Dear Editors,
Toxic epidermal necrolysis (TEN)/Stevens–Johnson syndrome (SJS), life-threatening drug reactions presenting with fever, diffuse erythema with mucosal involvement, and varying degrees of visceral damage, are differentiated according to the area of epidermal necrotic changes observed (In Japan, TEN is defined as involvement of >10% and SJS as <10% of the body surface area). Anti-programmed cell death protein 1 (anti-PD-1) antibodies enhancing the immune response against tumor cells can induce severe cutaneous adverse reactions (SCARs) as immune-related adverse events (irAEs) [1]. Herein, we report a case of TEN caused by nivolumab administration for intraperitoneal cancer.
A Japanese man in his 80s undergoing regular hemodialysis for chronic renal failure presented with conjunctival hyperemia, pharyngalgia, fever, body-wide coalescent edematous erythema, and a positive Nikolsky sign (Figure 1A). He had been treated for an unresectable intraperitoneal cancer that was probably derived from a poorly differentiated gastric adenocarcinoma. The treatment consisted of four courses of nivolumab (3 mg/kg biweekly) as third-line therapy after unsuccessful paclitaxel monotherapy and paclitaxel/ramucirumab combination therapy. Our patient developed the symptoms approximately 7 days after the last dose of nivolumab. Leukocytosis, elevated C-reactive protein levels, and elevated levels of biliary enzymes were detected, and the serotypes of class I human leukocyte antigen (HLA) were A24/33 and B58/60. Histopathological examination of the abdominal skin revealed moderate perivascular lymphocytic infiltration and diffuse epidermal necrosis with subepidermal blistering (Figures 1B, C). The infiltrating lymphocytes were mainly CD8 +, and the lesional epidermis was strongly positive for programmed death-ligand 1 (PD-L1) (Figures 1D, E). The clinical and laboratory findings led to a diagnosis of TEN. There were no imaging findings indicative of mycoplasma pneumonia, and no new medications, aside from nivolumab, had been initiated prior to appearance of patient’s skin rash. After a course of methylprednisolone pulse therapy (1000 mg/day for 3 days) followed by prednisolone 80 mg/day (i.e. 1.5 mg/kg/day), the eroded lesions re-epithelialized on day 21 without exacerbation of visceral and infectious symptoms. However, the patient died due to tumor progression on day 25.
FIGURE 1
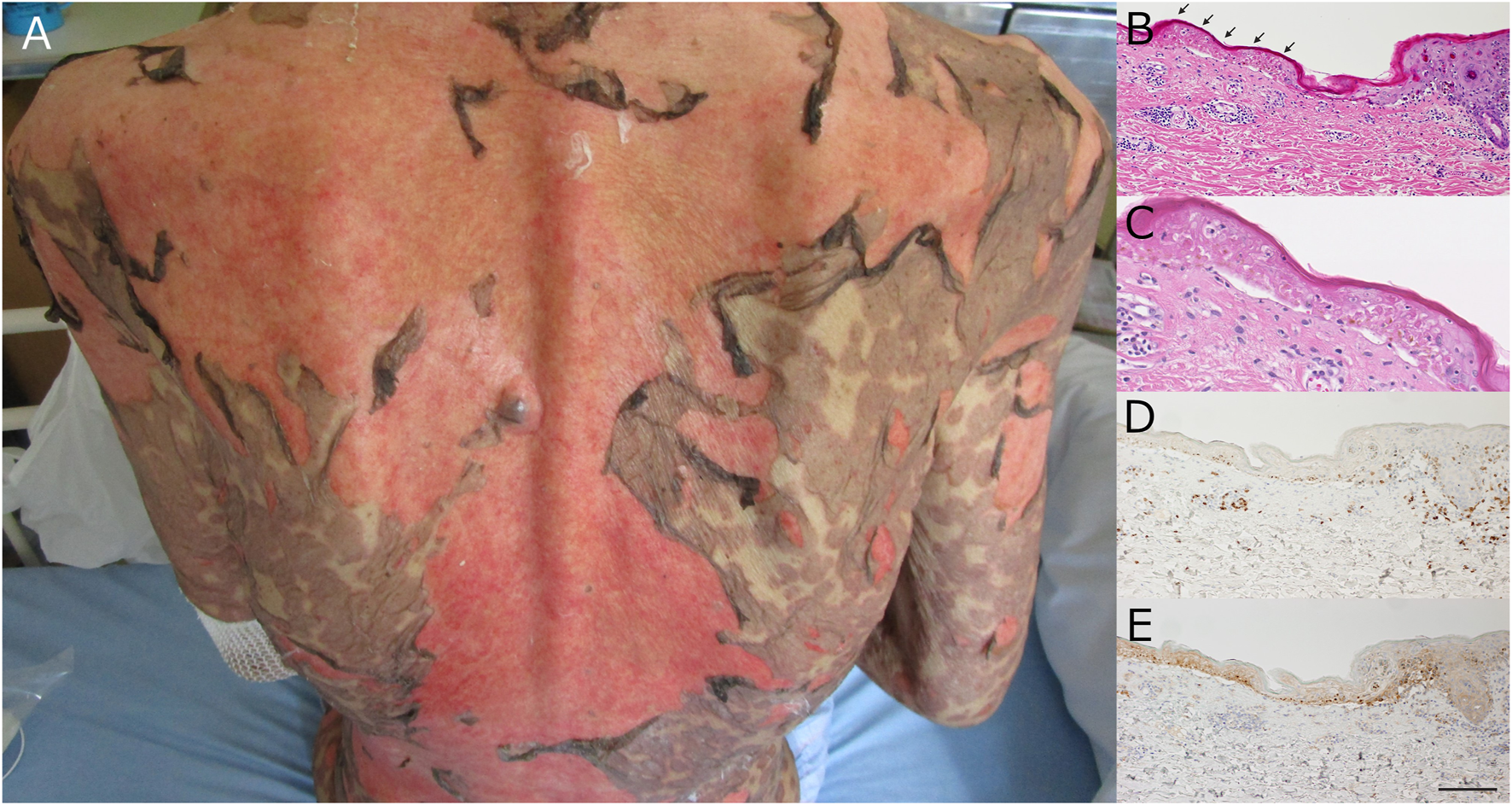
(A) Clinical appearance. Epidermal necrosis and erosion were evident on >30% of the body surface area, and the Nikolsky sign was positive. (B) Histopathology of the lesional edge. Epidermal necrosis can be observed throughout all layers (arrows) with perivascular lymphocytic infiltration. (C) Close-up View of the Arrowed Area in Figure (B). (D) Immunohistochemistry for CD8. The infiltrating lymphocytes were mainly CD8 positive. CD8, cluster of differentiation 8. (E) Immunohistochemistry for PD-L1. The necrotic epidermis is strongly positive for PD-L1. PD-L1, programmed death ligand 1 Scale bar: 200 µm.
