Abstract
Livestock farming is vital to global food systems, but the misuse of antibiotics in this sector has raised concerns about its role in the emergence of antimicrobial resistance - now a public health issue. Addressing the misuse of antibiotics requires an understanding of usage patterns and determinants among livestock farmers. This study investigated antibiotic use among livestock farmers in Uganda, examining the frequency of use and factors influencing usage with data from the 2018 Uganda Annual Agricultural Survey. Descriptive results showed that on average one in every three livestock keepers had used antibiotics in the previous year, with 15% of them using antibiotics at least every month in the previous year. Regression analysis results revealed that, among other factors, production systems (keeping cattle, keeping exotic livestock, and herd size) and keepers’ perceptions of the continued efficacy of antibiotics in controlling target diseases even with continuous use significantly influenced the decision to use antibiotics and the frequency with which they were used. The results of this study confirmed the growing concern that antibiotic use in Uganda is no longer limited to humans but has significantly expanded to the livestock sector too. The results further affirmed that antibiotic use in livestock production has reached significant levels that require streamlining from a public health perspective. The study recommends strengthening veterinary education and increasing public awareness of appropriate antibiotic use to combat wrong perceptions towards antibiotic use and encourage safe use.
Introduction
Uganda, as a low-income country, heavily relies on agriculture, including livestock farming, as a significant source of livelihoods and food security (Kaplan et al., 2019). Livestock is an important component of the livelihoods of 57% of the 70% of Ugandan households that depend on agriculture as a source of food, income, employment, and improved social status (Food and Agriculture Organization of the United Nations (FAO, 2019). Where the land terrain is favourable, livestock provide additional benefits in the form of draught power for cultivation and transport, especially in pastoral and agro-pastoral communities in the northern parts of the country. Nearly 90% of livestock keepers in Uganda are smallholder farmers producing at the subsistence level (FAO, 2019). For instance, although cattle are the main livestock in the country, only 8% and 2% of all cattle respectively, are kept in commercial ranching and semi-intensive systems highlighting the critical position of smallholder livestock keepers in the development of the sector.
Despite the number of people involved in livestock production, the sub sector contributed only 3.8% to the national GDP in 2019 (Uganda Bureau of Statistics (UBOS, 2020). Livestock rearing presents an opportunity to address high levels of poverty among smallholder farmers (Benson and Mugarura, 2013; FAO, 2019) if appropriate investments are made and persistent bottlenecks curtailing the growth of the sub sector are addressed. Persistent constraints to livestock production in Uganda include livestock parasites, infectious diseases, limited institutional and policy support for livestock farmers, and suboptimal management practices among farmers (Turner, 2005). These combined with emerging issues of climate change and turbulence in the international livestock markets continue to hold back the sector from reaching its true potential.
Livestock diseases such as contagious bovine pleuropneumonia, foot and mouth disease, Newcastle, or tick-borne diseases like east coast fever present the greatest challenge to producers due to their highly infectious and fast-spreading nature (Uganda National Academy of Sciences-(UNAS et al., 2015b). These have led to economic losses for farmers affecting the total supply of livestock products for both domestic consumption and sale. Of note, however, are bacterial diseases such as blackleg and mastitis in cattle, avian colibacillosis in poultry and salmonellosis-related diseases in all livestock in general, for which there has been a sharp increase in the use of antibiotics to abate them (Nayiga et al., 2020). Despite the undeniable importance of antibiotics in livestock health, their efficacy heavily depends on proper handling and judicious use (Chah, 2022). Anecdotal evidence has revealed that farmers administer antibiotics for conditions with fever symptoms (e.g., East Coast fever, tick fever, babesiosis or heartwater) even if they are not bacterial infections (UNAS et al., 2015a). In addition, the requirement to meet the current demand for food of animal origin in a cost-effective manner has made it necessary to ensure the production of early-maturing animal protein sources especially in poultry and piggery. Improvements in feed conversion ratios and disease management for optimal productivity have, to a significant extent, further driven the emerging demand for and increased use of antimicrobial agents (Mulchandani et al., 2023).
Antibiotic resistance has emerged as a critical global health crisis, posing a serious threat to the efficacy of existing medical interventions against infectious diseases and to public health management in general (Patel et al., 2020; Mulchandani et al., 2023). The rapid spread of antibiotic resistance has been primarily attributed to the inappropriate and excessive use of antibiotics in various sectors, such as agriculture, specifically in livestock production systems (Smith et al., 2021; García et al., 2022). Antibiotics are frequently employed in livestock farming to prevent disease, promote growth, and treat infections (Pew Commission on Industrial Farm Animal Production, 2020). While these practices may yield short-term benefits, they inadvertently contribute to the development of antibiotic resistance in both animals and humans (FAO, 2023; O'Neill, 2016). Moreover, inadequate regulatory frameworks and limited veterinary supervision in many low-resource settings exacerbate the misuse and overuse of antibiotics in livestock production (Dibner et al., 2018).
Uganda-specific estimates of commonly available antibiotics, quantities used, frequency, distribution channels and sources of information on antibiotic use are generally unavailable and when available they only cover a few districts (Kimera et al., 2020; Nayiga et al., 2020; Musoke et al., 2021), have limited samples, tend to be livestock specific (Sasanya et al., 2005; Ikwap et al., 2015) and are therefore not nationally representative. This is, in part, due to the poor documentation by both farmers and livestock health practitioners and the weak enforcement of policies on antimicrobial use (Musoke et al., 2021). This poses a great risk of drug misuse by farmers whose practices are largely unchecked leading to the emergence of antimicrobial resistance, a rising global threat to both human food safety and environmental conservation (Nayiga et al., 2020). Although Mikecz et al. (2020) showed that one in every three livestock farmers in Uganda use antibiotics, the extent, patterns, and implications of antibiotic use in livestock-keeping households in Uganda remain inadequately understood. In addition, previous research has primarily focused on antibiotic use in human healthcare settings, with limited attention given to the specific practices and drivers of antibiotic use in livestock production systems (Rwarimbuga et al., 2017).
Understanding the patterns and determinants of antibiotic use among livestock-keeping households is crucial for developing targeted interventions to mitigate the emergence and spread of antibiotic resistance in Uganda’s livestock sector by guiding the suitable use of antibiotics. Consequently, this study aims to address this knowledge gap by investigating the prevalence, patterns, and factors associated with antibiotic use among livestock-keeping households in Uganda. By investigating the prevalence, determinants, and frequency of antibiotic use among livestock farmers in Uganda, this study contributes to the understanding of AMR and provides insights for developing targeted interventions and policy measures. Understanding the factors influencing antibiotic use and promoting responsible practices can help mitigate the risks associated with AMR and ensure the long-term viability of livestock production systems in Uganda. These findings will inform strategies to improve veterinary education, strengthen regulatory frameworks, and increase public awareness regarding appropriate antibiotic use in livestock production. Ultimately, addressing the issue of antibiotic use in livestock-keeping households can significantly contribute to global efforts to combat antibiotic resistance and safeguard public health.
The rest of this paper consists of three main sections. The first section presents the methodological approach detailing aspects of the data used and the analytical path taken. The second section presents and interprets the results of the analysis. The final section provides a detailed discussion of the results, draws conclusions, and presents policy-relevant recommendations.
Materials and methods
Sources and type of data used
The study used the 2018 Annual Agricultural Survey (AAS) data set a nationally representative Household survey that was conducted by the Uganda Bureau of Statistics (UBOS) with technical support from FAO (https://microdata.ubos.org:7070/index.php/catalog/62/data-dictionary). Unlike the 2017 AAS and the other rounds of the AAS, in 2018, an antimicrobial use module was integrated into the survey to collect information on antibiotic use in livestock production. A detailed description of the AAS data especially the antimicrobial-related data has already been published by Mikecz et al. (2020). The AAS was administered to a sample of 7,157 agricultural households selected from all major regions of the country (UBOS, 2020). The livestock questionnaire in the AAS was administered during the post-harvest visit in the second season (March-May 2019) during which data on livestock stock, production and inputs were collected for the previous 12 months (UBOS, 2020). In the livestock module, five questions were asked that included the type of antibiotics used, the purpose of using antibiotics, the frequency of usage, who advises on the use of antibiotics, and the farmer’s opinion on whether frequent use of antibiotics can alter the efficacy of the drugs. These were the main questions used in this study and consequently both dependent variables (use and frequency of use) and some independent variables (knowledge and perceptions, and information seeking and access) were derived from these questions. Data were obtained from the UBOS microdata repository (online open access) and processed using Stata software version 17 (StataCorp, 2021). Data processing included cleaning, recoding of variables, calculation and generation of new variables to fit the purpose of the study. Table 1 presents the summary of the variables used in the analysis.
