Abstract
Alopecia areata (AA) is a chronic immune-mediated skin disorder characterized by non-scarring hair loss. Although clinical trials have shown the effectiveness and safety of baricitinib, there are limited reports that have shown real-world evidence of baricitinib in patients with AA. Herein, we present the real-world data of 17 Japanese adult patients with AA treated with baricitinib at Gunma University Hospital, Japan, for 60 weeks. All patients received 4 mg of baricitinib daily without interruption. The SALT20 achievement was 29.4% (5/17) at week 24, 52.9% (9/17) at week 36, 58.9% (11/17) at week 48, and 64.7% (11/17) at week 60. Patients were categorized as early responders (n = 1), gradual responders (n = 8), late responders (n = 3), and non-responders (n = 5). Exacerbations of alopecia occurred in only one patient. Eleven patients experienced improvement in scalp hair loss at week 12, leading to a significant reduction in the AA-IGA score. At week 24, improvements in eyebrows and eyelashes were noted in 12 and 11 patients, respectively, with significant reductions in their ClinRO scores. The duration of the current episode of AA exceeding 4 years significantly reduced the SALT20 achievement rate (p < 0.027). Common adverse effects included acne (n = 4, 23.5%) and mildly decreased renal function (n = 2, 11.7%). In conclusion, our findings indicate that baricitinib has significant potential for improving intractable severe AA.
Introduction
Alopecia areata (AA) is a chronic immune-mediated skin disorder characterized by non-scarring hair loss [1]. The disease burden of alopecia areata is significant, and as the severity of the condition increases, anxiety and depression also rise, leading to decreased work productivity and a deterioration in quality of life [2]. Recent surveys estimate the prevalence of alopecia areata in Japan to be between 1.45% and 2.18%, with approximately 38.90% of patients currently not receiving any treatment. This highlights the critical importance of promoting and establishing effective treatment methods [3]. In June 2022, baricitinib, a medication taken orally that specifically targets and temporarily inhibits Janus kinase 1/2, received approval in Japan for treating severe cases of AA in patients aged over 15 years. Although the effectiveness and safety of baricitinib have been demonstrated in clinical trials [4, 5], there are limited studies providing real-world evidence of baricitinib treatment for AA [6, 7]. This study examines the outcomes of 17 Japanese adult patients treated with baricitinib, providing real-world data on improvement rates.
Materials and methods
Patients with severe-to-very severe AA, who received treatment with baricitinib from June 2020 to September 2024 at Gunma University Hospital, Japan, were included. All patients completed the 60-week treatment with 4 mg baricitinib every day without interruption at our hospital. This retrospective cohort study was approved by the Institutional Review Board of Gunma University (HS2022-166) and conducted in accordance with the principles of the Declaration of Helsinki. Demographic and disease characteristics of AA (According to Alopecia Areata Investigator Global Assessment [AA-IGA] [8], Severity of Alopecia Tool [SALT] score [9], Clinician-Reported Outcome [ClinRO] measured for Eyebrow Hair Loss [0–3] and Eyelash Hair Loss [0–3]) [10], and adverse events were evaluated at baseline and weeks 12, 24, 36, 48, and 60. For analysis, the responder who obtained SALT30 was categorized into three subgroups: early responders who achieved SALT30 within 12 weeks; gradual responders who attained SALT30 after week 12 and up to week 36; and late responders who first achieved SALT30 after week 36 and up to week 52 based on previous report [11]. Statistical analyses for Fisher’s exact test, Wilcoxon matched-pairs signed rank test, and Mann Whitney test were performed using GraphPad Prism software (Version 10).
