- 1College of Veterinary Medicine, Jigjiga University, Jigjiga, Ethiopia
- 2School of Veterinary Medicine, Wolaita Sodo University, Wolaita Sodo, Ethiopia
- 3School of Veterinary Medicine, Ambo University, Guder, Ethiopia
Background: Camelpox is a common viral disease of camelids caused by camelpox virus, which is endemic in most camelid-breeding countries including Ethiopia, and causes major economic losses. This study aimed to quantify the seroprevalence of camelpox and identify associated risk factors to put into practice efficient control strategies for the disease in the study area.
Methods: A cross-sectional study was carried out between January and July of 2023. Blood samples were collected from 374 camels of 75 households residing in two districts in six peasant associations. A sandwich enzyme-linked immunosorbent assay was employed to detect camelpox-specific antibodies from sera samples. To identify potential risk factors, camel owners were asked in face-to-face interviews.
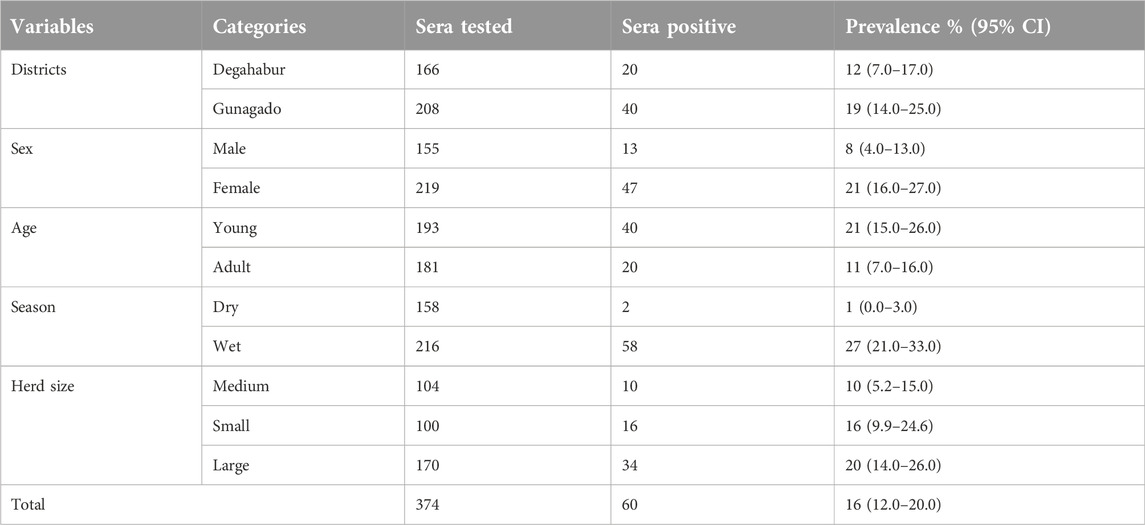
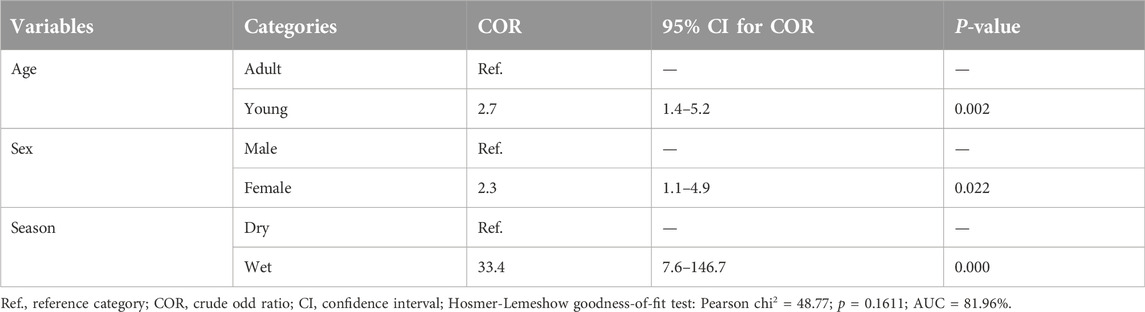
Results: The overall seroprevalence of camelpox in the study areas was 16.0% (95% confidence interval: 12.0%–20.0%). A multivariable logistic regression analysis revealed risk factors season, age, and sex had an association with seroprevalence of camelpox (p < 0.05). Sex was strongly associated with camelpox seropositivity, with female camels having a 3.2-fold higher risk of infection than male camels. Likewise, age-related vulnerability to infection was observed in young dromedaries, as they were 2.3 times more likely to become infected than adults.