TABLE 1
| Variable | Percent/Mean | Std. Dev. |
|---|---|---|
| Dependent variable | ||
| Use of any antibiotics in the household in the last 12 months (1 = Male; 0 = Female) | 34.27% | |
| Frequency of antibiotic use | ||
| Never | 61.62% | |
| Occasional (less than monthly) | 33.17% | |
| Regular (At least once a month or more frequent) | 4.45% | |
| Regular (At least once per week) | 0.08% | |
| Independent variables | ||
| Household head is a youth (aged 35 years or below) (1 = Yes; 0 = No) | 11.06% | |
| Sex of the household head (1 = Male; 0 = Female) | 76.97% | |
| Household kept cattle and pack animals (1 = Yes; 0 = No) | 65.00% | |
| Household kept pigs (1 = Yes; 0 = No)? | 30.18% | |
| Household kept poultry (1 = Yes; 0 = No) | 67.06% | |
| Total tropical livestock units of both local and exotic animals owned* | 1.39 | 4.53 |
| Number of livestock types kept by the household | 2.10 | 1.08 |
| Proportion of land under crop | 0.71 | 0.31 |
| Region | ||
| Central region | 15.70% | |
| Eastern region | 25.67% | |
| Northern region | 34.21% | |
| Western region | 24.43% | |
| Household has at least one exotic livestock species (1 = Yes; 0 = No) | 12.51% | |
| Household’s main economic activity is agriculture (1 = Yes; 0 = No) | 82.50% | |
| Household’s members belong to a farmers’ group (1 = Yes; 0 = No) | 13.65% | |
| Household head has completed at least secondary school (1 = Yes; 0 = No) | 25.87% | |
| Household believes that antibiotics will not become less effective even with continuous use (1 = Yes; 0 = No) | 16.08% | |
| Information about antibiotics comes from private/public extension services (1 = Yes; 0 = No) | 4.58% | |
| Information about antibiotics comes from farmer to farmer (1 = Yes; 0 = No) | 14.17% | |
| Information about antibiotics comes from word of mouth/other peers (1 = Yes; 0 = No) | 10.38% | |
| Distance to nearest input shop is greater than 5 km (1 = Yes; 0 = No) | 67.73% | |
| Farmer has sought information from veterinarians (1 = Yes; 0 = No) | 20.81% | |
| Farmer has not sought advice from any source--self-administered (1 = Yes; 0 = No) | 10.13% | |
| Household had access to loans for agricultural purposes (1 = Yes; 0 = No) | 11.71% | |
| Number of households in the sample keeping livestock | 4,407 | |
Summary of variables used in the analysis.
Tropical Livestock Units are livestock numbers converted into a common unit. Source: microdata from the 2018 AAS (UBOS).
Data analysis
The study used both descriptive and econometric methods of analysis to address the set objectives. All data analysis was carried out using Stata 17 (StataCorp, 2021) Software except for graphs and illustrations where Microsoft Excel software was used. The UBOS data set came with sampling weights thus weighted percentages or means were reported for descriptive data while model output coefficients, standard errors, and p-values were reported for econometric analysis. Where statistical tests of differences were performed, p-values were reported. All hypotheses were tested at the 95% and 99% levels of significance.
Although the study’s main objective was to determine the drivers of frequency of use, we found it fitting to start by investigating the correlates of the decision to use antibiotics by livestock keepers. The dependent variable was the use or non-use of antibiotics. Naturally, when a dependent variable is binary and takes on the values of zero and one with mutually exclusive and exhaustive outcomes, a Probit or logit model should be motivated (Cragg, 1971). The motivation for the binary Probit model for this study was the response probability as shown in Eq. 1where is the probability that a randomly chosen household has used antibiotics in the 12 months prior to the date of the interview conditioned on , a set of explanatory variables influencing the decision to use antibiotics. The study assumed that the response probability is linear in a set of parameters taking on the form (Eq. 2);where and assumed a standard normal cumulative distribution function (cdf) taking values between zero and one i.e., 0< for all real numbers. Hence the estimation of the parameters using a Probit model.
For livestock keepers who used antibiotics, we descriptively determined how frequently antibiotics were used and weighted sample percentages were reported. The frequency of antibiotic use was analysed at the household (production unit) level. Households were characterised based on how often (never, occasionally, or often) they used antibiotics-this was also the dependent variable in the model described below. This was disaggregated by the sex of the main decision maker in the household to understand the gender dynamics in the use of antibiotics. The same data were also disaggregated by age to identify any role of the young or the elderly in the use of antibiotics. Statistical tests of differences (student’s t-tests and chi-square tests) were conducted to confirm statistical significance where appropriate.
The study also sought to determine the factors that influence the frequency with which antibiotics are used once the decision to use antibiotics has been made. The dependent variable (frequency of use) had three possible outcomes, that is, never used, occasionally used (less than once a month), and frequently used (at least once a month or more). The outcomes were discrete but ordered so an ordered response model (Probit or Logit) could be appropriate for such a dependent variable. However, when exploring the relationship between the frequency of antibiotic use and the factors influencing it, it is critical to note that although the frequency of use assumes non-negative discrete and ordered values, it is also characterised by a considerable proportion of zeros—non-users (Fávero et al., 2021). This is because a significant number of livestock keepers (over 65%) were found not to use antibiotics (Mikecz et al., 2020) and these would register a zero in the dependent variable. Therefore, the data on the frequency of antibiotic use showed an over-representation of the zeros (non-users).
Due to the nature of livestock keepers with very few units (e.g., only three chickens), this study considered that the people in the non-user category may be structurally different. There may be a category of people who have never used antibiotics and may never use them -for instance, people who do not invest in livestock disease management. The remainder could be people who have used antibiotics in the past but were non-users at the time of the study or who have not yet used antibiotics for their livestock but may in the future—true non-users. The standard ordered Probit model would fit the behaviour of antibiotic users, taking the non-user category to be homogeneous. The zero inflation arises because the non-users category now includes those who have never used antibiotics and may never use them and those who have never used and may use them. The existence of the latter group could lead to an inflation of the proportion of non-users. Standard-ordered Probit models cannot account for the great number of zero observations when the zeros relate to an extra, distinct source (those who may never use antibiotics) Harris and Zhao (2007). Thus, the zero-inflated ordered Probit (ZIOP) model was used to determine the drivers of antibiotic use frequency. ZIOP models are used for ordered response variables when the data exhibit a high proportion of observations at the lowest end of the ordering (0 or non-use). The concept of zero inflation has its origin in Poisson models of count data with an overabundance of zeros. ZIOP applies this idea to ordinal data, where the numerical value of the lowest category need not be zero. The study used Stata’s zioprobit command to fit the model (Harris and Zhao, 2007). The literature review (Manyi-Loh et al., 2018; Nayiga et al., 2020; Musoke et al., 2021; Mikecz et al., 2020) guided on the identification of key variables to be used as independent variables in the model.
Specification of the zero-inflated ordered probit model
We adhered to specifications by Maddala (1983) for Eqs 1–5, and then by Harris and Zhao (2007) for Eq. 6 and onwards to specify the zero-inflated ordered Probit model. Let denote a binary variable indicating the split between Regime 0 (with for non-antibiotic users) and Regime 1 (with for antibiotic users). is related to a latent variable via mapping: for and for . The latent variable represents the extent of antibiotic use and is given by Eq. 3.where is a vector of covariates that determine the choice between the two regimes, is a vector of unknown coefficients and is the error term. Therefore, the probability that a livestock keeper is in Regime 1 is given by Eq. 4 (Maddala, 1983).where is the cumulative distribution function (c.d.f.) of the univariate standard normal distribution.
