- N. N. Blokhin National Medical Research Center of Oncology, Moscow, Russia
Numerous studies have shown that antitumor vaccines based on synthetic peptides are safe and can induce both CD8+ and CD4+ tumor-specific T cell responses. However, clinical results are still scarce, and such approach to antitumor treatment has not gained a wide implication, yet. Recently, particular advances have been achieved due to tumor sequencing and the search for immunogenic neoantigens caused by mutations. One of the most important issues for peptide vaccines, along with the choice of optimal adjuvants and vaccination regimens, is the search for effective target antigens. Extensive studies of peptide vaccines, including those on murine models, are required to reveal the effective vaccine constructs. The review presents transplantable murine tumors with the detected peptides that showed antitumor efficacy as a vaccine compound.
Introduction
Peptide-based antitumor vaccines include synthetic tumor-associated or tumor-specific peptides or combinations of peptides and are designed to activate peptide-specific T cells in vivo.
Peptide vaccines have a number of advantages over other types of vaccines, especially in terms of safety and simple manufacturing techniques. Unlike other antigen-specific therapies, such as CAR T cells, an important characteristic of peptide-based vaccines is that they can easily and economically combine several different antigens within a single injection. Thus, multipeptide vaccines may solve the problem of antigen loss and reduce the risk of tumor escape from the immune reactions, which often results from the received chemotherapy.
The main difficulties in creating peptide antitumor vaccines are the selection of the most optimal antigens and the search for effective but non-toxic adjuvants [1]. An ideal antigen for a tumor vaccine should be highly immunogenic and strongly expressed in all cancer cells (but not in normal cells), and the survival of cancer cells should depend on these antigens. Tumor antigens could be classified as tumor-associated antigens (TAA) and tumor-specific antigens. Tumor-associated antigens are also expressed in normal cells, though their expression in tumors is several times higher. TAA include differentiation, cancer-testis (CT) and overexpressed antigens. The limitation for TAA use in vaccines is potential elimination of the activated T-cells that recognize TAA due to the central immune tolerance of the thymus, which will eventually affect the vaccine efficacy. Moreover, TAAs are also expressed in non-malignant tissues, which increases the risk of vaccine-induced autoimmune toxicity. However, clinical studies of antitumor vaccines have demonstrated only rare autoimmune events [2–5].
Tumor-specific antigens include a number of antigens of viral origin and antigens emerging after mutations in cellular proteins (neoantigens). It is assumed that during tumor growth cancer cells accumulate a large number of somatic mutations which lead to the formation of neoantigens. Some of these neoantigens are highly immunogenic and may be regarded as target molecules for peptide vaccines. Neoantigens have original tumor specificity and therefore they are not affected by central or peripheral tolerance. A promising potential of neoantigen-based vaccines has been studied in preclinical and early clinical trials evaluating neoantigen-based peptide vaccines in a number of cancers [6–8]. Modern sequencing and bioinformatics technologies are now available for the development of personalized vaccines based on neoantigens. However, identification of immunogenic neoantigens is still a challenge and various bioinformatics tools are being designed to improve prediction and selection of candidate neoepitopes for antitumor vaccines. Besides, different tumors have a rather low mutational burden and selection of a necessary range of immunogenic neoantigens becomes a problem.
Preclinical studies of peptide vaccines involve various models of transplantable mouse tumors. Mouse models include a class of antigen-transfected tumors. For instance, mouse TC-1 tumor cells express E6 and E7 oncogenes derived from human papillomavirus (HPV-16). Thus, TC-1 cells may be considered as a mouse model of HPV-16-induced cervical cancer. Transfected cell lines have an expressed antigen as a target for an antitumor vaccine that can be used for studying new adjuvants.