Conclusion: This study revealed moderate seroprevalence of camelpox in the study area. Thus, effective preventative strategies, such as vaccination programs to stop the spread of camelpox and reduce the financial losses caused by the disease, should be encouraged.
Introduction
The one-humped camel (Camelus dromedarius) is thought to number 35 million in the world. Camels are major sources of milk and meat in many parts of the world including parts of Asia and Africa. Of the total population of Old-World camels, the one-humped camel makes up about 95% (Sazmand et al., 2019). More than 80% of camels worldwide are found in Africa, with 60% of these species found in the Horn of Africa, which includes Somalia, Sudan, Ethiopia, Kenya, Djibouti, and Eritrea. These countries have a greater percentage of one-humped camels than any other region in the world (Othieno et al., 2022). Ethiopia ranks sixth in Africa for number of camels, with an estimated population of more than 1.42 million (Kena, 2022). Camels are mainly found in the arid and semi-arid lowlands of the Somali, Afar, and Southern Oromia regional states, where nomadic herders make up the majority of the population (Kena, 2022; Keskes et al., 2013). Camels are highly valued in the Somali region and play crucial role in milk and meat production, transportation, and trade (Othieno et al., 2022; Kena, 2022). In the region’s dry and semi-arid rangelands, where true pastoralism is the predominant way of life, these animals are raised for their adaptation to the tough environment. However, infectious camel diseases, such as camelpox, are one of the biggest problems for the pastoralist communities in the Somali and Afar regions (Kena, 2022; Balamurugan et al., 2013).
Camelpox is a highly contagious disease found in Old-World camelids (Dahiya et al., 2016), with rare reports of human infection (Khalafalla and Abdelazim, 2017). It is caused by the camelpox virus (CMLV), which is a member of the Poxviridae family and the Orthopoxvirus genus. The disease is widespread in almost every country where camels are raised (Dahiya et al., 2016).
Camelpox is transmitted through either direct contact with infected animal, or indirect contact with a contaminated environment. Direct transmission occurs between infected and vulnerable animals by contact with skin lesions. Mechanical transfer is also possible by potential vectors such as biting flies and mosquitoes (Wernery and Kaaden, 2002). Afflicted camels may transmit the virus through scab materials and secretions (milk, saliva, ocular, and nasal discharges) shed to the environment including water, which can then infect susceptible animals (Wernery and Kaaden, 2002; Khalafalla and Ali, 2007). The virus can live for up to 4 months in dried scabs. Camelpox is a highly infectious and exceedingly transmissible skin disease in camels that can cause moderate skin lesions or severe systemic illnesses depending on the virus strain and the animal’s immune condition (Dahiya et al., 2016; Wernery and Kaaden, 2002). Camelpox signs range from acute to mild illness and may include fever, lymph node enlargement, face edema, lachrymation, pendulous lips, and pox lesions. Papules and vesicles first occur on the lips and nose, then spread to the entire head, neck, hindquarters, abdomen, legs, and groin (Khalafalla et al., 2015). The prevalence of camelpox is socioeconomically relevant since it results in large losses in terms of morbidity and mortality, abortion, weight loss, and milk output reductions. Camelpox epidemics generate substantial economic damage and necessitate quarantine and containment efforts to prevent the disease from spreading (Aregawi et al., 2018; Tefera and Gebreah, 2001).
To estimate the disease burden and develop effective control measures, prevalence studies and assessing risk factors are important. Some studies have been conducted to determine how common camelpox is in Ethiopia (Aregawi et al., 2018; Megersa, 2010; Ayelet et al., 2013). These studies used a range of approaches, such as molecular methods, clinical observations, and serological testing. For example, using clinical examination, Megersa (Megersa, 2010) stated that in Borana zone (the southernmost zone of Oromia region), the prevalence of camelpox was 0% during the dry season, 0.3% during the major wet season, and 14.2% during the minor wet season. Similarly, Aregawi (Aregawi et al., 2018) also reported seroprevalence of camelpox at 21.6% and 16.7% in Amibara and Awash Fentale of Afar region, North Eastern Ethiopia, respectively. Moreover, Ayelet et al. (Ayelet et al., 2013) reported the first camelpox virus isolation and molecular detection in Ethiopia.