Conditional on , frequency of use under Regime 1 is represented by a discrete variable that is generated by an ordered Probit model via a second underlying latent variable (Eq. 5):with z being a vector of explanatory variables with unknown weights , and an error term following a standard normal distribution. The mapping between and is given by Eq. 6where are boundary parameters to be estimated in addition to (unknown weight of parameters to be estimated), and we assume throughout the paper that . It should be noted that, importantly, Regime 1 also allows for zero consumption. Also, there is no requirement that . Under the assumption that is standard Gaussian, the ordered probit probabilities are specified as in Eq. 7 (Maddala, 1983).while and are not individually observable in terms of the zeros, they are observed via the criterion specified in Eq. 8.
That is, to observe a outcome we require either that (the individual is a non-user) or jointly that and (the individual is a zero-use user or a current non-user with the possibility of having used in the past or using in the future). To observe a positive , we require jointly that the individual is a user and . Under the assumption that and identically and independently follow standard Gaussian distributions, the full probabilities for y are given by Eq. 9.
In this way, the probability of a zero observation has been ‘‘inflated’’ as it is a combination of the probability of ‘‘zero use’’ from the ordered Probit process plus the probability of ‘‘non-use’’ from the split Probit model. Note that this specification is analogous to the zero-inflated/augmented count models, and that there may or may not be overlap with the variables in x and z.
Results
Use of antibiotics by livestock farmers in Uganda
Table 2 below shows the percentages of livestock keepers using antibiotics disaggregated by livestock type, animal breed (exotic or local) reared, and the region and sex of the household head. The results indicate that there were significantly more households using antibiotics for shoats (76.2%), and cattle and pack animals (72.6%). In addition, there were significantly more households with exotic breed animals in the category of antibiotic users (16.5%) compared to non-users (9.7%). The results also indicate that there were more male-headed households (78.3%) in the user category compared to the non-user category (75.6%). In terms of regions, the highest share of households using antibiotics was recorded in the North and Eastern regions (50% and 24% respectively). There were significantly more non-users than users in the Western and Central regions.
TABLE 2
| Variable | Use (%) | Non-use (%) | Z-statistic |
|---|---|---|---|
| Livestock kept | |||
| Cattle and pack animals | 72.6 | 30.9 | −25.591 |
| Shoats | 76.2 | 59.5 | −9.276 |
| Pigs | 25.9 | 30.7 | 1.856 |
| Poultry | 64.3 | 67.8 | −0.941 |
| Rabbits | 1.4 | 1.9 | 1.104 |
| At least one exotic animal | 16.5 | 9.7 | −7.495 |
| Region | |||
| Central | 12.7 | 16.4 | 1.321 |
| Eastern | 24.5 | 25.1 | −2.765 |
| Northern | 50.7 | 28.7 | −11.511 |
| Western | 12.1 | 29.8 | 12.790 |
| Mal head of household | 78.3 | 75.6 | −3.706 |
Level of antibiotic use-prevalence.
Source: 2018 AAS, microdata (UBOS).
Bold figures indicate variables significant at least 10% level of significance.
Factors that influence the decision to use antibiotics
This study used a Probit model to determine the correlates of antibiotic use status among livestock keepers in Uganda. The results (Table 3) indicate that production systems (keeping cattle, keeping exotic livestock, and herd size), socio economic and demographic (sex of the head of household and level of education) and regional/geographical, and institutional factors (access to information and distance to service providers) influenced the decision to use antibiotics. In addition, this study also found that livestock keepers who perceived antibiotics to retain their effectiveness even with continuous use were more likely to use antibiotics than their counterparts who perceived the opposite.
TABLE 3
| Household antibiotic use in the previous 12 months | Coefficient | std. err. | dy/dx | std. err. | P > t |
|---|---|---|---|---|---|
| Socioeconomic and Demographic factors | |||||
| Respondent is aged 35 years or younger | −0.017 | 0.129 | −0.002 | 0.019 | 0.896 |
| Head of household is a man | −0.260 | 0.083 | −0.038 | 0.012 | 0.002 |
| Main economic activity of household is agriculture | 0.080 | 0.116 | 0.012 | 0.017 | 0.491 |
| Household members belong to a farmer’s group | −0.036 | 0.098 | −0.005 | 0.014 | 0.712 |
| Education-Household head has completed at least secondary school | 0.212 | 0.093 | 0.031 | 0.014 | 0.023 |
| Production system and environment | |||||
| Household kept cattle and pack animals | 0.224 | 0.117 | 0.032 | 0.017 | 0.056 |
| Household kept pigs | −0.187 | 0.107 | −0.027 | 0.016 | 0.082 |
| Household kept poultry | 0.069 | 0.103 | 0.010 | 0.015 | 0.503 |
| Total tropical livestock units of local and exotic animals owned | 0.052 | 0.014 | 0.008 | 0.002 | 0.000 |
| Number of livestock species kept by the household | 0.119 | 0.071 | 0.017 | 0.010 | 0.096 |
| Proportion of land under crop | −0.169 | 0.139 | −0.024 | 0.020 | 0.227 |
| Household has at least one exotic livestock species | 0.208 | 0.105 | 0.030 | 0.015 | 0.049 |
| Region (Eastern region is the base category) | |||||
| Western Region | −0.009 | 0.102 | −0.001 | 0.015 | 0.930 |
| Central Region | 0.200 | 0.110 | 0.029 | 0.016 | 0.069 |
| Northern Region | 0.532 | 0.098 | 0.077 | 0.014 | 0.000 |
| Perceptions towards antibiotic use | |||||
| Household believes that antibiotics will not become less effective even with continuous use | 0.984 | 0.113 | 0.143 | 0.016 | 0.000 |
| Institutional factors | |||||
| Information about antibiotics comes from private/public extension services | 0.434 | 0.137 | 0.063 | 0.020 | 0.002 |
| Information about antibiotics comes from farmer to farmer | 0.416 | 0.101 | 0.060 | 0.015 | 0.000 |
| Distance to nearest input shop is greater than 5 km | −0.284 | 0.074 | −0.041 | 0.011 | 0.000 |
| Farmer sought information from a veterinarian | 2.160 | 0.082 | 0.314 | 0.009 | 0.000 |
| Farmer did not seek advice from any source--self-administered | 2.465 | 0.138 | 0.358 | 0.018 | 0.000 |
| Constant | −1.623 | 0.251 | 0.000 | ||
Probit model marginal effects of the factors influencing the decision to use antibiotics.
Source: 2018 AAS, microdata (UBOS).
Access to information from professional sources (veterinarians) or personal judgement positively influenced the decision to use antibiotics in livestock production. The marginal effects show a 35.8% likelihood of using antibiotics in a self-administered way compared to a 6.3% likelihood for those who sought advice on animal health-related issues from professional staff (Table 3). The results of our study indicated that believing that antibiotics remained effective even with continuous use significantly influenced the decision to use antibiotics. Household heads who believed that antibiotics remained effective even with frequent use were 14.3% more likely to use antibiotics than their counterparts who believed otherwise.
The study also found a negative association between the sex of the household head and the decision to use antibiotics. Male-headed households were significantly less likely (−3.8%) to have used antibiotics than their female counterparts. In addition, household heads who had completed at least lower secondary education (13 years or more of schooling) were also more likely (3.1%) to have used antibiotics.
Frequency of antibiotic use
This analysis used a sub-sample that included only those who reported antibiotic use in the previous 12 months to assess the frequency of use descriptively. Figure 1 shows the frequency of antibiotic use disaggregated by gender of the household head, region, production intensity and type of livestock kept. On average, most (87%) livestock keepers had occasionally (less than once a month) used antibiotics in their livestock production activities in the one-year period preceding the data collection phase. The results show that the most intensive users were among households keeping some exotic animals where 20% of the livestock keepers used antibiotics at least once a month or more frequently. This category is followed by households in the Eastern region (19%), households keeping pigs (18%) and households keeping poultry (14%). The least intensive use was observed in the northern and western regions both at 11%. We also noted that there were many users in the north, but they used antibiotics occasionally.
