Dear Editors,
Kidney transplant recipients are at high risk for severe COVID-19 disease or death in case of SARS-CoV-2 infection (1). There is growing evidence suggesting that anti-SARS-Cov2-antibody response is markedly blunted in kidney transplant patients after vaccination (2). Severe COVID- 19 and COVID-19-related death has been recently reported in kidney transplant recipients despite prior complete (two dose) vaccination with SARS-CoV-2 mRNA vaccines (3). (4).
In this retrospective cohort study involving 320 prevalent kidney transplant recipients from a single transplant center (Ordensklinikum Linz—Elisabethinen hospital), anti- Spike (S) protein IgG antibody titers were measured 3–6 weeks [BNT162b2: median 28 days (IQR: 6 days), mRNA1273: median 28 days (IQR: 8 days)] after administration of the second dose of either mRNA-1273 or BNT162b2 SARS-CoV-2 vaccine. Vaccinations took place between January 15th and June 8th, 2021 according to the Austrian national SARS-CoV-2 vaccination program. Vaccine doses were allocated by the Austrian government depending on availability. Patients were vaccinated ranked by age beginning with the oldest as soon as vaccines were available. Allocation to a certain vaccine (BNT162b2 or mRNA-1273) was therefore determined by the vaccination progress in our kidney transplant cohort and vaccine availability at that time. A two-dose vaccination regimen was applied, with 3-weeks (BNT162b2) and 4-weeks (mRNA-1273) intervals between the first and second vaccination, regardless of a history of prior infection with SARS-CoV-2.
Anti-SARS-CoV-2-antibodies directed against the receptor binding domain (RBD) of the S1 subunit of the Spike (S) protein were measured with the SARS-CoV-2 IgG II Quant assay (Abbott Ireland Diagnostics Division, Finisklin Business Park, Sligo, Ireland), which was reported to have high sensitivity and specificity for detection of anti-SARS-CoV-2 S-protein antibodies (5) and high correlations with anti-SARS-CoV-2 neutralizing antibodies (6). Results were reported in BAU/ml (binding antibody units). Differences between vaccine groups (mRNA-1273 vs BNT162b2) in S-antibody-positivity were tested for statistical significance using the Chi2-Test. To further investigate the impact of vaccine type on S-antibody-positivity, we computed a multivariate logistic regression model taking potential confounding factors of seroconversion after SARS-COV2 vaccination into account. Results are reported as odds ratios (OR) and 95% confidence intervals (95% CI). We performed a complete case analysis as covariate information was missing in one patient only for the multivariate model. The study was approved by the Ethics Committee of the Johannes Kepler University Linz (ID: 1100/2021). Patients provided written informed consent. Patient demographics and additional analyses are shown in the Supplementary Material.
Anti-S-antibody positivity was detected in 51% of the patients in our study cohort. A higher proportion of mRNA-1273 vaccinated patients achieved antibody-positivity compared to those vaccinated with BNT162b2 (61.6 vs 47.7%, p = 0.037, Chi2-test). After correction for age, diabetes status, sex, serum albumin and serum creatinine, the odds ratio for anti-S- antibody seroconversion was significantly higher for mRNA-1273 vaccinated patients compared to BNT162b2 in a multivariate regression analysis (odds ratio: 2.12, 95% confidence interval: 1.16 to 3.87, p = 0.013, Figure 1). After exclusion of patients with a history of prior SARS-CoV-2-infection [N = 21; 17 patients with BNT162b2, four patients with mRNA-1273; six patients with IgG antibodies directed against the nucleocapsid (N) protein, 15 Patients with positive SARS-CoV-2 polymerase chain reaction (PCR) test], results remained similar. In patients without prior SARS-CoV-2 infection (N = 299) a higher proportion of patients vaccinated with mRNA-1273 achieved seropositivity compared to patients vaccinated with BNT162b2 (59.4 vs 44.3%, p = 0.027, Chi2-test). The odds ratio for seroconversion was higher in mRNA-1273-vaccinated patients compared to BNT162b2-vaccinated patients in multivariate analysis (OR: 2.2, 95% CI: 1.19 to 4.08, p = 0.011).
FIGURE 1
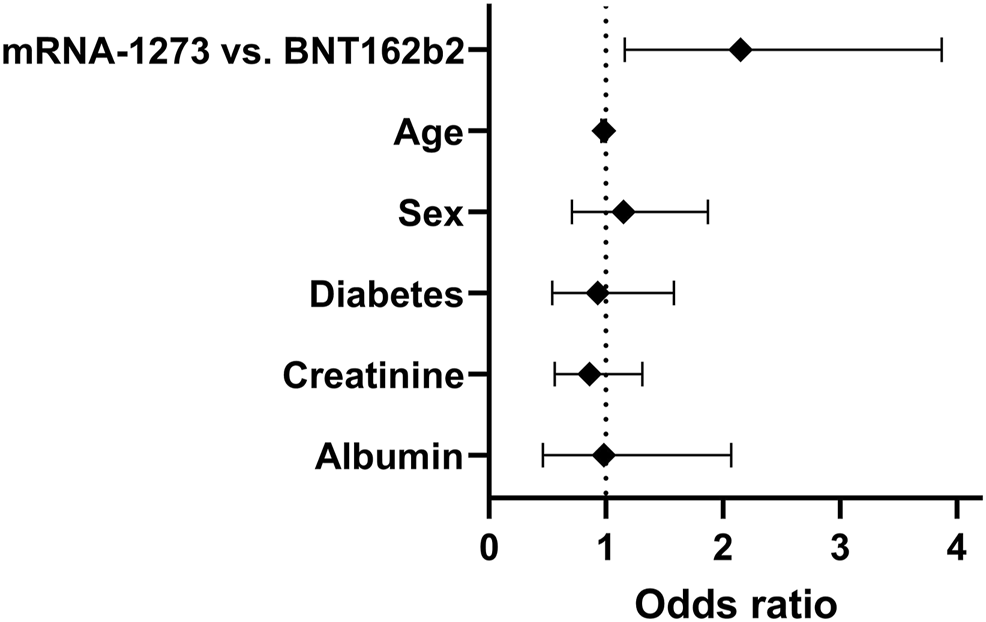
Odds ratios and 95% confidence intervals of a multivariate logistic regression analysis for anti-S-antibody seroconversion after 2 doses of SARS-CoV-2 mRNA vaccine in 320 prevalent kidney transplant patients irrespective of prior history of SARS-CoV-2 infection.