However, there is a dearth of information on camelpox in the Jarar zone of the Somali region in eastern Ethiopia, even if camelpox is acknowledged as an economically significant viral disease affecting camels in Ethiopia. Thus, this study aims to estimate the seroprevalence of camelpox infection and identify associated risk factors in the study area.
Materials and methods
Description of the study areas
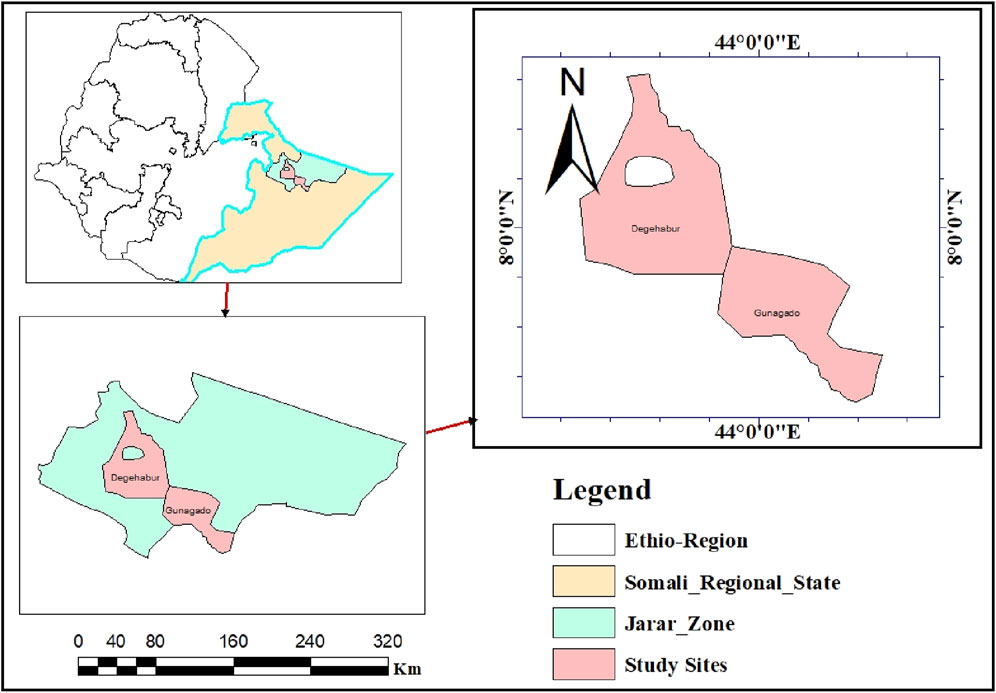
The study was carried out in Jarar zone, Somali Region, Ethiopia (Figure 1). Jarar zone is well-known for its sizable camel population and its hot, dry, semi-arid climate with frequent high temperatures and little precipitation. The Jarar zone people’s way of life and culture are fundamentally based on camel rearing (Hassen G. et al., 2022). For this study, Jarar zone and two districts in the zone, namely, Degahabur and Gunagado districts were chosen purposively based on their infrastructure, and camel population.
Degahabur district is located in Somali regional state in Eastern part of Ethiopia about 630 km from Addis Ababa, Ethiopia’s capital. The district has an elevation of 1,044 m above sea level. It is known for its large camel population and receives high temperatures and little rainfall, resulting in a dry and semi-arid environment. Camels are important to the local people’s livelihoods and culture, with an estimated population of approximately 116,790 (Hassen G. et al., 2022).
Similarly, Gunagado district is located in the southern section of Jigjiga city and encompasses a large territory, rising 1,640 m above sea level. It has two distinct rainy seasons: Gu’ (spring) and Deyr (fall). The spring rainy season runs from March to May, while the fall rainy season lasts from September to November. Gunagado district has an average annual temperature of 29°C and a rainfall range of 400–600 mm. The major style of production in this area is an extensive system in which more than 90% of the population moves from one location to another in search of pasture and water. The estimated number of camels is 137,521 (Hassen M. et al., 2022).
