- INSTM, and Department of Mechanical and Industrial Engineering, University of Brescia, Brescia, Italy
Mineral carbonation emerges as a promising technology to tackle a contemporary challenge: climate change. This method entails the interaction of carbon dioxide with metal-oxide-bearing materials to produce solid carbonates resembling common substances (chalk, antacids, or baking soda). Given that steelmaking industries contribute to 8% of the global total emissions annually, the repurposing of their by-products holds the potential to mitigate CO2 production. Steel slag is a by-product of the metallurgical industry which is suitable for capturing CO2 due to its chemical composition, containing high CaO (24%–65%) and MgO (3%–20%) amounts, which increases the reactivity with the CO2. Moreover, the carbonation process can improve the hydraulic and mechanical properties of steel slag, making this by-product interesting to be reused in building materials. Different studies have developed in the last years addressing the possibilities of reducing the environmental impact of steel products, by CO2 sequestration. This study is dedicated to reviewing the basics of mineral carbonation applied to steel slag, along with recent advancements in research. Special emphasis is placed on identifying parameters that facilitate the reactions and exploring potential applications for the resulting products. The advantages and disadvantages of steel slag carbonation for the industrialization of the process are also discussed.
Introduction
In recent decades, a critical challenge facing modern society is the surge in greenhouse gas (GHG) emissions, leading to climate change with a global temperature increase of 0.8°C over the last century. Anthropogenic activities, including deforestation, fossil fuel consumption, and the meat industry, primarily contribute to the rise in these gases, notably CO2. With its concentration reaching 419 ppm in 2021 (compared to 360 ppm in the 19th century), urgent measures are required to address this environmental concern (Azadi et al., 2019).
Given this scenario, it becomes imperative to explore alternative strategies for mitigating CO2 emissions and reducing dependence on fossil fuels. Carbon capture and storage (CSS) technology emerges as an economically feasible strategy, potentially preventing the emission of eight billion tonnes of CO2 by 2050 (IEA50, 2021). This technology encompasses geological, oceanic, and mineral storage, with carbon mineralization being a key component.
Carbon mineralization involves a reaction between CO2 and Ca and Mg-minerals, forming stable carbonated products (CaCO3 and MgCO3) and providing a permanent solution for CO2 storage. This process accelerates the natural weathering of rocks, transforming it from a millennia-long process to mere minutes or hours. The resulting by-products are stable solids with long-term storage capacity (Sanna et al., 2014).
Direct carbonation (Lackner et al., 1995) occurs in one step through a direct gas-solid or gas-liquid-solid reaction. While effective, it requires high temperature and pressure for acceleration. In contrast, indirect carbonation involves two reactions: the extraction of metal ions (mainly Mg and Ca) from corresponding containing phases and subsequent carbonation between CO2 and the resulting solution. This route is more viable owing to its gentle reaction conditions, elevated carbonation effectiveness, and the generation of high-value secondary materials (Lee et al., 2021).
Notably, industrial wastes like steel slag, with high alkalinity and reactivity, are suitable for this process. The carbonation potential of these wastes for CO2 sequestration was evaluated suggesting that mineral carbonation could reduce global anthropogenic CO2 emissions by 12.5% (Pan et al., 2020). Waste materials such as cement waste, red mud, municipal incinerator ash, and steel slag are viable for the carbonation reaction, addressing both environmental concerns and waste disposal challenges (Izumi et al., 2021).
Steel slag, a by-product of steel manufacturing, has garnered significant attention for its potential applications in various sectors, particularly in construction. The importance of steel slag lies in its ability to serve as an alternative to natural resources, which are becoming increasingly scarce due to overexploitation. Its utilization in construction offers a multifaceted approach to resource conservation and environmental sustainability (Sun and Wang, 2024). Substituting traditional aggregates can effectively conserve finite natural resources that are both unevenly distributed and rapidly diminishing (Zhang et al., 2024). This effort holds particular significance in the context of sustainable development, where the responsible stewardship of natural resources is paramount and practices to mitigating the environmental impact associated with conventional construction activities are fundamental. In addition, the extraction and processing of natural aggregate entails significant energy consumption and environmental disturbance, including habitat destruction and landscape alteration. By reducing reliance on these materials through the utilization of steel slag, construction projects can minimize their ecological footprint and lessen pressure on sensitive ecosystems. Finally, repurposing, and harnessing steel slag can yield significant economic advantages, curbing waste and diminishing expenses linked with industrial by-products management.
Steel slag is produced in large quantities, with availability depending on the production of steel in each region.
As per the U.S. Geological Survey, specific data regarding actual ferrous slag production in the U.S. is not available. However, it is estimated that domestic slag sales reached approximately 17 million tons, valued at around $460 million in 2021. This slag was processed by 28 companies serving operational iron and steel factories or reprocessing previously deposited slag wastes at approximately 124 dedicated plants across 33 states (Cris Candice Tuck, 2022). In the context of India, each tonne of steel production generates about 200 kg of steel slag (Ministry of India, 2023). Owing to the lack of efficient disposal methods for steel slag, substantial slag piles have emerged around steel plants, evolving into a significant source of water, air, and land pollution (Mayes et al., 2008).
In Europe, an annual generation of approximately 45 million tons of slag (originating from iron and steel waste) is reported (EUROSLAG, 2024). This manufacturing originates from the procedures of ore conversion into iron, the conversion of hot iron into steel, and the melting of scrap in an electric arc furnace, or from the subsequent refining of crude steel.
It is crucial to underline that the availability of steel slag can depend on various conditions, such as the rate of steel production, the type of steel being manufactured, and the methods employed for slag disposal. Consequently, the precise availability of steel slag may fluctuate over time and across different locations.
Calculations indicate that utilizing 4.7 tons of steel slag could result in the absorption of one ton of CO2, producing 2.3 tons of CaCO3. If the entirety of the slag were employed for CaCO3 production, it could lead to the consumption of 53 million tons of CO2 and the generation of 120 million tons of CaCO3 (Eloneva et al., 2012). Other projected statistical estimates suggest that utilizing all steel slag for mineral carbonation could result in the potential sequestration of between 138 and 209 million tons of CO2 annually, constituting approximately 9.1%–10.4% of the iron and steel industry’s total CO2 emissions (Pan et al., 2017) and evaluate that the total CO2 potential for mineralization from 2020 to 2,100 will fall within the range of 26–42 gigatons (Myers and Nakagaki, 2020).
Despite the promising potential of carbon mineralization, its industrial applications face obstacles related to energy and cost consumption. Overcoming these challenges requires a focus on recovering value-added by-products derived from carbonation and generating revenues. The carbonated products can find applications in construction materials, paper, and paint filler. This review focuses on offering a comprehensive overview of the scientific literature concerning steel slag carbonation. It delves into ex-situ mineral carbonation methods, analyses slag properties and examines their effects on dissolution and carbonation mechanisms. Additionally, it outlines the potential reuse of carbonated slag in the cement industry, emphasizing how the carbonation process can enhance the hydraulic properties of this by-product. Finally, it also highlights the advantages and challenges associated with the diffusion process. The work is not overly specialistic because it is directed towards all scientific communities engaged in exploring available circular economy strategies to mitigate greenhouse gas emissions.
Review Methodology
To investigate the literature data about steel slag carbonation, text research was addressed to academic journals and scientific publications by Scopus.
Relevant keywords in the abstract and paper titles “steel slag” and “carbonation” were used. 437 papers were found on January 18, 2024. Among them, 25 works are review papers.
Following the bibliographic search, a cluster analysis was conducted by VOSviewer software1 (van Eck and Waltman, 2010). This enables the exploration of co-occurring textual data derived from the bibliometric database, facilitating a systematic analysis of the literature. The results are reported in Figure 1, showing a two-dimensional depiction of the research field, where the keywords are represented as circles, with closely correlated terms positioned near each other. The size of the bubbles indicates the number of publications where the specified term (or keyword) is mentioned.
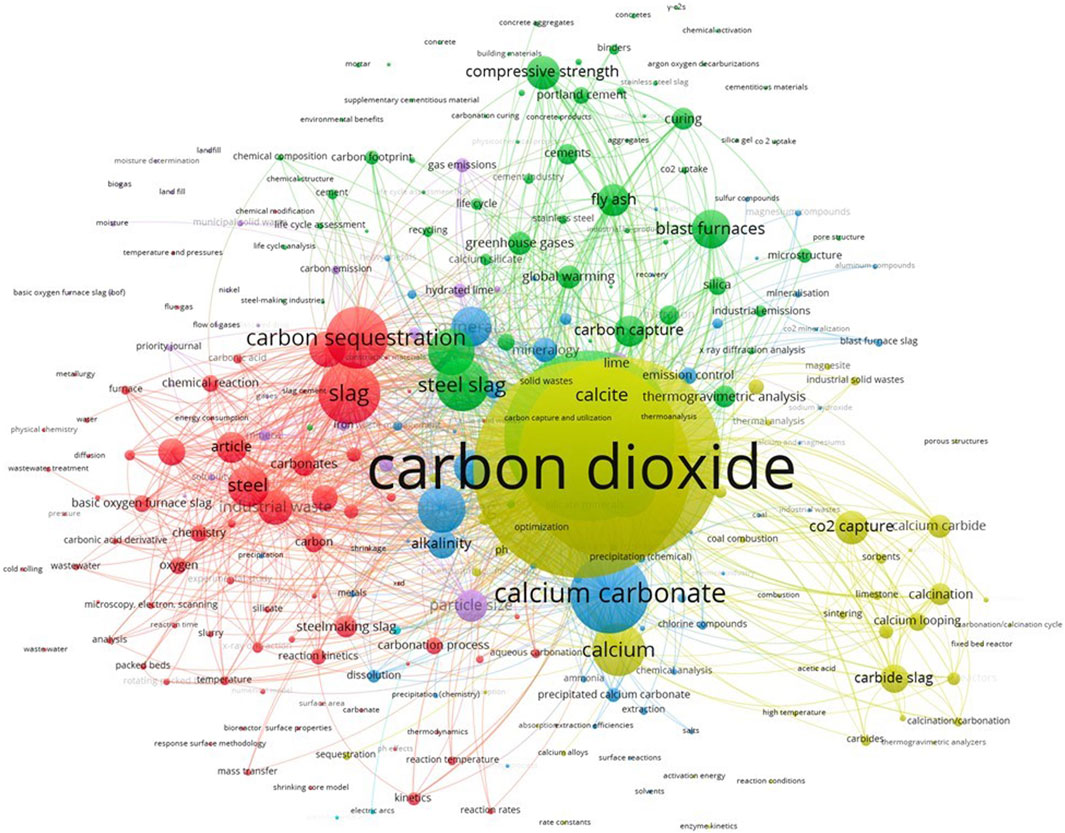
Figure 1. Cooccurrence of keywords for articles used for cluster analysis. Updated on January 18, 2024, by VOSviewer. In a two-dimensional depiction of a research field, closely correlated terms are positioned near each other. The size of the bubbles indicates the number of publications where the specified term (or keyword) is mentioned.
Figure 1 shows that keywords have been separated into 6 clusters. The initial group, depicted by red bubbles and comprising 75 items, primarily centers on carbonation reactions, with a focus on the kinetics mechanism. The second group (68 items) highlighted by green circles, is dedicated to the process’s environmental effects and the possibilities of reusing the by-products, by addressing them to the cement industry. The third cluster (59 items) represented by blue bubbles contains keywords addressed to contaminations and pollution, such as emission control and metal leachability. The fourth group (49 items), highlighted by yellow bubbles, is addressed to reaction optimization, with terms such as activation energy, concentration, composition, and enzyme kinetics.