Results
Baseline demographics and clinical features are presented in Tables 1, 2. Seventeen Japanese patients (10 males, 7 females; mean age ±standard deviation (SD), 33.9 ± 15.0 years) were included. The mean SALT score was 95.6 ± 10.4 (SD), while the mean ClinRO-Eyebrow and ClinRO-Eyelash scores were 2.5 ± 1.0 and 2.4 ± 1.2, respectively. The average duration of the current episode of AA was 3.6 ± 5.3 years. Two patients (11.8%) were classified as severe (50%–94% scalp hair loss), while 15 were very severe (95%–100% scalp hair loss) based on AA-IGA. Six patients (54.5%) presented with alopecia universalis, and eleven (45.5%) exhibited alopecia totalis. Clinical data at baseline and changes observed from weeks 12–60 are illustrated in Figure 1 and Table 3, and representative clinical photographs are presented in the chronological order for each group in Figure 2. The SALT20 achievement rate was 29.4% (5/17) at week 24, 52.9% (9/17) at week 36, 58.9% (11/17) at week 48, and 64.7% (11/17) at week 60. One patient (5.8%) was categorized as an early responder, nine (52.9%) as gradual responders, two (11.8%) as late responders, and five (29.4%) as non-responders (Figure 1A). Exacerbations of alopecia occurred in only one patient. Eleven patients experienced an improvement in their scalp hair loss at week 12 following the initiation of baricitinib, leading to a significant reduction in AA-IGA score (Figure 1B). At week 24 after the start of baricitinib treatment, improvements were observed in eyebrows and eyelashes for 12 and 11 patients, respectively, with significant reductions in ClinRO scores (Figures 1C, D; Table 3). As noted in previous reports [7], the duration of the current episode of AA over 4 years significantly reduced the SALT20 achievement rate (0/3 [0%] in >4 years and 9/11 [81%] in <4 years; p < 0.027, Fisher’s exact test) at week 60. Conversely, others did not did not affect the improvement in hair growth including, gender (6/10 [60%] in male and 5/7 [71.4%] in female; p > 0.99), AA predominant type (3/6 [50%] in universalis and 8/11 [72%] in totalis; p < 0.6), first history of AA (6/7 [85%] in first history and 5/10 [50%] in relapse history; p < 0.304), history of steroid pulse therapy (4/6 [67%] in positive and 7/11 [64%] in negative; p > 0.99), and atopic dermatitis (4/7 [57%]) in AD positive and 7/10 [70%] in AD-negative; p = 0.64: Fisher’s exact test), respectively (Table 4). Common adverse effects include acne (n = 4, 23.5%), mildly decreased renal function (n = 2, 11.7%), herpes zoster (n = 2, 11.7%), neutropenia (n = 1, 5.9%), anemia (n = 1, 5.9%), muscle pain (n = 1, 5.9%), dizziness (n = 1, 5.9%), elevated liver enzymes (n = 1, 9.1%). One patient switched treatment from baricitinib 4 mg–2 mg due to reduced renal function at week 60, and another patient temporarily discontinued the treatment due to atypical mycobacteriosis in the lung but later resumed.
TABLE 1
| Characteristic | Total patients (n = 17) |
|---|---|
| Age, years | |
| Mean (SD) | 33.9 (15) |
| Range | 16–54 |
| Gender | |
| Male | 10 (58.8%) |
| Female | 7 (41.2%) |
| Times since onset of AA, y | |
| Mean (SD) | 11.7 (14.9) |
| Range | 0.8–49 |
| Duration of current episode of AA (yr) | |
| Mean (SD) | 3.6 (5.3) |
| Range | 0.5–20 |
| AA predominant type, n (%) | |
| Universalis | 6 (54.5) |
| Totalis | 11 (45.5) |
| First history of AA, n (%) | 7 (41.1) |
| Histroy of steroid pulse therapy, n (%) | 6 (54.5) |
| Atopic dermatitis, n (%) | 7 (41.1) |
| SALT score | |
| Mean (SD) | 95.6 (10.4) |
| Range | 60–100 |
| ClinRo (Eyebrow) | |
| Mean (SD) | 2.5 (1) |
| Range | 0–3 |
| ClinRo (Eyelash) | |
| Mean (SD) | 2.4 (1.2) |
| Range | 0–3 |
Patient demographics and baseline disease characteristics.
