- 1Postgraduate Training Base at Shanghai Gongli Hospital, Ningxia Medical University, Shanghai, China
- 2Department of Dermatology, Shimane University Faculty of Medicine, Izumo, Japan
- 3Department of Dermatology, Gongli Hospital of Shanghai Pudong New Area, Shanghai, China
- 4Department of Clinical Trial Management, Clinical Research Center, Shimane University Hospital, Izumo, Japan
- 5Department of Hygiene and Preventive Medicine, Fukushima Medical University, Fukushima, Japan
- 6Department of Rhinolaryngology, Head and Neck Surgery, Matsue Red Cross Hospital, Matsue, Japan
Background: α-Gal syndrome is a general term for allergies to red meat, cetuximab, and flounder roe caused by tick bites. Among these, cetuximab allergy is the most severe, potentially leading to anaphylactic shock after the first infusion. In 2014, 4 of 13 patients with head and neck cancer at Matsue Red Cross Hospital developed severe anaphylactic shock after they received the first administration of cetuximab.
Objectives: To prevent cetuximab-induced anaphylaxis and establish a cutoff value for α-Gal-specific IgE.
Methods: Besides the 13 patients who received cetuximab without prior allergy testing (non-intervention group), the study also included 205 new patients with head and neck cancer who were potential candidates for cetuximab (intervention group). Of 205 new patients, 39 with α-Gal-specific IgE below 0.35 UA/mL by CAP-FEIA were administered cetuximab based on their cancer progression and general condition and were observed for anaphylaxis development.
Results: Four patients in the non-intervention group developed anaphylactic shock, all of whom had α-Gal-specific IgE levels ≥0.35 UA/mL (incidence 30.8%). Three of the 39 patients in the intervention group developed anaphylactic shock (incidence 7.7%). ROC analysis in all patients who received cetuximab in the non-intervention and intervention groups determined that the optimal cutoff value for α-Gal-specific IgE was 0.14 UA/mL, with a sensitivity of 86% and a specificity of 98%. Of the 218 participants, 20 tested positive for α-Gal-specific IgE (9.2%). A 0.14 UA/mL cutoff value revealed that 32 of 218 participants (14.7%) had detectable α-Gal specific IgE.
Conclusion: We must exercise heightened caution regarding the potential for α-Gal syndrome when using products derived from mammals and other products, particularly pharmaceuticals.
Introduction
α-Gal syndrome, caused by tick bites, is well-known and reported in numerous cases worldwide [1–5]. This condition occurs when repeated tick bites sensitize individuals to the sugar chain galactose-α-1,3-galactose (α-Gal) found in tick saliva, leading to allergies to red meat, cetuximab, and flounder roe [6, 7]. Red meat and flounder roe allergy, both components of the α-Gal syndrome, are characterized by delayed onset reactions [8, 9]. In contrast, since cetuximab is administered intravenously, patients with α-Gal-specific IgE experience anaphylaxis shortly after the first dose [10]. Cetuximab allergy is primarily caused by sensitization to α-Gal and can occasionally result in fatal outcomes [10, 11]. In recent years, the risk of allergy has been extended to other mammalian-derived products, including heart valves, gelatin-based plasma expanders, and pancreatic enzymes [12]. Serrier et al. reported a case of severe anaphylaxis caused by a gelatin-based colloid plasma substitute in a patient with α-Gal syndrome, suggesting that similar cases may go undiagnosed [13].
In the eastern part of Shimane Prefecture, Japan, where our hospital is located, ticks are prevalent, and we have treated numerous patients with α-Gal syndrome. In 2014, 4 out of 13 patients with head and neck cancer who received their first dose of cetuximab at the Department of Rhinolaryngology, Head and Neck Surgery, Matsue Red Cross Hospital, experienced severe anaphylactic shock. This study aimed to prevent anaphylaxis caused by cetuximab through pre-administration blood testing. Ultimately, we sought to establish a cutoff value for α-Gal-specific IgE levels to help prevent the onset of anaphylaxis.
Materials and methods
Participants
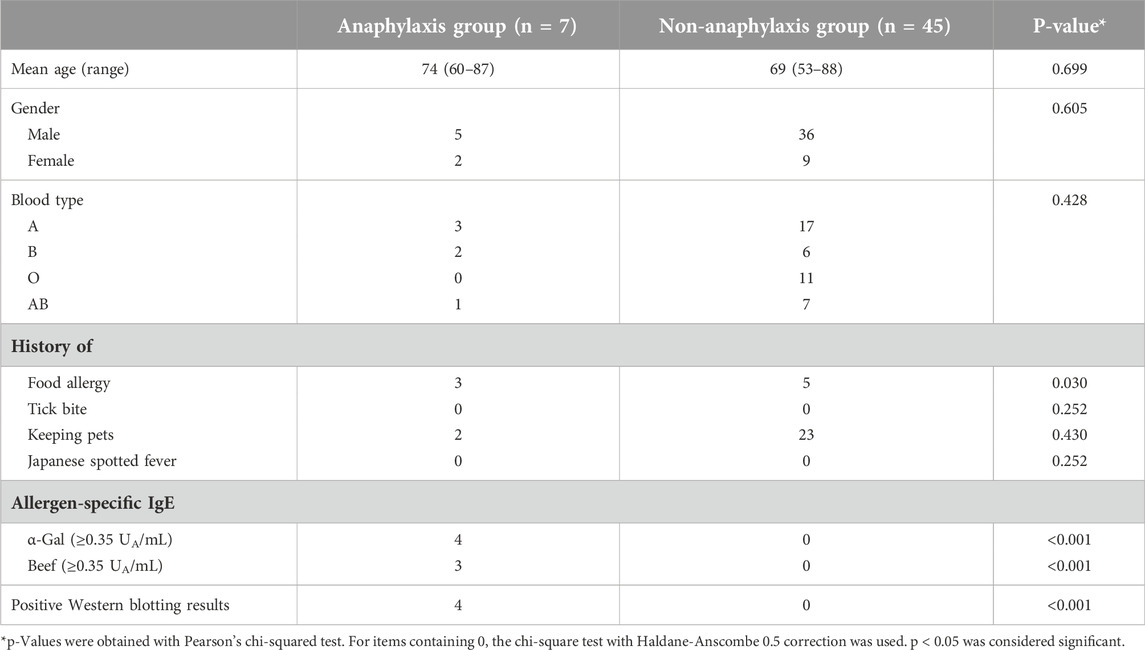
This study included 218 patients with head and neck cancer who visited the Department of Otorhinolaryngology, Head and Neck Surgery at Matsue Red Cross Hospital between 2014 and 2017. Among these, 13 patients had already received cetuximab treatment before the study and were classified as the non-intervention group (Figure 1). They completed a questionnaire and underwent blood tests after cetuximab treatment. Sera from four patients who developed anaphylactic shock were immediately frozen following the onset of anaphylaxis and later used for analysis with informed consent. For the remaining nine patients, sera collected more than 2 weeks after cetuximab administration were used for analysis. The other 205 patients formed the intervention group (Figure 1). They completed a questionnaire and underwent blood tests before starting cetuximab treatment. Although all 205 patients were potential future recipients of cetuximab, only 39 had the treatment by the time of this study, based on their cancer progression and overall condition. These 39 patients tested negative for α-Gal-specific IgE using CAP-fluorescent enzyme immunoassay (FEIA) (<0.35 UA/mL). Although cetuximab-specific IgE using Western blotting was only a reference finding, all 39 of these patients were negative. When we explained the test results and the benefits and risks of cetuximab treatment to all participants in the intervention group, those with positive α-Gal-specific IgE levels (≥0.35 UA/mL) declined cetuximab treatment. The alternate treatments were thoroughly explained to ensure these patients retained access to appropriate therapies and individualized treatment plans were developed to avoid limiting their therapeutic options.
