Abstract
In the last few years, several studies have analyzed sex and gender differences in liver transplantation (LT), but none have performed a disaggregated analysis of both mortality and causes of death. Data from 15,998 patients, 11,914 (74.5%) males and 4,069 (25.5%) females, transplanted between 2000 and 2016 were obtained from the Liver Transplantation Spanish Registry. Survival analysis was applied to explore recipient sex as a risk factor for death. The causes of death at different follow-up duration were disaggregated by recipient sex for analysis. Short-term survival was higher in males, whereas long-term survival was higher in females. Survival at 1, 5 and 10 years post-transplant was 87.43%, 73.83%, and 61.23%, respectively, in males and 86.28%, 74.19%, and 65.10%, respectively, in females (p = 0.05). Post-LT mortality related to previous liver disease also presented sex differences. Males had 37% increased overall mortality from acute liver failure (p = 0.035) and 37% from HCV-negative cirrhosis (p < 0.001). Females had approximately 16% increased mortality when the liver disease was HCV-positive cirrhosis (p = 0.003). Regarding causes of death, non-malignancy HCV+ recurrence (6.3% vs. 3.9% of patients; p < 0.001), was more frequently reported in females. By contrast, death because of malignancy recurrence (3.9% vs. 2.2% of patients; p = 0.003) and de novo malignancy (4.8% vs. 2.5% of patients; p < 0.001) were significantly more frequent in male recipients. Cardiovascular disease, renal failure, and surgical complications were similar in both. In summary, male patients have lower short-term mortality than females but higher long-term and overall mortality. In addition, the post-LT mortality risk related to previous liver disease and the causes of mortality differ between males and females.

Introduction
Liver transplantation (LT) is the best treatment for patients with end-stage liver disease. Advances in surgical techniques and medical management of patients have markedly improved outcomes. However, short-term mortality remains at 10%–15%, and no clear improvement in long-term mortality has been achieved in the last few years (1). Interest in causes of mortality after LT and how they vary with time is increasing. Boganate et al. (2) described short-term mortality occurring mainly due to infections and circulatory disease in the first 90 days after LT. Regarding long-term mortality, Watt et al. (3) analyzed a large cohort of patients and concluded that the most frequent causes of death were graft failure, malignancy, cardiovascular disease, and kidney failure. Subsequent studies corroborated these findings and, in recent years, special programs have been launched for the early detection and prevention of cardiovascular and cancerous diseases (4,5).
Sex- and gender-disaggregated data analyses are important for reducing health inequities in medicine and many recent studies have analyzed sex and gender differences in liver disease. For example, sex imbalances in MELD predictor, waiting list mortality, and survival after LT have been studied (6–8). However, no studies have analyzed mortality after LT from the perspective of sex and gender. Gathering sex-disaggregated mortality data could provide even greater insight into differential outcomes that are associated with biological differences and behaviors linked to gender norms. These studies are very likely essential to improving outcomes and developing sex and gender-specific short- and long-term risk prevention policies.
Sex depends on biological attributes and gender refers to social roles, behaviors and constructed identities. Given that databases typically collect only sex-related data, we will focus our analysis on sex differences. Therefore, the objective of this study was to analyze sex differences in short- and long-term mortality after LT in a large cohort of patients with long-term follow-up. In addition, we analyzed the specific causes of death from the perspective of sex recipient and calculated the cumulative incidence of mortality from specific causes in relation to the follow-up.
Patients and Methods
Study Design and Population
We retrospectively explored data collected from the Spanish Liver Transplant Registry (Registro Español de Trasplante Hepático, RETH). RETH is a multicenter registry that recruits data from all liver transplant units in Spain with periodic auditing. The inclusion criteria were transplants performed on patients older than 16 years, from January 2000 to December 2016 with follow-up to November 2017. Multi-visceral transplantations were excluded.
Data from the 15,998 liver transplant recipients were stratified by sex on the characteristics age of recipient, MELD, donor sex, age of donor, number of transplants, type of transplant, cold ischemia time, presence of hepatitis C virus (HCV), presence of HIV, and main liver disease (acute liver failure, cholestasis, cirrhosis HCV+, cirrhosis HCV-, liver cancer, or other causes).
Causes of death were captured in the post-transplant period, and the number of deaths in different periods were stratified by sex and cause. Causes of death were classified into the following categories: surgical complications, infections, recurrence of HCV-positive liver disease, recurrence of HCV-negative liver disease, tumor recurrence, de novo malignancy, circulatory disease, kidney failure, de novo liver disease, rejection, and others.
Statistical Analysis
Data were descriptively analyzed; continuous variables were summarized as median and interquartile range and categorical variables as absolute and relative frequencies. Significant differences by sex of the recipient were established by the Mann-Whitney or chi-squared test as appropriate.
Survival analysis was applied to analyze recipient sex as a risk factor for overall mortality. Kaplan-Meier curves and log-rank tests were used to study the differences between male and females recipients. Regarding predictors of mortality, we used univariate and multivariate Cox proportional regression models to estimate the hazard ratios and 95% confidence intervals for prognostic variables in order to predict 1 month (early), 1 year (short-term), 5 years (long-term) and overall mortality. We also performed a sub-analysis to study differences between the sexes by main disease (acute liver failure, cholestasis, cirrhosis, cirrhosis HCV-positive, cirrhosis HCV-negative, liver cancer, or other causes), sex and age of the recipient, MELD, sex and age of the donor, and the presence of HIV in the recipient in order to predict mortality. The significance of the differences between male and female recipients was determined by a test of proportions.
The causes of death at different follow-up duration (from 1 to 10 years) were analyzed overall and disaggregated by recipient sex.
In addition, the relationship between cause of mortality and main disease was analyzed using a heatmap showing the correlation between groups of both variables by sex.
Analyses were performed using R v.4.0.3 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Clinical characteristics of the recipients by sex are shown in Table 1. Our dataset consisted of 11,914 (74.5%) males and 4,069 (25.5%) females, with a longer median follow-up in females (4.6 years vs 4.2, p = 0.009). The median age of patients at time of LT [55 (IQR 49–61) and 56 (IQR 46–62) years, respectively] was not different. Donor sex was predominantly male (61.1%) among male recipients and female (50.2%) among female recipients (p < 0.001). Donation after circulatory death was infrequent in this series (1.1% of male and 0.9% of female; p = 0.282). The median donor age was significantly lower for female recipients than for male recipients (55 (IQR 40–68) vs. 57 (IQR 43–69) years, p < 0.001) and the MELD value at LT was slightly higher for females than males) (18 (IQR 12–22) vs. 17 (IQR 12–21), p = 0.022). A total of 860 (7.8%) men and 359 (8.8%) women received more than one liver transplant (p = 0.003). Regarding the urgency of the procedure, 5.8% and 12.1% of liver transplant procedures were urgent in males and female, respectively (p < 0.001). The median cold ischemia time was 365 min (IQR 290–471) in males and 360 min (IQR 280–470) in females (p = 0.007). More males than females had HIV infection (2.47% vs 1.65%; p = 0.003), but no differences in HCV-related liver disease were found. Differences were found in the distribution of the main liver disease (Table 1). The most frequent diseases were HCV-positive liver cirrhosis in women and HCV-negative liver cirrhosis in men.