FIGURE 1
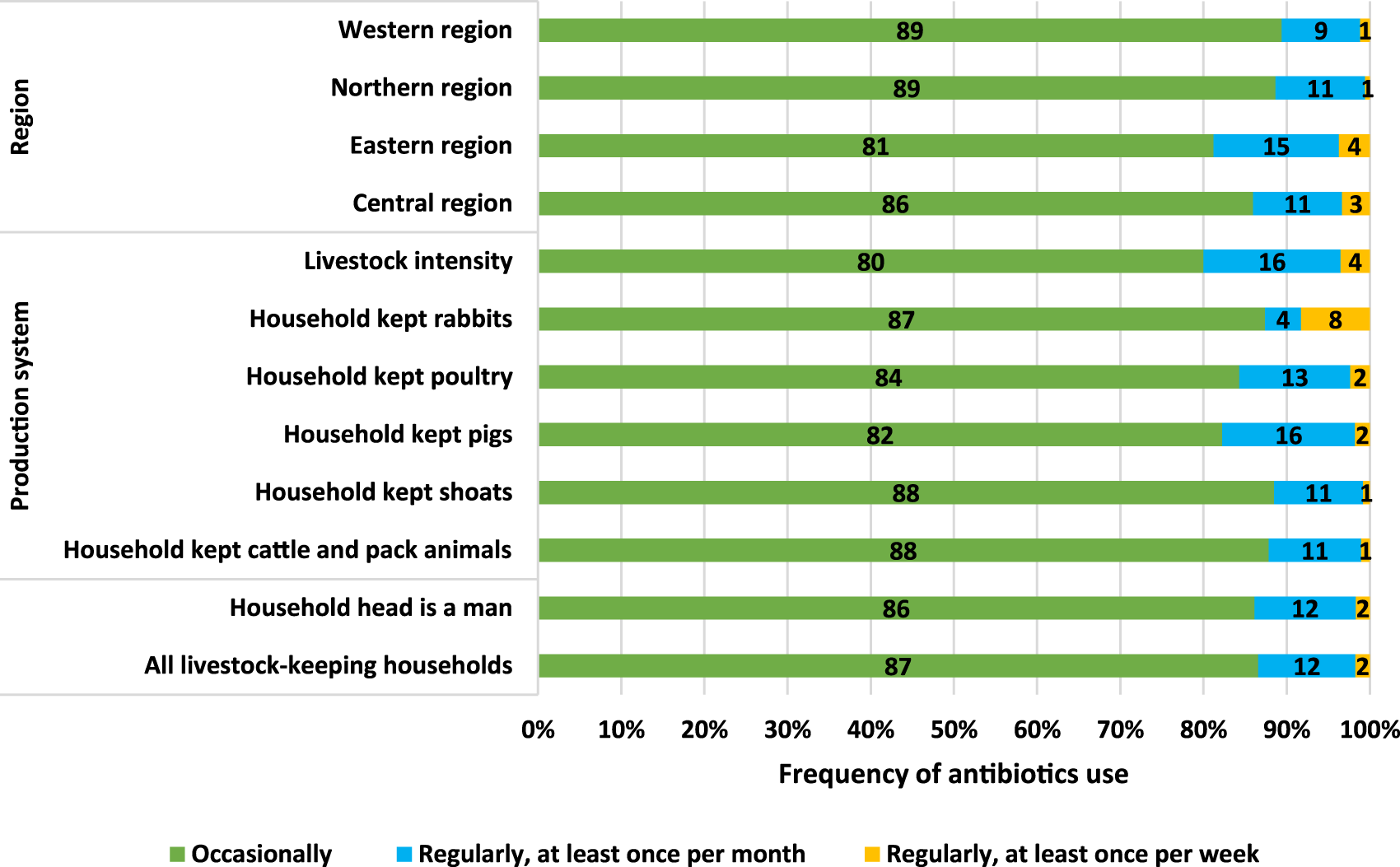
Frequency of antibiotic use (in percentage). Source: 2018 AAS microdata.
Purpose of antibiotic use among livestock-keeping households in Uganda
The purpose for which farmers use antibiotics is critical for decision making in interventions against the erratic use of antibiotics in the livestock sector. The results (Figure 2) of the analysis indicate that curative treatment (47%) was the main purpose for which households used antibiotics followed by disease prevention (33%) and vaccination (11%). Livestock-keeping households in the Western (18%) and Central regions reported mostly using antibiotics for growth promotion whereas only 1.6% of livestock keeping households in the Eastern region used antibiotics for growth promotion.
FIGURE 2
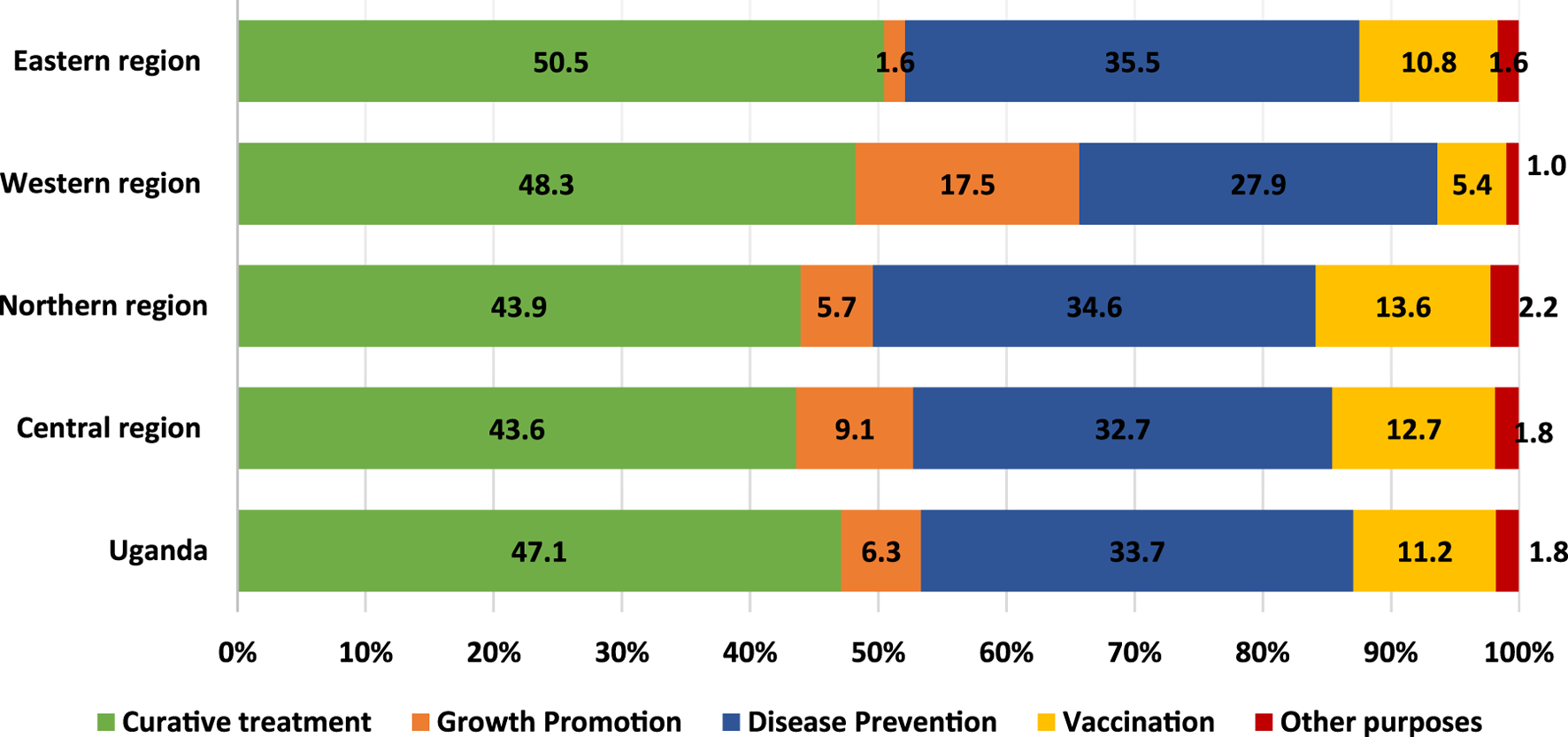
Purpose of antibiotic use among livestock keeping households. Source: 2018 AAS microdata.
