- 1Department of Dermatology, Kanazawa Medical University, Uchinada, Ishikawa, Japan
- 2Center for Clinical Genomics, Kanazawa Medical University, Uchinada, Ishikawa, Japan
Hypohidrotic ectodermal dysplasia (HED) is a rare disease. Patients with HED present with sparse hair on the head, dysplastic teeth and anhidrosis since childhood, along with atopic dermatitis-like skin manifestations. We report a 20-year-old male HED patient with atopic dermatitis-like skin who was successfully treated with dupilumab. Genetic analysis identified a splicing mutation in the EDA gene, NG_009809.2 (NM_001399.5):c.793 + 3A>C r.742_793del p.Pro248Ilefs Ter15, which has never been reported before. The subject presented to our department because of worsening generalized itching. Based on the distribution of the skin rash, the patient was diagnosed with atopic dermatitis and started on dupilumab. The skin rash became less severe in the third month of treatment. The potential of dupilumab in treating genodermatoses associated with Th2 immunity is known. Although the skin rash is related to atopic dermatitis-like skin manifestations of HED or independent atopic dermatitis is unclear, dupilumab may be a candidate for the treatment of HED with atopic dermatitis skin.
Introduction
Hypohidrotic ectodermal dysplasia (HED) is an inherited condition whose three main symptoms are anhidrosis, sparse hair and hypoplasia of the teeth. Patients also present with characteristic facial features (protruding forehead, thick lips, ectropion of the lower lip, auricular hypoplasia, low saddle nose and periocular pigmentation) [1]. Many patients are diagnosed at approximately 1 year of age, and it may be difficult then to determine whether the dental and hair findings are abnormal, but the aforementioned characteristic facial features and abnormal sweating are considered important findings for diagnosis. Thermal sweating tests generally show widespread anhidrosis and skin biopsies often show absent or hypoplastic sweat glands. The skin is described to be very dry and often presents with skin symptoms similar to those of atopic dermatitis. Inazawa-Terada et al. summarized the clinical characteristics of patients with anhidrotic/hypohidrotic ectodermal dysplasia (A/HED), the status of genetic aberrations and complications of A/HED in Japan and revealed that in A/HED patients there is a significantly higher incidence of atopic dermatitis-like cutaneous manifestations [2], bronchial asthma and food allergies compared to healthy controls [2]. In the present study, we report on a case of a HED due to a novel EDA splicing variant with concomitant severe atopic dermatitis-like cutaneous manifestations that were successfully treated with dupilumab.
Case report
A 20-year-old man presented to our hospital complaining of itching. The patient had a history of childhood asthma. From birth, sparse hair on the head, anhidrosis, abnormal teeth formation and generalized dryness were observed; at the age of 15, a genetic analysis revealed an EDA gene mutation and a diagnosis of HED was made. There was no family history of such a condition. At the same time, the patient was also diagnosed with atopic dermatitis for generalized dryness and itching, and various topical treatments centered on topical steroids were carried out by his doctor. However, as no improvement in the symptoms was observed, the patient was referred to our department.
The face was dark red throughout, with lichenification and erythema on the forehead, periocular and perioral areas (Figure 1A). Eyebrows were absent and the hair on the head was sparse. Hair remained in the occipital area. Facial features were characteristic: a depressed nasal bridge, a small mouth with thick lips, a protruding forehead, and brown pigmentation around the eyes. The posterior neck showed pale erythematous plaques (Figure 1B). The majority of the teeth were missing and conical (Figure 1C). Dry skin and lichenified erythema on the trunk (Figures 1D, E). Dry skin and lichenified erythema were also observed in both knee fossae (Figure 1F), with similar findings in both elbow fossae. Blood tests were as follows; WBC 6960/µL, Neu 69.2%, Lym 18.5%, Eos 5.3%, Baso 0.7%, RBC 498 × 106/µL, Hb 15.2 g/dL, Ht 47.2, CRP 0.14 mg/dL, TP 7.7 g/dL, Alb 4.7 g/dL, CK 148 U/L, Na 141 mEq/L, K 4.3 mEq/L, Cl 103 mEq/L, Ca 9.8 mg/dL, P 3.4 mg/dL, AST 23 U/L, ALT 33 U/L, ɤ-GTP 38 U/L, BUN 10 g/dL, Cre 0.76 mg/dL, IgE 2,167 (<170) IU/mL, TARC 358 pg/mL.