Study animal
The study animals were all dromedary camels of both sexes found in Degahabur and Gunagado districts of Jarar zone, Somali region. Ages were categorized as young (6 months–4 years old) and adults (>4 years old), and sex was divided into female and male categories. Seasons were divided into dry and wet seasons (Megersa, 2010).
Inclusion and exclusion criteria
To reduce the impact of herd migratory bias, animals included in the study were chosen for sampling only from specified districts in Jarar Zone, Somali Region. The study comprised unvaccinated male and female camels over the age of 6 months (Aregawi et al., 2018). The information on prior vaccination was gathered from the animal health sector of the Pastoral Agriculture and Rural Development Office of the districts, as well as camel owners, and the camel population in the study area was confirmed as having never been vaccinated against any diseases including camelpox.
Study design and methods
A cross-sectional study was carried out from January to July 2023. The objectives of the study were to estimate camelpox seroprevalence and its potential risk factors. The formula provided by Thrusfield (Thrusfield, 2018) was used to calculate the sample size for seroprevalence, by considering a 95% confidence level, a predicted prevalence of 14.2% (Megersa, 2010), and a 5% absolute precision.
Accordingly, the minimum sample size required was 187. To enhance the study’s precision and representativeness, samples was doubled. Thus, a total of 374 camels were sampled.
Jarar zone was selected purposively from Somali region. Similarly, the two districts, Degahabur and Gunagado, were chosen based on camel population density, geographic location, and accessibility. The six peasant associations (PAs), seventy-five households, and 374 study camels were chosen using systematic random sampling.
Bulale, Dogorjir and Dabile were the Pas chosen in Degahabur, and Balisaredo, Bulhan, and Kararo in Gunagado. Face-to-face interview was used to collect information about possible risk factors, namely, age, study location, and herd size. In this study, “herd of camel” means those groups of camels that share common grazing and watering points and were classified into large (>40 heads), medium (15–40 heads), and small (<15 heads). “PA” is the lowest administrative unit in Ethiopia.
Sample collection, and laboratory analysis
About 10 mL of blood was collected from camels’ jugular veins using sterile, plain vacutainer tubes. The blood was allowed to clot before being centrifuged at 3,000 rpm for 15 min to separate the serum. The recovered sera samples were transferred into sterile cryogenic tubes. The specimens were transported in an icebox with ice packs to Jigjiga Regional Laboratory and Diagnostic Center (JRLDC) and stored at −20°C until processed for the detection of camelpox antibodies.
A sandwich ELISA assay (Fisher Scientific, US) was used to analyze the sera samples for the detection of camelpox virus-specific antibodies following procedures recommended by the Office International des Epizooties (OIE) (OIE, 2014).
This sandwich ELISA test is a widely used immunoassay technique. In this assay, an antibody specific to camelpox virus is pre-coated onto a 96-well plate. Controls, test samples, and horseradish peroxidase (HRP)-conjugated reagents were added to the wells and incubated. After incubation, unbound conjugates were removed by washing the plate with a wash buffer. To quantify the HRP enzymatic reaction, a TMB substrate was added to each well. Only wells that contain sufficient camelpox virus antibodies will produce a blue-colored product, which later changes to yellow upon the addition of the acidic stop solution. The intensity of the yellow color was directly proportional to the amount of camelpox antibodies bound on the well. The optical density (OD) of the yellow color was measured spectrophotometrically at 450 nm using a microplate reader. By comparing the OD values of the test samples to the controls, the presence of camelpox virus antibodies was determined. The entire test procedure was conducted at Jigjiga Regional Laboratory and Diagnostic Center (JRLDC). The details of the test principle, materials and reagents used, the test procedure, and interpretation of the results were described (OIE, 2014; Aboul Soud et al., 2005).