TABLE 2
| Case | Age | Sex | Baseline | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Times since onset of AA (yr) | Duration of current episode of AA (yr) | Type of AA | Atopic Dermatis | SALT | ClinRO (Eyebrow) | ClinRO (Eyelash | |||
| #1 | 42 | M | 2 | 2 | Univarsalis | Yes | 100 | 3 | 3 |
| #2 | 23 | F | 2.3 | 2.3 | Totalis | Yes | 100 | 3 | 3 |
| #3 | 21 | M | 12 | 7 | Totalis | Yes | 100 | 2 | 3 |
| #4 | 27 | F | 0.8 | 0.8 | Totalis | No | 95 | 0 | 0 |
| #5 | 54 | M | 40 | 9 | Univarsalis | Yes | 100 | 3 | 3 |
| #6 | 16 | M | 1.9 | 1.9 | Totalis | No | 100 | 3 | 3 |
| #7 | 52 | F | 49 | 0.5 | Univarsalis | No | 100 | 3 | 3 |
| #8 | 28 | M | 1.9 | 1.7 | Totalis | No | 100 | 2 | 0 |
| #9 | 49 | F | 35 | 20 | Univarsalis | No | 100 | 3 | 3 |
| #10 | 38 | F | 9 | 0.5 | Totalis | Yes | 100 | 3 | 3 |
| #11 | 45 | F | 0.8 | 0.8 | Totalis | No | 80 | 2 | 2 |
| #12 | 18 | M | 8 | 0.6 | Totalis | Yes | 100 | 3 | 3 |
| #13 | 18 | M | 4.6 | 2.5 | Totalis | Yes | 98 | 3 | 3 |
| #14 | 23 | M | 1.3 | 1.3 | Totalis | No | 97 | 3 | 3 |
| #15 | 54 | M | 10 | ND | Univarsalis | No | 100 | 3 | 3 |
| #16 | 15 | F | 6 | ND | Totalis | No | 95 | 0 | 0 |
| #17 | 53 | M | 15 | ND | Univarsalis | No | 60 | 3 | 3 |
Details of 17 patients diagnosed with AA who underwent treatment with a 4 mg dosage of baricitinib.
FIGURE 1
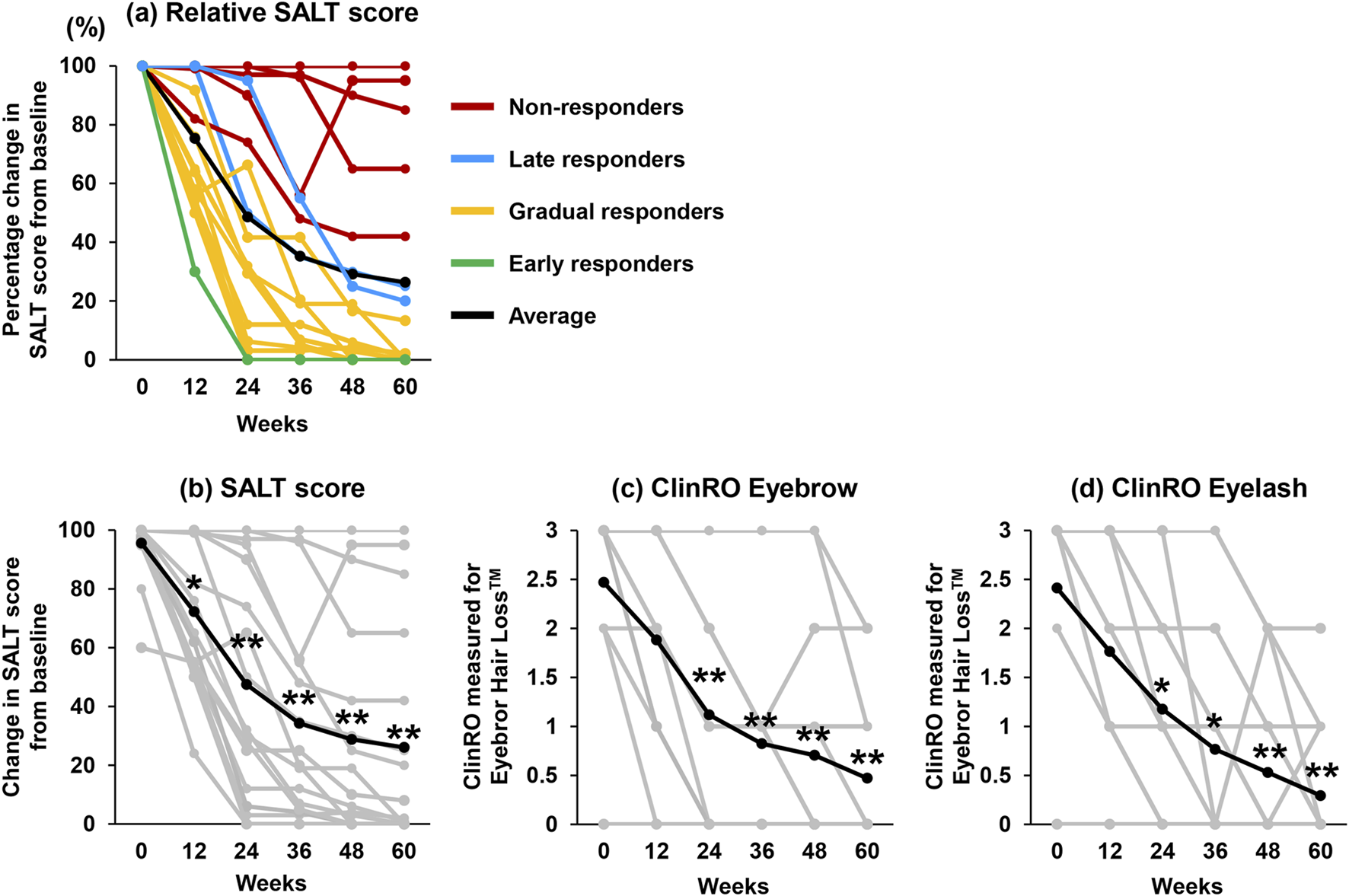
Change in efficacy results from baseline to 60 weeks. (A) Percentage change in SALT score. Nonresponder (red line), late responders (blue line), gradual responders (yellow line), early responders (green line), and average (black line) (n = 17). (B–D) The line chart reveals individual data (gray line) and average (black line) changes in each score at baseline to week 60 after treatment with baricitinib (n = 17). (B) SALT score, (C) ClinRO-Eyebrow score, and (D) ClinRO-Eyelash score. Statistical analyses were conducted with Wilcoxon matched-pairs signed rank test, and Mann Whitney test. *p < 0.05, **p < 0.01.