The review presents the description of mouse tumor lines for studying new adjuvants or combination of new peptide antigens with the previously identified peptides in antitumor vaccines. The purpose of the review is to summarize the information about the cell lines of transplantable mouse tumors used in preclinical studies of peptide antitumor vaccines.
Transplantable Mouse Tumor Models With the Available Immunogenic Peptides
The table below comprises a number of models of transplantable mouse tumors with the available immunogenic peptides (Table 1). The study reports describe antitumor functions of these peptides being the base for antitumor vaccines.
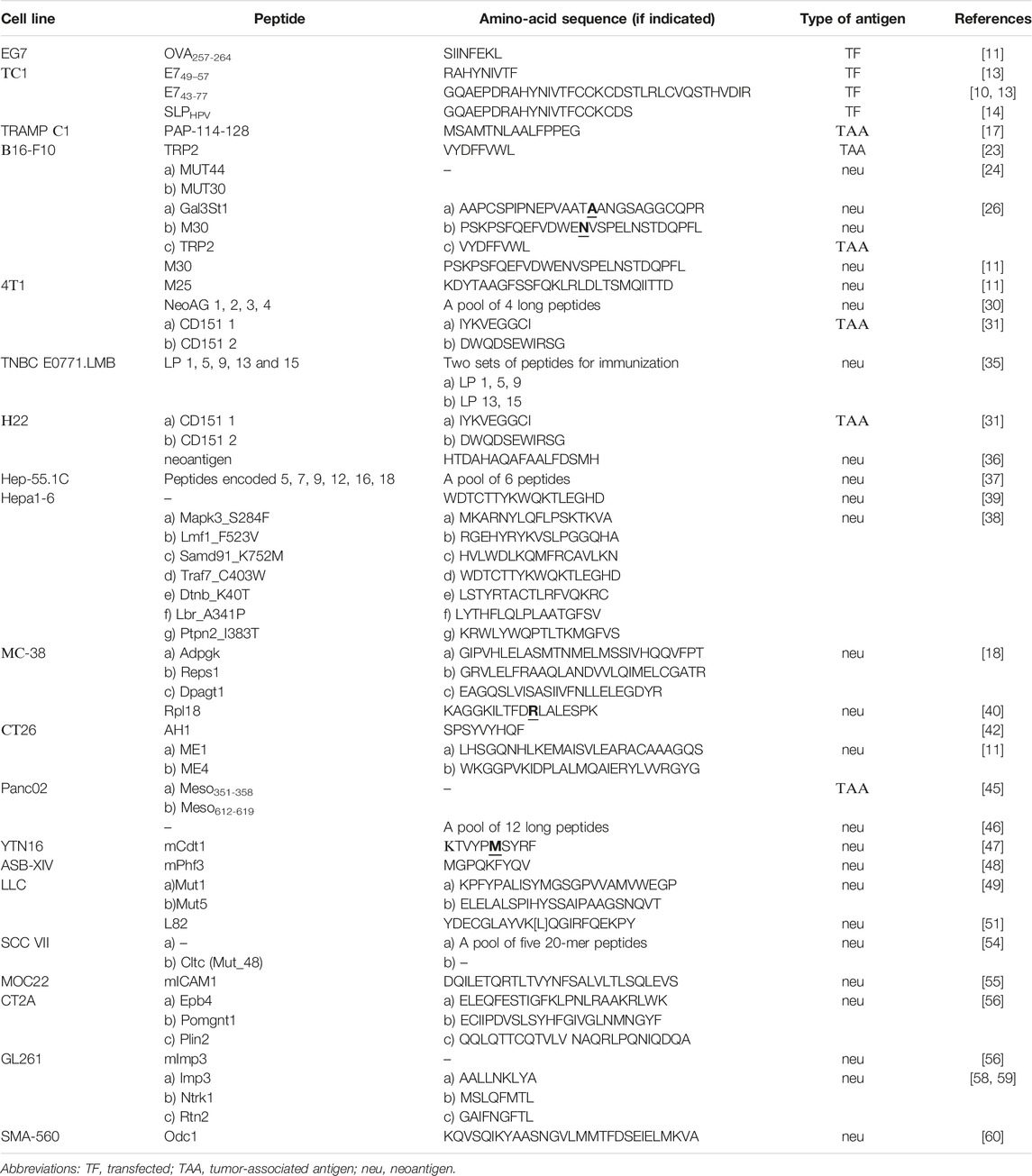
TABLE 1. Transplantable mouse tumor cell lines with the available peptides that have antitumor activity as a vaccine compound.
EG7
EG7 is a lymphoma cell line (derived from the EL4 line) that expresses ovalbumin and grows in mice C57BL/6. The line is often used to study new vaccine adjuvants, since it expresses a well-known antigen ovalbumin and antitumor vaccines and adjuvants usually show a pronounced effect towards EG7. M. Matsumoto et al. studied their original adjuvant in the EG7 model in the therapeutic mode with ovalbumin as an antigen. The study results showed a significant reduction of the tumor size [9]. Other authors, R. Heidenreich et al., also studied a new adjuvant as a vaccine component with the antigen ovalbumin in prophylactic and therapeutic modes. It should be noted that the researchers registered moderate inhibition of the tumor growth in the prophylactic mode, and after vaccination the tumors did not express ovalbumin. Probably, immune surveillance could have led to the selection of OVA-negative tumor cells [10]. Zh. Jing et al. reported a study of a vaccine construct containing OVA257-264 peptide that could significantly inhibit the EG7 tumor growth in both prophylactic and therapeutic regimens [11].
TC-1
The TC-1 cell line was derived from the primary lung epithelial cells of the C57BL/6 mice. They are transformed by the c-Ha-ras oncogene and consistently express E6 and E7 oncogenes derived from the human papillomavirus (HPV-16). These cells provide progressive tumor growth after infection with the oncogenic dose [12]. TC-1 cells might be regarded as a mouse model of HPV-16-induced cervical cancer. A study [13] showed that the therapeutic vaccination with long peptide E743-77 with an adjuvant had a significantly stronger antitumor effect than that with short peptide E749-57 and an adjuvant. Another study showed a very significant antitumor efficacy of long peptide E743-77 and an adjuvant used in the prophylactic and therapeutic regimens [10]. G.G. Zom et al. immunized the mice with transplantable TC-1 tumors by the vaccines obtained by conjugating an adjuvant with the SLPHPV peptide or by plain mixing an adjuvant and the SLPHPV peptide. The results showed that the conjugation, but not the mixture, of the adjuvant and SLPHPV significantly increased the survival of mice [14]. Other researchers, E.K. Duperret et al. performed a study to search for neoantigens in the tumor. The authors found that a DNA vaccine based on the detected neoantigens inhibited the tumor growth [15].
TRAMP C1
TRAMP C1 is a transgenic prostate adenocarcinoma of the C57BL/6 mice [16]. Patients with human prostate cancer have an overexpressed prostatic acid phosphatase (PAP), which is a tumor-associated antigen. Regarding an antitumor vaccine, it is important to note that PAP sequences have a high degree of homology between murine and human proteins. A vaccine with the PAP-114-128 peptide inhibited the growth of the TRAMP C1 mouse tumor in both prophylactic and therapeutic regimens [17]. Nevertheless, an attempt to find immunogenic tumor mutations by mass spectrometry and exome sequencing showed a small number of mutations in that cell line and no immunogenic neoantigens were found [18].
ID8
ID8 cell line is used as a syngeneic mouse model for ovarian cancer. The cell line was derived from the epithelial cells of the surface epithelium of the ovaries of C57BL/6 mice. S.D. Martin et al. searched for neoantigens in the ID8 cells and found just a small number of mutations. The authors used peptide vaccines based on the detected neoantigens, but the vaccination could not inhibit the progression of the tumor in either prophylactic or therapeutic regimens [19]. E.K. Duperret et al. vaccinated the animals with a prophylactic neoantigen-based DNA vaccine and achieved an increased survival of the mice [15].