TABLE 1
| Feature/sex | Male (N = 11,914) | Female (N = 4,069) | p-value |
|---|---|---|---|
| Follow-up, years | 0.009 | ||
| Median (IQR) | 4.189 (1.170–8.832) | 4.630 (1.109–9.663) | |
| Age of recipients, years | 0.349 | ||
| Median (IQR) | 55 (49–61) | 56 (46–62) | |
| MELD | 0.022 | ||
| Median (IQR) | 17 (12–21) | 18 (12–22) | |
| Donor sex | <0.001 | ||
| Male | 4,624 (38.88%) | 2,022 (49.79%) | |
| Female | 7,270 (61.12%) | 2,039 (50.21%) | |
| Age of donors | <0.001 | ||
| Median (IQR) | 57 (43–69) | 55 (40–68) | |
| Number of transplants | 0.004 | ||
| 1 | 11,054 (92.78%) | 3,710 (91.18%) | |
| 2 | 801 (6.72%) | 326 (8.01%) | |
| 3 | 54 (0.45%) | 31 (0.76%) | |
| 4 | 5 (0.04%) | 2 (0.05%) | |
| Type of transplant | |||
| Elective | 11,102 (94.18%) | 3,551 (87.94%) | <0.001 |
| Urgent | 686 (5.82%) | 487 (12.06%) | |
| Ischemia time, minutes | 0.007 | ||
| Median (IQR) | 365 (290–471) | 360 (280–470) | |
| HCV | 4,349 (39.06%) | 1,460 (38.37%) | 0.460 |
| HIV | 294 (2.47%) | 67 (1.65%) | 0.003 |
| Main disease | <0.001 | ||
| Acute liver failure | 273 (2.49%) | 385 (10.36%) | |
| Cholestasis | 293 (2.67%) | 514 (13.83%) | |
| HCV-positive Cirrhosis | 2,585 (23.60%) | 991 (26.67%) | |
| HCV-negative Cirrhosis | 4,149 (37.87%) | 815 (21.93%) | |
| Liver cancer | 3,314 (30.25%) | 711 (19.13%) | |
| Other | 341 (3.11%) | 300 (8.07%) |
Clinical characteristics of the patients by sex.
Survival Analysis
Patient survival according to recipient sex showed small differences in the short- and long-term. Short-term survival was higher in males, whereas overall and long-term survival were higher in females. Male survival at 1, 5, and 10 years post-transplant was 87.43%, 73.82%, and 61.23%, respectively, while that of female patients was 86.28%, 74.20%, and 65.10%, respectively. Sex-based survival probability after transplant is depicted as a Kaplan-Meier curve in Figure 1, which also provides the number of patients at risk. As shown, survival curves intersect in the follow-up period and the log-rank test shown no statistical significant differences between groups (p = 0.05).
FIGURE 1
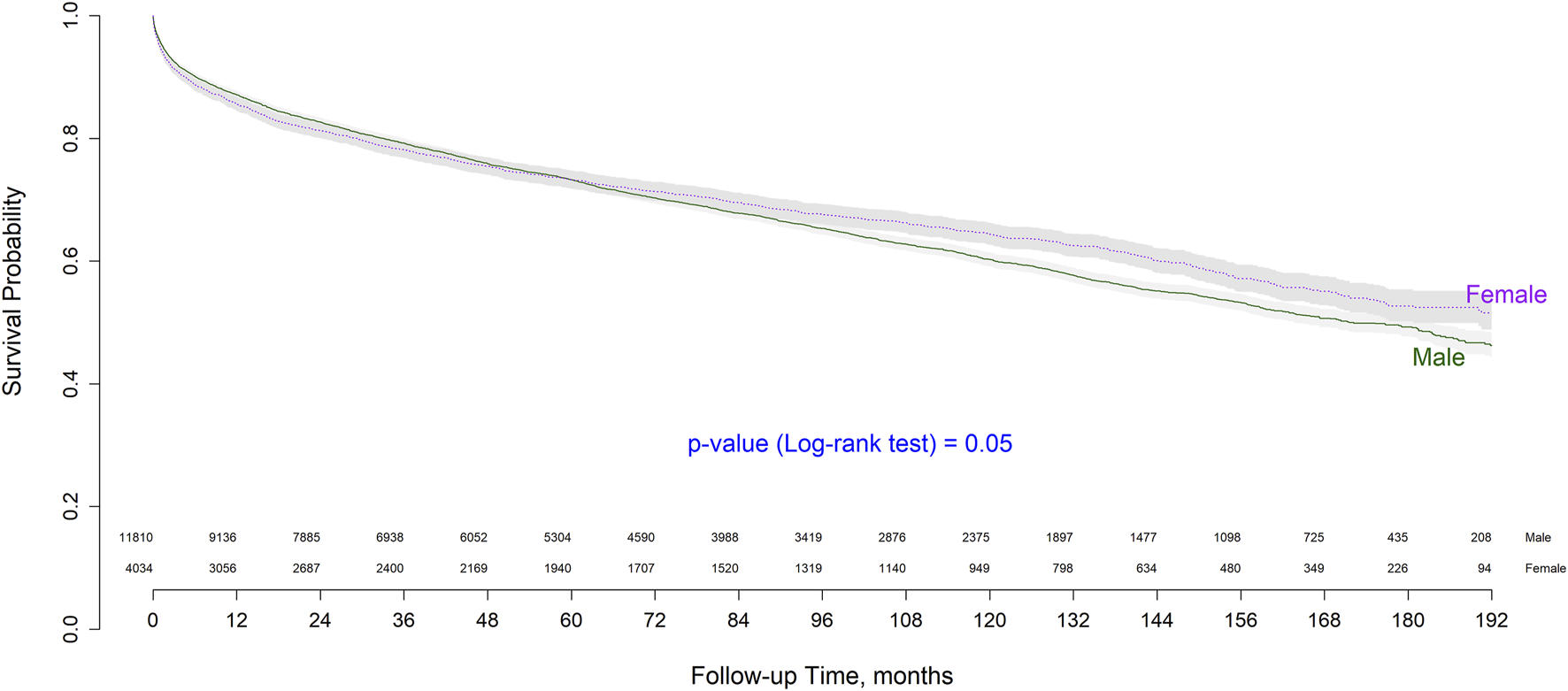
Kaplan-Meier overall survival curve by sex.