TABLE 3
| Case | Response type | SALT score | ClinRO eyebrow | ClinRO eyelash | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 12 | 24 | 36 | 48 | 60 | 0 | 12 | 24 | 36 | 48 | 60 | 0 | 12 | 24 | 36 | 48 | 60 (Weeks) | ||
| #1 | Gradual | 100 | 65 | 32 | 7 | 3 | 0 | 3 | 3 | 2 | 1 | 1 | 0 | 3 | 3 | 1 | 1 | 1 | 0 |
| #2 | Gradual | 100 | 76 | 30 | 19 | 19 | 0 | 3 | 1 | 0 | 0 | 0 | 0 | 3 | 1 | 0 | 0 | 0 | 0 |
| #3 | Late | 100 | 100 | 50 | 35 | 30 | 25 | 2 | 2 | 1 | 1 | 1 | 1 | 3 | 2 | 1 | 0 | 0 | 0 |
| #4 | Gradual | 95 | 52 | 3 | 3 | 4 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| #5 | Non | 100 | 82 | 74 | 48 | 42 | 42 | 3 | 2 | 1 | 1 | 0 | 0 | 3 | 2 | 1 | 0 | 0 | 0 |
| #6 | Gradual | 100 | 54 | 12 | 12 | 6 | 1 | 3 | 3 | 2 | 1 | 1 | 1 | 3 | 2 | 1 | 1 | 1 | 1 |
| #7 | Non | 100 | 100 | 100 | 100 | 100 | 100 | 3 | 3 | 3 | 3 | 3 | 2 | 3 | 3 | 3 | 3 | 2 | 0 |
| #8 | Non | 100 | 100 | 100 | 96 | 65 | 65 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| #9 | Non | 100 | 99 | 97 | 97 | 90 | 85 | 3 | 3 | 3 | 3 | 3 | 1 | 3 | 3 | 3 | 3 | 2 | 1 |
| #10 | Late | 100 | 100 | 95 | 55 | 25 | 20 | 3 | 3 | 2 | 1 | 1 | 1 | 3 | 3 | 2 | 1 | 0 | 0 |
| #11 | Early | 80 | 24 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 |
| #12 | Non | 100 | 100 | 90 | 56 | 95 | 95 | 3 | 3 | 2 | 1 | 2 | 2 | 3 | 3 | 3 | 0 | 2 | 2 |
| #13 | Gradual | 98 | 55 | 65 | 20 | 0 | 0 | 3 | 2 | 0 | 0 | 0 | 0 | 3 | 1 | 1 | 1 | 0 | 0 |
| #14 | Gradual | 97 | 62 | 6 | 4 | 0 | 0 | 3 | 2 | 1 | 1 | 0 | 0 | 3 | 2 | 2 | 1 | 0 | 0 |
| #15 | Gradual | 100 | 50 | 0 | 0 | 0 | 0 | 3 | 1 | 0 | 0 | 0 | 0 | 3 | 1 | 0 | 0 | 0 | 0 |
| #16 | Gradual | 95 | 55 | 28 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| #17 | Gradual | 60 | 55 | 25 | 25 | 10 | 8 | 3 | 3 | 2 | 1 | 0 | 0 | 3 | 3 | 2 | 2 | 1 | 0 |
Details of SALT score and ClinRO Eyebrow and Eyelash Scores for each case.