B16-F10
B16-F10 is a metastatic clone of the B16 cell line derived from spontaneous melanoma of C57BL/6 mice. B16-F10 cells have low immunogenicity, and vaccination with irradiated tumor cells could not protect the mice from tumor growth after transplantation of live B16-F10 cells [20, 21], which might be partly explained by low expression of MHC class I and II molecules [22]. M.B. Bloom et al. have found that tyrosinase-related protein 2 (TRP2) is a tumor rejection antigen for melanoma B16-F10. Their studies showed that cytotoxic lymphocytes stimulated in vitro with the TRP2 peptide could recognize B16-F10 tumor and had a therapeutic effect against established pulmonary metastases [23]. J.C. Castle et al. sequenced melanoma B16-F10 and found an antitumor effect associated with the two tested neoantigenic peptides MUT44 and MUT30. Each peptide included into a vaccine with an adjuvant was used in a therapeutic regimen and thus showed a decreased tumor growth compared with the control [24]. Another study showed an antitumor effect of an RNA vaccine including peptide sequence M30 [25]. Zh. Jing et al. used a vaccine construct with M30 peptide antigen in a prophylactic mode. The authors reported that the vaccine significantly inhibited the development of B16-F10 metastases in the mouse lungs [11]. In 2021 H. Lam et al. reported a study of the whole exome sequencing of melanoma B16-F10. It revealed another neoantigenic peptide Gal3St1, which was used in vaccination resulting in a moderate antitumor effect. On the other hand, vaccination with a set of peptides Gal3St1+M30+TRP2 with an adjuvant in the therapeutic mode led to a very strong antitumor effect. Studies on the B16-F10 model helped the authors to discover a phenomenon that some neoantigenic peptides may inhibit the immune responses. Immunization with such peptides led to accelerated tumor growth and canceled the effect of the protective vaccine [26]. Y. Zhang et al. proposed an original way to increase the immunogenicity of neoantigens. The authors modified 4 weak neoantigenic peptides B16-F10 and significant inhibition of tumor growth was observed in preventive and therapeutic regimens after immunization with this construct [27].
4T1
4T1 mammary carcinoma of BALB/C mice is highly tumorigenic and invasive and may have spontaneous metastases [28]. An RNA vaccine including peptide sequence M25 showed an essential antitumor effect [25]. Zh. Jing et al. immunized the mice with a vaccine construct including peptide M25 as an antigen in the prophylactic mode and noted a slight slowdown of the tumor growth [11]. M. Peterson et al. searched for the neoantigens that appeared due to the errors in the production of RNA resulting from the frameshift, but not due to the DNA mutations. The researchers tested the vaccines that included about 10 neoantigenic peptides with an adjuvant. The immunization was performed in the therapeutic regimen and the results showed a reduction in the incidence and rate of tumor growth, though it should be noted that the peptides were not studied individually [29]. M.O. Mohsen et al. performed a search for immunogenic neoantigens and selected 4 peptides for a more thorough study. Therapeutic immunization of mice with a vaccine construct including long peptides of these neoantigens led to inhibition of tumor growth [30]. W. Lin et al. completed a proteomic and bioinformatics analysis and identified protein CD151 as a potential tumor-associated antigen for a vaccine to be tested in the 4T1 and H22 cell lines. The preventive immunization with two peptides of protein CD151 with an adjuvant showed a lower rate of metastasis and fewer tumor nodes in the lungs of mice as compared with the control. Mice immunized with the peptide had a higher survival rate, as well [31]. C. Lhuillier et al. searched for potentially immunogenic neoantigens for this cell line and found only a small number of candidate genes. However, the expression of some candidate genes increased significantly after irradiation of 4T1 cells. The authors made a vaccine from an adjuvant and three peptides to those neoantigens (DHX58, CAND1 and ADGRF5-II). The antitumor effect was observed only when vaccination was combined with radiation therapy, but not when vaccination was used in mono-regimen [32]. L. Li et al. used sequencing and bioinformatics analysis to search for neoantigens for 4T1 cells and another murine mammary tumor E0771, though the mice were immunized with a DNA vaccine, but not with peptides [33].
TNBC E0771.LMB
Metastatic tumor EO771.LMB was derived from poorly metastatic mammary tumor cells EO771 of C57BL/6 mice [34]. R. Brito Baleiro et al. performed whole exome sequencing and bioinformatics analysis to search for immunogenic neoantigens of the TNBC E0771.LMB cell line. Then the authors immunized the mice with a pool of long peptides, an adjuvant and a PD-1 inhibitor. The experimental animals had slower tumor growth and longer survival compared to the untreated control mice or animal group receiving the adjuvant and a PD-1 inhibitor [35]. Other researchers, such as L. Li et al. searched for and studied neoantigens of the E0771 cells [33].
H22
H22 is a BALB/C mouse hepatocellular carcinoma cell line. As mentioned above, W. Lin et al. identified protein CD151 as a promising tumor-associated antigen for 4T1 and H22 murine transplantable tumors. When mice were vaccinated prophylactically with two CD151 peptides and an adjuvant, tumor growth significantly slowed down as compared to the control group [31]. D. Zhang et al. identified a neoantigen of the H22 cells. Prophylactic immunization with an adjuvant and the neoantigenic peptide inhibited the tumor growth. The authors designed a vaccine that prevented tumor growth in a part of experimental animals. Therapeutic vaccination with this construct led to slower tumor growth and improved survival [36].
Hep-55.1C
Hep-55.1C is a hepatocellular carcinoma cell line derived from C57BL/6 mice. S.F. Yang et al. revealed neoantigens in cell lines Hep-55.1C and Dt81 Hepa1-6 by comparing their whole exome sequences with those of normal mouse liver. Mice were vaccinated with an adjuvant and a mixture of six neoantigenic peptides. Treatment of mice with the vaccine in a therapeutic regimen resulted in slower tumor growth and improved survival compared to administration of an adjuvant alone. Combining vaccination with anti-PD-1 therapy resulted in more significant tumor regression as compared to that after monotherapy [37].
Hepa1-6
Hepa1-6 is a hepatoma cell line derived from C57L mice. H. Chen et al. performed tumor cell sequencing, bioinformatics analysis, and neoantigen screening and selected 7 peptides for immunization of mice. Therapeutic vaccination with a mixture of these peptides and the adjuvant Poly(I:C) (but not other adjuvants) led to the disappearance of tumors in 60% of mice. Immunization with each peptide separately showed lower antitumor efficacy [38]. Q. Zhao et al. included one neoantigenic peptide, specific to these tumor cells, into the vaccine construct and achieved a marked antitumor effect [39]. S.F. Yang et al. identified specific neoantigens for line Dt81 Hepa1-6 [37].