Clusters 5 (violet) and 6 (azure) are more devoted to metallurgical fields, with keywords related to steel production, such as arc furnace, emissions, and flue gas. These last two clusters highlighted correlations that are out of the scope of this work.
Drawing insights from the cluster analysis results (refer to Figure 1), an in-depth examination of the literature was conducted. The formulation of the work presented below emerged through a reading of the selected full-text articles.
Steel Slag Characterization section characterizes steel slag by mineralogical and chemical composition, Carbonation and Reaction Parameters section describes the carbonation reaction and the influence of reaction parameters, Kinetics Models section compares the two main kinetic models, proposed by literature data, Methodologies to Improve the Mineral Carbonation Efficiency section describes different methodologies to improve the carbonation degree and Carbonated Slag Application in the Cement Industry section takes into account the reuse of carbonated steel slag in the cement industry as aggregates and supplementary cementitious material and as soil stabiliser, Advantages and Limitations of Steel Slag Carbonation section resumes the advantages of carbonation process for steel slag and Conclusion and Perspectives section concludes the manuscript.
Steel Slag Characterization
Steel slag is a waste of steel plants, comprising various types such as Electric Arc Furnace (EAF) slag, Argon Oxygen Decarburization (AOD) slag, Basic Oxygen Furnace (BOF) slag, Ladle Refining Furnace (LF) slag, and Blast Furnace (BF) slag (Zhang et al., 2023). The categorization is based on the diverse steelmaking procedures employed. Figure 2 shows what the steel slag looks like after its production. Indeed, the chemical and physical properties of steel slag are influenced by different production conditions and feedstock inputs. For example, in the process of EAF steelmaking and oxygen converter steelmaking, CaO and MgO are added to the smelting furnace to remove dangerous elements such as P and S in molten steel. Oxygen is used to remove impurities and carbon into Basic-Oxygen Furnaces and Electric Arc Furnaces. By this treatment, elements such as Al, Si, Mn, and P are oxidized to respective oxides. The last step of the steelmaking process is the refining by desulfurization process to remove impurities, oxygen, nitrogen, hydrogen, and carbon in the ladle furnace, where LF slag is a carbon by-product (Humbert and Castro-Gomes, 2019; Luo and He, 2021; Zhang et al., 2023).
Steel slag encompasses various valuable components, including 24%–65% CaO, 10%–32% SiO2, 3%–20% Al2O3, 0%–37% Fe2O3, and 3%–20% MgO and the chemical composition, based on the type of slag, is reported in Table 1. As illustrated, the chemical composition is influenced by the origin of slag. EAF and AOD slag may have elevated levels of Cr, posing a risk of significant pollution if it is directly discharged into soil and water resources (Chen et al., 2021a; Zhang et al., 2023).
CO2 sequestration is influenced by the mineralogical composition of steel by-products (see Table 2).
Except for BF slag, which is completely amorphous, other slags contain mainly Ca and Mg-phases as larnite, periclase and Ca-Mg-Al silicate. The mineral composition of steel slag primarily consists of 15%–25% dicalcium silicate (Ca2SiO4, C2S), 20%–25% tricalcium silicate (Ca3SiO5, C3S), 40%–45% RO (inert phases and combination between Mg, Fe and Ca), along with small quantities of free CaO and MgO (f-CaO and f-MgO). The primary constituents of Electric Arc Furnace slag (EAF) are CaO, SiO2, and Fe2O3. In BOF and EAF slag Fe-phases are identified as FeO or Fe2O3 due to high FeO quantity. Argon Oxygen Decarburization slag (AOD) contains a substantial amount of Cr2O3, and its direct discharge poses a significant threat to water and soil resources (Chen et al., 2021b).
Mineral compositions of steel slag have a considerable influence on CO2 sequestration and the main reactive species are periclase (MgO), calcium silicate (C3S and C2S) and merwinite. Thermodynamics calculations demonstrate that the carbonation of these phases is a spontaneous process (∆G is negative) at standard temperature and pressure as reported in the formula (1) (Ragipani et al., 2021; Zhang et al., 2023):
Carbonation and Reaction Parameters
Carbonation reaction occurs in nature but its kinetic is slow, also requiring millions of years. It is divided into two pathways, as shown in Figure 3. Direct carbonation happens when a step reaction can be highlighted, whereas, in the indirect process, two subsequent steps can be enhanced: extraction of alkaline metals by acidic solvents from steel slags and carbonation.
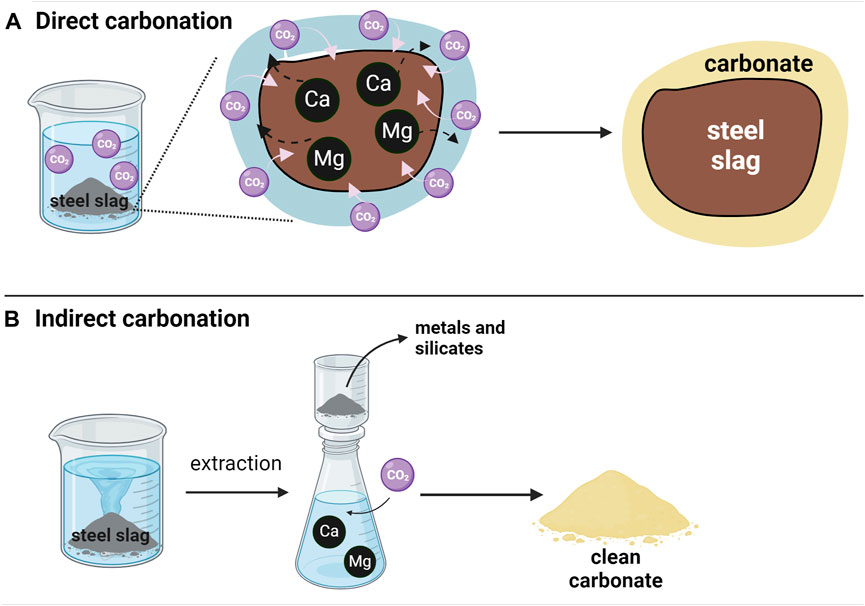
Figure 3. (A) The direct carbonation process occurs in one step through a direct gas-solid or gas-liquid-solid reaction. (B) The indirect carbonation process involves two reactions: the extraction of metal ions (mainly Mg and Ca) from corresponding containing phases and subsequent carbonation between CO2 and the resulting solution.
Direct Carbonation
Direct Gas-Solid Carbonation
First proposed by Lackner (Lackner et al., 1995), this method closely resembles the natural weathering process. The reactions entail oxides and hydroxides containing calcium and magnesium reacting with CO2 to form calcite (CaCO3), magnesite (MgCO3), and/or dolomite (CaMg(CO3)2) as shown in the formulas 2–4:
Moreover, some studies have demonstrated that also larnite (Ca2SiO4) and merwinite (Ca3Mg(SiO4)2), contained in steel slag, can react with CO2 as follows (Chen et al., 2021a; Yadav and Mehra, 2021) in the Eqs 5, 6:
The capacity CaO and MgO to generate carbonates depends on the degree of chemisorption of carbonate ions, the solubility product constant in aqueous carbonation, and the presence of alkaline active sites on the particle surface (Rahmanihanzaki and Hemmati, 2022).
Under specific conditions, the direct gas-solid pathway can achieve a maximum sequestration capacity of more than 10 g of CO2 per kilogram of steel slag at room temperature (Rushendra Revathy et al., 2016).
This value can increase if the temperature increases (Tian et al., 2013). Nevertheless, the reaction rate might be sluggish owing to the restricted interaction between the gas and solid phases, necessitating elevated temperatures for the process to proceed at a practical rate (Sanna et al., 2014).
Despite these obstacles, the direct gas-solid carbonation method holds the advantage of simplicity and the prospect of achieving high carbonation efficiency. This area remains an active focus of research in the field of CO2 sequestration.
Direct Aqueous Carbonation
Direct aqueous carbonation is an extensively studied method. It is a more complex process in comparison to direct gas-solid carbonation because it involves some steps: dissolution of carbon dioxide (CO2) in water, the formation of carbonic acid and the reaction with metal oxides to produce carbonates (Veetil and Hitch, 2020).
The reaction can be represented as follows in the formula 7:
Where M represents a divalent metal cation, such as calcium (Ca) or magnesium (Mg), commonly present in steel slag.
This reaction can also involve calcium species other than CaO, such as C3S and C2S (β-C2S and γ-C2S) which hydrate forming Ca(OH)2 and calcium-silicate-hydrates (C-S-H) (Chen et al., 2021a). At the beginning, the pH value of this system is alkaline but, when the CO2 is dissolved in water, carbonic acid is formed at equilibrium and the fully dissociation of CO32- decreases the pH (Yadav and Mehra, 2021). The anion reacts with Ca2+ or Mg2+ ions forming Ca(or Mg)CO3 (Schnabel et al., 2021) as shown in following reactions 8–10:
a) The leaching of minerals:
b) The carbon dioxide dissolution leads to the liberation of carbonate ions:
c) The initiation and expansion of mineral carbonate formations:
These three steps occur concurrently within a single reactor.
The procedure is conducted at relatively low temperatures and pressures, making it less energy-intensive compared to alternative carbonation methods. However, the reaction rate may be sluggish due to the limited solubility of CO2 in water and the passivation of the metal oxide surface by the formed carbonates.
To address these challenges, various process modifications have been suggested as the use of chelating agents, some pre-treatments, autoclave curing and continuous wet carbonation (Wu et al., 2017; Yadav and Mehra, 2021).
Despite the obstacles, direct aqueous carbonation has demonstrated a carbonation efficiency higher than 30% under specific conditions. During accelerated aqueous carbonation, steel slag has exhibited an impressive capacity for carbon fixation, reaching effective CO2 storage ranging from 130 to 330 g CO2 (kg slag)−1 (Wang F. et al., 2021).
Influence of Reaction Parameters
Direct-solid carbonation is primarily influenced by temperature and pressure, where higher pressure and temperature enhance process efficiency by favouring equilibrium shifts to free CO2 (Chen et al., 2021a). However, several parameters such as temperature, particle size, reaction time, carbon dioxide pressure, pH, and liquid-to-solid ratio (L/S) influence aqueous carbonation (Xiao et al., 2014):
Reaction Time
Initially, carbonation is a rapid process, but the rate diminishes over time until reaching equilibrium. This trend is attributed to the high concentration of reactive species (such as CaO and calcium silicates) and the alkaline pH (approximately 11) at the start, facilitating the reaction with CO2. Unfortunately, when the time passes, the concentration of free Ca ions decreases, and the pH decreases (around 6.5) making the carbonation process. Additionally, the formation of calcite creates a layer around the dissolved steel slag particles, influencing sequestration (Chen et al., 2021a).
Reaction Temperature
In the aqueous carbonation process, both the thermodynamic equilibrium and rate constants, as well as CO2 dissolution, are influenced by temperature. If it increases, this parameter has a dual effect: it enhances the solubility of cations (such as Mg and Ca) but impacts CO2 dissolution. It is crucial to emphasize the importance of finding the “optimum temperature,” which is contingent on material characteristics and experimental conditions. Temperatures ranging from 10°C to 40°C promote cation release but decrease the solubility of CO2, which is not a limiting step, in this condition. Additionally, low temperatures facilitate CaCO3 formation on both the solid and liquid slag surfaces due to the high dissolution of CO2, whereas at high temperatures, CaCO3 forms on gas-water interfaces owing to the increased dissolution of Ca ions in the liquid phase (Chiang and Pan, 2017; Chen et al., 2021a; Wang J. et al., 2021; Khudhur et al., 2022). This is in accord with some authors (Ukwattage et al., 2017; Li Z. et al., 2023; Huang et al., 2024) reporting that the “optimum temperature” for steel slag is below 50°C–60°C.