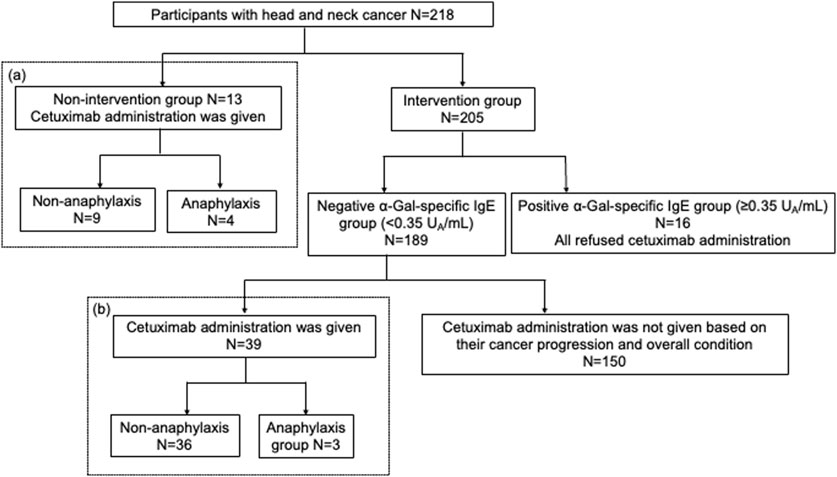
Figure 1. A flowchart of the study. A total of 218 patients with head and neck cancer consented and participate in the study. This included 13 patients in the non-intervention group and 205 in the intervention group. In the intervention group, 39 patients with α-Gal-specific IgE levels below 0.35 UA/mL received cetuximab based on their cancer progression and general condition. The incidence of anaphylaxis was compared between the non-intervention and intervention groups.
Evaluation of anaphylaxis
An otolaryngology head and neck surgeon evaluated adverse reactions following cetuximab administration. The occurrence and severity of anaphylaxis were assessed based on the grading criteria outlined in the severity grading system for acute allergic reactions: a multidisciplinary Delphi study [14]. This grading system is classified as grade 1 through grade 5. Grade 1 is the mildest allergic reaction with mild symptoms of skin, gastrointestinal, and mucosal/angioedema, and grade 5 is the most severe life-threatening allergic reaction with severe symptoms of cardiovascular, neurologic and respiratory.
Questionnaire items
As in our previous cohort study of subclinical sensitization against α-Gal in Japan [15], we asked the following questions: age, sex, blood type, history of food allergies, tick bites, history of Japanese Spotted Fever, and pet ownership.
Blood test
Serum allergen-specific IgE value
As in our previous study [15], serum samples obtained from participants were stored at −20°C until analysis. Allergen-specific IgE values for α-Gal- and beef-specific IgE antibodies were measured using a CAP-FEIA system (ImmunoCAP; Thermo Fisher Scientific). The results were expressed in units of allergen per milliliter (UA/mL). A ≥0.35 UA/mL value was considered positive for allergen-specific IgE.
IgE immunoblotting
As in our previous study [15], cetuximab (Erbitux®; Merck) was electrophoresed at 2 μg per lane via sodium dodecyl sulfate-polyacrylamide gel electrophoresis using a 7.5% polyacrylamide gel. The electrophoresed proteins were transferred to polyvinylidene difluoride membranes (PVDFs; Immobilon-P; Millipore). After blocking the PVDF membrane with 0.6% polyvinylpyrrolidone in Tris-buffered saline containing 0.1% Tween-20 (TBS-T) for 1 h, the membrane was incubated with serum diluted 1:20 for 20 h at room temperature (15°C–25°C). The membrane was washed three times with TBS-T and incubated with horseradish peroxidase-conjugated mouse monoclonal anti-human IgE Fc (ab99806; Abcam) for 1 h at room temperature. After washing with TBS-T, cetuximab-binding IgE antibodies were visualized on Super RX (FUJIFILM Co.) using an Amersham ECL-Primekit (GE Healthcare UK Ltd.).
Statistical analysis
Receiver Operating Characteristic (ROC) curve analysis was performed using IBM SPSS Statistics version 27 (IBM Corp., Armonk, NY, United States) to evaluate the diagnostic accuracy of allergen-specific IgE tests. The area under the ROC curve (AUC) was calculated to assess the performance, with values closer to 1 indicating better discriminative ability. Sensitivity and specificity were determined at various threshold levels, and the optimal cutoff point was identified using the Youden Index (J = Sensitivity + Specificity - 1). Confidence intervals (CI) for the AUC were calculated using a non-parametric approach, and statistical significance was set at p < 0.05.
Pearson’s chi-squared test was used to examine the association of clinical factors with cetuximab-induced anaphylaxis and the association of clinical factors with positivity for α-Gal-specific IgE. The chi-square test with the Haldane-Anscombe 0.5 correction was applied for items containing zero. Statistical analysis was conducted using SPSS software version 25 (SPSS Inc.), and p < 0.05 was considered statistically significant.