Data management and analysis
Epi data entry version 3.0 was used to record and save the field data. The statistical software STATA® version 14.0 was then used to import the data and compute odds ratios and seroprevalence. The relationship between possible risk factors and the seroprevalence of camelpox was assessed using logistic regression analysis. The prevalence was used as an outcome variable in the logistic regression analysis against the hypothesized explanatory variables (districts, sex, age, herd size, and season). The final multiple logistic regression models were created manually, using a forward inclusion criterion. A confounder was defined as a variable that impacted the coefficient of the significant variables by more than 25%. Kruskal gamma statistics were used to analyze the predictors’ multicollinearity in the models. The final multivariate logistic regression models were used to compute the odds ratio (OR) and 95% confidence interval (CI) of the factors influencing the outcome variables. Significant differences were considered at a p-value < 0.05. Hosmer and Lemeshow statistics, as well as the Receiver Operating Curve (ROC), were used to assess model fit and validity (Dohoo et al., 2003).
Results
Seroprevalence of camelpox
Of 374 dromedary camels tested using a sandwich ELISA test, 60 camels were positive with an overall seroprevalence of camelpox at 16.0% (95% CI: 12.0%–20.0%). The seroprevalence of camelpox across study districts, sex, season, age, and herd size is presented in Table 1.
Risk factors for camelpox
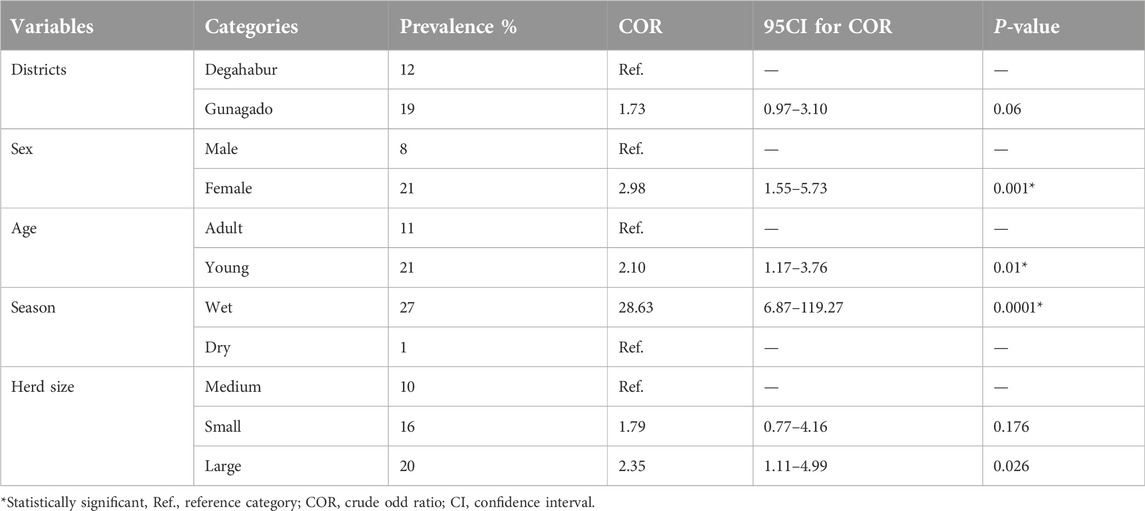
The potential risk factors considered for the seropositive of camelpox include age, sex, season, herd size, and study districts (Table 2).
In multivariable logistic regression analysis only season, age, and sex were substantially associated to the occurrence of camelpox virus (p < 0.05). Higher odds ratio (OR) of 2.7 (95% CI: 1.4–5.0) indicated that young were more likely than adult camels to contract camelpox. In addition, the chance of camelpox occurring during the wet season was 33.4 times ((95% CI: 7.6–146.7) higher than during the dry season (Table 3).
Discussion
A seroepidemiological study of camelpox was carried out in selected areas of Jarar zone in Somali Region of Ethiopia using sandwich ELISA test. This study revealed that overall the seroprevalence of camelpox in the study area was 16%. The current finding is consistent with Aregawi et al. (2018), who reported 19.3% seroprevalence of camelpox in the Afar region, Ethiopia.
However, Ayelet et al. (2013) and Housawi (2007) observed a lower prevalence of 4.50% in Afar and 9.14% in Saudi Arabia, respectively. On the other hand, larger prevalence values of neutralizing antibodies have been recorded from similarly unvaccinated camel populations following disease outbreaks in 72.5% of camels in Sudan (Khalafalla et al., 1998) and 100% of clinical cases and in-contact seemingly healthy camels in Egypt (Mahmoud et al., 2012). These disparities could be attributed to differences in agro-geographical location, sample size (Ayelet et al., 2013), and animal herding practices.