Particle Size
The particle size importantly influences the carbonation process: smaller particles provide a greater surface area favouring the reaction. Following carbonation, the specific surface area of steel slag is enhanced, caused by two main factors: enhancement of slag’s surface porosity due to the removal of calcium ions from the solid layer, and irregular layers of calcite formed (Ibrahim et al., 2019).
CO2 Pressure
Generally, when the temperature is constant, the amount of dissolved CO2 in the liquid is dependent on the carbon dioxide partial pressure, following Henry’s law. This occurs when CO2 solubilization is the rate-limiting step. In such cases, enhancing pressure increases the solubilisation of the mineral and the precipitation of carbonate minerals. However, when the rate-limiting step is calcium extraction, the effect of pressure can be different and less apparent. High pressure may have negative effects, such as unfavourable pH conditions or the rapid formation of calcite, leading to the accelerated presence of a protective layer around the slag, reducing the contact area between the gas and the matrix (Chen et al., 2021a; Khudhur et al., 2022).
pH
pH has a variable role in the carbonation process: at lower pH, the carbonation process enhances the leaching of metals (cations), which are the reactants of the reaction. Conversely, in a more alkaline environment (higher pH), the carbonation process favours the precipitation of calcium carbonate (Khudhur et al., 2022).
Liquid to Solid (L/S) Ratio
When the L/S ratio is lower than the optimum value, the steel slag is not properly dissolved in water and the interaction of CO2 and calcium ions decreases. When it is higher, CaO reacts directly with CO2 forming CaCO3 and CaO dissolves in water producing Ca(OH)2 which forms calcite. However, when L/S overcomes a critical value, the excessive water can become a mass transfer barrier, or the concentration of calcium ions and ionic strength are reduced (Chen et al., 2021a; Wang J. et al., 2021).
Table 3 reports literature results in terms of the CO2 percentages, as a function of representing the amount of gas sequestration per unit of steel slag, with the corresponding reaction parameters (temperature, carbon dioxide pressure, reaction time, liquid-to-solid ratio (L/S), particle size, carbon dioxide pressure, and pH), found in the literature for direct aqueous carbonation.
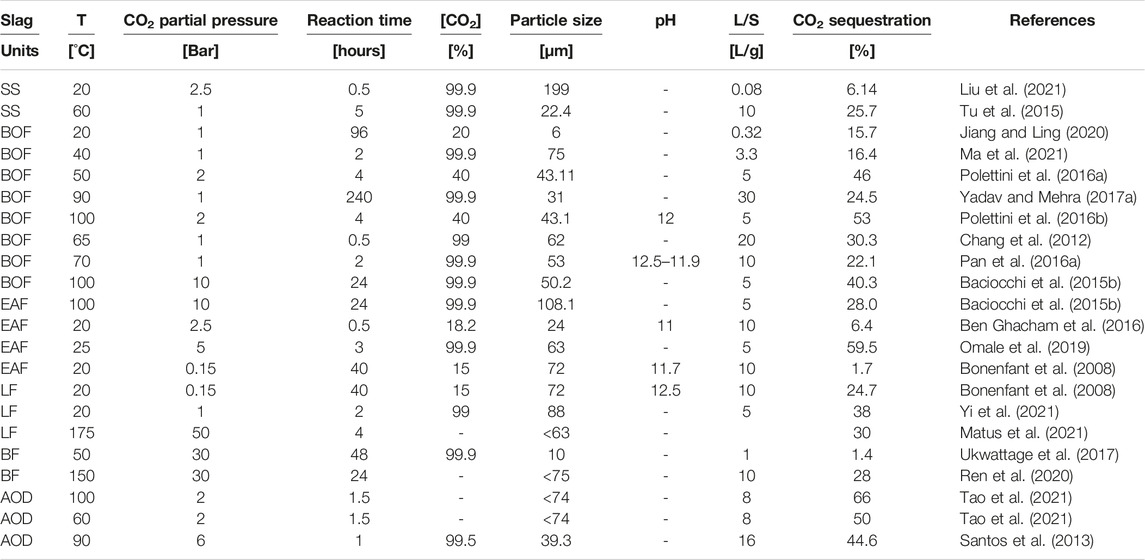
Table 3. CO2 percentages (amount of gas sequestration per unit of steel slag), with the corresponding reaction parameters (temperature, carbon dioxide pressure, reaction time, liquid-to-solid ratio (L/S), particle size, carbon dioxide pressure, and pH), found in the literature for direct aqueous carbonation.
Typically, better results are achieved with a particle size distribution between approximately 20–200 μm, and with carbonation durations of around 1 h, especially, when steel slag is ground below 100 μm to enhance their reactivity. Regarding temperature, around 100 °C generally supports carbonation, aligning with industrial waste heat ranges. Moreover, a high liquid-to-solid ratio (L/S > 10) is recommended for the reaction; however, significant improvements diminish beyond L/S > 15. Additionally, increasing CO2 partial pressure enhances CO2 sequestration, and this is particularly evident at lower gas concentrations due to enhanced solubility through pressurization (He et al., 2023).
Indirect Carbonation
Unlike direct carbonation, where CO2 directly interacts with the solid material, indirect carbonation involves a two-step process. Initially, reactive species, such as Ca and Mg ions are pulled out from minerals using solvents, and then they are transformed into respective oxides or hydroxides. Subsequently, these compounds react with carbon dioxide to produce carbonated species. While indirect carbonation offers advantages such as the purity and economic value of the carbonated product and operates under milder reaction conditions (atmospheric pressure and lower temperature), its drawback lies in the use of additional chemical solvents, leading to increased economic costs and necessitating efficient and low-cost recovery methods, which can impact industrial scale-up (Chen et al., 2021a; Yadav and Mehra, 2021; Rahmanihanzaki and Hemmati, 2022).
Indirect Gas-Solid Carbonation
In general, the reaction between CO2 and Ca and Mg oxides and hydroxides is faster than the direct pathway of respective silicates. Unfortunately, oxides and hydroxides are not present in steel slag. For this reason, their production from silicates is necessary and this is the basis of indirect carbonation. By adding solvent (as HCl) the reactive species are formed (Huijgen et al., 2005; Yadav and Mehra, 2021) as shown in the Eqs 11–13:
Indirect Aqueous Carbonation
This procedure involves two successive phases: the extraction of metal ions followed by carbonation in aqueous environments. The advantage is the optimization of each step. The extraction of ions is optimized using different solvents (acids or bases):
- Strong acids: they are better, in comparison to weak acids, at extracting metal elements from steel slags. Under suitable conditions, the yield of magnesium and calcium solubilisation can result in 46% and 9% respectively (Chen et al., 2021a; Yadav and Mehra, 2021).
- Acetic acid: the limited corrosive properties of this solvent render carbonation a more viable option compared to using strong acids. However, pH of the leaching solution can be adjusted before initiating the carbonation reaction. The acetic acid can be recovered after the extraction process and SiO2 can be separated by a thickener as shown in the following reactions 14, 15 (Chen et al., 2021a; Yadav and Mehra, 2021):
At 1 bar pressure and 25°C of temperature, the conversion is 40% and it can be improved to 75% increasing pressure until 30 bar.
- Ammonium salts: the use of acidic solvents requires a pH adjustment of the solution before the carbonation reaction by the addition of alkaline substances. The crucial aspect of indirect carbonation lies in recuperating chemical solvents with minimal energy consumption, thereby enhancing the process’s economic viability. For this reason, Satoshi et al. (Wang and Maroto-Valer, 2011) evaluated the “pH-swing process” which is based on NH4Cl use, and extraction and carbonation steps to obtain alkaline and acid conditions without any other chemicals. NH4Cl exhibits greater selectivity but reduced leachability for the extraction of Ca and Mg ions. In BOF slag the calcium silicate is the more reactive species, and it reacts with NH4Cl as shown in the following reactions 16, 17 (Chen et al., 2021a; Yadav and Mehra, 2021):
Influence of Reaction Parameters
Solvents Typology and Concentration
In the indirect carbonation process, the solvent plays a key role in the process. The nature (base or acidic) of the solvent influences the solubility of the metals as follows: strong acids (such as H2SO4; HCl, and HNO3) > acetate (such as CH3COOH and HCOOH) > ammonium salts (NH4Cl and NH4NO3)> alkali (NaOH and KOH). In fact, a lower pH increases the solubility of metal ions whereas a higher pH favours the precipitation of calcite (Chang et al., 2011).
Reaction Temperature
The effect of temperature is opposing: some studies show temperature has a kinetic effect, while others report that increasing temperature enhances the yield of magnesium and calcium extraction. As for direct carbonation, there exists an optimum value of temperature, under it the speed of the reaction is limited but when it is higher the CO2 solubility decreases (Chen et al., 2021a).
Particle Size
Particle size has an important role in the metal solubility in steel slag. In fact, the small particle size favours the solubilization of Mg and Ca ions due to their higher surface area (Chen et al., 2021a).
Liquid to Solid (L/S) Ratio
The L/S ratio mostly influences the extraction step. At higher values, the extraction rate of Mg and Ca ions increases, and it becomes constant when this parameter increases. On the contrary, reducing the liquid-to-solid (L/S) ratio may decrease the reaction rate, but the increase of solvent concentration can solve this effect (Chen et al., 2021a).
Kinetics Models
Generally, there are models used to depict the carbonation reaction, namely, the surface coverage model and the shrinking core model as shown in Figure 4 (Pan et al., 2014; Li et al., 2018; Chen et al., 2020b).
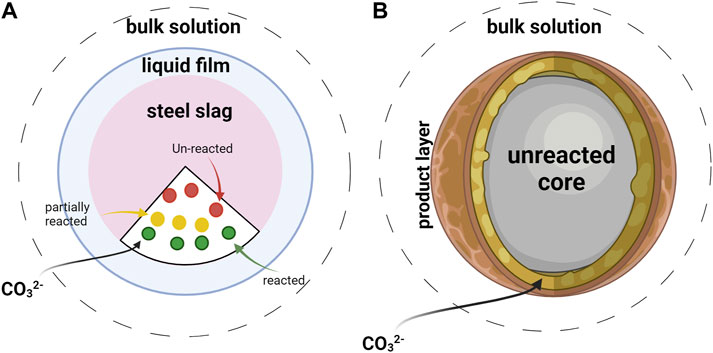
Figure 4. Kinetics models of carbonation reaction: (A) The surface coverage model shows that the reaction takes place exclusively at unreacted surface sites. In this model, the phase containing calcium reacts with CO2, forming calcium carbonate that covers the active surface of basic oxygen furnaces, leading to surface coverage. As the reaction progresses the active surface area remains uncovered by reaction products until reaching maximum conversion. (B) The shrinking core model shows that an initially unreacted core progressively diminishes in size as the reaction proceeds. Furthermore, the creation of small calcite crystals on the surface of the solid forms a protective layer that envelops the reactive particles. This layer serves to shield the particles from further reactions.
The coverage model proposes that the reaction rate is directly linked to the portion of the surface covered by the reactant. As the reaction progresses over time, the active surface area remains uncovered by reaction products until reaching maximum conversion.
The phase containing calcium reacts with CO2, forming calcium carbonate that covers the active surface of basic oxygen furnaces, leading to surface coverage.
The model shows how ions compete when they dissolve or form solids, how the area that can react changes over time, and how the reactions on the surface affect the movement of matter between the liquid and the solid (Ragipani et al., 2021; Wang et al., 2023a). Additionally, it accounts for the influence of variability in size distribution and diverse morphology in shaping the changes in the active surface area over time.
On the other hand, the shrinking core model has been employed to determine the step that predominantly controls the rate of particle reactions. (Castellote et al., 2008). The primary premise of this model is that the reaction initiates on the outer surface of the solid material, advances through a narrow front, and eventually permeates the entire solid, forming a fully reacted product known as the “ash” layer.
