Abstract
Hematologic malignancies, diabetes, and the development and widespread use of novel anticancer and immunosuppressive agents have introduced a new dimension of immunosuppression, raising concerns about emerging opportunistic infections. Here, we report a case of mucormycosis in a patient with therapy-related acute myeloid leukemia, encountered in our department. The patient presented with a necrotic lesion around the right eyelid. In this case, the condition and skin lesions rapidly progressed, and the patient passed away within a few days of the appearance of necrotic plaques. The causative organism was identified as Rhizopus microsporus. Mucormycosis is a severe invasive fungal infection that can rapidly worsen the prognosis of immunocompromised patients. Cutaneous mucormycosis is classified into primary and secondary forms. Based on the findings and the underlying disease, secondary cutaneous mucormycosis was suspected. Familiarity with the characteristic clinical features of mucormycosis may be the key factor in initiating timely treatment.
Introduction
Hematologic malignancies, the increasing prevalence of diabetes, chemotherapy for cancer treatment, and the global use of newly approved immunosuppressive therapies are the causative factors contributing to the growing number of immunocompromised patients worldwide [1]. These conditions make patients more vulnerable to opportunistic infections caused by organisms that have traditionally received little attention or were previously regarded as innocuous [2]. One such infection is mucormycosis, a serious fungal disease. Mucormycosis poses a significant threat to immunocompromised patients, particularly because it progresses rapidly and leaves physicians with limited time for diagnosis and intervention [3]. The infection can severely compromise patient outcomes, and recognizing the clinical features of secondary mucormycosis is critical for timely management. Here, we report a case that underscores the importance of early identification and awareness of this emerging opportunistic infection.
Case description
A 60-year-old Japanese male with a history of diffuse large B-cell lymphoma, multiple rounds of chemotherapy, and autologous peripheral blood stem cell transplantation presented to our department with a necrotic lesion around the right eyelid. He had been diagnosed with therapy-related acute myeloid leukemia 3 months earlier and underwent chemotherapy. The patient did not have diabetes mellitus. After 6 weeks of treatment, a necrotic plaque with swelling and pain developed in the right periorbital area, suggesting secondary cutaneous mucormycosis. Plain computed tomography (CT) performed 2 weeks before the onset of skin symptoms revealed findings suggestive of sinusitis in the right maxillary sinus, indicating possible direct invasion from the rhinocerebral type. At the time of appearance of skin symptoms, white blood cell count was 0.1 × 103/µL, red blood cell count was 3.31 × 106/µL, platelet count was 4.0 × 103/µL, C reactive protein was 20.58 mg/dL, and β-D-glucan was 7.5 pg/mL. Chest x-ray revealed no lung abnormalities. Blood cultures detected Escherichia coli. Due to pancytopenia and the patient’s poor overall condition, a skin biopsy could not be performed. The previous physician initiated intravenous posaconazole (300 mg every 12 h), but the condition rapidly progressed to edema and necrosis, extending from the right nose to the cheek (Figure 1). This was followed by left conjugate deviation, hypoxia, and the patient’s passing on the fifth day.
FIGURE 1
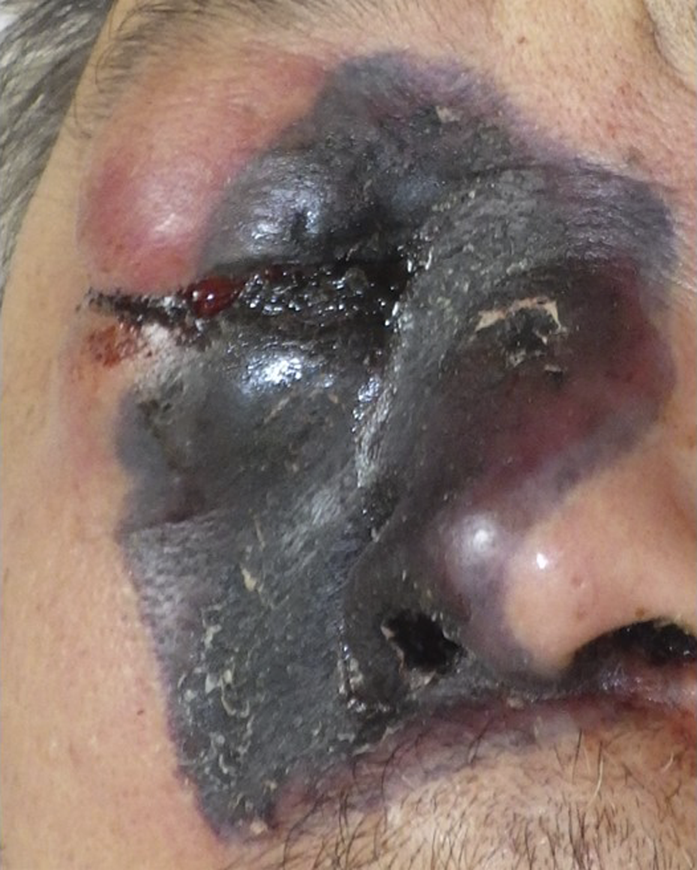
Clinical manifestation. Edema and necrosis of the right nose to the cheek.