The findings indicated that camelpox occurrences and camelpox antibody seropositivity were significantly linked with age, sex, and season. Accordingly, seroprevalence in young was considerably (p < 0.05) greater than in adult camels. The declining maternal antibodies make young camels more susceptible to camelpox infection than adults (Mahmoud et al., 2012; Allan et al., 2023). In general, young animals under the age of 4 years in a herd suffer from a more severe and generalized form of the disease due to the loss of acquired maternal immunity after 5–8 months (Mahmoud et al., 2012; Joseph et al., 2021).
On the other hand, the present findings contrast with an earlier study carried out in the Afar region, in that adult age groups (>4 years) had a considerably greater seroprevalence of camelpox than did younger age groups (>6 months and <4 years) (Narnaware et al., 2021; Zhugunissov et al., 2021). This disparity might be attributed to the distribution of the two age groups proportions during sampling (Mahmoud et al., 2012).
According to the current study, camelpox antibodies were more common in females (21%). This difference was statistically significant (p < 0.05). These might be attributed to hormonal underpinnings because increased prolactin and progesterone levels have been shown to impair immunological responses, which may impact the body’s capacity to prevent camelpox infections and increase the susceptibility of females to infection (Khalafalla et al., 2021). Stress associated with pregnancy and nursing may also make female camels more vulnerable to the camelpox virus.
Likewise, this study indicated that there is a seasonal variation in the prevalence of camelpox, with the disease being more common in the wet season (p < 0.05) at 27.0% as opposed to the dry season (1.0%). The report of a previous study by Aregawi et al. (2018) in Ethiopia’s Afar pastoral region is similar to our observation. They found that camelpox is endemic to the area and that it frequently appears during the minor and major rainy seasons. Comparably, research has shown that camelpox outbreaks are more common during the rainy season, when the disease manifests in a more severe form. Wet seasons may offer more conducive environment for virus reproduction or enhance the survival of viral particles outside the host, which could result in increased seropositivity rates during such times. This difference would also be related to arthropods like biting flies and mosquitoes which are vectors of camelpox transmission. The density of these vectors varies with seasons. As a result, increasing vector activity during the rainy seasons may raise the virus’s rate of transmission and raise the seropositivity of camelpox (Zhugunissov et al., 2021). The higher seropositivity of camelpox during the rainy season than the dry is supported by works of many researchers (Wernery and Kaaden, 2002; Khalafalla and Ali, 2007; Megersa, 2010).
Seropositivity was not significantly influenced by herd size and study districts. This is supported by Aregawi et al. (2018). These findings can be related to the infectious nature of the camelpox virus, which transmits within the same herd or across herds when camels congregate for grazing and watering. Furthermore, the pastoral production system itself, which is characterized by camel herders’ migratory nature combined with the sharing of communal grazing and watering points, as well as the proximity of the two districts, contributes to the observed lack of statistically significant variations between districts. Herds cannot be considered separate units in a pastoral production system; instead, they are part of a wider animal production ecology (Aregawi et al., 2018).
The current researchers did not perform molecular characterizations and detect active clinical cases of the camelpox due to a lack of manpower and funds.
Conclusion
The results of the current study revealed that the prevalence of camelpox in Jarar zone of Ethiopia’s Somali Region was 16%. Sex, age, and season were the only predictor factors for the seroprevalence of the camelpox in the study area. This highlights necessity for efficient disease management and control strategies to mitigate the financial loss due to camelpox. Thus, the genetic diversity of the camelpox viruses circulating in the area needs to be investigated through further research.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal studies were approved by Jigjiga University, College of Veterinary Medicine- Research Review Committee (Protocol No. JJU/CVM/clis/022/14). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
Designed the work: HAA and HFG; Data collection: HAA and AAA; Data entry: HAA, AAA, and HFG; Writing–original draft, analyzing and interpreting the data: HAA and HFG, Data curation, analyzing, interpretation, and reviewing: IAK. All authors contributed to the article and approved the submitted version.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors would like to acknowledge Jigjiga Regional Veterinary Laboratory and Diagnostic Center (JRVLDC) for their help with materials used for the study, sample preparation, and analysis. The authors also greatly acknowledge camel owners in the study area for their willingness and voluntariness. NB: This manuscript is solely deposited at bioRxiv (Arog et al., 2024); rather than this, it is an original that has not been considered elsewhere.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
CMLV, Camelpox virus; JRLDC, Jigjiga regional laboratory diagnostic center; OD, Optical density; ELISA, Sandwich Enzyme-Linked Immune-sorbent Assay.
