- 1Division of Cutaneous Science, Department of Dermatology, Nihon University School of Medicine, Tokyo, Japan
- 2Division of Hematology and Oncology, Department of Internal Medicine, Nihon University School of Medicine, Tokyo, Japan
Dear Editors,
Pyoderma gangrenosum (PG) is a rare neutrophilic dermatosis characterized by painful, rapidly progressing ulcers, and often associated with systemic diseases such as inflammatory bowel disease, rheumatoid arthritis, and hematologic malignancies. However, cases of PG concomitant with myelofibrosis are particularly uncommon. Here, we present a case of PG complicated by myelofibrosis that was successfully treated with systemic corticosteroid, adalimumab, and ruxolitinib.
A 57-year-old man was referred to our department with a fever and a painful ulcer of the right upper back that occurred 1 month previously. His medical history included JAK2V617F mutation-positive myelofibrosis, glaucoma, and hyperuricemia. Upon examination, a round-shaped, 7 × 8 cm-sized yellowish ulcer with mound-like edges and surrounding erythema was observed on the upper right back. (Figure 1A). Laboratory examination revealed elevated white blood cell count (19,800/µL), platelet count (576,000/µL), and C-reactive protein (145 mg/L). Myeloblasts and erythroblasts were also identified in the peripheral blood. Computed tomography showed splenomegaly. Histopathology of the lesion revealed neutrophil infiltration in the dermis (Figures 1B, C). Based on these findings, the patient was diagnosed with pyoderma gangrenosum (PG) associated with myelofibrosis. Three-week treatment with 30 mg/day of oral prednisolone failed to reduce the size of the ulcer (Figure 1D). Therefore, anti-tumor necrosis factor (TNF)-α antibody adalimumab therapy (40 mg/week) was combined. Then, the ulcer began to epithelialize, with epithelialization rates of 50% at 7 weeks, 90% at 12 weeks, and 100% at 16 weeks after the initiation of adalimumab therapy (Figure 1E). However, because thrombocytosis caused by myelofibrosis deteriorated, 10 mg/day of oral ruxolitinib, a Janus kinase (JAK) 2 inhibitor, was started while adalimumab was reduced to 40 mg biweekly. Ruxolitinib was gradually increased to 40 mg/day due to myelofibrosis progression, and adalimumab was reduced to 40 mg every 3-4 weeks and eventually discontinued. He died 22 months after his first visit due to myelofibrosis progression, with no recurrence of skin ulcers during this period (Figure 1F).
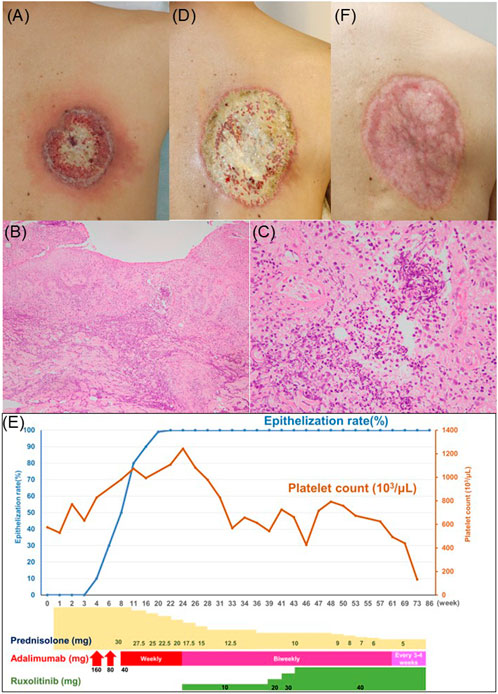
Figure 1. (A) Clinical appearance of the ulcer at the first visit. (B) Low power view of histopathology (Hematoxylin-Eosin Stain; ×40). (C) High power view of histopathology (Hematoxylin-Eosin Stain; ×200). (D) Clinical appearance of the ulcer at 3 weeks after 30 mg/day of oral prednisolone therapy. (E) Clinical appearance of the ulcer at 16 weeks after the initiation of adalimumab therapy. (F) Schematic description of the clinical course.
PG is a relatively rare inflammatory skin disease characterized by hyperplastic and gangrenous ulcers in which neutrophils play a major role [1]. In Japanese patients, underlying systemic diseases are present in 50.3% of the patients. These include ulcerative colitis (23.5%), Crohn’s disease (7.2%), arthritis (9.7%), hematologic malignancies (8.7%), and Takayasu’s arteritis (1.2%) [1]. Most of the associated hematologic malignancies are myelodysplastic syndromes, but myelofibrosis is observed in rare cases [2, 3].