The analyses of recipient sex as a risk factor for mortality, or stratified by main disease are shown in Table 2. Female sex was found to be a risk factor for early (HR = 1.219, p = 0.019) and short term mortality (HR = 1.131, p = 0.014), while male was a risk factor (HR = 1.065, p = 0.050) for overall mortality, specifically when the main disease was acute liver failure (HR = 1.370, p = 0.035) and HCV-negative cirrhosis (HR = 1.375, p < 0.001). Male sex was a protective factor (HR = 0.884, p = 0.014), particularly when the main liver disease was HCV-positive cirrhosis (HR = 0.759; p = 0.002), In all other main liver diseases, no significant values were obtained. All of these results are depicted in detail in Table 2 and the forest plot in Figure 2.
TABLE 2
| Risk factor | 1-month hazard ratio (95% CI) | p-value | 1-year hazard ratio (95% CI) | p-value | 5-year hazard ratio (95% CI) | p-value | Overall hazard ratio (95% CI) | p-value |
|---|---|---|---|---|---|---|---|---|
| Sex (male:female) | 0.820 (0.695–0.968) | 0.019 | 0.884 (0.802–0.975) | 0.014 | 0.972 (0.902–1.046) | 0.445 | 1.065 (1.000–1.137) | 0.050 |
| Main disease (male:female) | ||||||||
| Acute liver failure | 1.811 (1.149–2.857) | 0.011 | 1.511 (1.061–2.152) | 0.022 | 1.549 (1,123–2,134) | 0.008 | 1.370 (1.023–1.836) | 0.035 |
| Cholestasis | 1.419 (0.821–2.452) | 0.210 | 1.345 (0.879–2.058) | 0.171 | 1.299 (0.911–1,852) | 0.148 | 1.295 (0.954–1.758) | 0.096 |
| HCV positive-Cirrhosis | 0.914 (0.657–1.272) | 0.594 | 0.759 (0.638–0.902) | 0.002 | 0.788 (0.692–0.897) | 0.003 | 0.842 (0.751–0.943) | 0.003 |
| HCV negative-Cirrhosis | 1.132 (0.740–1.730) | 0.568 | 1.153 (0.902–1.472) | 0.256 | 1.223 (1,011–1,478) | 0.038 | 1.375 (1.176–1.607) | <0.001 |
| Liver cancer | 0.665 (0.427–1.034) | 0.070 | 0.803 (0.637–1.010) | 0.061 | 0.867 (0.744–1,010) | 0.068 | 0.931 (0.815–1.065) | 0.299 |
| Other | 1.623 (0.803–3.280) | 0.177 | 1.782 (1.13–2.805) | 0.013 | 1.796 (1,233–2,615) | 0.002 | 1.691 (1.220–2.345) | 0.016 |
Survival analysis according to recipient sex considering the main liver diseases.
Comparison of 1-month, 1-year, 5-year, and overall.
FIGURE 2
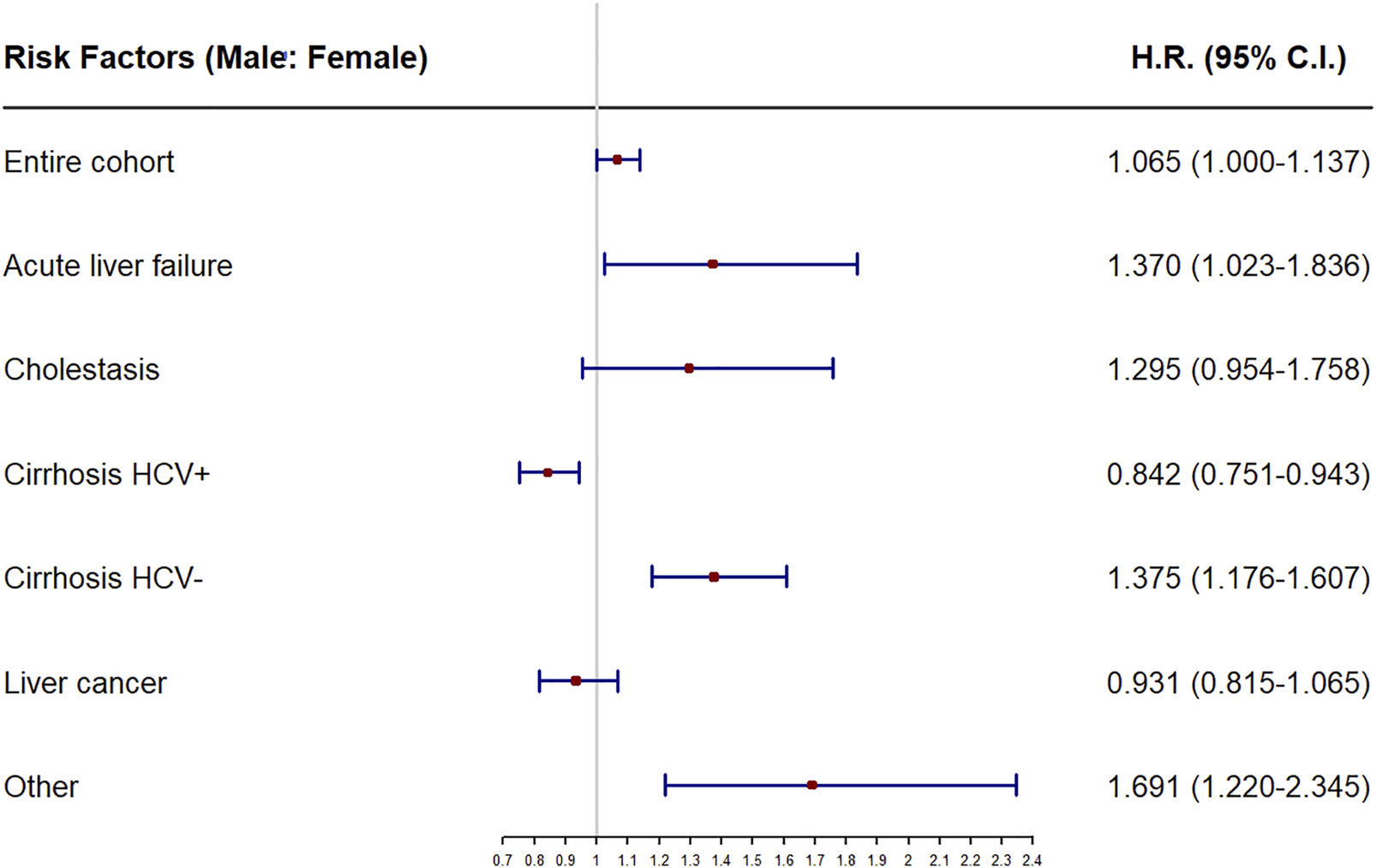
Forest plot of recipient sex as prognostic factor of overall mortality for the entire cohort and the subcohorts stratifying by main disease. HR, hazard ratios.