Common antibiotics used by livestock types
In the 2018 AAS farmers were asked about the types of antibiotics used on their farms. The results presented in Table 4 indicate that the most used antibiotic among livestock farmers was Oxytetracycline (46.3%), which was most commonly used by cattle-keeping households (50%) followed by households keeping small ruminants (40%). Alamycin (16.8%) and Hitet 120 (16.4%) were the next most commonly used antibiotics reported. Betamox LA, Gentamycin and Limox were reported to be used only on cattle whereas Asampro and Oxytravet Powder were used specifically on poultry.
TABLE 4
| S/No. | Antibiotics used | Cattle | Small ruminants | Poultry | Total |
|---|---|---|---|---|---|
| 1 | Alamycin | 64.2 | 31.6 | 4.2 | 16.8 |
| 2 | Asampro | 0.0 | 0.0 | 100.0 | 2.0 |
| 3 | Betamox LA | 100.0 | 0.0 | 0.0 | 4.1 |
| 4 | Dipen | 59.9 | 33.9 | 6.2 | 12.4 |
| 5 | Gentamycin | 100.0 | 0.0 | 0.0 | 6.7 |
| 6 | Hitet 120 | 66.0 | 33.7 | 0.3 | 16.4 |
| 7 | Limox | 100.0 | 0.0 | 0.0 | 1.5 |
| 8 | Norodine | 49.4 | 47.5 | 3.1 | 8.9 |
| 9 | Oxystar | 51.4 | 38.5 | 10.1 | 8.1 |
| 10 | Oxytet | 56.8 | 29.6 | 13.6 | 10.9 |
| 11 | Oxytetracycline | 50.1 | 39.6 | 10.3 | 46.3 |
| 12 | Oxytravet Powder | 0.0 | 0.0 | 100.0 | 1.6 |
| 13 | Penstrep | 63.2 | 36.5 | 0.4 | 15.2 |
| 14 | Tetroxy | 38.9 | 30.0 | 31.1 | 4.9 |
| 15 | Tylosin | 46.5 | 43.4 | 10.1 | 5.4 |
| 16 | Other specify | 39.6 | 35.1 | 25.4 | 7.3 |
Types of Antibiotics used by livestock-keeping households.
Factors driving antibiotic use frequency in livestock production in Uganda
A zero-inflated ordered Probit (ZIOP) model was used to determine the drivers of antibiotic use frequency among livestock-keepers in Uganda (Table 5). Two models were used to ensure the robustness of the estimates, an ordinary ordered model (results are in Appendix I) and a zero-inflated ordered Probit model. Bayesian information criterion estimates indicated that the ZIOP model performed better (with lower Bayesian information criterion estimates) than the ordinary ordered Probit model (results are in Appendix II). The model parameters revealed a significant model thus rejecting the null hypothesis that none of the included regressors determined the frequency of antibiotic use. The ZIOP results (Table 3) were used for the remainder of this section.
TABLE 5
| Variable | Parameter estimates | Marginal effects | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Zero-inflated ordered probit model | Never | Occasionally | Frequently | ||||||
| Coef. | std. err. | p-value | dy/dx | std. err. | dy/dx | std. err. | dy/dx | std. err. | |
| Socioeconomic and demographic factors | |||||||||
| Respondent is aged 35 years or younger | −0.070 | 0.155 | 0.653 | 0.000 | 0.000 | 0.005 | 0.012 | −0.005 | 0.012 |
| Head of household is a man | −0.098 | 0.128 | 0.444 | −0.031 | 0.011 | 0.033 | 0.014 | −0.002 | 0.010 |
| Main economic activity of household is agriculture | 0.053 | 0.134 | 0.691 | 0.000 | 0.000 | −0.004 | 0.010 | 0.004 | 0.010 |
| Household members belong to a farmer’s group | 0.113 | 0.119 | 0.343 | 0.000 | 0.000 | −0.009 | 0.009 | 0.009 | 0.009 |
| Head of household has completed at least secondary school | 0.029 | 0.102 | 0.777 | −0.021 | 0.009 | 0.016 | 0.011 | 0.006 | 0.008 |
| Production system and environmental factors | |||||||||
| Household kept cattle and pack animals | −0.017 | 0.130 | 0.895 | −0.012 | 0.009 | 0.012 | 0.013 | 0.001 | 0.010 |
| Household kept pigs | 0.392 | 0.139 | 0.005 | −0.008 | 0.008 | −0.023 | 0.013 | 0.031 | 0.010 |
| Household kept poultry | 0.398 | 0.134 | 0.003 | −0.012 | 0.010 | −0.020 | 0.013 | 0.032 | 0.010 |
| Total tropical livestock units of local and exotic animals owned | 0.012 | 0.007 | 0.094 | −0.008 | 0.002 | 0.006 | 0.002 | 0.002 | 0.001 |
| Number of livestock species kept by the household | −0.232 | 0.093 | 0.013 | 0.000 | 0.000 | 0.018 | 0.007 | −0.018 | 0.007 |
| Proportion of land under crop | 0.377 | 0.165 | 0.022 | 0.000 | 0.000 | −0.028 | 0.012 | 0.028 | 0.012 |
| Household has at least one exotic livestock species | 0.483 | 0.133 | 0.000 | −0.022 | 0.012 | −0.018 | 0.013 | 0.040 | 0.010 |
| Region | |||||||||
| Eastern (base) | |||||||||
| Western | −0.499 | 0.166 | 0.003 | 0.060 | 0.011 | −0.012 | 0.015 | −0.048 | 0.013 |
| Central | −0.533 | 0.180 | 0.003 | 0.053 | 0.013 | −0.004 | 0.017 | −0.049 | 0.014 |
| Northern | −0.019 | 0.118 | 0.875 | 0.012 | 0.011 | −0.008 | 0.013 | −0.003 | 0.009 |
| Perceptions of antibiotic use | |||||||||
| Household believes that antibiotics will not become less effective even with continuous use | 0.303 | 0.104 | 0.004 | −0.695 | 0.050 | 0.557 | 0.046 | 0.138 | 0.020 |
| Institutional factors | |||||||||
| Information about antibiotics comes from private/public extension services | −0.308 | 0.164 | 0.060 | −0.028 | 0.017 | 0.047 | 0.018 | −0.019 | 0.013 |
| Information about antibiotics comes from farmer to farmer | −0.510 | 0.138 | 0.000 | −0.028 | 0.011 | 0.062 | 0.015 | −0.034 | 0.010 |
| Distance to nearest input shop is greater than 5 km | −0.173 | 0.106 | 0.100 | 0.012 | 0.008 | 0.003 | 0.010 | −0.015 | 0.008 |
| Farmer sought information from a veterinarian | −0.172 | 0.127 | 0.176 | −0.777 | 0.055 | 0.661 | 0.050 | 0.116 | 0.017 |
| Farmer did not seek advice from any source--self-administered | −0.195 | 0.166 | 0.240 | −0.768 | 0.055 | 0.656 | 0.051 | 0.113 | 0.018 |
| Household accessed loans for agricultural purposes | −0.204 | 0.153 | 0.183 | −0.027 | 0.014 | 0.038 | 0.017 | −0.011 | 0.011 |
| Constant | −1.189 | 0.262 | 0.000 | ||||||
Zero-inflated Probit model estimates of the factors influencing the intensity of antibiotic use in livestock production in Uganda.
Coef. = coefficient; std. err = Standard error; dy/dx = Coefficient of marginal effect.
Bold figures indicate variables significant at least 10% level of significance.
The coefficient on the belief that antibiotics remained effective even with continuous use was found to be positive and significant at the 1% level. This indicates that households holding this belief were more likely to use antibiotics frequently than their counterparts who believed otherwise. The marginal effects show that households holding this belief were 69.5% less likely to fall into the never-use category but 55.7% and 13.8% more likely to fall into the occasionally and frequently use categories, respectively.