FIGURE 2
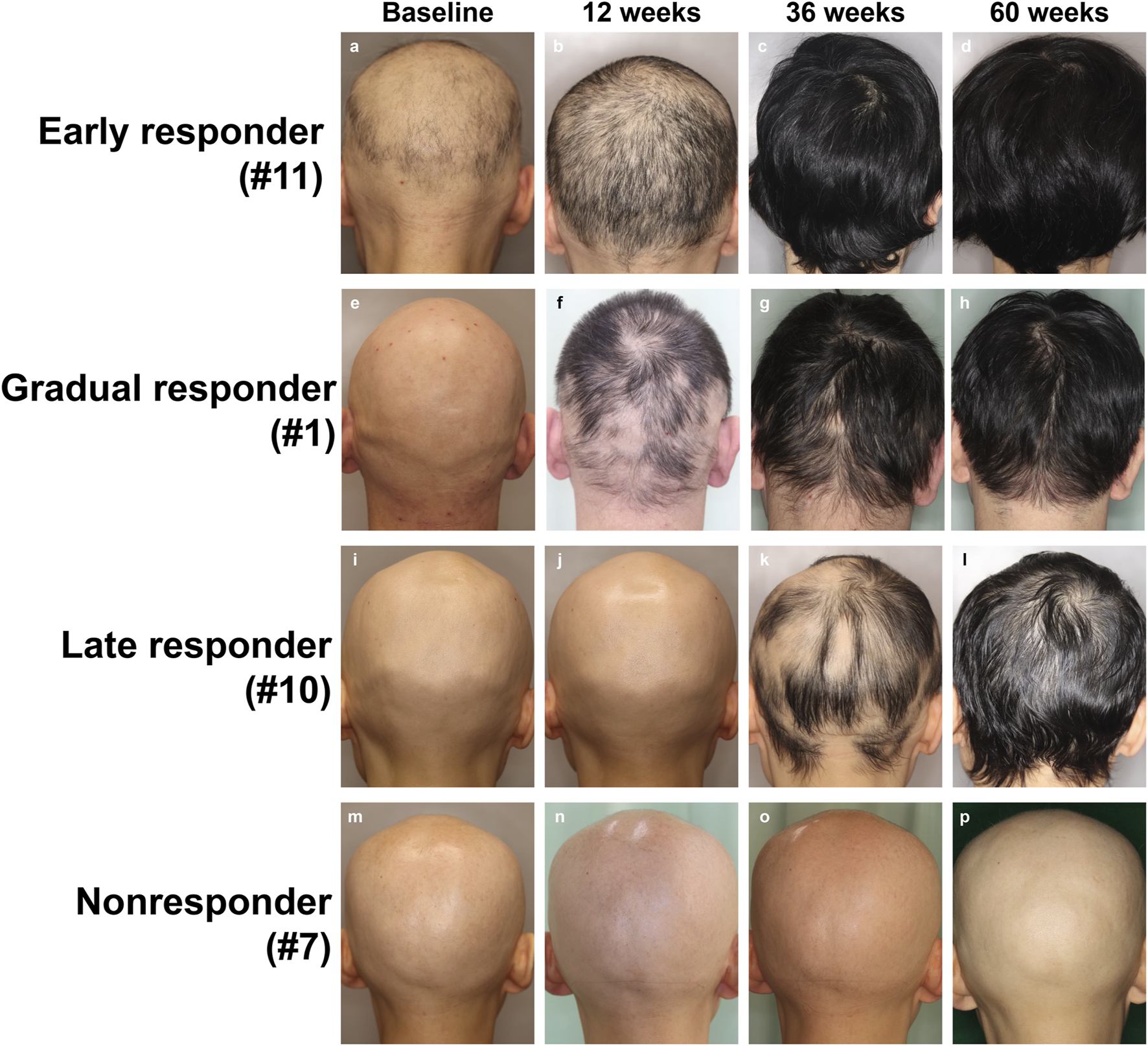
Visual images of each group treated with baricitinib 4 mg from baseline through to week 60. (A–D) Eary responder, (E–H) gradual responder, (I–L) late responder, and (M–P) non-resonder.