Subsequently, the rate of the reaction is controlled by either the chemical reaction transpiring at the interface between the reacted and unreacted sections or the diffusion of reactants through the product layer.
Within the shrinking core model, an initially unreacted core progressively diminishes in size as the reaction proceeds (Tu et al., 2015). Furthermore, the creation of small calcite crystals on the surface of the solid forms a protective layer that envelops the reactive particles. This layer serves to shield the particles from further reactions (Wei et al., 2021). Initially, the chemical reaction governs the process, particularly in the initial phase. However, as the reaction progresses, it transitions into diffusion through the porous layer, becoming the rate-limiting step. Carbonate conversion occurs at a notably slower rate compared to leaching conversion and is found to be constrained by diffusion through the calcium carbonate layer that forms. The apparent activation energy for the aqueous carbonation of steel slag is determined to be 4.8 kJ mol−1. This indicates the amount of energy required to initiate the reaction (Tu et al., 2015).
Moreover, both models are general models, then the actual kinetics may vary depending on the specific conditions and composition of the steel slag (Tu et al., 2015; Ragipani et al., 2021; Wang et al., 2023a).
Methodologies to Improve the Mineral Carbonation Efficiency
The main methodologies to improve the efficiency of mineral carbonation are the use of additives (chelating agents, HCl, CH3COOH, and HCOOH) and altering conditions such as change of solid-to-liquid ratio, carbon dioxide concentration, pressure, temperature, and particle size (Wu et al., 2017; Yadav and Mehra, 2021). These factors can accelerate the kinetic of mineral dissolution, which is the lowest step, favouring the silicate dissolution.
Chelating Agents
These agents are molecules capable of establishing multiple bonds with a single metal ion, effectively capturing the ion, and maintaining it in solution (Chen et al., 2023).
They can augment the solubilization of metal ions from the raw materials, thereby improving the efficiency of the carbonation process. Once the metal ions are leached into the solution, they can engage in a reaction with the dissolved CO2, resulting in the formation of solid carbonates.
Frequently used chelating agents encompass ethylenediaminetetraacetic acid (EDTA) and its derivatives, etidronic acid, galactaric acid, sodium metasilicate, as well as phosphate derivatives (Chen et al., 2023). As an example, in a study focusing on indirect carbonation, three chelating agents (adipate salts, malonate, and citrate) were used as solvents under ambient temperature and atmospheric pressure conditions. The study found that the concentration of the solvent was the crucial factor affecting calcium extraction from cement kiln dust (CKD), an alkaline industrial by-product (Castellote et al., 2008).
A research study titled “Investigation into the Accelerated Carbonation Behavior of Steel Slag Used as Cementitious Materials with EDTA Effects” delves into the accelerated carbonation process of steel slag in the presence of EDTA (Chen et al., 2023).
The findings indicate that the carbonation process efficiently decreases the free calcium oxide content in steel slag, leading to a significant enhancement in the volume stability of carbonated steel slag (CSS)-based cementitious materials. The presence of EDTA accelerates calcium leaching and acts as a catalyst for the carbonation reaction, leveraging its chelating effect. Steel slag treated with EDTA achieves a CO2 sequestration rate exceeding 14% within a 60-min carbonation period.
However, it’s fundamental to underline that the inclusion of chelating agents may introduce complexity to the process, as there might be a need for the recovery and reuse of these agents to ensure economic viability (Castellote et al., 2008).
Pre-treatment Process
Enhancing the carbonation process is achievable also through the pretreatment of slags (Quaghebeur et al., 2015). Improving CO2 absorption can be achieved by milling or grinding steel slags into smaller particle sizes, as this increases their specific surface area (Wang et al., 2023a). Literature shows that the carbonation of the finest particles results in a nearly complete conversion of free lime to carbonate (or portlandite) under the given reaction conditions. In contrast, larger particles consistently retain free lime, possibly due to carbonate mineral precipitation on the free lime particles, creating a layer of a carbonate phase that hinders fluid from reaching the free lime unreacted core of the particle (Quaghebeur et al., 2015).
Other studies revealed that decreasing the slag particle size and enhancing the porosity of the original slag can notably elevate the carbonation efficiency (Polettini et al., 2016a).
Carbonation in an autoclave, also increasing the temperature, can represent an alternative method to enhance the steel slag carbonation (Quaghebeur et al., 2015). Furthermore, elevating the partial pressure of CO2 not only facilitates CO2 dissolution in water but also lowers the pH value, representing a not favourable condition for carbonate precipitation.
Finally, the repetitive effectiveness of the continuous wet carbonation process is also possible, to improve the efficiency of the process (Chen et al., 2023). The research findings reveal that despite a decline in CO2 sequestration efficiency with each cycle, it consistently outperformed the control group.
Carbonated Slag Application in the Cement Industry
The cement industry is one of the major contributors to the environmental impact of energy consumption and emission of GHGs, including carbon dioxide (Mei et al., 2022). This industrial sector contributes 7.4% of global CO2 emissions since production of 1 kg of cement releases 0.5–0.7 kg of CO2 into the atmosphere (Kim et al., 2022; Younsi, 2022). Since cement is an essential component of concrete, its production is responsible for at least 70% of GHGs from the manufacture of concrete. To reduce the CO2 emissions from concrete products, cement industries have implemented many solutions such as using supplementary cementitious materials (SCMs) as partial replacement for clinker, the main component of Portland cement, or cement during concrete production. Supplementary cementitious materials can be different by-products such as fly ash, steel slag and cement wastes (Younsi, 2022).
Steel slag has been proposed to be employed for road filler production. Nevertheless, the inclusion of f-CaO and f-MgO in steel slag and the low content of hydraulic components, such as C2S and C3S, can induce delayed expansion and cracking in cement-based materials, leading to prolonged volume instability (Li et al., 2022). Moreover, recent studies have indicated significant alterations in the mechanical properties of steel slag and volume instability and a decrease of heavy metals leaching, following carbonation treatment, making it a possible supplementary cementing material or aggregate in concrete and cement applications (Mo et al., 2017; Dong et al., 2021). The accelerated carbonation eliminates the expansive compounds like f-CaO/MgO in fresh slag enabling the potential of these by-products as building materials (Li and Wu, 2022; Wang et al., 2023).
Some opportunities for reuse in building applications are reported in the following.
Supplementary Cementitious Materials
One possible use of carbonated slag is as a partial replacement of Portland cement. The first results in existing studies are encouraging but the research activities are still ongoing. Chen et al. (2016) demonstrated that the mechanical compressive strength of cement with 10% carbonated BOF slag has higher values than uncarbonated slag in a short time. Moreover, the carbonated BOFs have a better hydration activity than the starting material at 3 days and 28 days increased by 97% and 16% respectively (Wang et al., 2023b). Mechanical properties increase due to the activity of limestone and hydrated products. CaCO3 densify the microstructure of steel slag, reduces the pores size and increases hydration activity providing a better surface for the growth and nucleation of hydrated products (Zajac et al., 2020; Song et al., 2021). Moreover, in the cement matrix, calcite can react with C3A to form C3A•CaCO3•xH2O which shows better stability and hardness compared with sulphoaluminate, present in the cement (Pan et al., 2016b; Song et al., 2021; Wang et al., 2023b). Another improvement of carbonated steel slag is the reduction free species (CaO and MgO) and consequently the increase in their stability (Song et al., 2021). Peng et al. (Liu et al., 2021) show the positive effects of carbonation on increasing compressive strength. As Figure 5 shows, mortar mixes with respectively 15% and 30% of carbonated slag (CSG15 and CSG30) have higher compressive strength compared to mixes with uncarbonated slag. Otherwise, it is important to underline that the introduction of this by-product decreases the mechanical properties compared to PC mortar due to the presence of non-hydraulic phases such as γ-C2S, Fe-containing solid solutions, C2F, C12A7 (Liu et al., 2021). The compressive strength decreases when the steel slag percentage is enhanced.
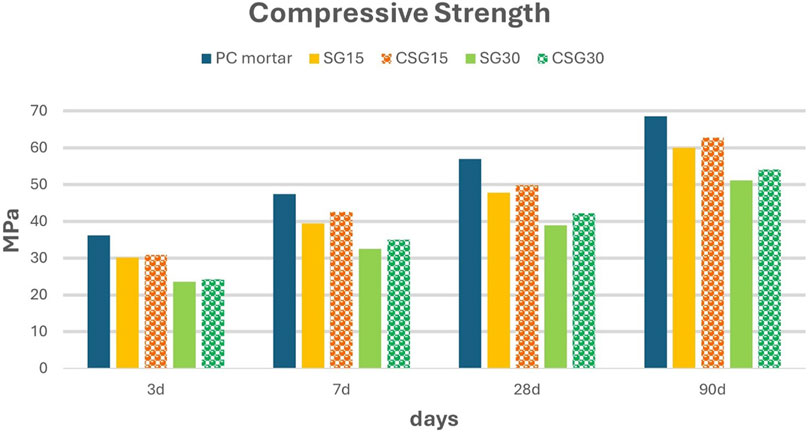
Figure 5. Compressive strength values of mixes blended uncarbonated and carbonated steel slag (Liu et al., 2021). Mortar mixes with respectively 15% and 30% of carbonated slag (CSG15 and CSG30) have higher compressive strength compared to mixes with uncarbonated slag. Otherwise, it is important to underline that the introduction of this by-product decreases the mechanical properties compared to PC mortar due to the presence of non-hydraulic phases as γ-C2S, Fe-containing solid solutions, C2F, C12A7.
Srivastava et al. show the role of carbonation conditions on compressive strength, using 30% of carbonated BOF slag. Varying the CO2 pressure, the calcite content and consequently the mechanical performance change. The sample carbonated at 1 bar (less calcite formed) reaches a double value compared to the mix with slag carbonated at 5 bar (high calcite content) (Srivastava et al., 2023).
Also, particle size can influence the compressive strength of the mix. Bodor et al. show that mix with the finest (<0.08 mm) and the coarsest (<1.6 mm) untreated and carbonated BOFs have lower compressive strength compared to Portland Clinker (Bodor et al., 2013). Otherwise, using a ternary system, as Zhang et al. (2011) proposed, can produce high-performance cement by using a huge quantity of SCMs. This system is a combination of fine and coarse slag to have a discontinuous particle size distribution (Pan et al., 2016b).
Aggregates
Another use of carbonated steel slag is as a sand substitute for cementitious building materials (Zhang et al., 2011; Wang et al., 2023b). Mo et al. (2020) use carbonated slag as artificial aggregates in a mix with Portland cement and fly ash. The concrete prepared with carbonated slag exhibits high compressive strengths in the range of 36.2–45.5 MPa at 28 days. If this mix is compared to natural limestone aggregates, the internal humidity is enhanced and autogenous shrinkage of concrete decreases. Mo et al. (2017) show the positive influence of the CO2 curing process in concrete samples with steel slag as coarse aggregates. Concrete containing steel slag shows better mechanical properties (like higher compressive strength) than natural aggregate concrete due to the higher content of mineralogical phases that could react with CO2.
In conclusion, using carbonated steel slag in the cement takes some advantages such as 1) increase in volume stability, 2) reduction of heavy metal leaching, 3) improvement of mechanical properties and 4) decrease of corrosion due to sulfates, salts, and acid attacks. Otherwise, the use of uncarbonated slag reduces the compressive and tensile strengths and durability and favors a volume expansion (Li and Wu, 2022).
Furthermore, the carbonation process applied to steel slag seems to involve additional energy consumption, contributing to CO2 emissions either directly or indirectly (Wang et al., 2023a). As a result, the widespread industrial adoption of carbonation technology for steel slag remains considerably constrained. Future research efforts should concentrate on developing more efficient.