Necrotic tissue samples collected during the initial visit were inoculated on Sabouraud’s dextrose agar and yielded a white-gray mold (Figure 2), identified as Rhizopus microsporus through internal transcribed spacer region sequence analysis. Treatment with posaconazole was initiated when cutaneous mucormycosis was suspected; however, the patient had already succumbed to the disease by the time the causative microorganism was identified.
FIGURE 2
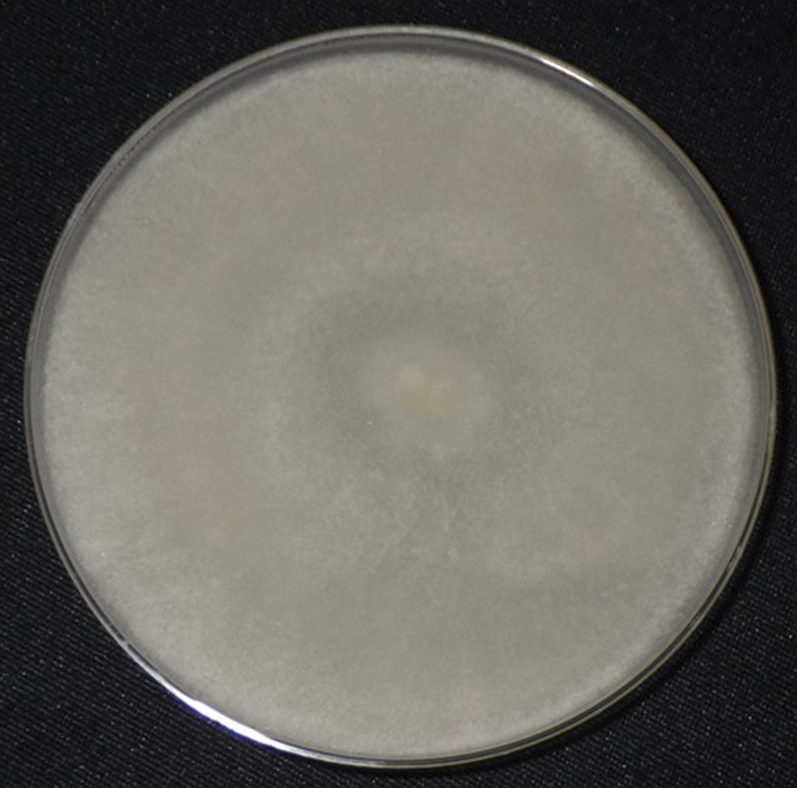
Colonies on a Sabouraud’s dextrose agar medium incubated at 25°C for 3 days. White-gray mold yielded.
Discussion
Cutaneous mucormycosis is an emerging fungal infection caused by fungi of the phylum Glomeromycota [4]. It presents in two clinical forms: primary and secondary cutaneous mucormycosis [5]. Primary cutaneous mucormycosis, often resulting from trauma, is more common in immunosuppressed patients and generally has a favorable prognosis when diagnosed early [5].
Secondary cutaneous mucormycosis, which typically arises from either rhinocerebral or disseminated mucormycosis, is more common in patients with uncontrolled diabetes or severe immunosuppression [5]. Its rapid progression and high mortality rate make early diagnosis particularly challenging [4]. The fatal outcome is primarily due to the severe infection, compounded by the patient’s underlying disease. Rhinocerebral mucormycosis often presents with initial symptoms such as headache, fever, periocular pain, and facial swelling [6]. This type results from direct invasion of mucosal epithelium by fungal spores from the external environment. Hematogenous dissemination from rhinocerebral mucormycosis is rare, making progression to the disseminated type uncommon. Consequently, fungal pathogens are rarely detected in blood cultures in cases of rhinocerebral mucormycosis. Plain computed tomography (CT) imaging usually reveals nonspecific findings, including unilateral sinus mucoperiosteal thickening [6]. In contrast, disseminated mucormycosis involves two or more noncontiguous organs, with CT imaging often demonstrating abscesses or nodules in the affected organs [1]. Secondary cutaneous mucormycosis may originate from either the rhinocerebral or disseminated type, depending on the primary site of infection.