TABLE 4
| Adjusted OR (95% CI) | P value | |
|---|---|---|
| Gender | 0.6 (0.08–5.01) | >0.999 |
| Duration of current episode of AA, <4 years | 0 (0.00–0.49) | 0.027* |
| AA predominant type | 0.37 (0.06–2.47) | 0.6 |
| First history of AA | 6 (0.58–80.61) | 0.304 |
| Histroy of steroid pulse therapy | 1.14 (0.11–8.07) | >0.999 |
| Atopic dermatitis | 0.57 (0.09–3.52) | 0.643 |
Analysis of risk factors for refractory alopecia areata following baricitinib treatment.
Statistical analyses were conducted using Fisher’s exact test. *p < 0.05. CI, confidence interval; AA, alopecia areata.
Discussion
In clinical trials, the rate of patients achieving a SALT20 response at week 36 was reported as 35.9%–38.8% [4], and at week 52 as 36.8%–40.9% [5], among those treated with 4 mg of baricitinib. In this retrospective study, SALT20 achievement rate at week 48 (58.8%) was notably higher than that observed in clinical trials. Furthermore, the proportion of patients achieving a ClinRO for eyebrow and eyelash score of 0 or 1 and had 2-point or higher improvement from baseline, was reported as 35.2%–38.9% and 36.2%–36.8% at week 36 [4], and 39.4%–49.7% and 40.7%–50.7% at week 52 in clinical trial [5]. In contrast, our study showed that 80% of patients (12/15) achieved these improvements by week 48, indicating superior outcomes compared to clinical trials. Interestingly, a recent report from China showed significant improvement in AA by baricitinib 2 mg/day treatment at weeks 12 and 24 after treatment. The researchers suggested that these enhanced outcomes may be related to less severe baseline disease and the use of adjunct treatments like topical minoxidil in some patients [12]. These findings imply that baricitinib may be more effective in Japanese or Asian patients than in other racial groups. In a subanalysis of two phase III trials (BRAVE-AA1 and AA2), 23% of the 340 patients were classified in the slow response group, while 9% were in the late response group. In both groups, the treatment response curves did not reach a plateau at 36 weeks, indicating that a longer evaluation period is necessary compared to early responders [11]. The proportion of gradual responders in this study was 47%, which appeared to be higher than that observed in clinical trials. Six out of eight patients (75.0%) achieved a SALT score of 30 or less at 24 weeks, and all achieved a SALT score of 0 at 60 weeks, suggesting that it may be possible to predict subsequent treatment response as early as 24 weeks in this group. On the other hand, late responders still had a SALT score of approximately 20 at 60 weeks, indicating that, beyond requiring long-term evaluation, there may be inherent differences between late responders and gradual responders in terms of their final response to treatment.
Various factors, including autoimmune diseases, are known risk factors for the development of alopecia areata, with atopic dermatitis being particularly significant [13]. As of October 2024, baricitinib remains the only JAK inhibitor indicated for both atopic dermatitis and alopecia areata. In our study, the rate of achieving EASI 20 rate was slightly lower in patients with atopic dermatitis, although this difference was not statistically significant. Previous reports have also demonstrated the efficacy of baricitinib in cases involving both atopic dermatitis and alopecia areata [14, 15]. Therefore, it would be reasonable to prioritize the use of baricitinib in patients with concomitant atopic dermatitis.