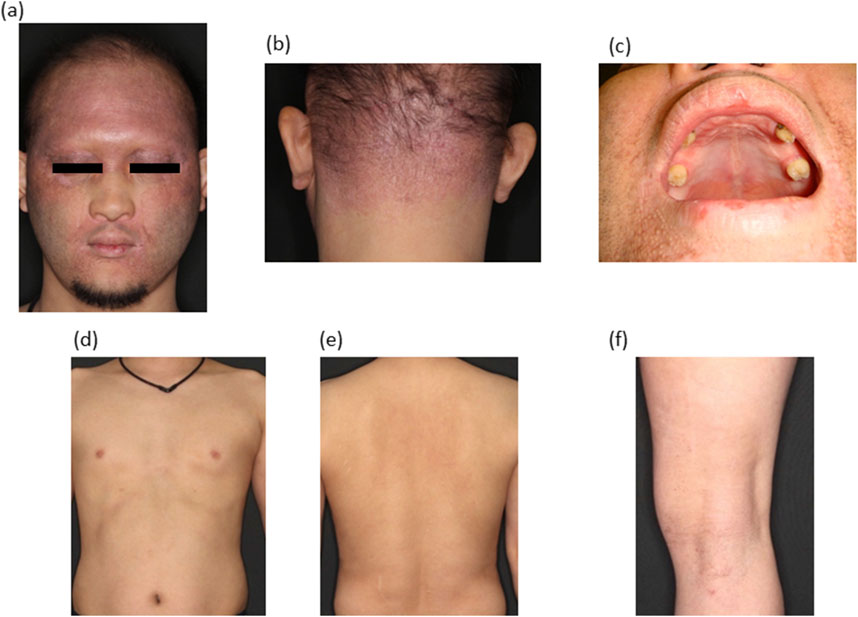
Figure 1. Clinical manifestations. (A) Frontal view of the face. Lichenified erythema can be observed. (B) Posterior neck with lichenified erythema. (C) Numerous oral teeth are missing. Conical teeth are present. (D) Dry skin on the chest and abdomen. (E) Lichenified erythema on the back. (F) Dry skin and lichenified erythema on the popliteal fossa.
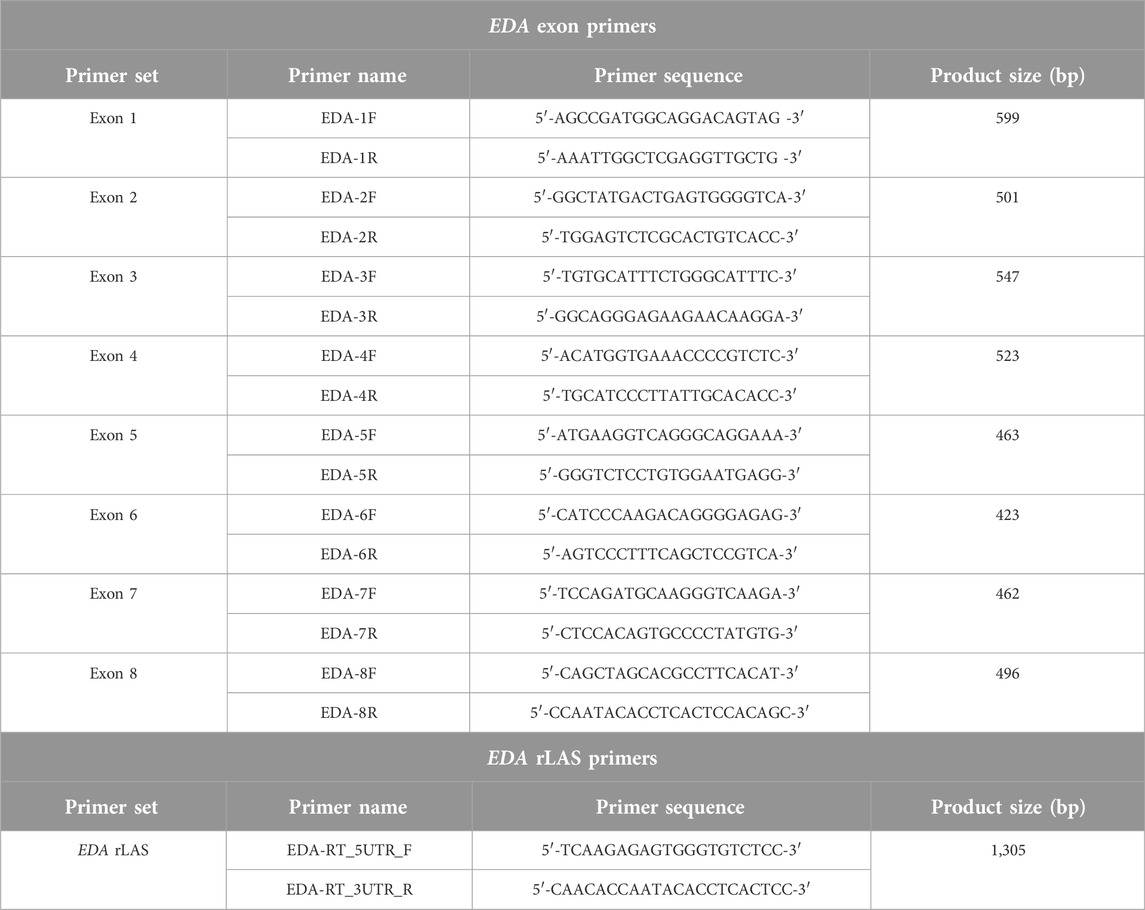
After written informed consent was obtained, genetic testing for the EDA gene was performed. The study design was approved by the ethics review board of Kanazawa Medical University. Direct sequencing of each exon revealed a heterozygous single base substitution at the +3 position of the exon 6 splice donor. We further analyzed the gene transcripts using reverse transcribed long amplicon sequencing (rLAS) as previously described [3] and identified exon 6 skipping in the gene, resulting in a premature termination codon, NG_009809.2 (NM_001399.5):c.793 + 3A>C r.742_793del p.Pro248IlefsTer15 (Figures 2A, B, PCR primers are listed in Table 1).