MC-38
MC-38 is a cell line of the colon adenocarcinoma of C57BL/6 mice. The line is a tumor model with a high mutational burden. Vaccination with three predicted neoantigenic peptides together with an adjuvant generated effective T-cell immunity for significant tumor inhibition in prophylactic and therapeutic regimens [18]. B.J. Hos et al. revealed another immunogenic neoantigen Rpl18. Mice vaccinated with this peptide in a therapeutic regimen had a significantly lower tumor size compared to the control animals that received only an adjuvant [40]. B. Schrors et al. showed that MC38 colorectal adenocarcinoma cell lines derived from two different sources had significant differences in transcriptome and mutanome expression. There were also differences in the previously described immunogenic neoantigens [41].
CT26
CT26 is a cell line of the colon carcinoma of BALB/C mice. The results of a study of a therapeutic RNA vaccine including a mixture of five neoantigenic peptides (pentatope 2) demonstrated a significant antitumor effect [25]. J.E. Slansky et al. showed that immunization with the AH1 (SPSYVYHOF) peptide, which is an epitope of the gp70 protein (a protein of the endogenous mouse virus) within a therapeutic dendritic cell vaccine resulted in the inhibition of tumor growth and an increase of the survival rate of mice [42]. Zh. Jing et al. designed a vaccine construct including 2 neoantigenic peptides ME1 and ME4. The researchers noted an inhibition of tumor growth after preventive and therapeutic vaccination. Besides, the therapeutic regimen showed improved survival of the experimental animals [11]. S. Feola et al. studied a vaccine construct consisting of an adenovirus coated with tumor antigen peptides that were similar to pathogen antigens due to molecular mimicry. The authors demonstrated the antitumor effectiveness of the designed vaccine. The peptide had a poly-K amino acid sequence at one end [43].
Panc02
The Panc02 cell line is a C57BL/6 mouse model of pancreatic adenocarcinoma. A study found that Panc02 cells and human tumor samples overexpressed mesothelin [44]. I.C. Leao et al. used a prophylactic dendritic cell vaccine, where the antigen included two peptides to mesothelin Meso351-358 and Meso612-619. The vaccination increased the survival rate of the mice [45]. H.L. Kinkead et al. performed the whole exome sequencing, RNA sequencing (RNASeq), and the NetMHC immunogenicity prediction algorithm to develop a neoantigenic Panc02 tumor vaccine. Therapeutic immunization with an adjuvant and 12 long peptides resulted in delayed tumor growth and increased survival of the mice. However, the researchers did not study individual peptides [46].
YTN16
YTN16 is a transplantable cell line from gastric cancer of C57BL/6 mice. K. Nagaoka et al. identified the immunogenic neoantigenic peptide mCdt1 in these tumor cells. A therapeutic vaccine based on dendritic cells loaded with this peptide showed an effective inhibition of the YTN16 tumor growth [47].
ASB-XIV
ASB-XIV is a BALB/c mouse lung carcinoma cell line. C. Sun et al. performed sequencing and bioinformatics analyses of the tumor and screening of neoepitope peptides, which revealed the immunogenic neoantigenic peptide mPhf3. Then the authors studied a preventive and therapeutic dendritic cell vaccine with the mPhf3 peptide and showed a significant inhibition of tumor growth compared to that of the control mice [48].
LLC
Lewis lung carcinoma (LLC) is a tumor cell line derived from spontaneous epidermoid carcinoma of the C57BL/6 mouse lungs. T. Chen et al. performed a search for immunogenic neoantigenic peptides of the tumor and showed that therapeutic vaccination with neoantigenic peptides Mut1 or Mut5 with an adjuvant resulted in delayed tumor growth and increased survival of the mice compared with the tumor growth and survival rate after the use of the adjuvant alone [49]. J. Sun et al. also searched for immunogenic neoantigens for LLC and found 10 neoantigens. An RNA vaccine targeting these neoantigens showed a reasonable antitumor effect [50]. C. Sun et al. conducted the whole exome and transcriptome sequencing of LLC from the ATCC collection to predict the neoantigen expressions. The authors evaluated neoantigenic peptide candidates and identified the most effective peptide L82. The experimental animals received a dendritic cell (DC) vaccine based on L82 and an additional immunomodulator CpG in a prophylactic mode, which resulted in a partial inhibition of the LLC1 tumor growth [51]. H. Qin et al. performed a search for immunogenic neoantigens in the LLC cells. The immunization was carried out using adoptive transfer of the RNA vaccine-treated DC-induced T cells [52].
SCC VII
Squamous cell carcinoma VII (SCC VII) is a spontaneous tumor of C3H mice and it has similar characteristics to human head and neck squamous cell carcinoma (HNSCC) [53]. J.S. Dolina et al. performed a search for neoantigens in the cell line. Prophylactic immunization with a pool of five 20-mer peptides and an adjuvant led to inhibition of tumor growth. The authors studied the five peptides individually, as well. Only vaccination with Cltc peptide (Mut_48) showed a pronounced antitumor effect [54].
MOC22
MOC22 is an oral cancer cell line derived from C57BL/6 mice. P. Zolkind et al. identified the mICAM1 neoantigen expressed by MOC22 cells using a cancer immunogenomics pipeline and ELISPOT immunogenicity testing. Prophylactic immunization by neoantigen peptide with an adjuvant resulted in the rejection of MOC22 tumors in 80% of mice [55].
CT2A
CT2A is a glioma cell line established from C57BL/6 mice. С.J. Liu et al. performed whole-exome DNA sequencing and RNA sequencing to search for neoantigens in the CT2A cells. The authors selected 3 neoantigenic peptides: Epb4, Pomgnt1, and Plin2. Therapeutic vaccination with the three peptides in combination with anti-PD-L1 treatment led to increased survival compared to the vaccine alone or anti-PD-L1 monotherapy [56].