Treatment of PG is challenging, especially when it is associated with hematologic diseases, due to its complex pathophysiology involving neutrophilic inflammation and cytokine dysregulation. Standard treatments, such as oral corticosteroids and immunosuppressants, often provide limited effectiveness, particularly in refractory cases. In recent years, TNF inhibitors have been used in severe PG cases, and adalimumab is approved for PG in Japan. Although there was a case of PG with JAK2V617F mutation-positive myelofibrosis showing good response to systemic steroids, our patient did not show improvement by systemic steroid monotherapy [4]. Currently, there are no established treatment strategies for myelofibrosis-associated PG. This is the first reported case of PG complicated by myelofibrosis successfully treated with a TNF inhibitor in combination with systemic steroid. However, the influence of TNF inhibitors on the course of myelofibrosis remains unknown.
An activating somatic mutation JAK2V617F identified in our patient confers a proliferative and survival advantage to hematopoietic progenitor cells [4]. This mutation is found in 50%–60% of patients with myelofibrosis [4]. Ruxolitinib, a JAK inhibitor, targets JAK1 and JAK2 pathways, which play essential roles in hematopoiesis and immune regulation [5]. By inhibiting these pathways, ruxolitinib is able to reduce cellular proliferation in myelofibrosis. The JAK/STAT pathway is also known to be activated in neutrophilic dermatoses, including PG, suggesting that JAK inhibition might help control excessive neutrophil activity at inflammatory sites [1]. Interestingly, JAK2V617F mutation has been associated with constitutive and enhanced activation of neutrophils [4]. Because no recurrences were observed even after the dose reduction of adalimumab, ruxolitinib may have helped maintain PG remission in our patient. Indeed, PG with polycythaemia vera having JAK2V617F mutation has been successfully treated with ruxolitinib monotherapy [5].
This case showed successful treatment of PG complicated by myelofibrosis using a combination of systemic corticosteroid, adalimumab, and ruxolitinib. The effectiveness observed in this case suggests that targeting multiple inflammatory pathways using JAK and TNF inhibitors may be promising in managing PG associated with myelofibrosis positive for JAK2 mutation. Further research is needed to confirm this idea and evaluate the long-term safety and efficacy of combined JAK and TNF inhibition in similar cases.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
KH has received honoraria for speaker and consultancy from AbbVie, Eisai and Novartis. KM has received honoraria for speaker and consultancy from AbbVie, Eisai and Novartis. HF has received honoraria for speaker and consultancy from AbbVie, Eisai and Novartis.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
References
1. Yamamoto, T, Yamasaki, K, Yamanaka, K, Komine, M, Kawakami, T, Yamamoto, O, et al. Clinical guidance of pyoderma gangrenosum 2022. J Dermatol (2023) 50(9):e253–e275. doi:10.1111/1346-8138.16845
2. Montagnon, CM, Fracica, EA, Patel, AA, Camilleri, MJ, Murad, MH, Dingli, D, et al. Pyoderma gangrenosum in hematologic malignancies: a systematic review. J Am Acad Dermatol (2020) 82(6):1346–59. doi:10.1016/j.jaad.2019.09.032
3. Cox, NH. Pyoderma gangrenosum and myelofibrosis. Br J Dermatol (1999) 140(2):360. doi:10.1046/j.1365-2133.1999.02678.x
4. Gou, P, Zhang, W, and Giraudier, S. Insights into the potential mechanisms of JAK2V617F somatic mutation contributing distinct phenotypes in myeloproliferative neoplasms. Int J Mol Sci (2022) 23(3):1013. doi:10.3390/ijms23031013
Keywords: pyoderma gangrenosum, myelofibrosis, adalimumab, ruxolitinib, Janus Kinase 2
Citation: Shibuta K, Hayama K, Miura K and Fujita H (2025) Successful treatment of pyoderma gangrenosum complicated by JAK2V617F mutation-positive myelofibrosis with adalimumab and systemic steroid. J. Cutan. Immunol. Allergy 8:14079. doi: 10.3389/jcia.2025.14079
Received: 18 November 2024; Accepted: 07 January 2025;
Published: 24 January 2025.
Copyright © 2025 Shibuta, Hayama, Miura and Fujita. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Koremasa Hayama, aGF5YW1hLmtvcmVtYXNhQG5paG9uLXUuYWMuanA=
 Kyohei Shibuta1
Kyohei Shibuta1 Koremasa Hayama
Koremasa Hayama Hideki Fujita
Hideki Fujita