis.
Sjögren’s syndrome (SS) and systemic lupus erythematosus (SLE) are autoimmune diseases that can be associated with psoriasis [2]. SS presents dry eye and dry mouth symptoms, associated with positive anti-SSA or anti-SSB antibodies. SLE affects various organs, with the presence of multiple autoantibodies. Recent studies suggest that IL-17A might contribute to the pathogenesis of SS and SLE, indicating possible therapeutic effectiveness of anti-IL-17A antibodies for these diseases [3, 4].
We present the first case of psoriasis vulgaris concurrent with SS and incomplete SLE successfully treated with ixekizumab, a humanized monoclonal anti-IL-17A antibody. A 62-year-old Japanese man, presented with persistent scaly indurated erythema on the trunk and extremities (Figure 1A) and dry eye symptom. Blood tests showed an anti-nuclear antibody titer of 320 times (speckled) (normal reference: <40), anti-Sm antibody of 8.9 U/mL (normal reference: <7 U/mL), anti-ds-DNA antibody of 38.5 IU/mL (normal reference: <12 IU/mL), anti-SS-A antibody of 240 U/mL (normal reference: <10 U/mL), and anti-SS-B antibody of 14.6 U/mL (normal reference: <10 U/mL). CH50 was found to be less than 5 (normal reference: 30–45 U/mL), while C3 and C4 were within the normal range (C3: 80–140 mg/dL, C4: 11–34 mg/dL). The histopathology of scaly erythema on his right buttock showed acanthosis, parakeratosis, subcorneal neutrophilic pustules, loss of granular layers, and elongation of rete ridges of the epidermis, and lymphocytic infiltration around blood vessels in the dermis (Figure 1B). Papulosquamous eruptions associated with SS were considered a differential diagnosis; however, the histopathological findings were more consistent with psoriasis. Furthermore, the Schirmer test revealed a value of 2 mm/5 min (with a normal value being 10 mm or more) and a positive fluorescein eye stain test was observed. He was diagnosed with psoriasis vulgaris concurrent with SS and subclinical SLE. The diagnosis of SS was made using the revised Japanese criteria for Sjögren’s syndrome (SS) (Ministry of Health, Labour and Welfare research group, 1999). In this case, salivary gland biopsy, ultrasound, and sialography were not performed because the patient met the diagnostic criteria for SS. He did not meet the full ACR/EULAR 2019 criteria for SLE, as calculated by the SLE Risk Probability Index (SLERPI); however, he did present with anti-dsDNA antibody and reduced CH50 levels, which are part of the immunological criteria under ACR/EULAR 2019 classification. Regarding incomplete SLE, the patient did not show any clinical signs apart from abnormal blood test results, such as anti-dsDNA positivity and reduced CH50 levels. There was no significant improvement or deterioration of these findings after treatment with ixekizumab. After 16 weeks of treatment with ixekizumab, the patient’s psoriasis area and severity index reduced from 21.6 to 1.2 (Figure 1C), and his dry eye symptom and tear secretion were improved as evaluated by the Schirmer test. For dry eye, diquafosol sodium eye drops were administered at a dosage of one drop, 5–6 times a day. After the treatment, the patient’s dry eye symptoms were alleviated, with the Schirmer test showing an improvement to 8 mm/5 min. However, the specific autoantibody values remained largely unchanged. While the Schirmer test was used to monitor dry eye symptoms, it should be noted that this alone may not fully capture the impact of treatment on SS.