GL261
GL261 cells present a syngeneic animal glioma model of C57BL/6 mice. T.M. Johannes et al. identified tumor-specific neoantigens in the GL261 cells on the base of the whole exome sequencing of DNA and RNA [57]. Another study showed that therapeutic vaccination of mice with the neoantigen peptide mImp3 and an adjuvant resulted in significantly improved survival compared with the adjuvant alone [56]. T. Su et al. studied a vaccine construct including 3 neoantigen peptides (Imp3, Ntrk1, Rtn2) combined with checkpoint inhibitors in a therapeutic regimen. The results showed a slight improvement of the survival in mice with GL261 tumors [58]. Previously, L. Scheetz et al. demonstrated the antitumor efficacy of a vaccine construct with these three peptides in combination with anti-PD-L1 therapy [59].
SMA-560
Cell line SMA-560 was established from glioma of VM/Dk mice. T.M. Johannes et al. searched for neoantigens in the SMA-560 murine tumor model and, using tetramers, found T cells specific to the Odc1 neoantigen [57]. A.M. Swartz et al. showed that therapeutic vaccination of mice with a long peptide of neoantigen Odc1 inhibited tumor growth [60].
Discussion
Nowadays, a potential for antitumor peptide vaccination is widely studied. New algorithms of searching for effective antigens are being developed, and new adjuvants are being studied. The presented information on transplantable mouse tumors with the identified peptides could be helpful for preclinical studies of antitumor peptide vaccines. Transplantable tumor models provide an important basis for designing new methods for identification of immunogenic antigens and direct testing the antigen antitumor effectiveness in vivo. In particular, that refers to the neoantigens arising due to mutations. In addition, these models could be used for comparison of various approaches to vaccination, such as comparing different lengths of peptides, different adjuvants, etc. When the problems in antigen identification and adjuvant selection are solved, the potential for this method could add to clinical practice as an independent therapy or in combination with other methods. Combination with therapies that reduce immunosuppression of the tumor microenvironment, such as checkpoint inhibitors, could be especially effective.
Author Contributions
This work was carried out in collaboration between all authors. AP gathered the literature data, wrote the initial manuscript draft. IS, ZS, MB gathered the literature and refined the initial manuscript draft. VK put forward with the concept, reviewed and edited the final draft. All authors approved the submitted article.
Funding
The work and the article had financial support of the Ministry of Science and Higher Education of the Russian Federation as part of the research “Creation and development of a bioresource collection of genetically and phenotypically characterized cell lines and primary human tumors.” Research registration # 075-15-2021-1060.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Nelde, A, Rammensee, HG, and Walz, JS. The Peptide Vaccine of the Future. Mol Cell Proteomics (2021) 20:100022. doi:10.1074/mcp.R120.002309
2. Amos, SM, Duong, CP, Westwood, JA, Ritchie, DS, Junghans, RP, Darcy, PK, et al. Autoimmunity Associated With Immunotherapy of Cancer. Blood (2011) 118(3):499–509. doi:10.1182/blood-2011-01-325266
3. Walter, S, Weinschenk, T, Stenzl, A, Zdrojowy, R, Pluzanska, A, Szczylik, C, et al. Multipeptide Immune Response to Cancer Vaccine IMA901 After Single-Dose Cyclophosphamide Associates With Longer Patient Survival. Nat Med (2012) 18(8):1254–61. doi:10.1038/nm.2883
4. Rini, BI, Stenzl, A, Zdrojowy, R, Kogan, M, Shkolnik, M, Oudard, S, et al. IMA901, a Multipeptide Cancer Vaccine, Plus Sunitinib Versus Sunitinib Alone, as First-Line Therapy for Advanced or Metastatic Renal Cell Carcinoma (IMPRINT): A Multicentre, Open-Label, Randomised, Controlled, Phase 3 Trial. Lancet Oncol (2016) 17(11):1599–611. doi:10.1016/S1470-2045(16)30408-9
5. Feyerabend, S, Stevanovic, S, Gouttefangeas, C, Wernet, D, Hennenlotter, J, Bedke, J, et al. Novel Multi-Peptide Vaccination in Hla-A2+ Hormone Sensitive Patients With Biochemical Relapse of Prostate Cancer. The Prostate (2009) 69(9):917–27. doi:10.1002/pros.20941
6. Li, L, Goedegebuure, SP, and Gillanders, WE. Preclinical and Clinical Development of Neoantigen Vaccines. Ann Oncol (2017) 28(12):xii11–xii17. doi:10.1093/annonc/mdx681
7. Blass, E, and Ott, PA. Advances in the Development of Personalized Neoantigen-Based Therapeutic Cancer Vaccines. Nat Rev Clin Oncol (2021) 18(4):215–29. doi:10.1038/s41571-020-00460-2
8. Lybaert, L, Thielemans, K, Feldman, SA, van der Burg, SH, Bogaert, C, and Ott, PA. Neoantigen-Directed Therapeutics in the Clinic: Where Are We? Trends Cancer (2023) 9(6):503–19. doi:10.1016/j.trecan.2023.02.004
9. Matsumoto, M, Tatematsu, M, Nishikawa, F, Azuma, M, Ishii, N, Morii-Sakai, A, et al. Defined TLR3-Specific Adjuvant That Induces NK and CTL Activation Without Significant Cytokine Production In Vivo. Nat Commun (2015) 6:6280. doi:10.1038/ncomms7280
10. Heidenreich, R, Jasny, E, Kowalczyk, A, Lutz, J, Probst, J, Baumhof, P, et al. A Novel RNA-Based Adjuvant Combines Strong Immunostimulatory Capacities With a Favorable Safety Profile. Int J Cancer (2015) 137(2):372–84. doi:10.1002/ijc.29402
11. Jing, Z, Wang, S, Xu, K, Tang, Q, Li, W, Zheng, W, et al. A Potent Micron Neoantigen Tumor Vaccine GP-Neoantigen Induces Robust Antitumor Activity in Multiple Tumor Models. Adv Sci (2022) 9(24):e2201496. doi:10.1002/advs.202201496
12. Lin, KY, Guarnieri, FG, Staveley-O'Carroll, KF, Levitsky, HI, August, JT, Pardoll, DM, et al. Treatment of Established Tumors With a Novel Vaccine That Enhances Major Histocompatibility Class II Presentation of Tumor Antigen. Cancer Res (1996) 56(1):21–6.