Reasons for a higher rate of seroconversion after mRNA-1273 vaccination compared to BNT162b2 are currently uncertain. Possible explanations include differences in mRNA content per vaccine dose, differences in mRNA modification or differences in the lipid formulation between the vaccines, all of which may influence expression of spike (S)-proteins and therefore immunogenicity. A limitation of this study is the lack of data in cellular immune responses, which may underestimate the immunogenicity of the vaccines. Another limitation is the retrospective nature of this study. However, similar results were recently reported in another observational study on immunogenicity of the mRNA-1273 and BNT162b2 vaccines in patients on renal replacement therapy (7), corroborating our findings.
In conclusion, vaccination with mRNA-1273 is associated with higher odds of anti-S-antibody seroconversion compared to vaccination with BNT162b2 in prevalent kidney transplant recipients.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the Johannes Kepler University Linz, Austria. The patients/participants provided their written informed consent to participate in this study.
Author contributions
Research idea and study design: MH, RK, PA, HK, and DC. Data acquisition: RK, SL, HK, CB, and DC. Data analysis/interpretation: MH, RK, SL, PA, HK, CB, and DC. Statistical analysis: MH and DC. Supervision or mentorship: DC. Each author contributed important intellectual content during manuscript drafting or revision and agrees to be personally accountable for the individual’s own contributions and to ensure that questions pertaining to the accuracy or integrity of any portion of the work, even one in which the author was not directly involved, are appropriately investigated and resolved, including with documentation in the literature if appropriate.
Acknowledgments
We thank Mrs. Julia Panholzer for valuable administrative assistance and the staff of the renal outpatient clinic of the OKL-Elisabethinen hospital for their support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/ti.2021.10026/full#supplementary-material
References
1.
Jager KJ Kramer A Chesnaye NC Couchoud C Sánchez-Álvarez JE Garneata Lu et al Results from the ERA-EDTA Registry Indicate a High Mortality Due to COVID-19 in Dialysis Patients and Kidney Transplant Recipients across Europe. Kidney Int (2020) 98(6):1540–8. 10.1016/j.kint.2020.09.006
2.
Grupper A Rabinowich L Schwartz D Schwartz IF Ben‐Yehoyada M Shashar M et al Reduced Humoral Response to mRNA SARS-CoV-2 BNT162b2 Vaccine in Kidney Transplant Recipients without Prior Exposure to the Virus. Am J Transpl Off J Am Soc Transpl Am Soc Transpl Surg. August (2021) 21(8):2719–26. 10.1111/ajt.16615
3.
Tsapepas D Paget K Mohan S Cohen DJ Husain SA . Clinically Significant COVID-19 Following SARS-CoV-2 Vaccination in Kidney Transplant Recipients. Am J Kidney Dis Off J Natl Kidney Found (2021) 78(2):314–7. 10.1053/j.ajkd.2021.05.004
4.
Caillard S Chavarot N Bertrand D Kamar N Thaunat O Moal V et al Occurrence of Severe COVID-19 in Vaccinated Transplant Patients. Kidney Int August (2021) 100(2):477–9. 10.1016/j.kint.2021.05.011
5.
National SARS-CoV-2 Serology Assay Evaluation Group. Performance Characteristics of Five Immunoassays for SARS-CoV-2: a Head-To-Head Benchmark Comparison. Lancet Infect Dis Dezember (2020) 20(12):1390–400. 10.1016/S1473-3099(20)30634-4
6.
Patel EU Bloch EM Clarke W Hsieh Y-H Boon D Eby Y . A. Comparative Performance of Five Commercially Available Serologic Assays to Detect Antibodies to SARS-CoV-2 and Identify Individuals with High Neutralizing Titers. J Clin Microbiol 21 Januar (2021) 59(2):e02257–20. 10.1128/jcm.02257-20
7.
Stumpf J Siepmann T Lindner T Karger C Schwöbel J Anders L . Humoral and Cellular Immunity to SARS-CoV-2 Vaccination in Renal Transplant versus Dialysis Patients: A Prospective, Multicenter Observational Study Using mRNA-1273 or BNT162b2 mRNA Vaccine. Lancet Reg Health Eur Oktober (2021) 9:100178. 10.1016/j.lanepe.2021.100178
Summary
Keywords
Covid-19, Sars-CoV-2, kidney transplantation, vaccination, mRNA vaccine
Citation
Haller MC, Kaiser RA, Langthaler S, Brandstetter C, Apfalter P, Kerschner H and Cejka D (2022) Comparison of mRNA-1273 and BNT162b2 SARS-CoV-2 mRNA Vaccine Immunogenicity in Kidney Transplant Recipients. Transpl Int 35:10026. doi: 10.3389/ti.2021.10026
Received
06 September 2021
Accepted
22 November 2021
Published
04 January 2022
Volume
35 - 2022
Updates
Copyright
© 2022 Haller, Kaiser, Langthaler, Brandstetter, Apfalter, Kerschner and Cejka.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniel Cejka, daniel.cejka@ordensklinikum.at
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.