Soil Stabilization
Historically, several amounts of steel slag have been stockpiled in open-air environments, posing significant risks to safety and environmental integrity, including concerns such as land occupation and the leaching of heavy metal constituents into the surrounding soil (Gao et al., 2023).
Moreover, the possibility of incorporating steel slag into the soil is considered in the literature and presents a multifaceted approach to enhancing soil properties and environmental sustainability. Even with the limited amount of available work, some considerations can be proposed.
Basically, the addition of steel slag to soil is based on the idea of providing the joint application of industrial solid waste and mine voids for the enduring subterranean sequestration of CO2 as minerals. This method not only ensures the resourceful recycling of CO2 and the optimal use of subterranean voids but also aids in averting geologic calamities, enhancing the economic viability of the system, and holds great potential for broad-scale implementation (Kang et al., 2024). Enhanced soil aggregate stability is achieved through the binding action of CaCO3, particularly evident with steel slag incorporation, thereby improving soil resistance to erosion and enhancing overall structural integrity.
Steel slag modifies the soil’s geotechnical characteristics, leading to a reduction in liquid and plastic limits, thereby improving workability and structural integrity. Moreover, the addition of steel slag increases the soil’s specific gravity, indicating densification and enhanced particle packing, consequently bolstering soil strength and stability. Additionally, steel slag contributes to carbon sequestration by reacting with CO2 to form stable carbonates, with CaCO3 acting as a catalyst, expediting the process, and mitigating greenhouse gas emissions (Rowley et al., 2018). The alkaline nature of steel slag also aids in neutralizing acidic soils (Capobianco et al., 2014), promoting optimal pH levels essential for nutrient availability and plant growth, while CaCO3 helps maintain this balance. Furthermore, the immobilization of heavy metals present in steel slag is facilitated by CaCO3, reducing their mobility and potential environmental impact by forming stable complexes within the soil matrix (Cristina Fernandes Deus et al., 2019).
In summary, the synergistic effects of steel slag carbonation with soil must be studied in more detail, to better understand their pivotal role in soil stabilization, carbon sequestration, pH regulation, nutrient availability, heavy metal containment, and aggregate stability, underscoring the importance of their comprehensive utilization in environmental applications.
Table 4 summarizes the mechanical properties of cement that are impacted by carbonated steel slag addition.
Advantages and Limitations of Steel Slag Carbonation
Leveraging steel slag CO2 sequestration can provide economic, environmental, and social advantages:
1. Economical carbon capture: the steel slag reuse obviates the necessity of establishing a new carbon capture facility, thereby reducing the costs of CO2 sequestration (Sarperi et al., 2014).
2. Waste valorization: carbonation provides a means of valorizing an industrial waste product, contributing to a circular economy, and promoting sustainable practices.
3. Carbon-negative potential: through the carbonation process, waste can be used as a carbon sink, effectively sequestrating and immobilizing CO2. This carbon-negative aspect can contribute to broader climate change mitigation efforts.
4. Technologically feasible and energy-efficient: this procedure is recognized to be relatively simple, cost-effective, and technologically feasible.
5. Adaptability to existing infrastructure: integrating the process with steel slag is adaptable to existing steel mill infrastructure, minimizing the need for significant modifications or new construction.
6. Improved leaching properties: the process of carbonation improves the stability of heavy metals contained in steel slag, reducing concerns related to environmental impact (Boone et al., 2014).
7. Reduced raw material needs: by steel slag carbonation, there is a consequential reduction in the demand for fresh raw materials, contributing to overall sustainability and resource efficiency.
8. Recovering value-added by-products: products derived from carbonation can be reused and generate revenues.
9. Versatile applications of the obtained secondary materials: the carbonation process improves the properties of steel slag, making it more appropriate for use as aggregates in various construction applications, thus contributing to sustainable building practices.
10. Reduced environmental footprint: the on-site utilization of secondary materials derived from steel slag after carbon dioxide sequestration reduces the need for transporting materials over long distances, minimizing the environmental footprint associated with transportation.
11. Community and stakeholder acceptance: this technology may encounter greater community and stakeholder acceptance compared to establishing new carbon capture facilities.
Although promising advantages, some challenges necessitate careful consideration and innovative solutions:
1. Chemical heterogeneity: the chemical composition of steel slags is inherently diverse, introducing significant complexities in accurately modelling the dissolution kinetics. This heterogeneity poses a challenge to researchers seeking to understand and predict the behaviour of steel slag during carbonation, demanding sophisticated modelling approaches (Ragipani et al., 2021).
2. Slow carbonation kinetics: the carbonation kinetics, especially under atmospheric conditions, is a notable hurdle that ongoing research endeavours are aiming to overcome. Improving the speed of the carbonation reaction is crucial for enhancing the overall efficiency of the process and its feasibility on an industrial scale.
3. Incomplete understanding of rate-limiting steps: the identification and comprehension of rate-limiting steps, coupled with a nuanced understanding of the role played by product layers in reaction kinetics, are aspects still in the process of development (Ragipani et al., 2021).
4. Increased process costs: while indirect carbonation shows a promising leaching ratio of alkaline earth ions and a high carbonation conversion rate, the requirement for adding chemical reagents to accelerate the reaction can lead to increased process expenses (Luo and He, 2021).
5. Additional processes: the carbonation of natural minerals, including steel slag, involves supplementary processes such as mining, transportation, and grinding of raw metals. These additional steps can contribute to heightened overall costs and energy consumption (Wang et al., 2023a).
Addressing these challenges is critical for establishing steel slag carbonation as a viable and widely adopted industrial process.
To apply the carbonation process at an industrial scale, the sustainability and possible impacts of this technology must be studied focusing on the process’s energies. To quantify the impact a life cycle assessment is the instrument. However, only limited studies can be found (Suer et al., 2022; Watjanatepin et al., 2023) and generally, they are referred to as preliminary results.
Conclusion and Perspectives
The mineral carbonation process mimics and accelerates natural weathering, where rocks break down over an extended period due to weather conditions, including the reaction of CO2 with specific rocks to form carbonates. However, the natural process is too slow to significantly impact atmospheric CO2 levels in a human timescale. Additionally, the abundance of magnesium and calcium silicate deposits in the Earth’s crust makes mineral carbonation a potentially scalable solution for CO2 sequestration.
This study provides insights into the steel slag carbonation process, highlighting its advantages and challenges. While it emphasizes the potential for both CO2 sequestration and waste valorisation, it also underscores the need for additional research to address challenges and make the technology economically feasible.
Direct gas-solid carbonation is a simple process where CO2 gas reacts directly with solid metal oxides to produce carbonates. The specific reactions vary depending on the feedstock.
In contrast, indirect carbonation offers benefits in terms of the superior purity of the resulting product. However, it is a more intricate process that may demand more energy due to the supplementary leaching step. Additionally, the challenges of recycling extractants contribute to increase costs in the carbon mineralization process.
Various approaches to accelerate carbonation, such as employing high pressure and/or high temperature, increased stirring speeds, and additives, have been explored. However, these techniques may lead to higher energy consumption and could affect the carbonation reaction. Hence, optimizing the steel slag carbonation process with minimal heat and power usage is essential but presents a significant challenge for direct carbonization.
While research on the reuse and recycle of steel slag after carbonation is not diffuse, the fundamental chemistry of mineral carbonation is well understood. Challenges, including the requirement for substantial starting material, slow reaction rates of natural minerals, and energy demands, need to be addressed.
Prospective research efforts could centre on enhancing the carbonation process for steel slag and optimizing parameters for scaling up applications.
Another big challenge of this topic is the reuse of carbonated steel slag in the cement industry. As different studies show, the introduction of steel slag as SCMs reduces the compressive strength of the cement and this reduction is proportional to the percentage of replacement (Zhang et al., 2011; Srivastava et al., 2023). Otherwise, the carbonation treatment improves the hydraulic properties of steel slag making them suitable for this use. It is mandatory to study deeply the mechanism of hydration of the slag in the matrix cement focusing on the possible leaching of heavy metals and the compressive strength.
In conclusion, mineral carbonation holds promise for mitigating climate change through CO2 sequestration and adding an extra value to steel slag. Despite existing challenges, the potential benefits make it a compelling area for further research, and it is a way to favour a circular process.
Author Contributions
EB and GB realized bibliographic analysis and performed draft manuscript preparation. LD contributed to the manuscript review and improvement. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of Interest
GB acknowledges Heidelberg Material Italia Cementi S.p.A for their contribution to her Ph.D. scholarship.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Figures 3, 4 were realized with Biorender.