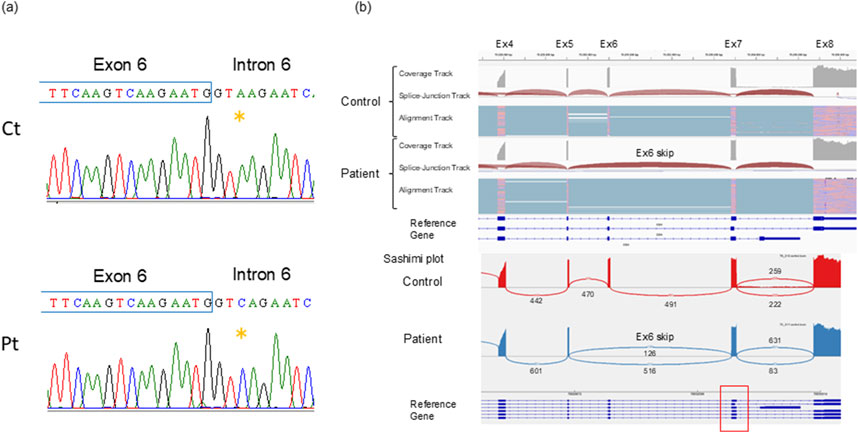
Figure 2. Genetic analyses (A) The splicing variant NG_009809.2(NM_001399.5):c.793 + 3A>C, detected by direct sequencing is indicated. (B) Integrative genome viewer image of the EDA rLAS. The patient has 100% exon 6 skipping. Exon 6 consists of 52 bases, so its deletion results in a premature termination codon due to a frameshift. The red square box indicates multiple mRNA variants of differing sizes of exon 7, but in the patient, all of them skip exon 6.
Based on the itching, eczematous lesions, skin rash distribution and genetic analysis results, the patient was diagnosed with atopic dermatitis-like cutaneous manifestations associated with HED. Given that the patient displayed the same condition as atopic dermatitis, we administered atopic dermatitis treatment. Based on the severity score (IGA score: 3 points, EASI score: 16.8 points), the patient was considered to be in a moderately or severely refractory state and was started on dupilumab (600 mg initially, followed by 300 mg every 2 weeks). Three months after treatment with dupilumab, the skin lesions had markedly improved (Figures 3A–E). Numerically, the IGA score improved from 3 to 2, the EASI score from 16.8 to 12.8 and the ADCT score from 13 to 8. The serum IgE levels decreased to 1,637 after 2 months and to 1,369 after 4 months. Currently, the pruritus and erythema are mildly relieved and there is a slight hair growth on the patient’s head.