Significant regional differences were observed in the frequency of antibiotic use. Specifically, households in the western and central regions were found to be significantly less likely to use antibiotics than their counterparts in the eastern region (base category) of the country. The marginal effects revealed that, compared to the eastern region, households from the western and central regions were 6% and 5% more likely to belong in the never use category, and 1% (both) and 5% (both) less likely to fall in the occasional and frequent use categories respectively.
The source of information was also found to influence the frequency of antibiotic use. The study found that information from any source (external to the farmer) significantly reduced the frequency of antibiotic use. Marginal effects show that livestock keepers who received information about antibiotics from private extension services or fellow farmers were 3% less likely to fall into the never-use category. This means that access to information increases the probability of falling into the use categories. The marginal effects further revealed that information from extension agent services increased the probability of falling into the occasional use category by approximately 5% while receiving information from fellow farmers increased the probability by approximately 6%. However, the same information sources reduced the probability of falling into the higher category of frequent use by 2% and 3% for extension and fellow farmer information sources respectively.
The frequency of antibiotic use was found to be driven by the type of livestock kept and the system in which the livestock was produced. The results in Table 3 indicate that owning poultry or pigs increased the frequency of antibiotic use which is the opposite of the factors influencing the decision to use antibiotics. This implies that once the decision was made to use antibiotics, households with poultry or pigs were more likely to use antibiotics frequently. Marginal effects show that households with poultry or pigs were associated with a 3% higher probability (for both) of falling into the frequent use category.
In addition, households that kept exotic animals were more likely to have used antibiotics frequently compared to those that kept only local animals. Households keeping exotic animals were associated with a 4% higher possibility of having used antibiotics frequently compared to households that were not keeping any exotic animals. Higher tropical livestock units (a higher number of animals) were also associated with an increased frequency of antibiotic use but the marginal effects reveal very small (less than 1%) but positive probabilities of increased antibiotic use following an increase in tropical livestock units by one. Increasing the diversity of livestock had significantly mixed results on the frequency of antibiotic use, holding all other factors constant. Increasing the diversity of livestock kept by one species marginally affected the probability of falling into the never-use category, and increased, or decreased the probabilities of falling into the occasional or frequent use category by 2%.
Discussion
The results of this study built on the initial work by Mikecz et al. (2020) about antibiotic use in Uganda. The results also constituted the first study, as far as the literature available can provide, on antibiotic use frequency at the country level. The results are nationally representative and come from rigorously collected and analysed data. These results can be used to draw conclusions at the national level and in some contexts at the level of Sub-Saharan Africa.
The results show that on average one in every three livestock keepers had used antibiotics in the previous year, and approximately three in every 20 livestock keepers had used antibiotics at least once a month in the previous 12 months. The use of antibiotics by livestock farmers could be attributed to several reasons including a higher disease burden in livestock (Kebirungi et al., 2022). Furthermore, the use of antibiotics in the livestock sector could be due to the fact that most farmers self-diagnose their animals and are likely to use the same drugs for diseases that present with similar symptoms. This may be attributed to the fact that farmers often have unrestricted access to antibiotics through drug shops which are usually not operated by trained veterinarians. The situation is no different from other low-income countries with large livestock numbers such as Ethiopia where farmers have easy access to veterinary drugs (Gemeda et al., 2020). This is further exacerbated by the likelihood of access to substandard or expired drugs which farmers may not be aware of at the time of purchase. The results of our study confirm the concerns of UNAS et al. (2015a) and UNAS et al. (2015b) that antibiotic use in Uganda is no longer limited to humans but has expanded to the livestock sector as well. Others such as Nayiga et al. (2020) studied whether households in an Eastern district (Tororo) and a Central district (Wakiso) in Uganda had ever used antibiotics to treat animals. Their study found 33% use in the Eastern district compared to 35% found in this study. However, their study found 99% use (over the period they could recall) in the Central district, which could be due to the small sample size (215) compared to this study. Regional differences in antibiotic use are attributable to the higher number of livestock kept in the Northern and Eastern regions which is also associated with a higher disease burden. These findings are similar to those by (Emes et al., 2023) who highlighted a higher disease burden in the Northern region compared to the Central region.
This study found that the highest share of households using antibiotics was recorded in the northern region = which was also where the least intensive use of antibiotics was observed. First, there are more households that keep livestock in the Eastern and Northern regions of Uganda compared to the Southern and Western regions (Mickez et al., 2020). In addition, the main reason for livestock production in the northern region is draft power for cultivation and food compared to commercially-oriented livestock production in the western and central regions (livestock production in the northern region is driven by the need for animals (Okello et al., 2021). Due to the high disease burden in the north (Dione et al., 2021; Emes et al., 2023) households may try to treat disease with antibiotics hence the high percentage of households using them. However, because animal production in the northern region is primarily for subsistence, there is less incentive to invest in the larger doses (comes with higher frequency) of antibiotics that are typically used in commercially-oriented livestock production.
As highlighted by Mshana et al. (2021) limited information on antibiotic use coupled with weak institutional regulations for all actors involved in the importation, sale and use of antibiotics continues to hinder any tangible information on antimicrobial misuse and subsequently AMR (UNAS et al., 2015b). Additionally, Dione et al. (2021) highlighted the downside of weak institutional monitoring and regulation as a likely cause of drug misuse and rising anti-microbial resistance. Therefore, there is a need to support the efforts of the government of Uganda to streamline the use of antibiotics not only in humans but in livestock production too (UNAS et al., 2015b).
Both professional sources (veterinarians) of information and personal judgement positively influenced the decision to use antibiotics in livestock production. The marginal effects show a 35.8% likelihood of using self-administered antibiotics compared to a 6.3% likelihood for those who sought advice from professional staff on animal health issues. These findings are consistent with those of Ekakoro et al. (2019) who found that livestock keepers admitted to relying on a mix of their own experience, knowledge, or judgement when deciding to use antimicrobials in their cattle, sometimes consulting other producers and then seeking veterinarians’ expertise if cases were difficult to manage. In addition, Musoke et al. (2021) found that some farmers who consulted veterinarians before using antibiotics would later use previous prescriptions to purchase and administer antibiotics to their ailing livestock. This implies that the number of farmers relying on personal judgement to use antibiotics could potentially be higher. Farmers who purchase antimicrobials without consulting veterinary practitioners are more likely to prescribe them incorrectly (Musoke et al., 2021) which could lead to higher levels of antimicrobial resistance.
This study found that the perception that antibiotics will remain effective even with continuous use significantly influenced both the decision to use and the frequency of antibiotic use. Livestock keepers who perceived that antibiotics would not become less effective even with continuous use were more likely to have used antibiotics compared to those who perceived otherwise. The perception of the continued efficacy of antibiotics even with continuous use could be related to previous experience using the drugs, and advise from fellow farmers and/or agro-input dealers. It could also be due to the limited choice of drugs available on the market as agro-input dealers may tend to stock up on the most commonly sought-after antibiotics. This underscores the importance of livestock keepers’ perceptions and thus the need for a mindset change to influence the sustainable use of antibiotics in livestock production.
This study also found a positive association between the sex of the household head and the decision to use antibiotics. Male-headed households were significantly more likely to have used antibiotics than their female counterparts. In addition, household heads who had completed at least lower secondary education (13 or more years in school) were also more likely to have used antibiotics. Manyi-Loh et al. (2018) and Kahunde et al. (2023) also found that antibiotic use was positively correlated with farmers’ level of education and socioeconomic status. This could be attributed to their ability to read and understand drug labels in addition to their purchasing power compared to their less educated counterparts.
Conclusion and recommendation
The study contributes to the understanding of antibiotic use patterns, determinants, and implications among Ugandan livestock keepers and provides valuable evidence for policymakers, stakeholders, and researchers involved in livestock health management and public health domains. The study reveals a high prevalence of antibiotic use in livestock production and thus an increasing reliance on antibiotics in the livestock sector. The analysis indicates that socio-economic and demographic factors, regional/geographical variations, and institutional factors are significant in shaping antibiotic use practices. Furthermore, the findings reveal varying levels of intensity in antibiotic usage between different livestock production systems.