FIGURE 1
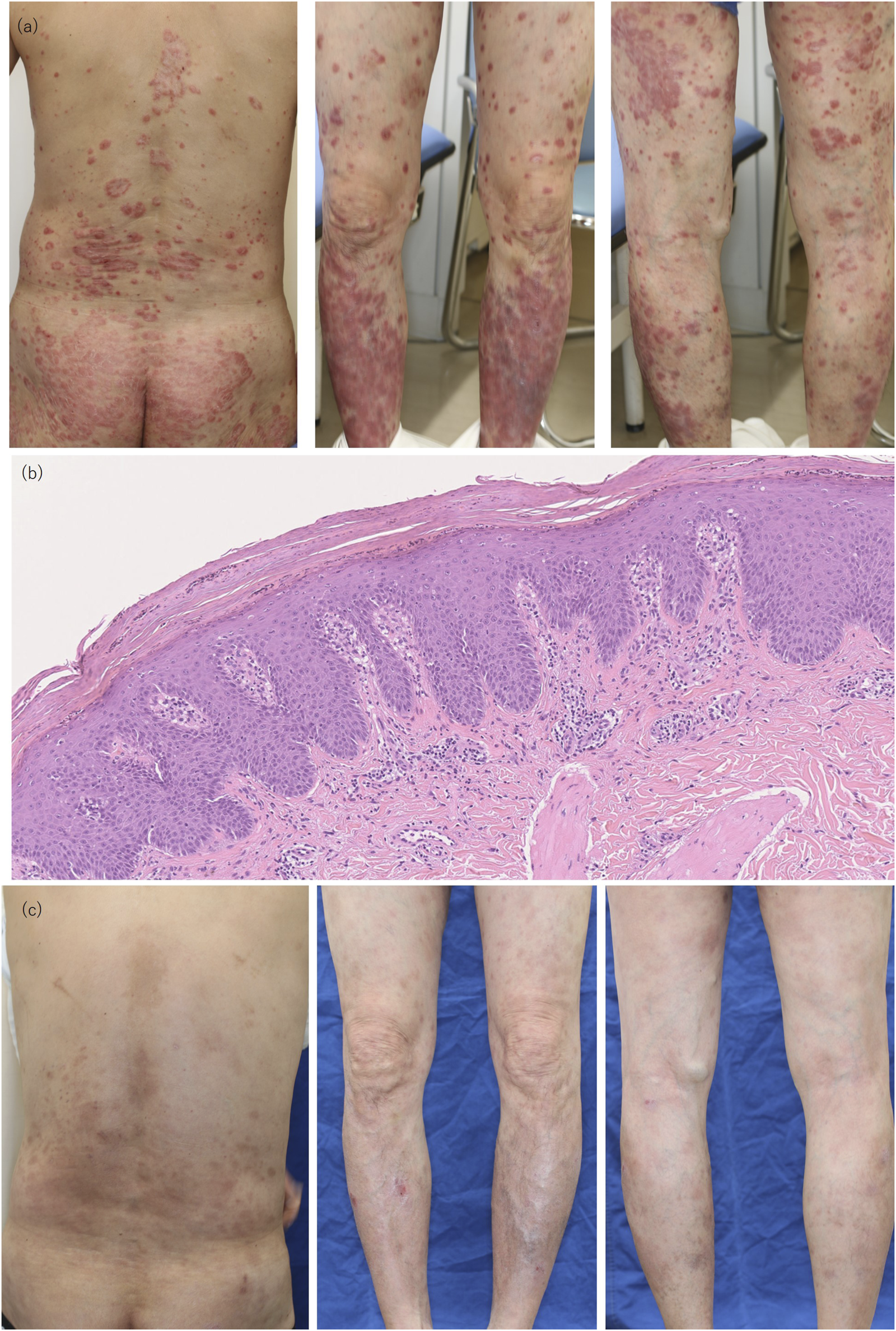
Clinical and Pathological Findings. (A) Pretreatment findings showing scaly erythema on the back, buttock, anterior, and posterior legs. (B) Pathologic findings with H and E staining; magnification ×100, showing acanthosis, parakeratosis, and subcorneal neutrophilic pustules. (C) Posttreatment findings on the back, anterior, and posterior legs at 16 weeks of ixekizumab treatment.