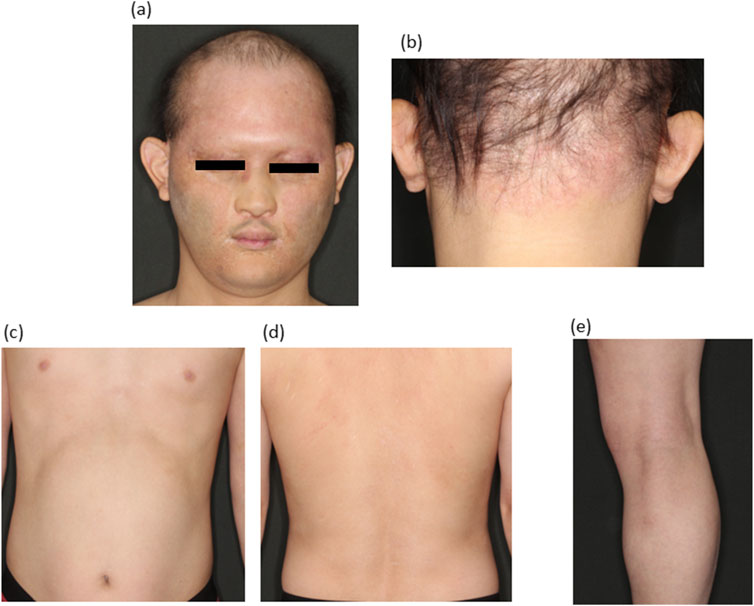
Figure 3. Clinical manifestations after dupilmab administration (A–E). Erythema and lichenification were remarkably improved.
Discussion
HED is most commonly an X-linked recessive condition and is rarely seen to be inherited as an autosomal recessive or dominant trait. The mechanism of ectoderm formation is that the EDA gene encodes the EDA protein, which is expressed in hair, nails, sweat glands and teeth, and the EDA protein specifically binds to the EDAR encoded by the Ectodysplasin A receptor (EDAR) gene [1]. Although we did not confirm the expression of EDA protein in the skin, genetic testing showed a novel splicing variant with a premature termination codon that would be a null allele.
Atopic-like skin rashes resulting from ectodermal dysplasia are different from atopic dermatitis in some respects. Ceramide composition is similar to atopic dermatitis, but only ceramide 1 is overproduced in ectodermal dysplasia [4], and the data show that filaggrin expression is normal in ectodermal dysplasia [5]. Serum IgE levels are known to be high in HED patients [1], and were found to be elevated in this patient, but TARC is in the normal range. A/HED patients tended to present with skin manifestations that were indistinguishable from Atopic dermatitis. Periorbital dermatitis may be characteristic of A/HED skin lesions and may differ from normal atopic dermatitis and is referred to as atopic dermatitis-like cutaneous manifestations in this case report.
An overseas case similar to our case report was that of a 66-year-old woman with severe atopic dermatitis associated with ectodermal dysplasia who had used several immunological agents and biologics with no effect, and dupilumab was the only agent that showed improvement in her symptoms [6, 7]. To date, this is the only case of response to dupilumab. However, this is a brief report from a conference and the patient’s mutation and other details are unclear. No other original papers have been published and, as far as we could find, this case is probably the first. There is also a report that the skin lesion of HED treated as atopic dermatitis.
Wu et al reported that dupilumab may have a potential therapeutic role in certain Th2-immune-biased genodermatoses, such as Netherton syndrome, epidermolysis bullosa pruriginosa, hyper-IgE syndrome, Hailey-Hailey disease, and severe eczema associated with some genetic disorders [7]. As mentioned above, filaggrin expression is normal in HED, but it has been suggested that reduced sweating may lead to reduced skin barrier function [5]. Skin barrier failure is also part of the pathophysiological triad [8, 9], and we believe that the Th2 shift in this disease also results from reduced skin barrier function. In line with this, as in our case, Koguchi et al. also noted AD skin in 5/6 of Japanese HED patients and elevated IgE in 2/3 [5]. Therefore, we believe that dupilumab was effective in this case due to the Th2 shift caused by skin barrier failure resulting from reduced sweating in HED.
It is also important to consider how drugs should be selected for these patients in the future. The reason why dupilumab was used in this patient is because we frequently used dupilumab when selecting drugs for this patient. Safety reasons, including side-effect measures, were the most important. In Japan, the following drugs are currently available for the treatment of severe atopic dermatitis: dupilumab, tralokinumab, lebrikizumab and nemolizumab as injectable drugs, and JAK inhibitors: upadacitinib, baricitinib and abrocitinib. In fact, an effective case of tofacitinib for atopic dermatitis in HED has been reported in a recent report [10], and treatment with JAK inhibitors may also be effective enough.
In recent years, genetic analysis of amniotic fluid has reported that a fetus with an EDA gene mutation showed normal sweating function and other symptoms after birth by injecting recombinant ectodysplasin A1 protein into the amniotic fluid [11]. In utero protein therapy is a promising treatment and this encourages us to identify mutations in patients. The authors also showed long-term results of short-term perinatal EDA1 replacement and stated that treatment before birth is feasible [12]. This therapy may apply to neonatal patients and the detection of mutations in HED patients should be more crucial to in utero protein therapy.