Ethics approval
The Shimane University Faculty of Medicine Ethics Committee approved the studies involving human participants. All procedures were conducted following local legislation and institutional requirements. Written informed consent for participation in this study was obtained from all participants.
Results
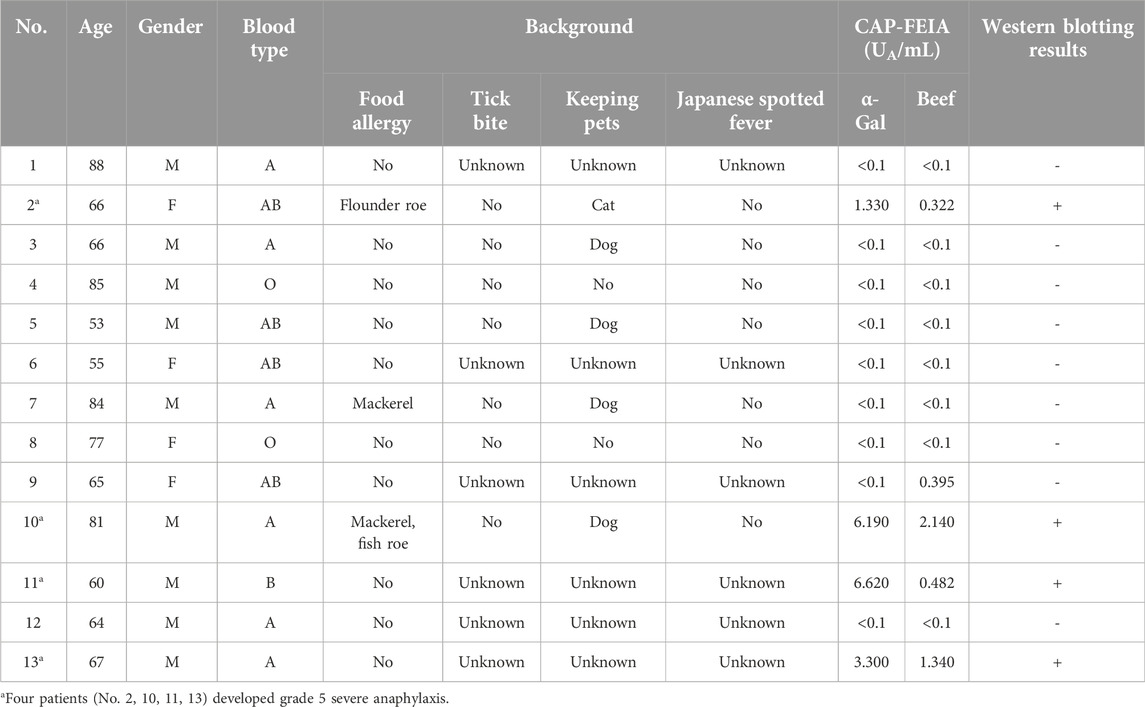
A flowchart of the study is shown in Figure 1. Among the 13 patients in the non-intervention group, four (No. 2, 10, 11, 13) developed grade 5 severe anaphylactic shock [14], and the incidence of anaphylaxis was 30.8% (Figure 2). All four patients with anaphylaxis tested positive for α-Gal-specific IgE and cetuximab-specific IgE by Western blotting, whereas the nine patients without anaphylaxis had no positive reaction in these tests (Table 1; Supplementary Figure S1).
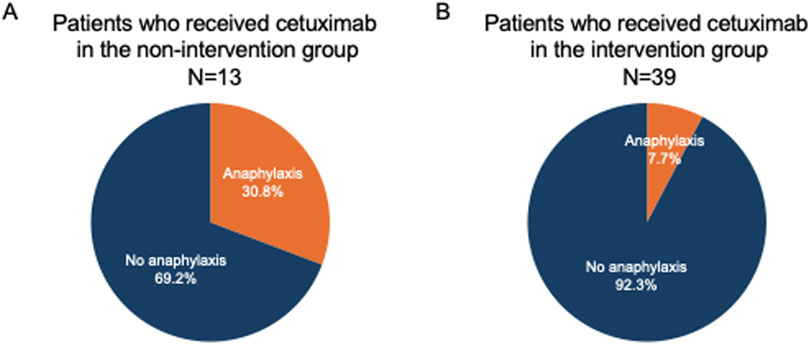
Figure 2. Differences in the incidence of anaphylaxis in the non-intervention and intervention groups. In the non-intervention group, the incidence of anaphylactic shock with the first dose of cetuximab was 30.8% (A). In contrast, it decreased to 7.7% in the intervention group (B).
Of the 205 patients in the intervention group, 16 patients tested positive for α-Gal-specific IgE. All 16 patients refused cetuximab treatment after detailed consultation with their doctor-in-charge (Figure 1). Of these 16 patients, 11 were also positive for cetuximab-specific IgE by Western blotting (Supplementary Table S1; Supplementary Figure S2). Among the 189 patients with negative α-Gal-specific IgE results, 39 patients chose to receive cetuximab based on the progression of their cancer and overall condition. Among the 189 patients who tested negative for α-Gal-specific IgE by CAP-FEIA, eight had cetuximab-specific IgE detected by Western blotting (Supplementary Figure S3). These patients were not administered cetuximab based on their cancer progression and overall condition. Their clinical characteristics and blood test results are shown in Supplementary Table S2.
Cetuximab-specific IgE was also negative in all 39 patients who received cetuximab. The clinical characteristics and laboratory results of the 39 patients who received cetuximab after intervention are shown in Table 2. As a result, three patients developed grade 5 anaphylactic shock after the first dose of cetuximab, and the incidence of anaphylaxis was 7.7% (Table 2; Figure 2). The four patients who developed anaphylactic shock in the non-intervention group and the three patients in the intervention group who developed anaphylactic shock recovered after cetuximab was discontinued and an intramuscular injection of adrenaline was administered. A statistical analysis of factors associated with the development of anaphylaxis is presented in Table 3. Factors contributing to the development of anaphylaxis after the first dose of cetuximab included a history of food allergy, α-Gal specific IgE greater than or equal to 0.35 UA/mL, beef-specific IgE greater than or equal to 0.35 UA/mL, and positive Western blotting for cetuximab.