Regarding the interaction between sex of recipient and MELD, urgent transplantation, donor age, recipient age, cold ischemia time, and HIV positivity, results are shown in Table 3. MELD score was a predictive risk factor for early and overall mortality (HR = 1.030, p = 0.014 and HR = 1.013, p = 0.017, respectively), but the interaction of MELD with recipient sex was not significant, thus we did not found differences by sex in the association of MELD with mortality.
TABLE 3
| Risk factor | 1-month HR (95%CI) | p-value | 1-year HR (95%CI) | p-value | 5-year HR (95%CI) | p-value | Overall HR (95%CI) | p-value |
|---|---|---|---|---|---|---|---|---|
| Age of recipient | 0.986 (0.994–1.003) | 0.121 | 1.010 (1.006–1.015) | <0.001 | 1.016 (1.012–1.020) | <0.001 | 1,021 (1.018–1.025) | <0.001 |
| Age of recipient: Sex (female: male) | 1.004 (1.001–1.007) | 0.011 | 1.002 (1.001–1.004) | 0.007 | 1.001 (1.000–1.002) | 0.211 | 0,999 (0.997–1.000) | 0.106 |
| MELD | 1.030 (1.006–1.055) | 0.014 | 1.026 (1.012–1.040) | <0.001 | 1.015 (1.005–1.027) | 0.006 | 1.013 (1.001–1.024) | 0.017 |
| MELD: Sex (female: male) | 1.012 (0.995–1.030) | 0.164 | 1.003 (0.992–1.013) | 0.621 | 0.997 (0.988–1.006) | 0.473 | 0.998 (0.989–1.006) | 0.563 |
| Age of donor | 0.998 (0.994–1.003) | 0.508 | 1.005 (1.003–1.008) | <0.001 | 1.008 (1.006–1.010) | <0.001 | 1.010 (1.008–1.012) | <0.001 |
| Age of donor: Sex (female: male) | 1.004 (1.001–1.007) | 0.006 | 1.002 (1.001–1.004) | 0.004 | 1.001 (1.000–1.003) | 0.045 | 1 (0.998–1.001) | 0.633 |
| HIV (positive: negative) | 0.923 (0.508–1.678) | 0.793 | 0.932 (0.641–1.360) | 0.714 | 1.080 (0.882–1.323) | 0.455 | 1.266 (1.038–1.544) | 0.020 |
| HIV = positive: Sex (female: male) | 1.174 (0.328–4.209) | 0.805 | 1.056 (0.461–2.418) | 0.897 | 0.735 (0.445–1.217) | 0.232 | 0.769 (0.476–1.243) | 0.284 |
| Type of transplant (urgent: elective) | 0.971 (0.612–1.542) | 0.900 | 1.123 (0.879–1.434) | 0.353 | 1.167 (1.012–1.340) | 0.029 | 1.571 (1.386–1.780) | <0.001 |
| Type of transplant = urgent: Sex (female: male) | 2.173 (1.219–3.876) | 0.009 | 1.311 (0.932–1.844) | 0.120 | 1.072 (0.875–1.313) | 0.502 | 0.662 (0.538–0.816) | <0.001 |
| Ischemia time | 1.001 (1–1.001) | <0.001 | 1 (1–1.001) | 0.002 | 1 (1–1.001) | <0.001 | 1.0003 (1–1,001) | <0.001 |
| Ischemia time: Sex (female: male) | 1 (1–1.001) | 0.081 | 1 (1–1.001) | 0.057 | 1 (0.999–1) | 0.542 | 0.999 (0.998–1) | 0.067 |
Interaction of main risk factors with recipient sex.
Each row shows the hazard ratio (HR) associated with each risk factor, and below is the HR added to that risk factor because of an interaction with recipient sex.
Other potential risk factors and their interaction with recipient sex were also analyzed. Recipient sex showed a significant interaction with the age of the recipient (HR = 1.004, p = 0.011), age of the donor (HR = 1.004, p = 0.006) and urgency of transplant (HR = 2.173, p = 0.009) on early mortality. Regarding overall mortality we only found a significant interaction with recipient sex in the urgency of transplant (HR = 0.662, p < 0.001). All of these results are depicted in detail in Table 3.
In the multivariate analysis for the predictors of overall mortality (Table 4), recipient and donor age, number of transplants and the presence of HCV remained as independent predictors of mortality in both sexes while MELD was, prognostic factor only in the male population.
TABLE 4
| Risk Factor | Male (C-index = 0.60) | Female (C-index = 0.64) | ||
|---|---|---|---|---|
| Coefficient (95% CI) | p-value | Coefficient (95% CI) | p-value | |
| Age of recipient | 1.388 (1.221–1.577) | <0.001 | 1.263 (1.144–1.393) | <0.001 |
| MELD | 1.210 (1.080–1.356) | <0.001 | — | n.s. |
| Age of donor | 1.321 (1.158–1.507) | <0.001 | 1.480 (1.350–1.624) | <0.001 |
| Number of retransplants transplants= 1 | Ref | — | Ref | — |
| 2 | 2.132 (1.587–2.862) | <0.001 | 1.746 (1.434–2.126) | <0.001 |
| ≥3 | 11.834 (3.977–35.209) | <0.001 | 5.953 (3.768–9.405) | <0.001 |
| HCV-negative | Ref | — | ref | — |
| HCV-positive | 1.524 (1.291–1.789) | <0.001 | 1.964 (1.738–2.219) | <0.001 |
Multivariate Cox regression model of long-term mortality prognosis.
ref, reference category; n.s., non significant.
In Table 5, we show the results for the predictors of early mortality (1 month); number of transplants was an independent prognostic factor for mortality in both men and women. In addition recipient age, ischemia time and main disease were risk factors in the male population whereas MELD was a risk factor among females.