Footnotes
References
Araizi, P. K., Hills, C. D., Maries, A., Gunning, P. J., and Wray, D. S. (2016). Enhancement of Accelerated Carbonation of Alkaline Waste Residues by Ultrasound. Waste Manag. 50, 121–129. doi:10.1016/j.wasman.2016.01.006
Azadi, M., Edraki, M., Farhang, F., and Ahn, J. (2019). Opportunities for Mineral Carbonation in Australia’s Mining Industry. Sustainability 11, 1250. doi:10.3390/su11051250
Baciocchi, R., Costa, G., Di Gianfilippo, M., Polettini, A., Pomi, R., and Stramazzo, A. (2015b). Thin-Film Versus Slurry-Phase Carbonation of Steel Slag: CO2 Uptake and Effects on Mineralogy. J. Hazard Mater 283, 302–313. doi:10.1016/J.JHAZMAT.2014.09.016
Baciocchi, R., Costa, G., Polettini, A., and Pomi, R. (2015a). Effects of Thin-Film Accelerated Carbonation on Steel Slag Leaching. J. Hazard Mater 286, 369–378. doi:10.1016/j.jhazmat.2014.12.059
Ben Ghacham, A., Pasquier, L.-C., Cecchi, E., Blais, J.-F., and Mercier, G. (2016). CO2 Sequestration by Mineral Carbonation of Steel Slags Under Ambient Temperature: Parameters Influence, and Optimization. Environ. Sci. Pollut. Res. 23, 17635–17646. doi:10.1007/s11356-016-6926-4
Biava, G., Zacco, A., Zanoletti, A., Sorrentino, G. P., Capone, C., Princigallo, A., et al. (2023). Accelerated Direct Carbonation of Steel Slag and Cement Kiln Dust: An Industrial Symbiosis Strategy Applied in the Bergamo–Brescia Area. Materials 16, 4055. doi:10.3390/ma16114055
Bodor, M., Santos, R., Gerven, T., and Vlad, M. (2013). Recent Developments and Perspectives on the Treatment of Industrial Wastes by Mineral Carbonation — A Review. Open Eng. 3. doi:10.2478/s13531-013-0115-8
Bonenfant, D., Kharoune, L., Sauve´, S., Hausler, R., Niquette, P., Mimeault, M., et al. (2008). CO2 Sequestration Potential of Steel Slags at Ambient Pressure and Temperature. Ind. Eng. Chem. Res. 47, 7610–7616. doi:10.1021/ie701721j
Boone, M. A., Nielsen, P., De Kock, T., Boone, M. N., Quaghebeur, M., and Cnudde, V. (2014). Monitoring of Stainless-Steel Slag Carbonation Using X-Ray Computed Microtomography. Environ. Sci. Technol. 48, 674–680. doi:10.1021/es402767q
Capobianco, O., Costa, G., Thuy, L., Magliocco, E., Hartog, N., and Baciocchi, R. (2014). Carbonation of Stainless Steel Slag in the Context of In Situ Brownfield Remediation. Min. Eng. 59, 91–100. doi:10.1016/j.mineng.2013.11.005
Castellote, M., Andrade, C., Turrillas, X., Campo, J., and Cuello, G. J. (2008). Accelerated Carbonation of Cement Pastes In Situ Monitored by Neutron Diffraction. Cem. Concr. Res. 38, 1365–1373. doi:10.1016/j.cemconres.2008.07.002
Chang, E.-E., Chen, C.-H., Chen, Y.-H., Pan, S.-Y., and Chiang, P.-C. (2011). Performance Evaluation for Carbonation of Steel-Making Slags in a Slurry Reactor. J. Hazard Mater 186, 558–564. doi:10.1016/j.jhazmat.2010.11.038
Chang, E.-E., Pan, S.-Y., Chen, Y.-H., Tan, C.-S., and Chiang, P.-C. (2012). Accelerated Carbonation of Steelmaking Slags in a High-Gravity Rotating Packed Bed. J. Hazard Mater 227–228, 97–106. doi:10.1016/j.jhazmat.2012.05.021
Chang, J., Wang, D., and Fang, Y. (2018). Effects of Mineralogical Changes in BOFS During Carbonation on pH and Ca and Si Leaching. Constr. Build. Mater 192, 584–592. doi:10.1016/J.CONBUILDMAT.2018.10.057
Chang, R., Kim, S., Lee, S., Choi, S., Kim, M., and Park, Y. (2017). Calcium Carbonate Precipitation for CO2 Storage and Utilization: A Review of the Carbonate Crystallization and Polymorphism. Front. Energy Res. 5. doi:10.3389/fenrg.2017.00017
Chen, B., Yoon, S., Zhang, Y., Han, L., and Choi, Y. (2019). Reduction of Steel Slag Leachate pH via Humidification Using Water and Aqueous Reagents. Sci. Total Environ. 671, 598–607. doi:10.1016/J.SCITOTENV.2019.03.362
Chen, K.-W., Pan, S.-Y., Chen, C.-T., Chen, Y.-H., and Chiang, P.-C. (2016). High-Gravity Carbonation of Basic Oxygen Furnace Slag for CO2 Fixation and Utilization in Blended Cement. J. Clean. Prod. 124, 350–360. doi:10.1016/j.jclepro.2016.02.072
Chen, T., Xue, Y., Zhao, X., and Liu, J. (2023). Effects of EDTA on the Accelerated Carbonation Behavior of Steel Slag Used as Cementitious Materials. J. Mater Cycles Waste Manag. 25, 1498–1508. doi:10.1007/s10163-023-01622-x
Chen, T.-L., Jiang, W., Shen, A.-L., Chen, Y.-H., Pan, S.-Y., and Chiang, P.-C. (2020b). CO2 Mineralization and Utilization Using Various Calcium-Containing Wastewater and Refining Slag via a High-Gravity Carbonation Process. Ind. Eng. Chem. Res. 59, 7140–7150. doi:10.1021/acs.iecr.9b05410
Chen, T.-L., Xiong, Y.-X., Chen, Y.-H., Chiang, P.-C., and Chen, Y.-H. (2020a). Performance Evaluation and Process Simulation for Synergetic Removal of NOx, CO2 and PM Using Green Alkaline Solution in a High-Gravity Rotating Packed Bed. Fuel 280, 118643. doi:10.1016/j.fuel.2020.118643
Chen, Z., Cang, Z., Yang, F., Zhang, J., and Zhang, L. (2021a). Carbonation of Steelmaking Slag Presents an Opportunity for Carbon Neutral: A Review. J. CO2 Util. 54, 101738. doi:10.1016/j.jcou.2021.101738
Chen, Z., Li, R., and Liu, J. (2021c). Preparation and Properties of Carbonated Steel Slag Used in Cement Cementitious Materials. Constr. Build. Mater 283, 122667. doi:10.1016/J.CONBUILDMAT.2021.122667
Chen, Z., Li, R., Zheng, X., and Liu, J. (2021b). Carbon Sequestration of Steel Slag and Carbonation for Activating RO Phase. Cem. Concr. Res. 139, 106271. doi:10.1016/j.cemconres.2020.106271
Costa, G., Polettini, A., Pomi, R., and Stramazzo, A. (2016). Leaching Modelling of Slurry-Phase Carbonated Steel Slag. J. Hazard Mater 302, 415–425. doi:10.1016/J.JHAZMAT.2015.10.005
Cristina Fernandes Deus, A., Marques de Almeida Bertani, R., Constantino Meirelles, G., de Aquino Vidal Lacerda Soares, A., Lorena Queiroz Moreira, L., Theodoro Büll, L., et al. (2019). “The Comprehensive Utilization of Steel Slag in Agricultural Soils,” in Recovery and Utilization of Metallurgical Solid Waste (IntechOpen). doi:10.5772/intechopen.81440
De Windt, L., Chaurand, P., and Rose, J. (2011). Kinetics of Steel Slag Leaching: Batch Tests and Modeling. Waste Manag. 31, 225–235. doi:10.1016/j.wasman.2010.05.018
Dong, Q., Wang, G., Chen, X., Tan, J., and Gu, X. (2021). Recycling of Steel Slag Aggregate in Portland Cement Concrete: An Overview. J. Clean. Prod. 282, 124447. doi:10.1016/j.jclepro.2020.124447
Eloneva, S., Said, A., Fogelholm, C.-J., and Zevenhoven, R. (2012). Preliminary Assessment of a Method Utilizing Carbon Dioxide and Steelmaking Slags to Produce Precipitated Calcium Carbonate. Appl. Energy 90, 329–334. doi:10.1016/j.apenergy.2011.05.045
EUROSLAG (2024). EUROSLAG. Available at: https://www.euroslag.com (Accessed January 21, 2024).
Gao, W., Zhou, W., Lyu, X., Liu, X., Su, H., Li, C., et al. (2023). Comprehensive Utilization of Steel Slag: A Review. Powder Technol. 422, 118449. doi:10.1016/j.powtec.2023.118449
Gurtubay, L., Gallastegui, G., Elias, A., Rojo, N., and Barona, A. (2014). Accelerated Ageing of an EAF Black Slag by Carbonation and Percolation for Long-Term Behaviour Assessment. J. Environ. Manage 140, 45–50. doi:10.1016/j.jenvman.2014.03.011
He, B., Zhu, X., Cang, Z., Liu, Y., Lei, Y., Chen, Z., et al. (2023). Interpretation and Prediction of the CO2 Sequestration of Steel Slag by Machine Learning. Environ. Sci. Technol. 57, 17940–17949. doi:10.1021/acs.est.2c06133
Hou, J., Liu, Q., Liu, J., and Wu, Q. (2018). Material Properties of Steel Slag-Cement Binding Materials Prepared by Precarbonated Steel Slag. J. Mater. Civ. Eng. 30. doi:10.1061/(ASCE)MT.1943-5533.0002370
Huang, X., Zhang, J., and Zhang, L. (2024). Accelerated Carbonation of Steel Slag: A Review of Methods, Mechanisms and Influencing Factors. Constr. Build. Mater 411, 134603. doi:10.1016/j.conbuildmat.2023.134603
Huijgen, W. J. J., Witkamp, G.-J., and Comans, R. N. J. (2005). Mineral CO2 Sequestration by Steel Slag Carbonation. Environ. Sci. Technol. 39, 9676–9682. doi:10.1021/es050795f
Humbert, P. S., and Castro-Gomes, J. (2019). CO2 Activated Steel Slag-Based Materials: A Review. J. Clean. Prod. 208, 448–457. doi:10.1016/j.jclepro.2018.10.058
Ibrahim, M., El-Naas, M., Benamor, A., Al-Sobhi, S., and Zhang, Z. (2019). Carbon Mineralization by Reaction With Steel-Making Waste: A Review. Processes 7, 115. doi:10.3390/pr7020115
IEA50 (2021). Is Carbon Capture Too Expensive? Available at: https://www.iea.org/commentaries/is-carbon-capture-too-expensive (Accessed February 19, 2024).
Izumi, Y., Iizuka, A., and Ho, H.-J. (2021). Calculation of Greenhouse Gas Emissions for a Carbon Recycling System Using Mineral Carbon Capture and Utilization Technology in the Cement Industry. J. Clean. Prod. 312, 127618. doi:10.1016/j.jclepro.2021.127618
Jiang, Y., and Ling, T.-C. (2020). Production of Artificial Aggregates From Steel-Making Slag: Influences of Accelerated Carbonation During Granulation And/or Post-Curing. J. CO2 Util. 36, 135–144. doi:10.1016/j.jcou.2019.11.009
Kang, J., Yu, Z., Zhang, Y., Xu, T., Guo, L., and Hao, S. (2024). Optimization of CO2 Sequestration in Alkaline Industrial Residues: The Enhancement Mechanism of Saline Soil. Chem. Eng. J. 486, 150402. doi:10.1016/j.cej.2024.150402
Khudhur, F. W. K., MacDonald, J. M., Macente, A., and Daly, L. (2022). The Utilization of Alkaline Wastes in Passive Carbon Capture and Sequestration: Promises, Challenges and Environmental Aspects. Sci. Total Environ. 823, 153553. doi:10.1016/j.scitotenv.2022.153553
Kim, M.-S., Sim, S.-R., and Ryu, D.-W. (2022). Supercritical CO2 Curing of Resource-Recycling Secondary Cement Products Containing Concrete Sludge Waste as Main Materials. Materials 15, 4581. doi:10.3390/ma15134581
Ko, M. S., Chen, Y. L., and Jiang, J. H. (2015). Accelerated Carbonation of Basic Oxygen Furnace Slag and the Effects on its Mechanical Properties. Constr. Build. Mater 98, 286–293. doi:10.1016/J.CONBUILDMAT.2015.08.051
Lackner, K. S., Wendt, C. H., Butt, D. P., Joyce, E. L., and Sharp, D. H. (1995). Carbon Dioxide Disposal in Carbonate Minerals. Energy 20, 1153–1170. doi:10.1016/0360-5442(95)00071-N
Lee, Y. H., Eom, H., Lee, S. M., and Kim, S. S. (2021). Effects of pH and Metal Composition on Selective Extraction of Calcium From Steel Slag for Ca(OH)2 Production. RSC Adv. 11, 8306–8313. doi:10.1039/D0RA08497B
Li, H., Zhang, S., Wang, K., Zhang, X., and Jiang, Y. (2023a). Effect of CO2 Capture on the Performance of CaO-Activated Slag Pastes and Their Acid Resistance. Constr. Build. Mater 365, 130039. doi:10.1016/j.conbuildmat.2022.130039
Li, L., Jiang, Y., Pan, S.-Y., and Ling, T.-C. (2021). Comparative Life Cycle Assessment to Maximize CO2 Sequestration of Steel Slag Products. Constr. Build. Mater 298, 123876. doi:10.1016/j.conbuildmat.2021.123876
Li, L., Ling, T.-C., and Pan, S.-Y. (2022). Environmental Benefit Assessment of Steel Slag Utilization and Carbonation: A Systematic Review. Sci. Total Environ. 806, 150280. doi:10.1016/j.scitotenv.2021.150280
Li, L., and Wu, M. (2022). An Overview of Utilizing CO2 for Accelerated Carbonation Treatment in the Concrete Industry. J. CO2 Util. 60, 102000. doi:10.1016/j.jcou.2022.102000
Li, Y., Pei, S., Pan, S.-Y., Chiang, P.-C., Lu, C., and Ouyang, T. (2018). Carbonation and Utilization of Basic Oxygen Furnace Slag Coupled With Concentrated Water From Electrodeionization. J. CO2 Util. 25, 46–55. doi:10.1016/j.jcou.2018.03.003
Li, Z., Chen, J., Lv, Z., Tong, Y., Ran, J., and Qin, C. (2023b). Evaluation on Direct Aqueous Carbonation of Industrial/Mining Solid Wastes for CO2 Mineralization. J. Industrial Eng. Chem. 122, 359–365. doi:10.1016/j.jiec.2023.02.036
Librandi, P., Nielsen, P., Costa, G., Snellings, R., Quaghebeur, M., and Baciocchi, R. (2019). Mechanical and Environmental Properties of Carbonated Steel Slag Compacts as a Function of Mineralogy and CO2 Uptake. J. CO2 Util. 33, 201–214. doi:10.1016/J.JCOU.2019.05.028
Liu, P., Zhong, J., Zhang, M., Mo, L., and Deng, M. (2021). Effect of CO2 Treatment on the Microstructure and Properties of Steel Slag Supplementary Cementitous Materials. Constr. Build. Mater 309, 125171. doi:10.1016/j.conbuildmat.2021.125171
Luo, Y., and He, D. (2021). Research Status and Future Challenge for CO2 Sequestration by Mineral Carbonation Strategy Using Iron and Steel Slag. Environ. Sci. Pollut. Res. 28, 49383–49409. doi:10.1007/s11356-021-15254-x
Ma, M., Mehdizadeh, H., Guo, M.-Z., and Ling, T.-C. (2021). Effect of Direct Carbonation Routes of Basic Oxygen Furnace Slag (BOFS) on Strength and Hydration of Blended Cement Paste. Constr. Build. Mater 304, 124628. doi:10.1016/j.conbuildmat.2021.124628
Matus, C., Stopić, S., and Friedrich, B. (2021). Carbonation of Minerals and Slags Under High Pressure in an Autoclave. Vojnoteh. Glas. 69, 486–498. doi:10.5937/vojtehg69-30203
Mayes, W. M., Younger, P. L., and Aumônier, J. (2008). Hydrogeochemistry of Alkaline Steel Slag Leachates in the UK. Water Air Soil Pollut. 195, 35–50. doi:10.1007/s11270-008-9725-9
Mei, K., Gu, T., Zheng, Y., Zhang, L., Zhao, F., Gong, P., et al. (2021). Effectiveness and Microstructure Change of Alkali-Activated Materials During Accelerated Carbonation Curing. Constr. Build. Mater 274, 122063. doi:10.1016/j.conbuildmat.2020.122063
Mei, X., Zhao, Q., Min, Y., Liu, C., Saxén, H., and Zevenhoven, R. (2022). Phase Transition and Dissolution Behavior of Ca/Mg-Bearing Silicates of Steel Slag in Acidic Solutions for Integration With Carbon Sequestration. Process Saf. Environ. Prot. 159, 221–231. doi:10.1016/j.psep.2021.12.062
Ministry of India (2023). Steel Slag Road Technology Fulfilling the Prime Minister’s ‘Waste to Wealth’ Mission: Sh. Faggan Singh Kulaste.