13. Zwaveling, S, Mota, SCF, Nouta, J, Johnson, M, Lipford, GB, Offringa, R, et al. Established Human Papillomavirus Type 16-Expressing Tumors Are Effectively Eradicated Following Vaccination With Long Peptides. J Immunol (2002) 169(1):350–8. doi:10.4049/jimmunol.169.1.350
14. Zom, GG, Willems, MMJHP, Khan, S, van der Sluis, TC, Kleinovink, JW, Camps, MGM, et al. Novel TLR2-Binding Adjuvant Induces Enhanced T Cell Responses and Tumor Eradication. J ImmunoTherapy Cancer (2018) 6(1):146. doi:10.1186/s40425-018-0455-2
15. Duperret, EK, Perales-Puchalt, A, Stoltz, RGHH, Gh, H, Mandloi, N, Barlow, J, et al. A Synthetic DNA, Multi-Neoantigen Vaccine Drives Predominately MHC Class I CD8+ T-Cell Responses, Impacting Tumor Challenge. Cancer Immunol Res (2019) 7(2):174–82. doi:10.1158/2326-6066.CIR-18-0283
16. Foster, BA, Gingrich, JR, Kwon, ED, Madias, C, and Greenberg, NM. Characterization of Prostatic Epithelial Cell Lines Derived From Transgenic Adenocarcinoma of the Mouse Prostate (TRAMP) Model. Cancer Res (1997) 57(16):3325–30.
17. Saif, JM, Vadakekolathu, J, Rane, SS, McDonald, D, Ahmad, M, Mathieu, M, et al. Novel Prostate Acid Phosphatase-Based Peptide Vaccination Strategy Induces Antigen-Specific T-Cell Responses and Limits Tumour Growth in Mice. Eur J Immunol (2014) 44(4):994–1004. doi:10.1002/eji.201343863
18. Yadav, M, Jhunjhunwala, S, Phung, QT, Lupardus, P, Tanguay, J, Bumbaca, S, et al. Predicting Immunogenic Tumour Mutations by Combining Mass Spectrometry and Exome Sequencing. Nature (2014) 515(7528):572–6. doi:10.1038/nature14001
19. Martin, SD, Brown, SD, Wick, DA, Nielsen, JS, Kroeger, DR, Twumasi-Boateng, K, et al. Low Mutation Burden in Ovarian Cancer May Limit the Utility of Neoantigen-Targeted Vaccines. PLoS One (2016) 11(5):e0155189. doi:10.1371/journal.pone.0155189
20. Dranoff, G, Jaffee, E, Lazenby, A, Golumbek, P, Levitsky, H, Brose, K, et al. Vaccination With Irradiated Tumor Cells Engineered to Secrete Murine Granulocyte-Macrophage Colony-Stimulating Factor Stimulates Potent, Specific, and Long-Lasting Anti-Tumor Immunity. Proc Natl Acad Sci U S A (1993) 90(8):3539–43. doi:10.1073/pnas.90.8.3539
21. Overwijk, WW, and Restifo, NP. B16 as a Mouse Model for Human Melanoma. Curr Protoc Immunol (2001) Chapter 20:Unit 20.1. doi:10.1002/0471142735.im2001s39
22. Seliger, B, Wollscheid, U, Momburg, F, Blankenstein, T, and Huber, C. Characterization of the Major Histocompatibility Complex Class I Deficiencies in B16 Melanoma Cells. Cancer Res (2001) 61(3):1095–9.
23. Bloom, MB, Perry-Lalley, D, Robbins, PF, Li, Y, el-Gamil, M, Rosenberg, SA, et al. Identification of Tyrosinase-Related Protein 2 as a Tumor Rejection Antigen for the B16 Melanoma. J Exp Med (1997) 185(3):453–60. doi:10.1084/jem.185.3.453
24. Castle, JC, Kreiter, S, Diekmann, J, Lower, M, van de Roemer, N, de Graaf, J, et al. Exploiting the Mutanome for Tumor Vaccination. Cancer Res (2012) 72(5):1081–91. doi:10.1158/0008-5472.CAN-11-3722
25. Kreiter, S, Vormehr, M, van de Roemer, N, Diken, M, Lower, M, Diekmann, J, et al. Mutant MHC Class II Epitopes Drive Therapeutic Immune Responses to Cancer. Nature (2015) 520(7549):692–6. doi:10.1038/nature14426
26. Lam, H, McNeil, LK, Starobinets, H, DeVault, VL, Cohen, RB, Twardowski, P, et al. An Empirical Antigen Selection Method Identifies Neoantigens That Either Elicit Broad Antitumor T-Cell Responses or Drive Tumor Growth. Cancer Discov (2021) 11(3):696–713. doi:10.1158/2159-8290.CD-20-0377
27. Zhang, Y, Lin, Z, Wan, Y, Cai, H, Deng, L, and Li, R. The Immunogenicity and Anti-Tumor Efficacy of a Rationally Designed Neoantigen Vaccine for B16F10 Mouse Melanoma. Front Immunol (2019) 10:2472. doi:10.3389/fimmu.2019.02472
28. Pulaski, BA, and Ostrand-Rosenberg, S. Mouse 4T1 Breast Tumor Model. Curr Protoc Immunol (2001) Chapter 20:Unit 20.2. doi:10.1002/0471142735.im2002s39
29. Peterson, M, Murphy, SN, Lainson, J, Zhang, J, Shen, L, Diehnelt, CW, et al. Comparison of Personal and Shared Frameshift Neoantigen Vaccines in a Mouse Mammary Cancer Model. BMC Immunol (2020) 21(1):25. doi:10.1186/s12865-020-00350-3
30. Mohsen, MO, Speiser, DE, Michaux, J, Pak, H, Stevenson, BJ, Vogel, M, et al. Bedside Formulation of a Personalized Multi-Neoantigen Vaccine Against Mammary Carcinoma. J Immunother Cancer (2022) 10(1):e002927. doi:10.1136/jitc-2021-002927