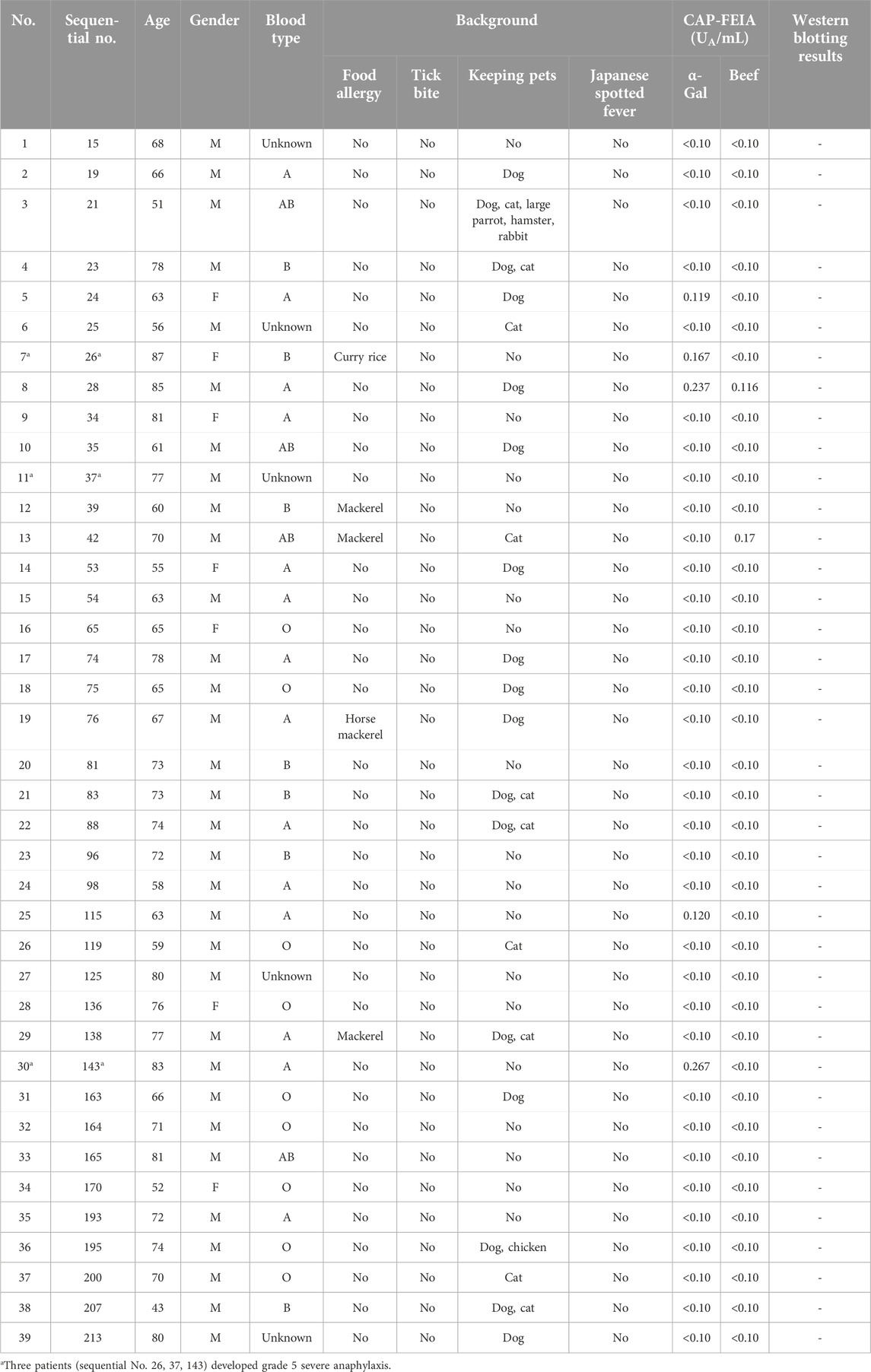
Table 2. Clinical characteristics and blood test results of patients who received cetuximab in the intervention group.
According to the ROC analysis in the non-intervention group (Group (a) in Figure 1, n = 13), the cutoff value for α-Gal-specific IgE in predicting anaphylaxis onset was 0.72 UA/mL (Figure 3A). At this threshold, the positive predictive value (PPV) was 100% (true positives/test positives), and the negative predictive value (NPV) was 100% (true negatives/test negatives), with a sensitivity of 100% and a specificity of 100%. Additionally, the AUC was 1.00. Similarly, the cutoff value for beef-specific IgE was 0.21 UA/mL, displaying a PPV of 100%, a NPV of 100%, a sensitivity of 100%, a specificity of 89%, and an AUC of 0.97 (CI: 0.89–1.00). In the intervention group (Group (b) in Figure 1, n = 39), the cutoff value for α-Gal-specific IgE in predicting anaphylaxis onset was 0.14 UA/mL, as determined by the ROC analysis (Figure 3B). At this threshold, the PPV was 67%, the NPV was 97%, with a sensitivity of 67% and a specificity of 97%. Additionally, the AUC was 0.81 (CI: 0.47–1.00). Similarly, the cutoff value for beef-specific IgE was 0.14 UA/mL, displaying a PPV of 0%, a NPV of 92%, a sensitivity of 0%, a specificity of 97%, and an AUC of 0.47 (CI: 0.14–0.80). The cutoff value for α-Gal-specific IgE in predicting anaphylaxis onset in all participants who received cetuximab (n = 52) was 0.14 UA/mL, determined by ROC analysis (Figure 3C). At this threshold, the PPV was 86%, the NPV was 98%, with a sensitivity of 86%, and a specificity of 98%. Additionally, the AUC was 0.92 (CI: 0.76–1.00). Similarly, the cutoff value for beef-specific IgE was 0.25 UA/mL, displaying a PPV of 80%, a NPV of 94%, a sensitivity of 57%, a specificity of 98%, and an AUC of 0.77 (CI: 0.53–1.00).