Mo, L., Yang, S., Huang, B., Xu, L., Feng, S., and Deng, M. (2020). Preparation, Microstructure and Property of Carbonated Artificial Steel Slag Aggregate Used in Concrete. Cem. Concr. Compos 113, 103715. doi:10.1016/j.cemconcomp.2020.103715
Mo, L., Zhang, F., Deng, M., Jin, F., Al-Tabbaa, A., and Wang, A. (2017). Accelerated Carbonation and Performance of Concrete Made With Steel Slag as Binding Materials and Aggregates. Cem. Concr. Compos 83, 138–145. doi:10.1016/j.cemconcomp.2017.07.018
Mohammed, A. M. A. (2021). Sequestration of Carbon Dioxide Using Ground Granulated Blast Furnaces Slag and Kaolin Mixtures. Glob. Nest J. 23(1):97–103. doi:10.30955/gnj.003487
Mohammed, A. M. A., Mohd Yunus, N. Z., Hezmi, M. A., Rashid, A. S. A., and Horpibulsuk, S. (2021). Carbonated Ground Granulated Blast Furnace Slag Stabilising Brown Kaolin. Environ. Sci. Pollut. Res. 28, 57308–57320. doi:10.1007/s11356-021-14718-4
Moon, E.-J., and Choi, Y. C. (2018). Development of Carbon-Capture Binder Using Stainless Steel Argon Oxygen Decarburization Slag Activated by Carbonation. J. Clean. Prod. 180, 642–654. doi:10.1016/j.jclepro.2018.01.189
Moon, E.-J., and Choi, Y. C. (2019). Carbon Dioxide Fixation via Accelerated Carbonation of Cement-Based Materials: Potential for Construction Materials Applications. Constr. Build. Mater 199, 676–687. doi:10.1016/j.conbuildmat.2018.12.078
Myers, C., and Nakagaki, T. (2020). Direct Mineralization of Atmospheric CO2 Using Natural Rocks in Japan. Environ. Res. Lett. 15, 124018. doi:10.1088/1748-9326/abc217
Omale, S. O., Choong, T. S. Y., Abdullah, L. C., Siajam, S. I., and Yip, M. W. (2019). Utilization of Malaysia EAF Slags for Effective Application in Direct Aqueous Sequestration of Carbon Dioxide Under Ambient Temperature. Heliyon 5, e02602. doi:10.1016/j.heliyon.2019.e02602
Pan, S.-Y., Adhikari, R., Chen, Y.-H., Li, P., and Chiang, P.-C. (2016b). Integrated and Innovative Steel Slag Utilization for Iron Reclamation, Green Material Production and CO2 Fixation via Accelerated Carbonation. J. Clean. Prod. 137, 617–631. doi:10.1016/j.jclepro.2016.07.112
Pan, S.-Y., Chen, Y.-H., Fan, L.-S., Kim, H., Gao, X., Ling, T.-C., et al. (2020). CO2 Mineralization and Utilization by Alkaline Solid Wastes for Potential Carbon Reduction. Nat. Sustain 3, 399–405. doi:10.1038/s41893-020-0486-9
Pan, S.-Y., Chiang, P.-C., Chen, Y.-H., Tan, C.-S., and Chang, E.-E. (2014). Kinetics of Carbonation Reaction of Basic Oxygen Furnace Slags in a Rotating Packed Bed Using the Surface Coverage Model: Maximization of Carbonation Conversion. Appl. Energy 113, 267–276. doi:10.1016/j.apenergy.2013.07.035
Pan, S.-Y., Chung, T.-C., Ho, C.-C., Hou, C.-J., Chen, Y.-H., and Chiang, P.-C. (2017). CO2 Mineralization and Utilization Using Steel Slag for Establishing a Waste-To-Resource Supply Chain. Sci. Rep. 7, 17227. doi:10.1038/s41598-017-17648-9
Pan, S.-Y., Liu, H.-L., Chang, E.-E., Kim, H., Chen, Y.-H., and Chiang, P.-C. (2016a). Multiple Model Approach to Evaluation of Accelerated Carbonation for Steelmaking Slag in a Slurry Reactor. Chemosphere 154, 63–71. doi:10.1016/j.chemosphere.2016.03.093
Park, B., Moon, E.-J., and Choi, Y. C. (2020). Investigation of Microstructure and Mechanical Performance of Carbon-Capture Binder Using AOD Stainless Steel Slag. Constr. Build. Mater 242, 118174. doi:10.1016/j.conbuildmat.2020.118174
Polettini, A., Pomi, R., and Stramazzo, A. (2016a). Carbon Sequestration Through Accelerated Carbonation of BOF Slag: Influence of Particle Size Characteristics. Chem. Eng. J. 298, 26–35. doi:10.1016/j.cej.2016.04.015
Polettini, A., Pomi, R., and Stramazzo, A. (2016b). CO2 Sequestration Through Aqueous Accelerated Carbonation of BOF Slag: A Factorial Study of Parameters Effects. J. Environ. Manage 167, 185–195. doi:10.1016/j.jenvman.2015.11.042
Quaghebeur, M., Nielsen, P., Horckmans, L., and Van Mechelen, D. (2015). Accelerated Carbonation of Steel Slag Compacts: Development of High-Strength Construction Materials. Front. Energy Res. 3. doi:10.3389/fenrg.2015.00052
Ragipani, R., Bhattacharya, S., and Akkihebbal, S. K. (2020). Understanding Dissolution Characteristics of Steel Slag for Resource Recovery. Waste Manag. 117, 179–187. doi:10.1016/j.wasman.2020.08.008
Ragipani, R., Bhattacharya, S., and Suresh, A. K. (2021). A Review on Steel Slag Valorisation via Mineral Carbonation. React. Chem. Eng. 6, 1152–1178. doi:10.1039/D1RE00035G
Rahmanihanzaki, M., and Hemmati, A. (2022). A Review of Mineral Carbonation by Alkaline Solidwaste. Int. J. Greenh. Gas Control 121, 103798. doi:10.1016/j.ijggc.2022.103798
Ren, E., Tang, S., Liu, C., Yue, H., Li, C., and Liang, B. (2020). Carbon Dioxide Mineralization for the Disposition of Blast-furnace Slag: Reaction Intensification Using NaCl Solutions. Greenh. Gases Sci. Technol. 10, 436–448. doi:10.1002/ghg.1837
Rowley, M. C., Grand, S., and Verrecchia, É. P. (2018). Calcium-Mediated Stabilisation of Soil Organic Carbon. Biogeochemistry 137, 27–49. doi:10.1007/s10533-017-0410-1
Rushendra Revathy, T. D., Palanivelu, K., and Ramachandran, A. (2016). Direct Mineral Carbonation of Steelmaking Slag for CO2 Sequestration at Room Temperature. Environ. Sci. Pollut. Res. 23, 7349–7359. doi:10.1007/s11356-015-5893-5
Salman, M., Cizer, Ö., Pontikes, Y., Santos, R. M., Snellings, R., Vandewalle, L., et al. (2014). Effect of Accelerated Carbonation on AOD Stainless Steel Slag for its Valorisation as a CO2-Sequestering Construction Material. Chem. Eng. J. 246, 39–52. doi:10.1016/j.cej.2014.02.051
Sanna, A., Uibu, M., Caramanna, G., Kuusik, R., and Maroto-Valer, M. M. (2014). A Review of Mineral Carbonation Technologies to Sequester CO2. Chem. Soc. Rev. 43, 8049–8080. doi:10.1039/C4CS00035H
Santos, R. M., Van Bouwel, J., Vandevelde, E., Mertens, G., Elsen, J., and Van Gerven, T. (2013). Accelerated Mineral Carbonation of Stainless Steel Slags for CO2 Storage and Waste Valorization: Effect of Process Parameters on Geochemical Properties. Int. J. Greenh. Gas Control 17, 32–45. doi:10.1016/j.ijggc.2013.04.004
Sarperi, L., Surbrenat, A., Kerihuel, A., and Chazarenc, F. (2014). The Use of an Industrial By-Product as a Sorbent to Remove CO2 and H2S From Biogas. J. Environ. Chem. Eng. 2, 1207–1213. doi:10.1016/j.jece.2014.05.002
Schnabel, K., Brück, F., Pohl, S., Mansfeldt, T., and Weigand, H. (2021). Technically Exploitable Mineral Carbonation Potential of Four Alkaline Waste Materials and Effects on Contaminant Mobility. Greenh. Gases Sci. Technol. 11, 506–519. doi:10.1002/ghg.2063
Song, Q., Guo, M.-Z., Wang, L., and Ling, T.-C. (2021). Use of Steel Slag as Sustainable Construction Materials: A Review of Accelerated Carbonation Treatment. Resour. Conserv. Recycl 173, 105740. doi:10.1016/j.resconrec.2021.105740
Srivastava, S., Cerutti, M., Nguyen, H., Carvelli, V., Kinnunen, P., and Illikainen, M. (2023). Carbonated Steel Slags as Supplementary Cementitious Materials: Reaction Kinetics and Phase Evolution. Cem. Concr. Compos 142, 105213. doi:10.1016/j.cemconcomp.2023.105213
Suer, J., Traverso, M., and Jäger, N. (2022). Review of Life Cycle Assessments for Steel and Environmental Analysis of Future Steel Production Scenarios. Sustainability 14, 14131. doi:10.3390/su142114131
Suer, P., Lindqvist, J.-E., Arm, M., and Frogner-Kockum, P. (2009). Reproducing Ten Years of Road Ageing — Accelerated Carbonation and Leaching of EAF Steel Slag. Sci. Total Environ. 407, 5110–5118. doi:10.1016/j.scitotenv.2009.05.039
Sun, R., and Wang, D. (2024). The Property, Structure, and Phase Evolution of a Binary Cementitious Material Derived From Sintering Flue Gas Desulphurization Ash and Steel Slag. J. Build. Eng. 86, 108908. doi:10.1016/j.jobe.2024.108908
Tao, M.-J., Wang, Y.-J., Li, J.-G., Zeng, Y.-N., Liu, S.-H., and Qin, S. (2021). Slurry-Phase Carbonation Reaction Characteristics of AOD Stainless Steel Slag. Processes 9, 2266. doi:10.3390/pr9122266
Tian, S., Jiang, J., Chen, X., Yan, F., and Li, K. (2013). Direct Gas–Solid Carbonation Kinetics of Steel Slag and the Contribution to In Situ Sequestration of Flue Gas CO 2 in Steel-Making Plants. ChemSusChem 6, 2348–2355. doi:10.1002/cssc.201300436