31. Lin, W, Liu, J, Chen, J, Li, J, Qiu, S, Ma, J, et al. Peptides of Tetraspanin Oncoprotein CD151 Trigger Active Immunity Against Primary Tumour and Experimental Lung Metastasis. EBioMedicine (2019) 49:133–44. doi:10.1016/j.ebiom.2019.10.025
32. Lhuillier, C, Rudqvist, NP, Yamazaki, T, Zhang, T, Charpentier, M, Galluzzi, L, et al. Radiotherapy-Exposed CD8+ and CD4+ Neoantigens Enhance Tumor Control. J Clin Invest (2021) 131(5):e138740. doi:10.1172/JCI138740
33. Li, L, Zhang, X, Wang, X, Kim, SW, Herndon, JM, Becker-Hapak, MK, et al. Optimized Polyepitope Neoantigen DNA Vaccines Elicit Neoantigen-Specific Immune Responses in Preclinical Models and in Clinical Translation. Genome Med (2021) 13(1):56. doi:10.1186/s13073-021-00872-4
34. Johnstone, CN, Smith, YE, Cao, Y, Burrows, AD, Cross, RS, Ling, X, et al. Functional and Molecular Characterisation of EO771.LMB Tumours, a New C57BL/6-Mouse-Derived Model of Spontaneously Metastatic Mammary Cancer. Dis models Mech (2015) 8(3):237–51. doi:10.1242/dmm.017830
35. Brito Baleeiro, R, Liu, P, Chard Dunmall, LS, Di Gioia, C, Nagano, A, Cutmore, L, et al. Personalized Neoantigen Viro-Immunotherapy Platform for Triple-Negative Breast Cancer. J Immunother Cancer (2023) 11(8):e007336. doi:10.1136/jitc-2023-007336
36. Zhang, D, Lin, Z, Wu, M, Cai, Z, Zheng, Y, He, L, et al. Cytosolic Delivery of Thiolated Neoantigen Nano-Vaccine Combined With Immune Checkpoint Blockade to Boost Anti-Cancer T Cell Immunity. Adv Sci (2021) 8(6):2003504. doi:10.1002/advs.202003504
37. Yang, SF, Weng, MT, Liang, JD, Chiou, LL, Hsu, YC, Lee, YT, et al. Neoantigen Vaccination Augments Antitumor Effects of Anti-PD-1 on Mouse Hepatocellular Carcinoma. Cancer Lett (2023) 563:216192. doi:10.1016/j.canlet.2023.216192
38. Chen, H, Li, Z, Qiu, L, Dong, X, Chen, G, Shi, Y, et al. Personalized Neoantigen Vaccine Combined With PD-1 Blockade Increases CD8+ Tissue-Resident Memory T-Cell Infiltration in Preclinical Hepatocellular Carcinoma Models. J Immunother Cancer (2022) 10(9):e004389. doi:10.1136/jitc-2021-004389
39. Zhao, Q, Wang, Y, Zhao, B, Chen, H, Cai, Z, Zheng, Y, et al. Neoantigen Immunotherapeutic-Gel Combined With TIM-3 Blockade Effectively Restrains Orthotopic Hepatocellular Carcinoma Progression. Nano Lett (2022) 22(5):2048–58. doi:10.1021/acs.nanolett.1c04977
40. Hos, BJ, Camps, MGM, van den Bulk, J, Tondini, E, van den Ende, TC, Ruano, D, et al. Identification of a Neo-Epitope Dominating Endogenous CD8 T Cell Responses to MC-38 Colorectal Cancer. Oncoimmunology (2019) 9(1):1673125. doi:10.1080/2162402X.2019.1673125
41. Schrors, B, Hos, BJ, Yildiz, IG, Lower, M, Lang, F, Holtsträter, C, et al. MC38 Colorectal Tumor Cell Lines From Two Different Sources Display Substantial Differences in Transcriptome, Mutanome and Neoantigen Expression. Front Immunol (2023) 14:1102282. doi:10.3389/fimmu.2023.1102282
42. Slansky, JE, Rattis, FM, Boyd, LF, Fahmy, T, Jaffee, EM, Schneck, JP, et al. Enhanced Antigen-Specific Antitumor Immunity With Altered Peptide Ligands That Stabilize the MHC-Peptide-TCR Complex. Immunity (2000) 13(4):529–38. doi:10.1016/s1074-7613(00)00052-2
43. Feola, S, Chiaro, J, Martins, B, Russo, S, Fusciello, M, Ylösmäki, E, et al. A Novel Immunopeptidomic-Based Pipeline for the Generation of Personalized Oncolytic Cancer Vaccines. Elife (2022) 11:e71156. doi:10.7554/eLife.71156
44. Li, M, Bharadwaj, U, Zhang, R, Zhang, S, Mu, H, Fisher, WE, et al. Mesothelin Is a Malignant Factor and Therapeutic Vaccine Target for Pancreatic Cancer. Mol Cancer Ther (2008) 7(2):286–96. doi:10.1158/1535-7163.MCT-07-0483
45. Leao, IC, Ganesan, P, Armstrong, TD, and Jaffee, EM. Effective Depletion of Regulatory T Cells Allows the Recruitment of Mesothelin-Specific CD8+ T Cells to the Antitumor Immune Response Against a Mesothelin-Expressing Mouse Pancreatic Adenocarcinoma. Clin Translational Sci (2008) 1(3):228–39. doi:10.1111/j.1752-8062.2008.00070.x
46. Kinkead, HL, Hopkins, A, Lutz, E, Wu, AA, Yarchoan, M, Cruz, K, et al. Combining STING-Based Neoantigen-Targeted Vaccine With Checkpoint Modulators Enhances Antitumor Immunity in Murine Pancreatic Cancer. JCI Insight (2018) 3(20):e122857. doi:10.1172/jci.insight.122857