TABLE 5
| Risk Factor | Male (C-index = 0.68) | Female (C-index = 0.67) | ||
|---|---|---|---|---|
| Coefficient (95% CI) | p-value | Coefficient (95% CI) | p-value | |
| Age of recipient | 1.220 (1.074–1.387) | 0.002 | — | n.s. |
| MELD | — | n.s | 1.587 (1.103–2.285) | 0.010 |
| Number of retransplant transplants | ||||
| 1 | ref | — | Ref | — |
| 2 | 3.665 (2.834–4.731) | <0.001 | 2.935 (1.230–7.004) | 0.010 |
| ≥3 | 6.048 (2.831–12.923) | <0.001 | 36.123 (10.986–118.780) | <0.001 |
| Ischemia time | 1.226 (1.116–1,345) | <0.001 | — | n.s. |
| Main Disease | ||||
| Acute liver failure | 5.646 (3.779–8.437) | <0.001 | ref | — |
| Cholestasis | 2.661 (1.662–4.263) | <0.001 | — | n.s. |
| HCV positive -Cirrhosis | 1.451 (1.124–1.874) | 0.004 | — | n.s. |
| HCV negative-Cirrhosis | ref | — | — | n.s. |
| Liver cancer | 0.680 (0.507–0.911) | <0.001 | — | n.s. |
| Other | 2.235 (1.380–3.620) | <0.001 | — | n.s. |
Multivariate Cox regression model of early (1-month) mortality prognosis.
ref, reference category; n.s., non-significant.
Mortality Analysis
Mortality and overall causes of death are shown in Table 6. In our cohort, a total of 3,723 (31.5%) male patients and 1,241 (30.8%) female patients died, with important differences in the causes of mortality. The different causes of death throughout the follow-up and according to recipient sex are shown in Table 7. Surgical complications, infections, and cardiovascular diseases were the most frequent causes of mortality in the short-term while infections, recurrence of HCV-positive liver disease, and de novo malignancy were the most frequent causes of mortality in the long-term.
TABLE 6
| Feature/Sex | Male (N = 11,914) | Female (N = 4,069) | p-value |
|---|---|---|---|
| Mortality (≤ 1 year) | 1,543 (12.31%) | 553 (13.71%) | 0.023 |
| Mortality (≤3 years) | 2,214 (18.75%) | 801 (19.86%) | 0.127 |
| Mortality (≤ 5 years) | 2,692 (22.79%) | 943 (23.38%) | 0.461 |
| Mortality (≤ 10 years) | 2,908 (28.96%) | 1,126 (27.91%) | 0.212 |
| Mortality (overall) | 3,723 (31.52%) | 1,241 (30.76%) | 0.379 |
| Cause of death (overall) | <0.001 | ||
| Surgical complications | 306 (8.37%) | 104 (8.54%) | |
| Infection | 684 (18.70%) | 282 (23.15%) | |
| Rejection | 40 (1.09%) | 18 (1.48%) | |
| Non-malignancy recurrence HCV+ | 455 (12.44%) | 252 (20.69%) | |
| Non-malignancy recurrence HCV− | 62 (1.70%) | 33 (2.71%) | |
| De novo liver disease | 145 (3.96%) | 48 (3.94%) | |
| Cardiovascular disease | 370 (10.12%) | 116 (9.52%) | |
| Malignancy recurrence | 459 (12.55%) | 88 (7.22%) | |
| De novo malignancy | 561 (15.34%) | 101 (8.29%) | |
| Renal failure | 39 (1.07%) | 14 (1.15%) | |
| Other causes | 536 (14.66%) | 162 (13.30%) |
Mortality and overall causes of death disaggregated by recipient sex.
Significant p values are shown in bold.
TABLE 7
| Cause of death/follow-up | 1 year | 3 years | 5 years | 10 years | Overall (>10 years) | |
|---|---|---|---|---|---|---|
| Surgical complication | O | 1.98% | 2.27% | 2.41% | 2.55% | 2.59% |
| M | 1.94% | 2.23% | 2.38% | 2.55% | 2.59% | |
| F | 2.11% | 2.41% | 2.48% | 2.55% | 2.58% | |
| p-value | 0.956 | 0.451 | 0.413 | 0.728 | 0.386 | |
| Infection | O | 4.03% | 4.73% | 5.14% | 5.78% | 6.07% |
| M | 3.80% | 4.45% | 4.85% | 5.46% | 5.79% | |
| F | 4.69% | 5.53% | 5.97% | 6.69% | 6.99% | |
| p-value | 0.082 | 0.002 | <0.001 | <0.001 | 0.186 | |
| Rejection | O | 0.30% | 0.32% | 0.35% | 0.36% | 0.37% |
| M | 0.27% | 0.29% | 0.32% | 0.33% | 0.34% | |
| F | 0.37% | 0.42% | 0.45% | 0.45% | 0.45% | |
| p-value | 0.526 | 0.233 | 0.228 | 0.287 | 0.205 | |
| Non-malignancy recurrence HCV+ | O | 1.21% | 2.52% | 3.24% | 4.13% | 4.46% |
| M | 1.01% | 2.20% | 2.79% | 3.63% | 3.85% | |
| F | 1.79% | 3.45% | 4.56% | 5.60% | 6.25% | |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Non-malignancy recurrence HCV− | O | 0.04% | 0.14% | 0.27% | 0.47% | 0.60% |
| M | 0.03% | 0.12% | 0.22% | 0.39% | 0.53% | |
| F | 0.07% | 0.20% | 0.40% | 0.69% | 0.82% | |
| p-value | 0.593 | 0.332 | 0.058 | 0.012 | 0.671 | |
| De novo liver disease | O | 0.44% | 0.80% | 0.95% | 1.13% | 1.22% |
| M | 0.43% | 0.78% | 0.96% | 1.15% | 1.23% | |
| F | 0.47% | 0.84% | 0.94% | 1.07% | 1.19% | |
| p-value | 1.000 | 0.703 | 0.890 | 0.917 | 0.417 | |
| Cardiovascular disease | O | 1.33% | 1.79% | 2.08% | 2.71% | 3.07% |
| M | 1.38% | 1.84% | 2.14% | 2.82% | 3.13% | |
| F | 1.19% | 1.64% | 1.91% | 2.38% | 2.88% | |
| p-value | 0.2% | 0.517% | 0.707% | 0.295% | 1 | |
| Malignancy recurrence | O | 0.64% | 2.03% | 2.82% | 3.36% | 3.45% |
| M | 0.74% | 2.27% | 3.16% | 3.77% | 3.89% | |
| F | 0.37% | 1.34% | 1.83% | 2.16% | 2.18% | |
| p-value | 0.007 | <0.001 | <0.001 | <0.001 | 0.003 | |
| De novo malignancy | O | 0.35% | 1.30% | 2.05% | 3.48% | 4.18% |
| M | 0.42% | 1.51% | 2.40% | 4.01% | 4.75% | |
| F | 0.15% | 0.69% | 1.02% | 1.93% | 2.50% | |
| p-value | 0.010 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Renal failure | O | 0.16% | 0.21% | 0.22% | 0.29% | 0.34% |
| M | 0.16% | 0.20% | 0.22% | 0.30% | 0.33% | |
| F | 0.17% | 0.22% | 0.22% | 0.27% | 0.35% | |
| p-value | 1.000 | 0.934 | 1.000 | 1.000 | 0.246 | |
| Other causes | O | 1.97% | 2.57% | 3.00% | 3.95% | 4.41% |
| M | 1.97% | 2.56% | 2.98% | 4.09% | 4.54% | |
| F | 1.96% | 2.60% | 3.05% | 3.55% | 4.02% | |
| p-value | 0.589 | 0.793 | 0.454 | 0.307 | 0.506 |
Cause of death during follow-up by recipient sex. Data are reported as % over the entire dataset.