References
Aboul Soud, E. A., Sayed, M., Badawi, A. A., Taha, M. M., El-Ebiary, E. A., and Daoud, A. M. (2005). Preparation of diagnostic ELISA kits for detection of camelpox virus. J. Veterinary Med. Res. 15 (2), 227–231. doi:10.21608/jvmr.2005.77992
Allan, F. K., Wong, J. T., Lemma, A., Vance, C., Donadeu, M., Abera, S., et al. (2023). Interventions to reduce camel and small ruminant young stock morbidity and mortality in Ethiopia. Prev. Veterinary Med. 219, 106005. doi:10.1016/j.prevetmed.2023.106005
Aregawi, W. G., Agga, G. E., Gishe, J., and Abdi, R. D. (2018). Seroprevalence and participatory epidemiology of camelpox in Afar region of Ethiopia. Prev. veterinary Med. 161, 25–32. doi:10.1016/j.prevetmed.2018.10.003
Arog, H. A., Ahad, A. A., and Fesseha, H. (2024). Sero-epidemiological investigation and risk factors associated with camelpox in pastoral areas of the Somali region, eastern Ethiopia: A cross-sectional study. bioRxiv. 2024:2024-06. doi:10.1101/2024.06.05.597649
Ayelet, G., Jenberie, S., Belay, A., Mohammed, A., Mola, B., Gizaw, Y., et al. (2013). The first isolation and molecular characterization of camelpox virus in Ethiopia. Antivir. Res. 98 (3), 417–422. doi:10.1016/j.antiviral.2013.04.002
Balamurugan, V., Venkatesan, G., Bhanuprakash, V., and Singh, R. K. (2013). Camelpox, an emerging orthopox viral disease. Indian J. Virology 24, 295–305. doi:10.1007/s13337-013-0145-0
Dahiya, S. S., Kumar, S., Mehta, S. C., Narnaware, S. D., Singh, R., and Tuteja, F. C. (2016). Camelpox: a brief review on its epidemiology, current status, and challenges. Acta Trop. 158, 32–38. doi:10.1016/j.actatropica.2016.02.014
Hassen, G., Abdimahad, K., Tamir, B., Ma’alin, A., and Amentie, T. (2022). Identification and chemical composition of major camel feed resources in degahbur district of Jarar zone, Somali regional state, Ethiopia. Open J. Animal Sci. 12 (3), 366–379. doi:10.4236/ojas.2022.123028
Hassen, M., Amentie, T., Abdimahad, K., Ma’alin, A., and Mahamed, A. (2022). Hygienic production and post-harvest handling practices of raw camel milk in Degahbour district of Jarar zone, Somali regional state, Ethiopia. Open J. Animal Sci. 12 (2), 303–316. doi:10.4236/ojas.2022.122023
Housawi, F. M. T. (2007). Screening of domestic ruminants sera for the presence of anti-camel pox 464 virus neutralizing antibodies. Assiut Vet. Med. J. 53, 101–105.
Joseph, S., Kinne, J., Nagy, P., Juhász, J., Barua, R., Patteril, N. A., et al. (2021). Outbreak of a systemic form of camelpox in a dromedary herd (Camelus dromedarius) in the United Arab Emirates. Viruses 13 (10), 1940. doi:10.3390/v13101940
Kena, D. (2022). Review on camel production and marketing status in Ethiopia. Pastoralism 12 (1), 38. doi:10.1186/s13570-022-00248-2
Keskes, S., Ibrahim, M., Tessema, T. S., Tamir, B., Regassa, F., Kassa, T., et al. (2013). Production systems and reproductive performances of Camelus dromedarius in Somali regional state, eastern Ethiopia. J. Agric. Environ. Int. Dev. (JAEID) 107 (2), 243–266.
