Review
Published on 22 Jul 2024
From fasting to fat reshaping: exploring the molecular pathways of intermittent fasting-induced adipose tissue remodeling
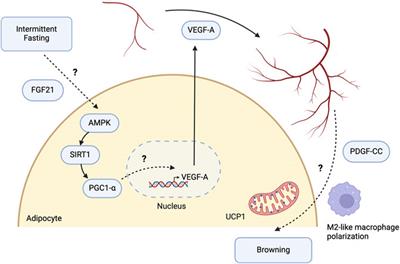
Review
Published on 22 Jul 2024

Review
Published on 17 Jul 2024
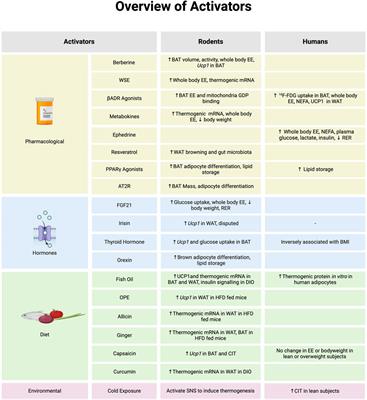
Review
Published on 16 Jul 2024
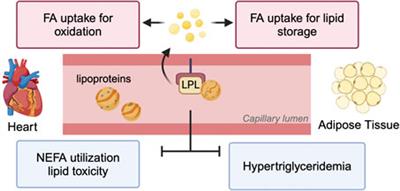
Review
Published on 28 Jun 2024
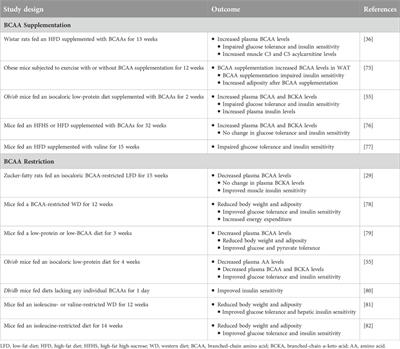
Original Research
Published on 28 Jun 2024
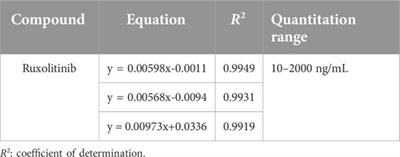
Mini Review
Published on 26 Jun 2024
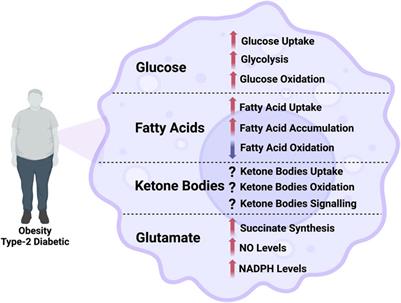
Review
Published on 13 Jun 2024
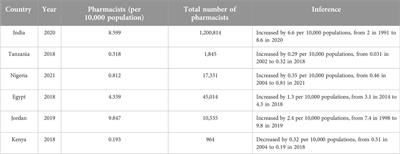
Brief Research Report
Published on 11 Jun 2024
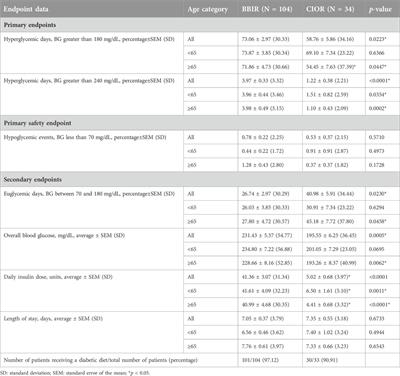
Original Research
Published on 10 Jun 2024
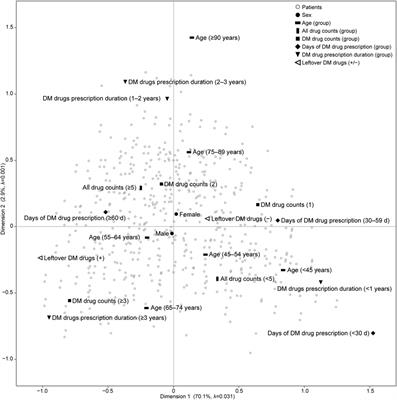
Mini Review
Published on 06 Jun 2024
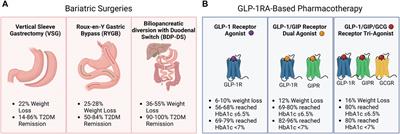
Mini Review
Published on 28 May 2024
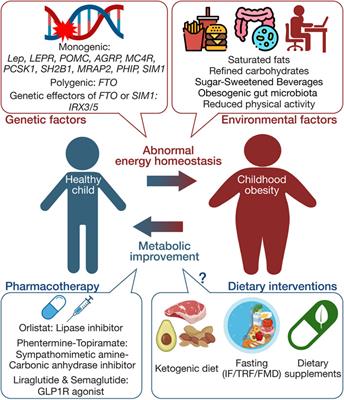
Review
Published on 19 Apr 2024
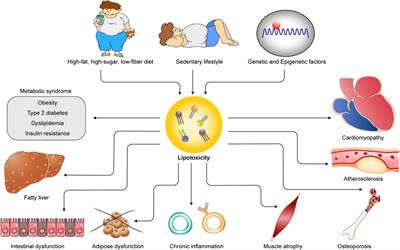
Original Research
Published on 28 Mar 2024
Review
Published on 21 Mar 2024
Review
Published on 20 Mar 2024
Review
Published on 15 Mar 2024