O, overall population; M, male population; F, female population. Significant p values are shown in bold.
By sex, the main causes of death were infections and non-malignancy HCV-positive recurrence in females (23.2% and 20.7% of events) and infections and de novo malignancy in males (18.7% and 15.3% of events).
The cumulative relative frequency of different causes of death for male and female recipients are presented in Figure 3. Non malignancy HCV-positive recurrence (6.3% vs 3.9% of patients; p < 0.001) was more frequent in female than male recipients. By contrast, death because of malignancy recurrence (3.9% vs 2.2%; p = 0.003) and de novo malignancy (4.8% vs 2.5%; p < 0.001) were significantly more frequent in male recipients. In turn, cardiovascular disease, renal failure, recurrence of HCV-negative liver disease and surgical complications were similarly distributed as causes of death in men and women. Importantly though, 35% of women and only in 11.7% of men with mortality due to recurrence of HCV-negative disease had been transplanted for a cholestastic disease (p < 0.0001).
FIGURE 3
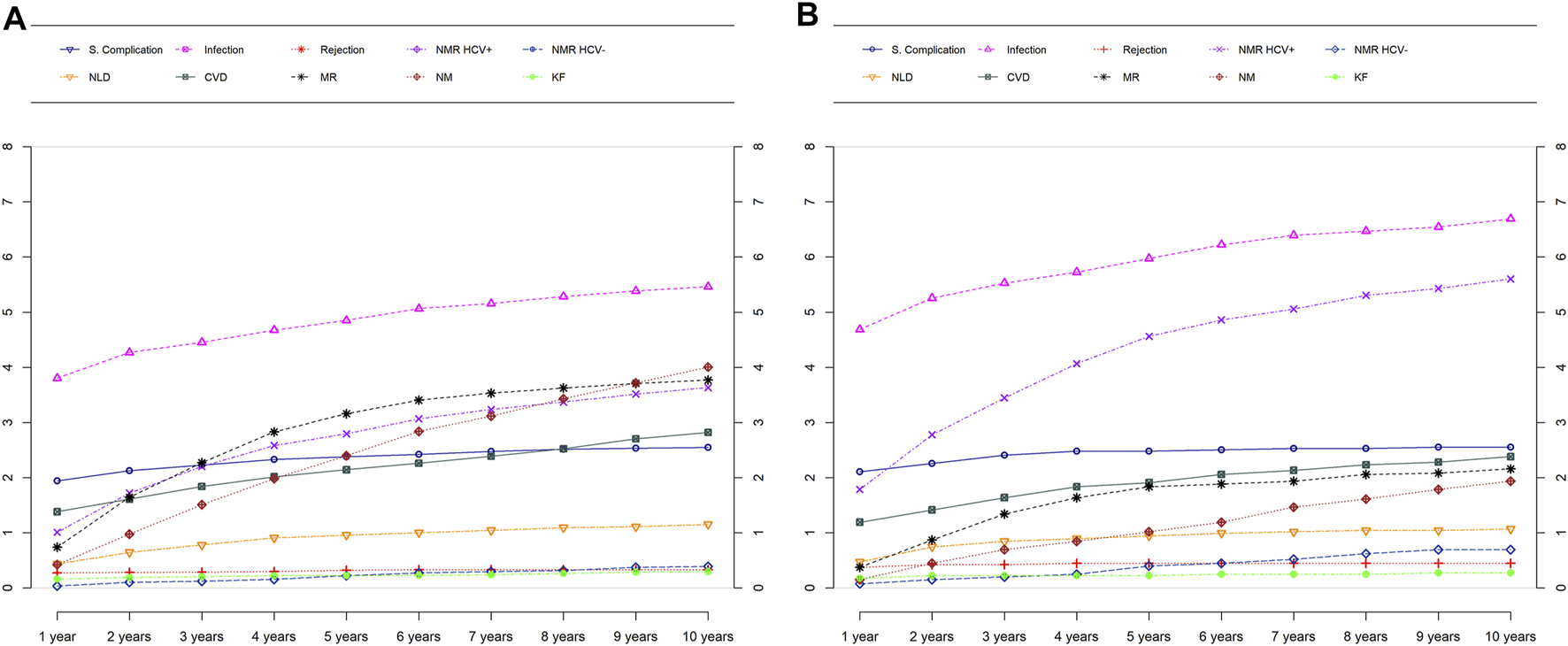
Causes of death over time by sex in males (A) and females (B). NMR, Non-malignancy recurrence; NLD, De Novo liver disease; CVD, Cardiovascular disease; MR, Malignancy recurrence; NM, De Novo malignancy; KF, Kidney failure.
We illustrate the relationship between causes of mortality and main diseases by sex in Figure 4. A heat map shows the differences by sex in the correlation between mortality and main disease; differences can be appreciated in the gradation of the color scale by sex.