In this case, the diagnosis of secondary cutaneous mucormycosis was made based on the remarkable clinical features and isolation of a fungus from the mucorales group, despite the absence of histopathological confirmation. We considered direct invasion from the rhinocerebral type. Ecthyma gangrenosum should be included in the differential diagnosis given the necrotic clinical presentation and the patient’s immunosuppressed state [7]. Unfortunately, treatment initiated after the appearance of skin lesions in secondary mucormycosis is often too late, and saving the patient’s life is difficult. Pathology and culture required for definitive diagnosis of fungal infections may not be available, and no commercially available screening tests specifically target mucormycosis [8, 9]. As a result, definitive diagnosis and early antifungal therapy are rare. Treatment typically involves debridement, management of the underlying immunosuppressive state, and antifungal therapy, with high-dose (5 mg/kg/day) liposomal amphotericin B (L-AMB) being the standard of care [8].
The rapidly progressing necrotic lesion in the periorbital region, with sharply demarcated borders in an immunocompromised host, is a hallmark of secondary cutaneous mucormycosis. Secondary cutaneous mucormycosis associated with rhinocerebral involvement carries a poor prognosis. Skin infections in patients with underlying disease should prompt suspicion of mucormycosis, requiring confirmation of characteristic clinical features and imaging studies. Therefore, it is crucial to continue raising awareness of this condition, which is the purpose of this report.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Care was taken to ensure that no personal information was identified in the study. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements because verbal consent was obtained and the patient died before written consent could be obtained. Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article because verbal consent was obtained and the patient died before written consent could be obtained.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
References
1.
Liang M Xu J Luo Y Qu J . Epidemiology, pathogenesis, clinical characteristics, and treatment of mucormycosis: a review. Ann Med (2024) 56:2396570. 10.1080/07853890.2024.2396570
2.
Gorensek MJ . The immunocompromised host. Dermatol Clin (1989) 7:353–68. 10.1016/s0733-8635(18)30605-3
3.
Bhaduri S Kurrle E Vanek E Spanel R . Mucormycosis in the immunocompromised host. Infection (1983) 11:170–2. 10.1007/BF01641299
4.
Castrejón-Pérez AD Welsh EC Miranda I Ocampo-Candiani J Welsh O . Cutaneous mucormycosis. An Bras Dermatol (2017) 92:304–11. 10.1590/abd1806-4841.20176614
5.
Bonifaz A Vázquez-González D Tirado-Sánchez A Ponce-Olivera RM . Cutaneous zygomycosis. Clin Dermatol (2012) 30:413–9. 10.1016/j.clindermatol.2011.09.013
6.
Scheckenbach K Cornely O Hoffmann TK Engers R Bier H Chaker A et al Emerging therapeutic options in fulminant invasive rhinocerebral mucormycosis. Auris Nasus Larynx (2010) 37:322–8. 10.1016/j.anl.2009.09.001
7.
Reich HL Williams Fadeyi D Naik NS Honig PJ Yan AC . Nonpseudomonal ecthyma gangrenosum. J Am Acad Dermatol (2004) 50(5 Suppl. l):S114–7. 10.1016/j.jaad.2003.09.019
8.
Kanda Y . Diagnosis and treatment of invasive mucormycosis. Med Mycol J (2023) 64:77–82. 10.11534/ishinkin.23.005
9.
Kakeya H . Exploratory research for new diagnostic markers of mucormycosis. Med Mycol J (2024) 65:29–32. 10.3314/mmj.24.002
Summary
Keywords
immnocompromised, mucormycosis, Rhizopus microspores , acute myeloid leukemia, infection
Citation
Higuchi M, Nishimoto K, Waseda T, Takenaka M and Murota H (2025) Case report: Mucormycosis due to Rhizopus microsporus: an important reminder of opportunistic infections in immunocompromised acute myeloid leukemia. J. Cutan. Immunol. Allergy 7:13995. doi: 10.3389/jcia.2024.13995
Received
28 October 2024
Accepted
09 December 2024
Published
03 January 2025
Volume
7 - 2024
Updates
Copyright
© 2025 Higuchi, Nishimoto, Waseda, Takenaka and Murota.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Misato Higuchi, micha3310@outlook.jp
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.