For non-responders and patient who experienced a relapse of AA, we are considering the use of ritlecitinib, a JAK3/tyrosine kinase expressed in hepatocellular carcinoma (TEC) family kinase inhibitor. This medication has been approved in Japan since September 2023 for severe cases of AA in individuals aged over 12 years. The potential for switching JAK inhibitors in AA treatment is notable, as successful cases of transitioning from one JAK inhibitor to another, particularly in atopic dermatitis, have been reported [16]. Kazami et al. presented the switching between tofacitinib, a partial and reversible JAK1/3 inhibitor, and baricitinib in patients with AA. Their findings suggested that individuals who did not experience hair regrowth with one JAK inhibitor were less likely to respond to a different JAK inhibitor [17]. The cytokines targeted by JAK1 inhibition cover a wide range, including IL-15, which involves JAK3. Interferon-γ also plays an important role in AA pathogenesis. JAK3 inhibition itself does not directly block interferon-γ signaling, which involves JAK1/2 [18]. However, the inhibition of JAK3 may suppress interferon-γ production by inhibiting the activation and proliferation of cytotoxic T cells because IL-15 receptor has JAK3 expressed on cytotoxic T cells. In fact, it has been reported that in C3H/HeJ mice, a mouse model of alopecia areata, retlecitinib treatment reduced the number of infiltrating CD8+ interferon-γ-producing cells in lesions with significant differences [19]. Recent meta-analysis findings have shown that ritlecitinib at a dose of 50 mg had a relatively lower odds ratio for achieving SALT50 compared to baricitinib at 4 mg (odds ratio: 3.2 vs. 7.7) [20]. On the other hand, TEC family kinase modulation of T-cell receptor signaling in immune cells, including CD8+ T-cells, presents a distinct mechanism compared to traditional JAK inhibition [21]. Thus, ritlecitinib may be effective in AA cases refractory to JAK1/2 inhibitors, although there is no current evidence of switching from baricitinib to ritlecitinib for non-responders. Our future research will carefully evaluate the efficacy of switching from JAK1/2 inhibitors to JAK3/TEC family kinase inhibitors.
In conclusion, our findings suggest that baricitinib has considerable potential in improving intractable severe AA in Japanese patients, with a higher response rate observed in our cohort compared to previous clinical trials. While no severe adverse effects were identified in our cases, long-term safety remains an important consideration. Determining the optimal timing for reducing drug doses in responders is also crucial, and should be evaluated on an individual basis as treatment progresses.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Institutional Review Board of Gunma University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
AU, TA, KK, and S-IM conducted the clinical work and collected information, AU and S-IM wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
|The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to thank members of the department of dermatology in Gunma University Hospital for their assistance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. The authors used ChatGPT for grammar check.
References
1.
Pratt CH King LE Jr Messenger AG Christiano AM Sundberg JP . Alopecia areata. Nat Rev Dis Primers (2017) 3:17011. 10.1038/nrdp.2017.11
2.
Edson-Heredia E Aranishi T Isaka Y Anderson P Marwaha S Piercy J . Patient and physician perspectives on alopecia areata: a real-world assessment of severity and burden in Japan. J Dermatol (2022) 49(6):575–83. 10.1111/1346-8138.16360
3.
Aranishi T Ito T Fukuyama M Isaka Y Mackie DS King-Concialdi K et al Prevalence of alopecia areata in Japan: estimates from a nationally representative sample. J Dermatol (2023) 50(1):26–36. 10.1111/1346-8138.16606
4.
King B Ohyama M Kwon O Zlotogorski A Ko J Mesinkovska NA et al Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med (2022) 386(18):1687–99. 10.1056/NEJMoa2110343
5.
Kwon O Senna MM Sinclair R Ito T Dutronc Y Lin CY et al Efficacy and safety of baricitinib in patients with severe alopecia areata over 52 weeks of continuous therapy in two phase III trials (BRAVE-AA1 and BRAVE-AA2). Am J Clin Dermatol (2023) 24(3):443–51. 10.1007/s40257-023-00764-w
6.
Moreno-Vílchez C Bonfill-Ortí M Bauer-Alonso A Notario J Figueras-Nart I . Baricitinib for the treatment of alopecia areata in adults: real-world analysis of 36 patients. J Am Acad Dermatol (2024) 90(5):1059–61. 10.1016/j.jaad.2023.09.089
7.
Numata T Irisawa R Mori M Uchiyama M Harada K . Baricitinib therapy for moderate to severe alopecia areata: a retrospective review of 95 Japanese patients. Acta Derm Venereol (2024) 104:adv18348. 10.2340/actadv.v104.18348
8.
Wyrwich KW Kitchen H Knight S Aldhouse NVJ Macey J Nunes FP et al The Alopecia Areata Investigator Global Assessment scale: a measure for evaluating clinically meaningful success in clinical trials. Br J Dermatol (2020) 183(4):702–9. 10.1111/bjd.18883
9.
Olsen EA Hordinsky MK Price VH Roberts JL Shapiro J Canfield D et al Alopecia areata investigational assessment guidelines--Part II. National Alopecia Areata Foundation. J Am Acad Dermatol (2004) 51(3):440–7. 10.1016/j.jaad.2003.09.032
10.