FIGURE 4
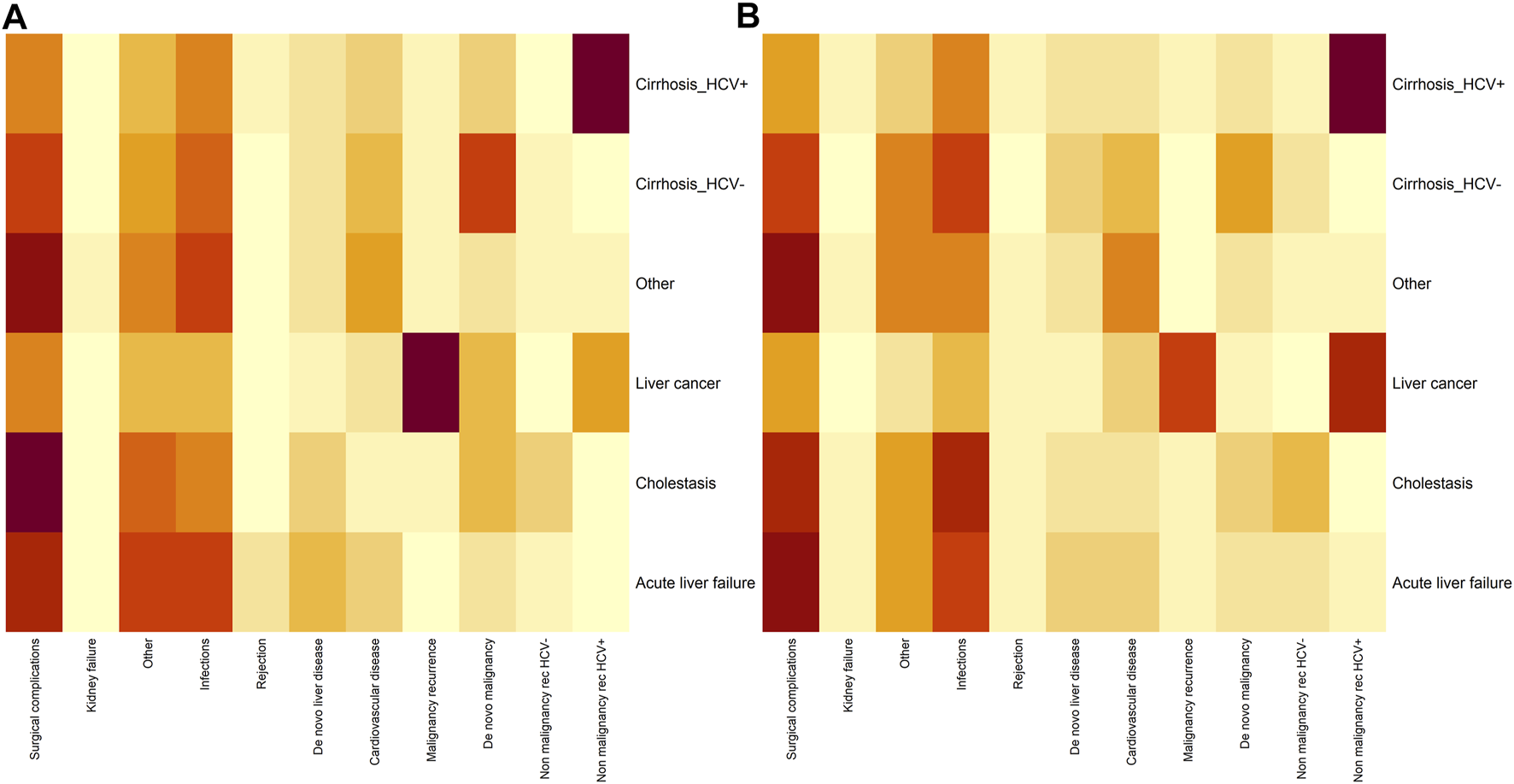
Heatmap analyzing the dependence between main disease (Y axis) and cause of mortality (X axis) in males (A) and females (B) Darker gray reflects a stronger relationship between the main disease and the cause of mortality.
Discussion
Our study analyzed mortality data disaggregated by sex after LT in a very large sample of patients with long follow-up. We found that patient survival varies significantly according to recipient sex and the time after LT. Male patients have lower short-term mortality than females but higher long-term and overall mortality. In addition, the post-LT mortality risk related to previous liver disease is different between male and female patients, with different causes of mortality.
Differences in Survival After Liver Transplantation
Our data show that although women have a significantly increased risk of early mortality after LT, with an overall 18% higher probability of dying in the first month after LT than males, they have better long-term survival, with males having a 6% overall higher probability of dying compared to females. A recent study based on the European Transplant Registry reported longer survival of transplanted women but did not find differences in short-term survival (9). In contrast, similar to the present study, Bruns et al. (10) reported higher mortality in women in the short-term after LT (OR 3.2), particularly among women with high MELD scores. Our results do not show a different impact of MELD according to sex on short-term mortality.
The multivariate analysis of risk factors for overall mortality found similar prognostic factors, with few exceptions. This may indicate that other factors not included in our registry, such as previous comorbidities and lifestyle, likely play an important role in mortality, mainly in the long-term.
Mortality Risk Related to Previous Liver Disease
On the other hand, there are important differences in the etiology of liver diseases (11) that may explain, in part, some of the differences in mortality. Different liver diseases have different outcomes after LT, but the role of sex in the prognosis of these diseases has not been thoroughly evaluated. Our findings demonstrate that differences exist in this context. For example, males have 50% increased 1-year mortality when LT is performed for acute liver failure and 37% increased overall mortality when it is due to HCV-negative cirrhosis, whereas females have approximately 15% increased overall mortality when the liver disease is HCV-positive cirrhosis. This finding was expected because more severe HCV recurrence and related mortality has been described in women after LT (12–14). However, HCV-related outcomes, including LT, have changed dramatically since the emergence of new antivirals (15). Data collection in our study extended until 2017, so the effect of these drugs on survival could not be observed, but it will undoubtedly be demonstrated in the analysis of subsequent years.
Conversely, outcomes of HCV-negative cirrhosis are worse in male than in female patients. In Spain, the leading etiology in patients with HCV-negative cirrhosis is alcohol related liver disease (ALD) (16). Tobacco use, sedentary lifestyle, and unhealthy diet are often associated with alcohol consumption, and all of them are risk factors for both cardiovascular and cancer mortality. ALD patients have been shown to have excess all-cause mortality, mainly mortality related to cardiovascular disease and cancer (17), and this excess mortality is higher in males than in females. In a large Danish cohort, Salhman et al. (18) found a significant excess of different cancers in males with ALD, with an overall standardized incidence ratio of 3.01 in males and 2.33 in females (p < 0.001). Other findings, such as higher mortality risk in transplanted males due to acute liver failure, are more unexpected. Although acute liver failure affects females more than males and it is also associated with lower short-term survival after LT, men have a greater probability of dying when being transplanted because of this indication. Several studies have investigated the outcomes of LT in patients with acute liver failure in Western countries (19,20), but only Nephew et al. analyzed mortality according to recipient sex in the UNOS database (21). They found differences in 1-year mortality, which was no longer significant when recipient age and underlying etiology were added to the model.
Our data combined with prior studies demonstrate that mortality risk after LT related to different liver diseases varies according to sex. This is an important finding that should be considered when designing post-LT survival models.
Causes of Mortality
Though causes of mortality have been described throughout the transplant follow-up, no sex-disaggregated analysis has been published previously. As in the non-transplanted population, there are important differences in the causes of mortality between men and women. Overall, infections are the most frequent cause of mortality in males and females, though they are significantly higher in females.
In our cohort, the main causes of mortality within the first year after transplantation were infections and surgical complications in both sexes. Although females were more frequently retransplanted, mortality due to surgical complications was similar in both. In contrast, death related to infections was significantly more common in females than in males and was evenly distributed across the different causes of liver disease, except for liver cancer. This may be explained by the clinical situation at the time of LT, crucial in explaining mortality from infections in the short-term (22). Differences in the prevalence and severity of infections between males and females vary depending on type of infection (23). Women have higher mortality in influenza A outbreaks (23), whereas male sex is a risk factor for developing severe SARS Cov-2 infection or sepsis (24,25). It seems that both immunological and hormonal factors play a role in these differences.