47. Nagaoka, K, Sun, C, Kobayashi, Y, Kanaseki, T, Tokita, S, Komatsu, T, et al. Identification of Neoantigens in Two Murine Gastric Cancer Cell Lines Leading to the Neoantigen-Based Immunotherapy. Cancers (Basel) (2021) 14(1):106. doi:10.3390/cancers14010106
48. Sun, C, Nagaoka, K, Kobayashi, Y, Maejima, K, Nakagawa, H, Nakajima, J, et al. Immunotherapies Targeting Neoantigens Are Effective in PD-1 Blockade-Resistant Tumors. Int J Cancer (2023) 152(7):1463–75. doi:10.1002/ijc.34382
49. Chen, T, Hu, R, Wan, Y, Sun, F, Wang, Z, Yue, J, et al. Comprehensive Mutanome Analysis of Lewis Lung Cancer Reveals Immunogenic Neoantigens for Therapeutic Vaccines. Biochem Biophysical Res Commun (2020) 525(3):607–13. doi:10.1016/j.bbrc.2020.02.132
50. Sun, J, Zhang, J, Hu, H, Qin, H, Liao, X, Wang, F, et al. Anti-Tumour Effect of Neo-Antigen-Reactive T Cells Induced by RNA Mutanome Vaccine in Mouse Lung Cancer. J Cancer Res Clin Oncol (2021) 147(11):3255–68. doi:10.1007/s00432-021-03735-y
51. Sun, C, Nagaoka, K, Kobayashi, Y, Nakagawa, H, Kakimi, K, and Nakajima, J. Neoantigen Dendritic Cell Vaccination Combined With Anti-CD38 and CpG Elicits Anti-Tumor Immunity Against the Immune Checkpoint Therapy-Resistant Murine Lung Cancer Cell Line LLC1. Cancers (Basel) (2021) 13(21):5508. doi:10.3390/cancers13215508
52. Qin, H, Hu, H, Liao, X, Zhao, P, He, W, Su, X, et al. Antitumor Effect of Neoantigen-Reactive T Cells Combined With PD1 Inhibitor Therapy in Mouse Lung Cancer. J Cancer Res Clin Oncol (2023) 149(10):7363–78. doi:10.1007/s00432-023-04683-5
53. Khurana, D, Martin, EA, Kasperbauer, JL, O'Malley, BW, Salomao, DR, Chen, L, et al. Characterization of a Spontaneously Arising Murine Squamous Cell Carcinoma (SCC VII) as a Prerequisite for Head and Neck Cancer Immunotherapy. Head Neck (2001) 23(10):899–906. doi:10.1002/hed.1130
54. Dolina, JS, Lee, J, Brightman, SE, McArdle, S, Hall, SM, Thota, RR, et al. Linked CD4+/CD8+ T Cell Neoantigen Vaccination Overcomes Immune Checkpoint Blockade Resistance and Enables Tumor Regression. J Clin Invest (2023) 133(17):e164258. doi:10.1172/JCI164258
55. Zolkind, P, Przybylski, D, Marjanovic, N, Nguyen, L, Lin, T, Johanns, T, et al. Cancer Immunogenomic Approach to Neoantigen Discovery in a Checkpoint Blockade Responsive Murine Model of Oral Cavity Squamous Cell Carcinoma. Oncotarget (2017) 9(3):4109–19. doi:10.18632/oncotarget.23751
56. Liu, CJ, Schaettler, M, Blaha, DT, Bowman-Kirigin, JA, Kobayashi, DK, Livingstone, AJ, et al. Treatment of an Aggressive Orthotopic Murine Glioblastoma Model With Combination Checkpoint Blockade and a Multivalent Neoantigen Vaccine. Neuro-Oncology (2020) 22(9):1276–88. doi:10.1093/neuonc/noaa050
57. Johanns, TM, Ward, JP, Miller, CA, Wilson, C, Kobayashi, DK, Bender, D, et al. Endogenous Neoantigen-Specific CD8 T Cells Identified in Two Glioblastoma Models Using a Cancer Immunogenomics Approach. Cancer Immunol Res (2016) 4(12):1007–15. doi:10.1158/2326-6066.CIR-16-0156
58. Su, T, Liu, X, Lin, S, Cheng, F, and Zhu, G. Ionizable Polymeric Nanocarriers for the Codelivery of Bi-Adjuvant and Neoantigens in Combination Tumor Immunotherapy. Bioactive Mater (2023) 26:169–80. doi:10.1016/j.bioactmat.2023.02.016
59. Scheetz, L, Kadiyala, P, Sun, X, Son, S, Hassani Najafabadi, A, Aikins, M, et al. Synthetic High-Density Lipoprotein Nanodiscs for Personalized Immunotherapy Against Gliomas. Clin Cancer Res (2020) 26(16):4369–80. doi:10.1158/1078-0432.CCR-20-0341
Keywords: antitumor vaccines, peptides, antigens, neoantigens, transplantable murine tumors
Citation: Ponomarev AV, Shubina IZ, Sokolova ZA, Baryshnikova MA and Kosorukov VS (2024) Transplantable Murine Tumors in the Studies of Peptide Antitumor Vaccines. Oncol. Rev. 17:12189. doi: 10.3389/or.2023.12189
Received: 05 October 2023; Accepted: 22 December 2023;
Published: 08 January 2024.
Edited by:
Mauro Cives, University of Bari Aldo Moro, ItalyReviewed by:
Nada Chaoul, University of Bari Aldo Moro, ItalyBarbara Mandriani, G. Pascale National Cancer Institute Foundation (IRCCS), Italy
Copyright © 2024 Ponomarev, Shubina, Sokolova, Baryshnikova and Kosorukov. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aleksandr V. Ponomarev, kl8546@yandex.ru
 Aleksandr V. Ponomarev
Aleksandr V. Ponomarev Irina Zh. Shubina
Irina Zh. Shubina Zinaida A. Sokolova
Zinaida A. Sokolova Maria A. Baryshnikova
Maria A. Baryshnikova Vyacheslav S. Kosorukov
Vyacheslav S. Kosorukov