The results underscore the urgent need for interventions to promote responsible antibiotic use in the livestock sector. Strengthening veterinary education, implementing robust regulatory frameworks, and increasing public awareness regarding appropriate antibiotic use are essential measures to mitigate the risks associated with antibiotic misuse. Restrictive use of antibiotics as has been the case in some developed countries (Dione et al., 2021) and was previously recommended in Uganda (Bashahun and Odoch, 2015) would require the support of a well-sensitive public that understands that restrictions are for their own good. Therefore, to regulate the problem, collaborative action with different relevant stakeholders is the most helpful strategy that we recommend. In addition to regulation and training on appropriate use, livestock keepers’ perceptions towards the continued use of antibiotics should also be addressed. This can be done by developing key messages on the potential effects of continuous, improper, and or non-medically prescribed use of antibiotics on animals, livestock and the environment in general. Programs campaigning for the judicious use of antibiotics need to be better targeted especially towards more educated livestock keepers, keepers of exotic livestock and households in the northern and central regions. These were associated with a higher likelihood of use and frequency of use.
Statements
Data availability statement
The data used in this study is publicly available from the World Bank Microdata Library. Access to the data requires users to create an account and register on the Microdata Library website. The data can be accessed at https://microdata.worldbank.org/index.php/catalog/3795, and users can register for an account at https://microdata.worldbank.org/index.php/auth/register.
Author contributions
CK: Verification of the idea conceptualized and the methods used; data processing; draft manuscript review and editing: coordination of manuscript finalization and submission, obtaining and managing the funds; CN: Verification of the idea conceptualized and the methods used; data processing; draft manuscript review and editing; RA: Conceptualization of the idea, methodology design: data processing and analysis; manuscript drafting. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by IFAD and the 50 × 2030 Initiative (Data Use Component).
Conflict of interest
Author CK was employed by Athari Lulu consults Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1
Bashahun D. Odoch T. (2015). Assessment of antibiotic usage in intensive poultry farms in Wakiso District, Uganda. Livest. Res. Rural. Dev.27, 247.
2
Benson T. Mugarura S. (2013). Livestock development planning in Uganda: identification of areas of opportunity and challenge. Land Use Policy35, 131–139. 10.1016/j.landusepol.2013.05.013
3
Chah J. N. S. (2022). Knowledge and practices regarding antibiotic use among small-scale poultry farmers in Enugu State, Nigeria. Heliyon7. 10.1016/j.heliyon.2022.e09342
4
Cragg J. G. (1971). Some statistical models for limited dependent variables with application to the demand for durable goods. Econometrica39, 829–844. 10.2307/1909582
5
Dibner J. J. Richards J. D. Knight C. D. (2018). Antibiotic growth promoters in agriculture: History and mode of action. Poult. Sci.98 (12), 5311–5323. 10.1093/ps/84.4.634
6
Dione M. M. Amia W. C. Ejobi F. Ouma E. A. Wieland B. (2021). Supply chain and delivery of antimicrobial drugs in smallholder livestock production systems in Uganda. Front. Veterinary Sci.8, 1–13. 10.3389/fvets.2021.611076
7
Ekakoro J. E. Zirintunda G. H. Omara R. Muwanguzi M. E. (2019). Socio-economic determinants of antimicrobial use practices among smallholder poultry farmers in Eastern Uganda. Livest. Res. Rural Dev.31 (7). 10.1186/s12917-018-1731-6
8
Emes E. Wieland B. Magnusson U. Dione M. (2023). How farm practices and antibiotic use drive disease incidence in smallholder livestock farms: evidence from a survey in Uganda. One Health17, 100627. 10.1016/j.onehlt.2023.100627
9
FAO (2019). The future of livestock in Uganda. Opportunities and challenges in the face of uncertainty. Rome: FAO.
10
FAO (2023). The use of antibiotics in livestock production and its impact on public health. Food Agric. Organ. U. N.
11
Fávero L. P. de Freitas Souza R. Belfiore P. Corrêa H. L. Haddad M. F. C. (2021). Count Data Regression Analysis: Concepts, Overdispersion Detection, Zero-inflation Identification, and Applications with R. Practical Assessment. Research and Evaluation26 (13), 1–22. 10.7275/44nn-cj68
12
García P. Martínez B. Rodríguez L. Rodríguez A. (2022). Antibiotic resistance in animal production. Veterinary Sci.9 (1), 4.
13
Gemeda B. A. Amenu K. Magnusson U. Dohoo I. Hallenberg G. S. Alemayehu G. et al (2020). Antimicrobial use in extensive smallholder livestock farming systems in Ethiopia: knowledge, attitudes, and practices of livestock keepers. Front. Vet. Sci.7, 55. 10.3389/fvets.2020.00055
14
Harris M. N. Zhao X. (2007). A zero-inflated ordered Probit model, with an application to modelling tobacco consumption. J. Econ.141, 1073–1099. 10.1016/j.jeconom.2007.01.002
15
Ikwap K. Erume J. Owiny D. O. Nasinyama G. W. Melin L. Bengtsson B. et al (2014). Salmonella species in piglets and weaners from Uganda: Prevalence, antimicrobial resistance and herd-level risk factors. Prev. Veterinary Med.115 (1–2), 39–47. 10.1016/j.prevetmed.2014.03.009
16
Kahunde M. A. Odoch T. Owiny D. O. Kankya C. Kaelin M. B. Hartnack S. (2023). Knowledge, attitudes and practices towards antibiotic use and resistance in Kyegegwa district, Uganda – a questionnaire study. medRxiv. 10.1101/2023.04.06.23288253
17
Kaplan R. Petersen T. Duquette J. (2019). Livestock and livelihoods in rural Uganda: a descriptive analysis of Uganda census of agriculture data. World Dev.115, 168–177.
18
Kebirungi P. Nyombi A. Omara T. Adaku C. Ntambi E. (2022). Oxytetracycline residues in bovine muscles, liver and kidney tissues from selected slaughter facilities in South Western Uganda. Bull. Natl. Res. Centre46 (1), 17. 10.1186/s42269-022-00702-6
19
Kimera Z. I. Mshana S. E. Rweyemamu M. M. Mboera L. E. G. Matee M. I. N. (2020). Antimicrobial use and resistance in food-producing animals and the environment: An african perspective. Antimicrob. Resist. Infect. Control9, 37. 10.1186/s13756-020-0697-x
20
Maddala G. S. (1983). Limited dependent and qualitative variables in econometrics. Cambridge, UK: Cambridge University Press.
21
Manyi-Loh C. E. Mamphweli S. N. Meyer E. L. Okoh A. I. Makaka G. (2018). Antibiotic use in agriculture and its consequential resistance in environmental sources: potential public health implications. Molecules23 (4), 795. 10.3390/molecules23040795
22
Mikecz R. Mendoza R. Smith J. (2020). Antibiotic use in Uganda: a comprehensive analysis. J. Public Health24 (2), 123–145. 10.1016/j.onehlt.2020.100165
23
Mshana S. E. Sindato C. Matee M. I. Mboera L. E. G. (2021). Antimicrobial use and resistance in agriculture and food production systems in africa: a systematic review. Antibiotics10 (8), 976. 10.3390/antibiotics10080976
24
Mulchandani R. Wang Y. Gilbert M. Van Boeckel T. P. (2023). Global trends in antimicrobial use in food-producing animals: 2020 to 2030. PLOS Glob. Public Health3 (2), e0001305. 10.1371/journal.pgph.0001305
25
Musoke D. Ssempebwa J. Abbo C. Opio A. (2021). Access, use and disposal of antimicrobials among humans and animals in Wakiso district, Uganda: a qualitative study. Journal of Pharmaceutical Policy and Practice, 14(1), 1–12. 10.1186/s40545-021-00361-4
26
Nayiga A. Ssewanyana I. Mugisha J. (2020). Antibiotic resistance in livestock farming: a systematic review of the literature. J. Veterinary Sci.12 (3), 345–367. 10.1093/jacamr/dlaa082
27
Okello W. O. MacLeod E. T. Muhanguzi D. Waiswa C. Shaw A. P. Welburn S. C. (2021). Critical linkages between livestock production, livestock trade and potential spread of human african trypanosomiasis in Uganda: bioeconomic herd modeling and livestock trade analysis. Front. Vet. Sci.8, 611141. 10.3389/fvets.2021.611141
28
O'Neill J. (2016). Tackling drug-resistant infections globally: final report and recommendations. The Review on Antimicrobial Resistance.