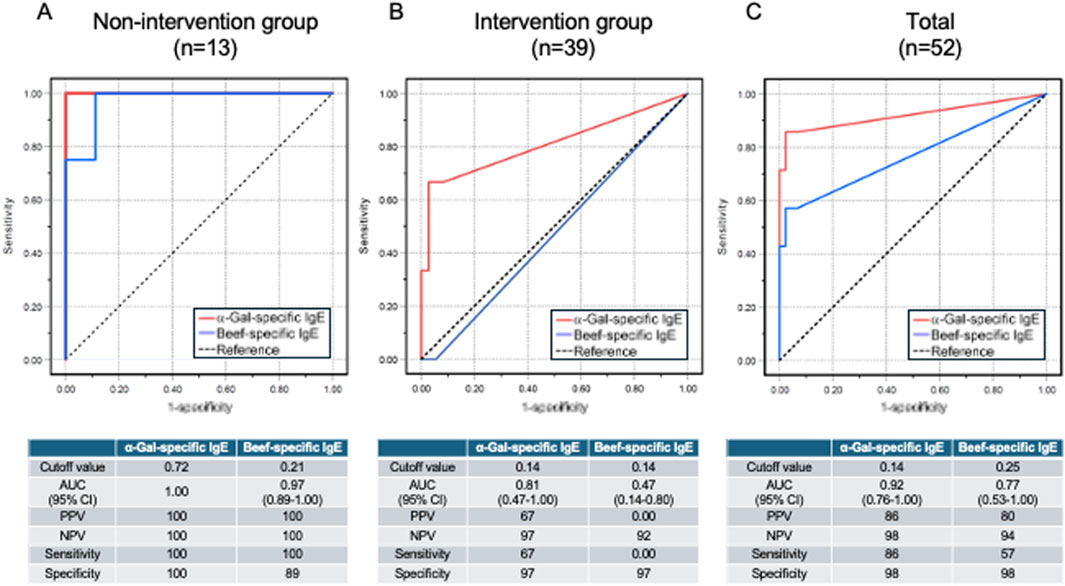
Figure 3. ROC curve analysis of allergen-specific IgE test. ROC curve analysis of α-Gal-specific and beef-specific IgE levels was performed for 13 patients in the non-intervention group (A), 39 patients in the intervention group (B), and all 52 patients who received cetuximab (C).
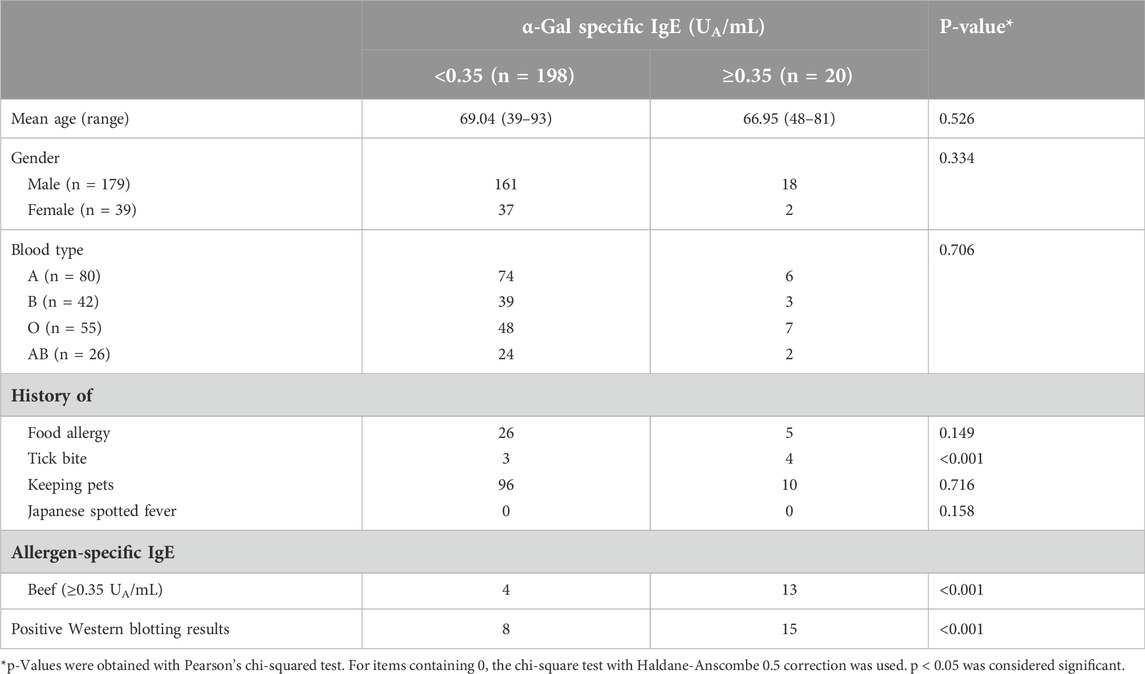
In this study, 20 patients had an α-Gal-specific IgE level of 0.35 UA/mL or higher out of 218 patients (sensitization rate, 9.2%) (Figure 4). A statistical analysis of the factors that may have contributed to the presence of α-Gal-specific IgE is presented in Table 4. Factors contributing to positive α-Gal-specific IgE (≥0.35 UA/mL) included a history of tick bites, positive beef-specific IgE (≥0.35 UA/mL), and positive Western blotting for cetuximab. Finally, with a cutoff value of 0.14 UA/mL, 32 of 218 patients had detectable α-Gal-specific IgE (sensitization rate, 14.7%) (Figure 4).
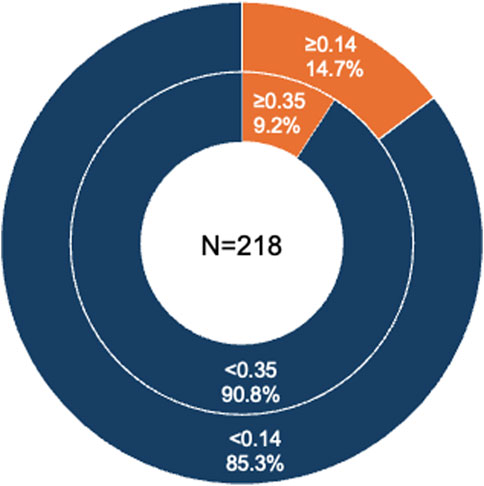
Figure 4. α-Gal-specific IgE retention in 218 participants. The percentage of patients with α-Gal-specific IgE levels greater than 0.35 UA/mL and 0.14 UA/mL.