Recent studies suggest the contribution of IL-17A to the pathogenesis of SS; IL-17A-producing CD4+T cells are increased in salivary glands of SS patients compared to the reference group, and IL-17A in vitro promotes the production of IL-6 and IL-8 in salivary gland cells [3]. In dry eye patients and model mice, the expression of IL-17A and matrix metalloproteinase (MMP)-9 or MMP-3 is elevated in lacrimal glands [5]. The administration of anti-IL-17A antibody reduced the expression of MMP-3 and MMP-9 in corneal epithelial cells together with the restoration of corneal epithelial barrier in the dry eye model mice [5], indicating IL-17A-induced expression of MMP-3/9. The observed improvement in dry eye symptoms by ixekizumab in our case might suggest therapeutic potential for SS, though this case does not establish a direct causal relationship. Furthermore, the improvement in dry eye symptoms may also be attributed to the use of diquafosol sodium eye drops, indicating the need for further detailed investigation.
In conclusion, this report demonstrates the therapeutic effectiveness of ixekizumab for psoriasis vulgaris in a patient with concurrent primary SS without extra-glandular involvement. Ixekizumab was particularly effective for the skin manifestations of psoriasis, with improvement in SS-associated dry eye symptoms.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the studies on humans in accordance with the local legislation and institutional requirements because only commercially available established cell lines were used. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
TH conceptualized the study, and mainly organized the manuscript. NK supervised the study. MO, HS, and EF revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
TH, HS, and NK received lecture fees from Eli Lilly. There was no involvement of a funder in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
Hagino T Saeki H Fujimoto E Kanda N . Effects of biologic therapy on laboratory indicators of cardiometabolic Diseases in patients with psoriasis. J Clin Med2023; 12(5):1934. 10.3390/jcm12051934
2.
Satoh Y Nakano K Yoshinari H Nakayamada S Iwata S Kubo S et al A case of refractory lupus nephritis complicated by psoriasis vulgaris that was controlled with secukinumab. Lupus (2018) 27(7):1202–6. 10.1177/0961203318762598
3.
Sakai A Sugawara Y Kuroishi T Sasano T Sugawara S . Identification of IL-18 and Th17 cells in salivary glands of patients with Sjögren's syndrome, and amplification of IL-17-mediated secretion of inflammatory cytokines from salivary gland cells by IL-18. J Immunol (2008) 181(4):2898–906. 10.4049/jimmunol.181.4.2898
4.
Vincent FB Northcott M Hoi A Mackay F Morand EF . Clinical associations of serum interleukin-17 in systemic lupus erythematosus. Arthritis Res Ther (2013) 15(4):R97. 10.1186/ar4277
5.
De Paiva CS Chotikavanich S Pangelinan SB Pitcher JD Fang B Zheng X et al IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunol (2009) 2(3):243–53. 10.1038/mi.2009.5
Summary
Keywords
psoriasis vulgaris, Sjögren’s syndrome, incomplete systemic lupus erythematosus, ixekizumab, IL-17A inhibitors
Citation
Onda M, Hagino T, Saeki H, Fujimoto E and Kanda N (2024) Successful treatment of psoriasis vulgaris with ixekizumab in a patient with concurrent Sjögren’s syndrome presenting with dry eye and incomplete SLE. J. Cutan. Immunol. Allergy 7:13804. doi: 10.3389/jcia.2024.13804
Received
14 September 2024
Accepted
22 October 2024
Published
31 October 2024
Volume
7 - 2024
Updates
Copyright
© 2024 Onda, Hagino, Saeki, Fujimoto and Kanda.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Teppei Hagino, teppei-hagino@nms.ac.jp
ORCID: Teppei Hagino, orcid.org/0000-0002-4183-9596; Hidehisa Saeki, orcid.org/0000-0002-1095-0355; Eita Fujimoto, orcid.org/0009-0002-5914-3244; Naoko Kanda, orcid.org/0000-0003-4389-2312
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.