Khalafalla, A. I., and Abdelazim, F. (2017). Human and dromedary camel infection with camelpox virus in Eastern Sudan. Vector-Borne Zoonotic Dis. 17 (4), 281–284. doi:10.1089/vbz.2016.2070
Khalafalla, A. I., Al-Busada, K. A., and El-Sabagh, I. M. (2015). Multiplex PCR for rapid diagnosis and differentiation of pox and pox-like diseases in dromedary Camels. Virology J. 12, 1–2. doi:10.1186/s12985-015-0329-x
Khalafalla, A. I., and Ali, Y. H. (2007). “Observations on risk factors associated with some camel viral diseases of camels in Sudan,” in Proceedings of the 12th International Conference of the Association of Institutions of Tropical Veterinary Medicine, Montpellier, France, August 20–22, 2007, 101–105.
Khalafalla, A. I., Hussein, M. F., and Khalafalla, A. I. (2021). Camel pox. Infectious diseases of dromedary camels: a concise guide. Cham: Springer, 23–32.
Khalafalla, A. I., Mohamed, M. E., and Agab, H. (1998). Serological survey in camels of the Sudan for prevalence of antibodies to camelpox virus using ELISA technique. J. Camel Pract. Res. 5 (2), 197–200.
Mahmoud, M. A., Abo-Elnag, T. R., Osman, W. A., Bassiouny, A. I., and Goda, A. S. (2012). Epidemiology and characterization of camel poxvirus in the northwest coastal area of Egypt. Glob. Vet. 9, 738–744.
Megersa, B. (2010). An epidemiological study of major camel diseases in the Borana lowland, Southern Ethiopia, 58. Oslo: Drylands Coordination Group.
Narnaware, S. D., Ranjan, R., Dahiya, S. S., Panchbuddhe, A., Bajpai, D., Tuteja, F. C., et al. (2021). Pathological and molecular investigations of systemic form of camelpox in naturally infected adult male dromedary camels in India. Heliyon 7 (2), e06186. doi:10.1016/j.heliyon.2021.e06186
OIE. Terrestrial Manual 2014 (2014). Chapter 2.9.2. Camelpox. Off. Int. Des. Epizoot. Available from: http://www.oie.int/fileadmin/Home/fr/Health_standards/tahm/2.09.02_CAMELPOX.pdf (Accessed May 9, 2023).
Othieno, J., Njagi, O., Masika, S., Apamaku, M., Tenge, E., Mwasa, B., et al. (2022). Knowledge, attitudes, and practices on camel respiratory diseases and conditions in Garissa and Isiolo, Kenya. Front. Veterinary Sci. 9, 1022146. doi:10.3389/fvets.2022.1022146
Sazmand, A., Joachim, A., and Otranto, D. (2019). Zoonotic parasites of dromedary camels: So important, so ignored. Parasites and vectors 12, 610–0. doi:10.1186/s13071-019-3863-3
Tefera, M., and Gebreah, F. (2001). A study on the productivity and diseases of camels in eastern Ethiopia. Trop. animal health Prod. 33, 265–274. doi:10.1023/a:1010580416485
Keywords: camelpox, Ethiopia, risk factors, seroprevalence, Somali region, Ethiopia
Citation: Arog HA, Ahad AA, Gebremeskel HF and Kebede IA (2024) Seroprevalence of camelpox and its associated risk factors in selected districts of Jarar zone, Somali Region, Ethiopia. Pastor. Res. Policy Pract. 14:13471. doi: 10.3389/past.2024.13471
Received: 01 July 2024; Accepted: 13 September 2024;
Published: 24 September 2024.
Edited by:
Derradji Harek, Algerian National Institute for Agronomic Research INRAA, AlgeriaCopyright © 2024 Arog, Ahad, Gebremeskel and Kebede. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Isayas Asefa Kebede, aXNheWFzYXNlZmFAYW1ib3UuZWR1LmV0
 Hassan Abdi Arog1
Hassan Abdi Arog1 Isayas Asefa Kebede
Isayas Asefa Kebede