Discussion
We found that the incidence of grade 5 anaphylactic shock was 30.8% in the non-intervention group. In contract, it was 7.7% in the intervention group, supporting the usefulness of the α-Gal-specific IgE test (<0.35 UA/mL) in preventing anaphylaxis due to potential α-Gal syndrome in cetuximab treatment. Importantly, all of the patients who developed allergies before and after the intervention had grade 5 severe anaphylactic shock. This may have been due to direct intravenous administration of the allergen, and warns of the dangers of allergy from injectable drugs. In the present study, we determined that the optimum cutoff value for preventing anaphylaxis was 0.14 UA/mL in the α-Gal-specific IgE test, with a sensitivity of 86%, and specificity of 98%. However, the present analysis has two limitations: First, 16 patients with α-Gal-specific IgE levels ≥0.35 UA/mL were not administered cetuximab in the intervention group. Consequently, it was impossible to clinically confirm whether anaphylaxis would occur in these patients with high α-Gal-specific IgE if treated with cetuximab. Thus, these cases could not be included in the ROC analysis. Second, in the non-intervention group, blood samples were obtained after cetuximab administration, resulting in differences in sampling timing conditions compared to that of the intervention group. We integrated data from the non-intervention and intervention groups where cetuximab was administered to address the first limitation. This approach allowed us to include participants with more natural α-Gal-specific IgE levels, enabling accurate calculations. Regarding the second limitation, blood samples from patients in the non-intervention group who developed anaphylaxis were collected immediately after symptom onset. Thus, it is unlikely that α-Gal-specific IgE levels differed significantly before and after cetuximab administration in these patients. For the remaining 9 patients in the non-intervention group who did not develop allergic reactions, a decrease in α-Gal-specific IgE cannot be ruled out, as their blood samples were collected more than 2 weeks after cetuximab administration. Nevertheless, since these 9 patients did not experience allergic reactions at the first dose, significant changes in α-Gal-specific IgE levels before and after administration are also unlikely. Based on these considerations, as shown in Figure 3C, we used the combined data from 52 participants in the non-intervention and intervention groups, where cetuximab was administered, for the analysis to determine the cutoff value. The rationale for the differing cutoff values between the groups is as follows: In the non-Intervention group, although 4 out of the 13 cases that received cetuximab experienced anaphylaxis, the small number of cases suggests that the data is insufficient to reliably calculate the cutoff value. In contrast, in the intervention group, α-Gal-specific IgE levels were measured for all subject being considered for cetuximab administration. Patients with α-Gal-specific IgE values of ≥0.35 UA/mL were identified as high-risk and informed of the risk of anaphylaxis; consequently, subjects with elevated α-Gal-specific IgE levels (≥0.35 UA/mL) chose not to receive cetuximab. The characteristics of the populations in the non-intervention and intervention groups differ as a result. In light of these limitations, we opted to combine the non-intervention and intervention groups to more accurately reflect the real-world scenario, subsequently analyzing the total group. The cutoff values were determined using both the Youden index and the minimum distance method, and these methods yielded consistent results. We believe that using cetuximab or other mammalian-derived agents in patients with α-Gal-specific IgE greater than or equal to 0.14 UA/mL should be carefully considered, including skin tests and basophil activation tests.
Although cutoff values for various tests worldwide have been proposed to prevent the development of α-Gal syndrome, particularly cetuximab allergy, this study examined their sufficiency. Serrier et al. validated an anti-α-Gal-IgE (CAP-FEIA) for screening patients at risk of severe anaphylaxis to cetuximab [16]. They concluded that a positive threshold of ≥0.525 UA/mL defined by ROC analysis or a conventional threshold of ≥0.35 UA/mL could be used to calculate a likelihood ratio, indicating a higher risk of severe immediate-type reactions when the FEIA test is positive and a lower risk when the test is negative. Kopaˇc et al. highlighted the importance of performing a basophil activation test (BAT) with cetuximab before administration [17]. They reported that IgE levels to α-Gal above 0.10 UA/mL are clinically significant, predicting a probable reaction to cetuximab. However, they noted that IgE are not associated with the reaction severity and emphasized needing a BAT to predict severity. Park et al. reported nationwide pharmacovigilance data for cetuximab-induced anaphylaxis and validated a predictive model using prospective-specific IgE detection [18]. The ImmunoCAP assay indicated significantly higher IgE concentrations for cetuximab, α-Gal, and beef in patients experiencing anaphylaxis (P < 0.001). Furthermore, based on the 0.35 UA/mL positive cutoff, the sensitivity, specificity, PPV, and NPV of beef, α-Gal, and cetuximab were all 100%.
Since allergen-specific IgE levels <0.35 UA/mL are considered negative by the ImmunoCAP method, we considered <0.35 UA/mL a safe predictive value for α-Gal-specific IgE in this study and proceeded with cetuximab administration. Since Western blotting cannot be performed uniformly worldwide, we used its results as reference findings after intervention. The results reduced the incidence of anaphylaxis from 30.8% to 7.7%, but did not eliminate it entirely. This outcome indicates that the <0.35 UA/mL cutoff value is insufficient to prevent cetuximab-induced anaphylaxis, the most severe form of α-Gal syndrome, even when α-Gal-specific IgE levels are below this threshold.
In this study, the optimum cutoff value of the α-Gal-specific IgE test to prevent cetuximab-induced anaphylaxis was 0.14 UA/mL, based on the blood test of non-intervention and intervention group, with a sensitivity of 86% and specificity of 98%. However, of the three patients who developed anaphylactic shock after the intervention, one had an α-Gal-specific IgE level below 0.10 UA/mL, indicating that even this optimized cutoff value might not entirely prevent allergic reactions. It has been reported that α-Gal-specific IgE levels increase with the number of tick bites [1, 19], suggesting that a tick might have recently bitten this patient and was developing higher IgE levels, although the exact details remain unknown. Alternatively, this patient may be allergic to the non-α-Gal portion of cetuximab, but again details remain unknown because Western blotting was negative. Conversely, one patient treated with cetuximab did not develop anaphylaxis despite having an α-Gal-specific IgE level of 0.237 UA/mL (Sequential number 28), suggesting that the 0.14 UA/mL cutoff value is not absolute but remains a valuable reference. The cutoff value for the beef-specific IgE test was 0.25 UA/mL, based on the blood test of non-intervention and intervention group, with an excellent specificity of 98% but a low sensitivity of 57%. We believe this test should be used as a reference finding. As an additional note, we did not ask about atopic predisposition (atopic dermatitis, allergic rhinitis, bronchial asthma, etc.) or concomitant medications in this study, so we cannot rule out the possibility that these factors influenced the antigen-specific IgE test results.