SJS/TEN is a rare irAE induced by immune checkpoint inhibitors (ICIs) in patients with SCARs. Although prednisone/methylprednisolone therapy at 1–2 mg/kg is recommended for grade 4 SCARs based on the ASCO-NCCN management guidelines for irAEs, a standard treatment strategy has not been established for cases of ICI-related SJS/TEN. Although these patients were mainly treated with corticosteroids and/or immunosuppressants, the unfavorable effects of immunosuppressive therapy on tumor progression cannot be eliminated. In most cases, however, the discontinuation of ICI therapy following SCARs might be more directly associated with the prognosis. Immunoglobulins can be metabolized into amino acids and other proteins without urinary excretion, and ICIs such as nivolumab and pembrolizumab have been safely and effectively used in patients receiving regular hemodialysis [2]. Therefore, renal failure is believed to have minimally affected the patient’s clinical course, and impaired renal function is not a restricting factor in monoclonal antibody therapy.
Although the detailed mechanism of SCARs has not been fully elucidated, dysregulated immunotolerance against autoantigens via the inhibition of the PD-1/PD-L1 interaction could be crucial. Interestingly, in this case, PD-L1 expression was upregulated in the lesional epidermis of the nivolumab-related TEN. Although upregulated PD-L1 expression can contribute to the protective effect on the tissue against excessively enhanced cytotoxic autoimmune reactions, represented by the lesional infiltration of CD8-positive cytotoxic T cells, the autoimmune reaction activated by PD-1 inhibition can overwhelm the increased epidermal expression of PD-L1 (Figure 1E) [3, 4]. We used monoclonal mouse anti-human CD8 (C8/144B, DakoCytomation) and rabbit monoclonal antibody PD-L1 (E1L3N®) XP® for each.
HLA serotypes may also be involved in the pathophysiology of irAEs. Recent progress in basic research has revealed that the response of ICIs against tumors can be influenced by HLA class I genotypes [5]. Therefore, the serotype of the HLA class detected in this case might have affected the pathomechanism of ICI-related TEN.
In general, TEN is a rare irAE that shortens the survival time of patients and its frequency can increase with ICI use. As life-threatening irAE-related SCAR and discontinuation of ICIs due to SCARs are both crucial factors that affect patient prognosis, the details of these cases should be recorded.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article because the patient died and had no relatives.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
References
1.
Brahmer JR Lacchetti C Atkins MB Brassil KJ Caterino JM Chau I et al Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol (2018) 36:1714–768. 10.1200/JCO.2017.77.6385
2.
Ishizuka S Sakata S Fujii K Takaki A Saeki S Nakamura K et al Successful treatment by pembrolizumab in a patient with end-stage renal disease with advanced non-small cell lung cancer and high PD-L1 expression. Respir Investig (2018) 56:361–4. 10.1016/j.resinv.2018.03.005
3.
Goldinger SM Stieger P Dummer R Micaletto S Contassot E French LE et al Cytotoxic cutaneous adverse drug reactions during anti-PD-1 therapy. Clin Cancer Res (2016) 22:4023–9. 10.1158/1078-0432.CCR-15-2872
4.
Vivar KL Deschaine M Seminario-Vidal L Divine JM Rabionet A Patel N et al Epidermal programmed cell death-ligand 1 expression in TEN associated with nivolumab therapy. J Cutan Pathol (2017) 44:381–4. 10.1111/cup.12876
5.
Chowell D Morris LGT Chan TA Weber JK Samstein RM Makarov V et al Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy. Science (2018) 359(6375):582–7. 10.1126/science.aao4572
Summary
Keywords
checkpoint inhibitors, adverse event, PD-1, PD-L1, hemodialysis
Citation
Nozaki H, Uehara J, Kato N, Honma M and Ishida-Yamamoto A (2025) Toxic epidermal necrolysis induced by nivolumab therapy for unresectable intraperitoneal cancer. J. Cutan. Immunol. Allergy 7:14014. doi: 10.3389/jcia.2024.14014
Received
31 October 2024
Accepted
17 December 2024
Published
06 January 2025
Volume
7 - 2025
Updates
Copyright
© 2025 Nozaki, Uehara, Kato, Honma and Ishida-Yamamoto.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hiroyoshi Nozaki, hnozaki@asahikawa-med.ac.jp
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.