Tong, Z., Ma, G., Zhou, D., Yang, G., and Peng, C. (2019). The Indirect Mineral Carbonation of Electric Arc Furnace Slag Under Microwave Irradiation. Sci. Rep. 9, 7676. doi:10.1038/s41598-019-44162-x
Tran, D. T., Lee, Y., Lee, H. S., Yang, H.-M., and Singh, J. K. (2021). Effects of γ-C2S on the Properties of Ground Granulated Blast-Furnace Slag Mortar in Natural and Accelerated Carbonation Curing. Sustainability 13, 357. doi:10.3390/su13010357
Tu, M., Zhao, H., Lei, Z., Wang, L., Chen, D., Yu, H., et al. (2015). Aqueous Carbonation of Steel Slag: A Kinetics Study. ISIJ Int. 55, 2509–2514. doi:10.2355/isijinternational.ISIJINT-2015-142
Ukwattage, N. L., Ranjith, P. G., and Li, X. (2017). Steel-Making Slag for Mineral Sequestration of Carbon Dioxide by Accelerated Carbonation. Measurement 97, 15–22. doi:10.1016/j.measurement.2016.10.057
van Eck, N. J., and Waltman, L. (2010). Software Survey: VOSviewer, a Computer Program for Bibliometric Mapping. Scientometrics 84, 523–538. doi:10.1007/s11192-009-0146-3
Veetil, S. P., and Hitch, M. (2020). Recent Developments and Challenges of Aqueous Mineral Carbonation: A Review. Int. J. Environ. Sci. Technol. 17, 4359–4380. doi:10.1007/s13762-020-02776-z
Wang, D., Chang, J., and Ansari, W. S. (2019). The Effects of Carbonation and Hydration on the Mineralogy and Microstructure of Basic Oxygen Furnace Slag Products. J. CO2 Util. 34, 87–98. doi:10.1016/j.jcou.2019.06.001
Wang, F., Dreisinger, D., Jarvis, M., and Hitchins, T. (2021b). Kinetic Evaluation of Mineral Carbonation of Natural Silicate Samples. Chem. Eng. J. 404, 126522. doi:10.1016/j.cej.2020.126522
Wang, J., Zhong, M., Wu, P., Wen, S., Huang, L., and Ning, P. (2021c). A Review of the Application of Steel Slag in CO2 Fixation. ChemBioEng Rev. 8, 189–199. doi:10.1002/cben.202000021
Wang, X., and Maroto-Valer, M. (2011). Integration of CO2 Capture and Storage Based on pH-Swing Mineral Carbonation Using Recyclable Ammonium Salts. Energy Procedia 4, 4930–4936. doi:10.1016/J.EGYPRO.2011.02.462
Wang, Y., Liu, J., Hu, X., Chang, J., Zhang, T., and Shi, C. (2023b). Utilization of Accelerated Carbonation to Enhance the Application of Steel Slag: A Review. J. Sustain Cem. Based Mater 12, 471–486. doi:10.1080/21650373.2022.2154287
Wang, Y., Tao, M., Li, J., Zhang, J., Qin, S., Liu, S., et al. (2023a). A Review of Use of Metallurgical Slag for Its Carbonation Products: Processes, Crystallization Behavior, and Application Status. J. Iron Steel Res. Int. 30, 2341–2365. doi:10.1007/s42243-023-01108-y
Wang, Y.-J., Tao, M.-J., Li, J.-G., Zeng, Y.-N., Qin, S., and Liu, S.-H. (2021a). Carbonation of EAF Stainless Steel Slag and its Effect on Chromium Leaching Characteristics. Cryst. (Basel) 11, 1498. doi:10.3390/cryst11121498
Watjanatepin, P., Steinwidder, L., de Schutter, A., Granata, G., Vicca, S., Van Gerven, T., et al. (2023). “Preliminary Environmental Assessment of Carbonated Slags as a Carbon Capture, Utilization, and Storage Materials (CCUS),” in RawMat 2023 (Basel Switzerland: MDPI), 36. doi:10.3390/materproc2023015036
Wei, C., Dong, J., Zhang, H., and Wang, X. (2021). Kinetics Model Adaptability Analysis of CO2 Sequestration Process Utilizing Steelmaking Slag and Cold-Rolling Wastewater. J. Hazard Mater 404, 124094. doi:10.1016/j.jhazmat.2020.124094
Wu, L., Li, H., Mei, H., Rao, L., Xia, Y., and Dong, Y. (2023). A Novel Approach to Accelerate Carbon Dioxide Sequestration of Ladle Furnace Slag Using Sodium Bicarbonate Solution. Min. Eng. 204, 108374. doi:10.1016/J.MINENG.2023.108374
Wu, X., Yu, B., Xu, W., Fan, Z., Wu, Z., and Zhang, H. (2017). Stabilization of Carbon Dioxide and Chromium Slag via Carbonation. Environ. Technol. 38, 1997–2002. doi:10.1080/09593330.2016.1244566
Xiao, L.-S., Wang, R., Chiang, P.-C., Pan, S.-Y., Guo, Q.-H., and Chang, E. E. (2014). Comparative Life Cycle Assessment (LCA) of Accelerated Carbonation Processes Using Steelmaking Slag for CO2 Fixation. Aerosol Air Qual. Res. 14, 892–904. doi:10.4209/aaqr.2013.04.0121
Xu, B., and Yi, Y. (2022a). Treatment of Ladle Furnace Slag by Carbonation: Carbon Dioxide Sequestration, Heavy Metal Immobilization, and Strength Enhancement. Chemosphere 287, 132274. doi:10.1016/j.chemosphere.2021.132274
Xu, B., and Yi, Y. (2022b). Immobilization of Lead (Pb) Using Ladle Furnace Slag and Carbon Dioxide. Chemosphere 308, 136387. doi:10.1016/J.CHEMOSPHERE.2022.136387
Yadav, S., and Mehra, A. (2017a). Experimental Study of Dissolution of Minerals and CO2 Sequestration in Steel Slag. Waste Manag. 64, 348–357. doi:10.1016/j.wasman.2017.03.032
Yadav, S., and Mehra, A. (2017b). Dissolution of Steel Slags in Aqueous Media. Environ. Sci. Pollut. Res. 24, 16305–16315. doi:10.1007/s11356-017-9036-z
Yadav, S., and Mehra, A. (2021). A Review on Ex Situ Mineral Carbonation. Environ. Sci. Pollut. Res. 28, 12202–12231. doi:10.1007/s11356-020-12049-4
Yi, Y. R., Lin, Y., Du, Y. C., qi Bai, S., le Ma, Z., and guang Chen, Y. (2021). Accelerated Carbonation of Ladle Furnace Slag and Characterization of its Mineral Phase. Constr. Build. Mater 276, 122235. doi:10.1016/J.CONBUILDMAT.2020.122235
Younsi, A. (2022). Long-Term Carbon Dioxide Sequestration by Concretes With Supplementary Cementitious Materials Under Indoor and Outdoor Exposure: Assessment as Per a Standardized Model. J. Build. Eng. 51, 104306. doi:10.1016/j.jobe.2022.104306
Zajac, M., Skocek, J., Durdzinski, P., Bullerjahn, F., Skibsted, J., and Ben Haha, M. (2020). Effect of Carbonated Cement Paste on Composite Cement Hydration and Performance. Cem. Concr. Res. 134, 106090. doi:10.1016/j.cemconres.2020.106090
Zeng, T., Hu, Z., Huang, C., and Chang, J. (2023). Influence of Carbonation on the Properties of Steel Slag–Magnesium Silicate Hydrate (MSH) Cement. Materials 16, 6737. doi:10.3390/ma16206737
Zhang, F., Wang, D., Cannone Falchetto, A., and Cao, Y. (2024). Microwave Deicing Properties and Carbon Emissions Assessment of Asphalt Mixtures Containing Steel Slag Towards Resource Conservation and Waste Reuse. Sci. Total Environ. 912, 169189. doi:10.1016/j.scitotenv.2023.169189
Zhang, H., Dong, J., and Wei, C. (2022). Material Properties of Cement Doped With Carbonated Steel Slag Through the Slurry Carbonation Process: Effect and Quantitative Model. Metallurgical Mater. Trans. B 53, 1681–1690. doi:10.1007/s11663-022-02477-7
Zhang, S., Ghouleh, Z., Liu, J., and Shao, Y. (2021). Converting Ladle Slag Into High-Strength Cementing Material by Flue Gas Carbonation at Different Temperatures. Resour. Conserv. Recycl 174, 105819. doi:10.1016/j.resconrec.2021.105819
Zhang, T., Yu, Q., Wei, J., Li, J., and Zhang, P. (2011). Preparation of High Performance Blended Cements and Reclamation of Iron Concentrate From Basic Oxygen Furnace Steel Slag. Resour. Conserv. Recycl 56, 48–55. doi:10.1016/j.resconrec.2011.09.003
Zhang, Y., Yu, L., Cui, K., Wang, H., and Fu, T. (2023). Carbon Capture and Storage Technology by Steel-Making Slags: Recent Progress and Future Challenges. Chem. Eng. J. 455, 140552. doi:10.1016/j.cej.2022.140552
Zhong, J., Cao, L., Li, M., Wang, S., Liu, F., Lv, X., et al. (2023). Mechanical Properties and Durability of Alkali-Activated Steel Slag–Blastfurnace Slag Cement. J. Iron Steel Res. Int. 30, 1342–1355. doi:10.1007/s42243-023-01003-6
Keywords: carbon sequestration, accelerated carbonation, steel slag, construction materials, SCMs
Citation: Biava G, Depero LE and Bontempi E (2024) Accelerated Carbonation of Steel Slag and Their Valorisation in Cement Products: A Review. Span. J. Soil Sci. 14:12908. doi: 10.3389/sjss.2024.12908
Received: 27 February 2024; Accepted: 08 April 2024;
Published: 23 April 2024.
Edited by:
Avelino Núñez-Delgado, University of Santiago de Compostela, SpainCopyright © 2024 Biava, Depero and Bontempi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elza Bontempi, ZWx6YS5ib250ZW1waUB1bmlicy5pdA==
 Giada Biava
Giada Biava Elza Bontempi
Elza Bontempi