In this report, we showed a case of HED associated with atopic dermatitis-like cutaneous manifestations that was successfully treated with dupilumab. Since there is no fundamental cure for skin lesions in HED, dupilumab may have a potential therapeutic role. Further cases are needed to conclude that dupilumab is effective in atopic dermatitis-like cutaneous manifestations of HED.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
The studies involving humans were approved by Ethics review board of Kanazawa Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
All authors participated in the design, interpretation of the studies and analysis of the data and review of the manuscript; ST, HU, and YN conducted the genetic testing, EU, YN, and AS wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Wright, JT, Fete, M, Schneider, H, Zinser, M, Koster, MI, Clarke, AJ, et al. Ectodermal dysplasias: classification and organization by phenotype, genotype and molecular pathway. Am J Med Genet A (2019) 179(3):442–7. doi:10.1002/ajmg.a.61045
2. Inazawa-Terada, M, Namiki, T, Omigawa, C, Fujimoto, T, Munetsugu, T, Ugajin, T, et al. An epidemiological survey of anhidrotic/hypohidrotic ectodermal dysplasia in Japan: high prevalence of allergic diseases. J Dermatol (2022) 49(4):422–31. doi:10.1111/1346-8138.16278
3. Togi, S, Ura, H, and Niida, Y. Optimization and validation of multimodular, long-range PCR-based next-generation sequencing assays for comprehensive detection of mutation in tuberous sclerosis complex. J Mol Diagn (2021) 23(4):424–46. doi:10.1016/j.jmoldx.2020.12.009
4. Jungersted, JM, Hogh, JK, Hellgren, LI, Agner, T, and Jemec, GB. Ceramide profile in hypohidrotic ectodermal dysplasia. Clin Exp Dermatol (2012) 37(2):153–5. doi:10.1111/j.1365-2230.2011.04200.x
5. Koguchi-Yoshioka, H, Wataya-Kaneda, M, Yutani, M, Murota, H, Nakano, H, Sawamura, D, et al. Atopic diathesis in hypohidrotic/anhidrotic ectodermal dysplasia. Acta Derm Venereol (2015) 95(4):476–9. doi:10.2340/00015555-1978
6. Ge, L, Monaro, G, and Weninger, W. Severe atopic dermatitis with ectodermal dysplasia: a case report. Australas J Dermatol (2016) 57:51–2.
7. Wu, PC, Dai, YX, Li, CL, Chen, CC, Chang, YT, and Ma, SH. Dupilumab in the treatment of genodermatosis: a systematic review. J Dtsch Dermatol Ges (2023) 21(1):7–17.
8. Kabashima, K. New concept of the pathogenesis of atopic dermatitis: interplay among the barrier, allergy, and pruritus as a trinity. J Dermatol Sci (2013) 70(1):3–11. doi:10.1016/j.jdermsci.2013.02.001
9. Nakajima, S, Nakamizo, S, Nomura, T, Ishida, Y, Sawada, Y, and Kabashima, K. Integrating multi-omics approaches in deciphering atopic dermatitis pathogenesis and future therapeutic directions. Allergy (2024) 79(9):2366–79. doi:10.1111/all.16183
10. Li, X, Wu, X, Elston, DM, Zhang, J, and Zhou, C. Hypohidrotic ectodermal dysplasia with c.28delG mutation in ectodysplasin A gene and severe atopic dermatitis treated successfully with tofacitinib. Acta Derm Venereol (2021) 101(1):adv00352. doi:10.2340/00015555-3693
11. Schneider, H, Faschingbauer, F, Schuepbach-Mallepell, S, Korber, I, Wohlfart, S, Dick, A, et al. Prenatal correction of X-linked hypohidrotic ectodermal dysplasia. N Engl J Med (2018) 378(17):1604–10. doi:10.1056/NEJMoa1714322
Keywords: hypohidrotic ectodermal dysplasia (HED), EDA gene, atopic dermatitis, dupilumab, genetic analysis
Citation: Uchiyama E, Takeda K, Ono H, Shimizu A, Togi S, Ura H and Niida Y (2025) Case report: Atopic dermatitis-like skin manifestations in hypohidrotic ectodermal dysplasia caused by a novel splice site mutation of the EDA gene successfully treated with dupilumab. J. Cutan. Immunol. Allergy 8:13813. doi: 10.3389/jcia.2025.13813
Received: 17 September 2024; Accepted: 16 January 2025;
Published: 31 January 2025.
Copyright © 2025 Uchiyama, Takeda, Ono, Shimizu, Togi, Ura and Niida. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Akira Shimizu, YXNoaW1penVAa2FuYXphd2EtbWVkLmFjLmpw
 Eri Uchiyama1
Eri Uchiyama1 Akira Shimizu
Akira Shimizu