With a cutoff value of 0.14 UA/mL of the α-Gal-specific IgE test, the positive rate was 14.7%. This sensitization rate was much higher than the potential α-Gal sensitization rate in Shimane Prefecture, which we previously examined in a cohort study [15]. This difference may partly result from variations in the average age (mean, range) of the patients in the previous study (57.8, 19–92) and in the present study (68.9, 40–91). However, this suggests that 14.7% of adults in Shimane Prefecture may develop α-Gal syndrome. Clinicians must exercise caution when using food allergens and some mammalian-derived products, such as heart valves, gelatin-based plasma expanders, and pancreatic enzymes. The observation that patients with a history of tick bites and those with beef-specific IgE ≥0.35 UA/mL tend to have α-Gal-specific IgE levels ≥0.35 UA/mL aligns with previous reports.
An interesting aspect of this study is that, of the seven patients who developed anaphylaxis after the first dose of cetuximab, six had known blood types: three were blood type A, and three were blood type B or AB. It has been reported that the sugar chain structure of the blood group B antigen is similar to the sugar chain α-Gal, which makes individuals with the B antigen less likely to develop red meat allergy [2, 20]. In our previous report on patients with red meat allergy caused by α-Gal syndrome, 27 of the 28 patients with known blood type were blood type A or O, one was blood type B, and none were type AB [6]. Although the reason of 50% of the anaphylaxis cases associated with the first dose of cetuximab occurring in patients with the B antigen is unclear, these findings suggest that cetuximab-induced anaphylaxis can still occur in individuals with the B antigen. Therefore, clinicians must exercise extreme caution when using mammalian-derived products, regardless of whether the patient has the B antigen.
Another point to note is that a history of food allergy was identified as a factor contributing to the development of anaphylaxis when cetuximab was administered. Two of the seven patients who developed grade 5 anaphylactic shock after receiving cetuximab had a history of fish roe allergy, suggesting that fish roe allergy is a predictor of α-Gal syndrome. This finding supports our previous study, which indicated that patients with α-Gal syndrome should exercise caution when consuming flounder roe [7]. On the other hand, although one patient with an allergy to curry rice, none of the seven patients who developed grade 5 anaphylactic shock due to cetuximab administration had a clear history of red meat allergy. This observation suggests that allergic symptoms in patients with α-Gal syndrome were more severe when they consumed flounder roe than when they consumed red meat. However, the most severe allergic reactions in the patients with α-Gal syndrome are likely to occur during the use of cetuximab and other drugs.
Since this study was conducted after 13 patients had already received cetuximab, the dosing conditions differed between the 13 patients treated before the intervention and the 39 patients treated after the intervention. Following the intervention, a stricter cutoff value was implemented, which may have been closer to the strict and appropriate cutoff value.
In conclusion, pre-measurement of α-Gal specific IgE before cetuximab administration will likely reduce the incidence of cetuximab-induced anaphylactic shock when using a cutoff value of 0.14 UA/mL. Although this is not a perfect value, it is a significant benchmark for preventing the development of α-Gal syndrome, particularly when supported by prospective studies. We universally recommend cautious consideration of cetuximab or other mammalian-derived agents in patients with α-Gal-specific IgE greater than or equal to 0.14 UA/mL, both in urban and rural areas. Such considerations should include additional diagnostic methods like skin and basophil activation tests.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Shimane University Faculty of Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
LT and YC performed the experiments and drafted the manuscript. KI, KN, and SI contributed to data collection. LT, KK, YN, and TK conducted statistical analyses. OY, XB, and EM assisted with preparing the study and manuscript. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We sincerely thank all participants in this trial. We particularly thank Kiyoe Ueda for her assistance in organizing the data. The IgE levels to α-Gal and beef (ImmunoCAP) were kindly measured by Thermo Fisher Diagnostics (Tokyo, Japan).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/jcia.2025.14348/full#supplementary-material
SUPPLEMENTARY FIGURE S1 | Results of IgE immunoblotting with cetuximab in the non-intervention group. Cetuximab (2 μg/lane) was separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotted with participant sera. The protein band at approximately 50 kDa corresponded to the α-Gal-bound heavy chain of cetuximab. Patients 2, 10, 11, and 13 developed anaphylactic shock during cetuximab infusion.
SUPPLEMENTARY FIGURE S2 | Results of IgE immunoblotting with cetuximab in patients positive for α-Gal-specific IgE by CAP-FEIA in the intervention group. Among patients positive for α-Gal-specific IgE by CAP-FEIA in the intervention group, 11 had cetuximab-specific IgE detected by Western blotting.
SUPPLEMENTARY FIGURE S3 | Results of IgE immunoblotting with cetuximab in patients negative for α-Gal-specific IgE by CAP-FEIA in the intervention group. Among the 189 patients who were negative for α-Gal-specific IgE by CAP-FEIA in the intervention group, eight had cetuximab-specific IgE detected by Western blotting.
Abbreviations
α-Gal, Galactose-α-1,3-galactose; BAT, Basophil activation test; FEIA, Fluorescent enzyme immunoassay; NPV, Negative predictive value; PPV, Positive predictive value.