Wyrwich KW Kitchen H Knight S Aldhouse NVJ Macey J Nunes FP et al Development of clinician-reported outcome (ClinRO) and patient-reported outcome (PRO) measures for eyebrow, eyelash and nail assessment in alopecia areata. Am J Clin Dermatol (2020) 21(5):725–32. 10.1007/s40257-020-00545-9
11.
King B Shapiro J Ohyama M Egeberg A Piraccini BM Craiglow B et al When to expect scalp hair regrowth during treatment of severe alopecia areata with baricitinib: insights from trajectories analyses of patients enrolled in two phase III trials. Br J Dermatol (2023) 189(6):666–73. 10.1093/bjd/ljad253
12.
Bi L Wang C Du Y Su T Zhao M Lin X et al Effectiveness and safety of baricitinib in patients with moderate-to-severe refractory alopecia areata in real world: an open-label, single-center study. J Cosmet Dermatol (2023) 23(4):1417–21. 10.1111/jocd.16123
13.
Chanprapaph K Mahasaksiri T Kositkuljorn C Leerunyakul K Suchonwanit P . Prevalence and risk factors associated with the occurrence of autoimmune diseases in patients with alopecia areata. J Inflamm Res (2021) 14:4881–91. 10.2147/JIR.S331579
14.
Fang H Zhang F Lin W Jiang Y Liu Q Yang D . Case report: sequential therapy with dupilumab and baricitinib for severe alopecia areata with atopic dermatitis in children. Front Immunol (2024) 15:1395288. 10.3389/fimmu.2024.1395288
15.
Uchida H Kamata M Nagata M Fukaya S Hayashi K Fukuyasu A et al Baricitinib improved alopecia areata concomitant with atopic dermatitis: a case report. J Dermatol (2021) 48(9):e472–e473. 10.1111/1346-8138.16024
16.
Hagino T Yoshida M Hamada R Saeki H Fujimoto E Kanda N . Effectiveness of switching from baricitinib 4 mg to upadacitinib 30 mg in patients with moderate-to-severe atopic dermatitis: a real-world clinical practice in Japan. J Dermatolog Treat (2023) 34(1):2276043. 10.1080/09546634.2023.2276043
17.
Kazmi A Moussa A Bokhari L Bhoyrul B Joseph S Chitreddy V et al Switching between tofacitinib and baricitinib in alopecia areata: a review of clinical response. J Am Acad Dermatol (2023) 89(6):1248–50. 10.1016/j.jaad.2023.03.041
18.
Sardana K Bathula S Khurana A . Which is the ideal JAK inhibitor for alopecia areata - baricitinib, tofacitinib, ritlecitinib or ifidancitinib - revisiting the immunomechanisms of the JAK pathway. Indian Dermatol Online J (2023) 14(4):465–74. 10.4103/idoj.idoj_452_22
19.
Dai Z Chen J Chang Y Christiano AM . Selective inhibition of JAK3 signaling is sufficient to reverse alopecia areata. JCI Insight (2021) 6(7):e142205. 10.1172/jci.insight.142205
20.
Husein-ElAhmed H Husein-ElAhmed S . Comparative efficacy of oral Janus kinase inhibitors and biologics in adult alopecia areata: a systematic review and Bayesian network meta-analysis. J Eur Acad Dermatol Venereol (2024) 38(5):835–43. 10.1111/jdv.19797
21.
Gravano DM Hoyer KK . Promotion and prevention of autoimmune disease by CD8+ T cells. J Autoimmun (2013) 45:68–79. 10.1016/j.jaut.2013.06.004
Summary
Keywords
alopecia areata, baricitinib, real-world data, retrospective study, Japanese
Citation
Uchiyama A, Araki T, Kosaka K and Motegi S-I (2025) Real-life effectiveness and safety of baricitinib in 17 Japanese patients with alopecia areata: a 60-week single center study. J. Cutan. Immunol. Allergy 7:13890. doi: 10.3389/jcia.2024.13890
Received
06 October 2024
Accepted
26 December 2024
Published
20 January 2025
Volume
7 - 2025
Updates
Copyright
© 2025 Uchiyama, Araki, Kosaka and Motegi.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Akihiko Uchiyama, akihiko1016@gunma-u.ac.jp
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.