29
Patel S. J. Wellington M. Shah R. M. Ferreira M. J. FerreiraJ M. (2020). Antibiotic stewardship in food-producing animals: challenges, progress, and opportunities. Clin. Ther.42 (9), 1649–1658. 10.1016/j.clinthera.2020.07.004
30
Pew Commission on Industrial Farm Animal Production (2020). Antibiotic resistance in food animal production: the public health impact. Pew Charit. Trusts.
31
Rwarimbuga A. A. Kambarage D. M. Karimuribo E. D. Mdegela R. H. (2017). Prevalence, risk factors, and antimicrobial resistance profiles of thermophilic Campylobacter species in cattle from selected small-scale farms in the Iringa and Tanga regions of Tanzania. Trop. Animal Health Prod.49 (4), 791–797.
32
Sasanya J. J. Okeng J. W. O. Ejobi F. Muganwa M. (2005). Use of sulfonamides in layers in Kampala district, Uganda and sulfonamide residues in commercial eggs. Afr. Health Sci.5 (1), 33–39.
33
Smith R. D. Coast J. Millar M. R. (2021). “Antibiotics and farm animal welfare,” in Antibiotic resistance economics and governance (Springer), 209–228.
34
StataCorp (2021). Stata statistical software: release 17. College Station, TX: StataCorp LLC.
35
Turner L. R. (2005). Livestock, liberalization and democracy: constraints and opportunities for rural livestock producers in a reforming Uganda. Policy Brief. Availabe at: https://www.fao.org/3/bp250e/bp250e.pdf.
36
Uganda National Academy of Sciences (UNAS) Maaif Ministry of Agriculture Animal Industry and Fisheries (MAAIF) Uganda National Council for Science and Technology (UNCST) (2015a). Sustainable use of antibiotics in the livestock sector in Uganda: situation analysis and recommendations. Kampala, Uganda: UNAS.
37
Uganda Bureau of Statistics (UBOS) (2020). Uganda annual agricultural survey 2018. Kampala, Uganda: UBOS.
38
UNAS CDDEP Garp-Uganda, MpairweY.WamalaS. (2015b). Antibiotic resistance in Uganda: situation analysis and recommendations. Kampala, Uganda: Uganda National academy of Sciences; Center for Disease Dynamics, Economics and Policy, 107.
Appendix I: Ordered probit model estimates
TABLE A1
| Variable | Ordered probit model | ||
|---|---|---|---|
| Coef. | std. err. | P > t | |
| Respondent is aged 35 years or younger | −0.031 | 0.102 | 0.759 |
| Head of household is a man | 0.171 | 0.067 | 0.010 |
| Household kept cattle and pack animals | −0.172 | 0.095 | 0.069 |
| Household kept pigs | −0.130 | 0.088 | 0.142 |
| Household kept poultry | −0.099 | 0.088 | 0.263 |
| Total tropical livestock units of local and exotic animals owned | 0.020 | 0.007 | 0.007 |
| Number of livestock species kept by the household | 0.218 | 0.059 | 0.000 |
| Proportion of land under crop | −0.103 | 0.110 | 0.348 |
| region==Western Region | −0.658 | 0.088 | 0.000 |
| region==Central Region | −0.470 | 0.102 | 0.000 |
| region==Northern Region | −0.228 | 0.080 | 0.004 |
| Household has at least one exotic livestock species | 0.330 | 0.096 | 0.001 |
| Household’s main economic activity is agriculture | 0.108 | 0.090 | 0.227 |
| Household members belong to a farmer’s group | 0.043 | 0.089 | 0.627 |
| Household head has completed at least secondary school | 0.129 | 0.073 | 0.077 |
| Household believes that antibiotics will not become less effective even with continuous use | 1.021 | 0.087 | 0.000 |
| Information about antibiotics comes from private/public extension services | 0.043 | 0.114 | 0.704 |
| Information about antibiotics comes from farmer to farmer | 0.021 | 0.086 | 0.806 |
| Information about antibiotics comes from word of mouth/other peers | −0.238 | 0.100 | 0.017 |
| Distance to nearest input shop is greater than 5 km | −0.213 | 0.066 | 0.001 |
| Farmer sought information from veterinarians | 2.160 | 0.107 | 0.000 |
| Farmer did not seek advice from any source--self-administered | 2.012 | 0.114 | 0.000 |
| Household accessed loans for agricultural purposes | 0.106 | 0.095 | 0.265 |
| /cut1 | 0.890 | 0.196 | |
| /cut2 | 3.430 | 0.241 | |
| Number of observations | 4407.000 | ||
| Design degrees of freedom | 4393.000 | ||
| F (23, 4371) | 48.510 | ||
| Prob > F | 0.000 | ||
Ordered Probit model estimates for the factors influencing the intensity of antibiotic use in livestock production in Uganda.
Appendix II: Bayesian information criterion estimates for ordered- and zero inflated- probits
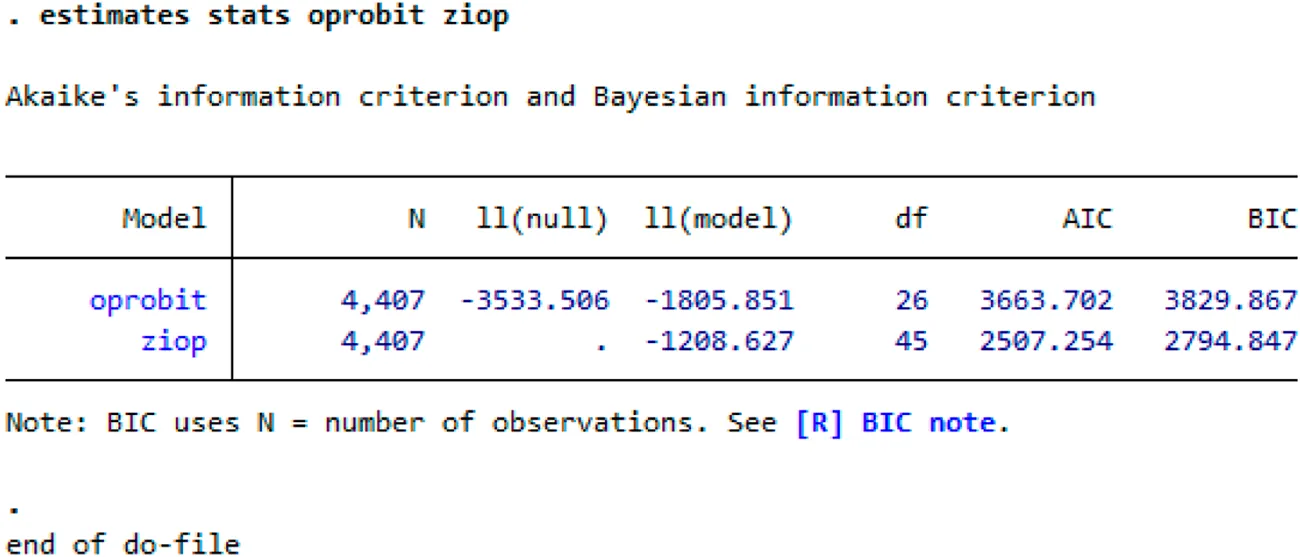
Summary
Keywords
antimicrobial resistance, frequency of use, Uganda, patterns, livestock
Citation
Kibooga C, Nakiyemba C and Asiimwe R (2024) Antibiotic use in Uganda’s livestock-keeping households: prevalence, patterns, and determinants. Pastor. Res. Policy Pract. 14:13017. doi: 10.3389/past.2024.13017
Received
21 March 2024
Accepted
11 June 2024
Published
17 July 2024
Volume
14 - 2024
Edited by
Carol Kerven, University College London, United Kingdom
Updates
Copyright
© 2024 Kibooga, Nakiyemba and Asiimwe.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Charity Kibooga, c.kibooga@alcuganda.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.