References
1. Commins, SP, James, HR, Kelly, LA, Pochan, SL, Workman, LJ, Perzanowski, MS, et al. The relevance of tick bites to the production of IgE antibodies to the mammalian oligosaccharide. J Allergy Clin Immunol (2011) 127(5):1286–93. doi:10.1016/j.jaci.2011.02.019
2. Hamsten, C, Tran, TAT, Starkhammar, M, Brauner, A, Commins, SP, Platts-Mills, TAE, et al. Red meat allergy in Sweden: association with tick sensitization and B-negative blood groups. J Allergy Clin Immunol (2013) 132(6):1431–4. doi:10.1016/j.jaci.2013.07.050
3. Wen, L, Zhou, J, Yin, J, Sun, JL, Sun, Y, Wu, K, et al. Delayed anaphylaxis to red meat associated with specific IgE antibodies to galactose. Allergy Asthma Immunol Res (2015) 7(1):92–4. doi:10.4168/aair.2015.7.1.92
4. Villalta, D, Cecchi, L, Farsi, A, Chiarini, F, Minale, P, Voltolini, S, et al. Galactose-α-1,3-galactose syndrome: an Italian survey. Eur Ann Allergy Clin Immunol (2017) 49(6):263–9. doi:10.23822/EurAnnACI.1764-1489.35
5. Joshi, L, Ramjith, J, Falcone, FH, Horsnell, W, Levin, ME, Cunningham, S, et al. Ascaris lumbricoides and ticks associated with sensitization to galactose α1,3-galactose and elicitation of the alpha-gal syndrome. J Allergy Clin Immunol (2022) 149(2):698–707.e3. doi:10.1016/j.jaci.2021.07.018
6. Chinuki, Y, Ishiwata, K, Yamaji, K, Takahashi, H, and Morita, E. Haemaphysalis longicornis tick bites are a possible cause of red meat allergy in Japan. Allergy (2016) 71(3):421–5. doi:10.1111/all.12804
7. Chinuki, Y, Takahashi, H, and Morita, E. IgE antibodies to galactose-α-1,3-galactose, an epitope of red meat allergen, cross-react with a novel flounder roe allergen. J Investig Allergol Clin Immunol (2022) 32(4):324–6. doi:10.18176/jiaci.0754
8. Commins, SP, Satinover, SM, Hosen, J, Mozena, J, Borish, L, Lewis, BD, et al. Delayed anaphylaxis, angioedema, or urticaria after consumption of red meat in patients with IgE antibodies specific for galactose-alpha-1,3-galactose. J Allergy Clin Immunol (2009) 123(2):426–33. doi:10.1016/j.jaci.2008.10.052
9. Román-Carrasco, P, Lieder, B, Somoza, V, Ponce, M, Szépfalusi, Z, Martin, D, et al. Only α-Gal bound to lipids, but not to proteins, is transported across enterocytes as an IgE-reactive molecule that can induce effector cell activation. Allergy (2019) 74(10):1956–68. doi:10.1111/all.13873
10. Chung, CH, Mirakhur, B, Chan, E, Le, QT, Berlin, J, Morse, M, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med (2008) 358(11):1109–17. doi:10.1056/NEJMoa074943
11. Chinuki, Y, and Morita, E. Alpha-Gal-containing biologics and anaphylaxis. Allergol Int (2019) 68(3):296–300. doi:10.1016/j.alit.2019.04.001
12. Platts-Mills, TAE, Li, RC, Keshavarz, B, Smith, AR, and Wilson, JM. Diagnosis and management of patients with the α-gal syndrome. J Allergy Clin Immunol Pract (2020) 8(1):15–23. doi:10.1016/j.jaip.2019.09.017
13. Serrier, J, Khoy, K, Ollivier, Y, Gervais, R, Le Moel, G, Lafosse, M, et al. Recurrent anaphylaxis to a gelatin-based colloid plasma substitute and to cetuximab following sensitisation to galactose-alpha-1,3-galactose. Br J Anaesth (2021) 126(6):e200–e202. doi:10.1016/j.bja.2021.02.013
14. Dribin, TE, Schnadower, D, Spergel, JM, Campbell, RL, Shaker, M, Neuman, MI, et al. Severity grading system for acute allergic reactions: a multidisciplinary Delphi study. J Allergy Clin Immunol (2021) 148(1):173–81. doi:10.1016/j.jaci.2021.01.003
15. Nakagawa, Y, Chinuki, Y, Ogino, R, Yamasaki, K, Aiba, S, Ugajin, T, et al. Cohort study of subclinical sensitization against galactose-α-1,3-galactose in Japan: prevalence and regional variations. J Dermatol (2022) 49(12):1268–77. doi:10.1111/1346-8138.16570
16. Serrier, J, Davy, JB, Dupont, B, Clarisse, B, Parienti, JJ, Petit, G, et al. Validation of an anti-α-Gal IgE fluoroenzyme-immunoassay for the screening of patients at risk of severe anaphylaxis to cetuximab. BMC Cancer (2023) 23(1):32. doi:10.1186/s12885-023-10501-5
17. Kopač, P, Koren, A, Bidovec-Stojkovič, U, Košnik, M, Dejanović, L, Mesti, T, et al. Basophil activation test predicts cetuximab anaphylaxis severity in alpha-gal IgE-positive patients. Diagnostics (Basel) (2024) 14(13):1403. doi:10.3390/diagnostics14131403
18. Park, KH, Lee, J, Beom, SH, Shin, SJ, Ahn, JB, Kim, SR, et al. Nationwide pharmacovigilance data for cetuximab-induced anaphylaxis and predictive model validation using prospective specific IgE detection. World Allergy Organ J (2021) 14(7):100553. doi:10.1016/j.waojou.2021.100553
19. Hashizume, H, Fujiyama, T, Umayahara, T, Kageyama, R, Walls, AF, and Satoh, T. Repeated Amblyomma testudinarium tick bites are associated with increased galactose-α-1,3-galactose carbohydrate IgE antibody levels: a retrospective cohort study in a single institution. J Am Acad Dermatol (2018) 78(6):1135–41. doi:10.1016/j.jaad.2017.12.028
Keywords: anaphylaxis, specific IgE, α-Gal syndrome, cetuximab, cutoff value
Citation: Tian L, Chinuki Y, Kohno K, Nakagawa Y, Kakamu T, Ito K, Nakajima K, Ichihashi S, Yamasaki O, Bu X and Morita E (2025) Establishment of α-Gal-specific IgE cutoff value to prevent cetuximab-induced allergic reactions in potential α-Gal syndrome. J. Cutan. Immunol. Allergy 8:14348. doi: 10.3389/jcia.2025.14348
Received: 16 January 2025; Accepted: 25 February 2025;
Published: 01 April 2025.
Copyright © 2025 Tian, Chinuki, Kohno, Nakagawa, Kakamu, Ito, Nakajima, Ichihashi, Yamasaki, Bu and Morita. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuko Chinuki, eWNoaW51a2lAbWVkLnNoaW1hbmUtdS5hYy5qcA==
 Linjie Tian
Linjie Tian Yuko Chinuki
Yuko Chinuki Kunie Kohno4
Kunie Kohno4 Osamu Yamasaki
Osamu Yamasaki Eishin Morita
Eishin Morita