More differences were found in short-term mortality. Mortality because of recurrence of HCV infection was significantly higher in females, and mortality due to recurrence of hepatocarcinoma and de novo cancer was more frequent in males.
These differences increased with follow-up, so that in the long-term (>10 years), mortality due to infections, including HCV recurrence, was 40% higher in women than in men and mortality due to de novo neoplasms was almost twice as high in men as in women. Though the latter accounted for more than 15% of mortality in males, it accounted for only 8.3% in females. When we added mortality because of tumor recurrence, cancer was the leading cause of overall mortality in males, accounting for 27.9% of events and a cumulative relative frequency of 8.6% of patients, but it was the third leading cause of death in women (15.5% of events) and approximately half of the cumulative relative frequency. Hepatocellular carcinoma (HCC) is overrepresented in males, resulting in a higher number of deaths because of HCC recurrence among this population. Nevertheless, higher recurrence risk was also recently described among males. Cullaro et al. found an independent effect of sex on the risk of HCC recurrence post-LT (26). Mortality because of liver cancer recurrence increases in the first 6 years after LT and subsequently stabilizes, whereas mortality due to de novo cancer follows an upward trend over time.
Circulatory diseases and kidney disease are important, but not different causes of death after LT in men and women. Approximately 3% of patients globally die from circulatory disease after LT and slightly more than a third of them die in the first year after LT. A careful analysis of cardiovascular risk factors before transplantation is mandatory, as detecting patients at risk of early mortality from circulatory disease is important to avoid futile transplantation.
As expected, we found an association between some causes of mortality and certain liver diseases prior to LT. For women, the strongest association was found between acute liver failure and mortality due to surgical complications. HCV cirrhosis was associated with mortality due to non-tumor recurrence in both men and women. However, when the transplant was due to liver cancer, the strongest association was found between mortality due to tumor recurrence in men and non-tumor recurrence in women.
Mortality in LT patients is mainly related to immunosuppression. Both infections and cancer, two sides of the same coin, are related to immunosuppressive treatment. However, our data show that they are distributed differently in both sexes. Though infections result in higher mortality among females, neoplasms affect predominantly males. Knowledge of these differences is important to improve the management of patients in both the short- and long-term. In recent years, special immunosuppression protocols and surveillance programs have been proposed for the prevention or early detection of de novo cancer (5, 27). These results could be important to designing suitable and more cost-effective protocols according to the sex of the recipient.
Finally, although it was not the objective of our research, the imbalance found between male and female transplant recipients is remarkable. Many end-stage liver diseases affect predominantly males, and sex differences among transplant patients have been increasing over the years. From 2000 to 2016, only j 25.5% of LT patients were female. Sex differences in our registry are higher than described in other registries (9,28). These differences could reflect disparities in listing patients or in waiting-list mortality (8, 29,30). Further studies are needed to clarify this. LT is a medical process strongly influenced by sex and gender issues such that disaggregated analyses at all levels of the procedure should be mandatory to avoid disparities.
The limitations of the present study are mainly derived from its retrospective nature. Although the data entered in RETH were standardized and periodically audited, the information, as well as the consistency between sites, cannot be guaranteed. As with most studies using data from record collections, the current study may have been susceptible to practice variations and incompletely reported covariates. In addition, the definitions for causes of death may vary due to different interpretations between different teams. However, the data source is a national registry with a large number of cases that allows robust statistical analyses of a nationally representative dataset. On the other hand, due to the difficulty of national registries to rapidly adapt to changing epidemiological scenarios, we have not been able to analyze the impact of new diseases such as non-alcoholic steatohepatitis (NASH) on post-LT prognosis and causes of death. Thus, sex differences in this increasingly important disease could not be analyzed.
In summary, short- and long-term mortality and their causes are different between male and female liver transplant recipients. The risk of mortality after LT associated with different liver diseases also varies by sex. These findings are important and highlight the need for sex and gender-disaggregated analyses of clinical data.
Spanish Liver Transplant Registry Representatives
Carolina Almohalla, Gerardo Blanco, Andrea Boscà, Federico Castillo, Ramón Charco, Valentín Cuervas-Mons, Juan Fabregat, Carmen García, Miguel Ángel Gómez, Loreto Hierro, Carmelo Loinaz, Enrique Moneva, Antonio Poyato, José Ignacio Rivas, Gonzalo Rodríguez, Fernando Rotellar, Francisco Sánchez, Julio Santoyo, Santiago Tomé, Andrés Valdivieso, Juan José Vila, Jesús Villar
Statements
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: Are datasets belonging to Spanish Liver Transplant Society and managed and administered by the National Transplant Organization. Requests to access these datasets should be directed to www.ont.es.
Author contributions
Conception or design of the work: MTS, SS, LME and MS. Data collection: SL and LC. Data analysis and interpretation: MTS, SS, LME and MS. Drafting the article: MTS, SS, LME, MS, SL and LC. Critical revision of the article: MB, CF, GS-A, JN, GR. Final approval of the version to be published: MTS, SS, LME, MB, CF, SL, LC, GS-A, JN, GR and MS.
Funding
This work was supported by RIS3 program (LMP223-18), Gobierno de Aragon and FSETH (Fundación Sociedad Española de Trasplante Hepático).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
ALD, alcoholic liver disease; CVD, cardiovascular disease; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HR, hazard ratio; IQR, interquartile range; KF, kidney failure; LT, liver Transplantation; MELD, model for end-stage liver disease; MR, malignancy recurrence; NLD, De Novo liver disease; NM, De Novo malignancy; NMR, non-malignancy recurrence; OR, odds ratio; RETH, Registro Español de Trasplante Hepático.
References
1.
Durand F . How to Improve Long-Term Outcome after Liver Transplantation?Liver Int (2018) 38(Suppl. 1):134–8. 10.1111/liv.13651
2.
Baganate F Beal EW Tumin D Azoulay D Mumtaz K Black SM et al Early Mortality after Liver Transplantation: Defining the Course and the Cause. Surgery (2018) 164:694–704. 10.1016/j.surg.2018.04.039
3.
Watt KDS Pedersen RA Kremers WK Heimbach JK Charlton MR . Evolution of Causes and Risk Factors for Mortality Post-Liver Transplant: Results of the NIDDK Long-Term Follow-Up Study. Am J Transpl (2010) 10:1420–7. 10.1111/j.1600-6143.2010.03126.x
4.
Konerman MA Fritze D Weinberg RL Sonnenday CJ Sharma P . Incidence of and Risk Assessment for Adverse Cardiovascular Outcomes after Liver Transplantation. Transplantation (2017) 101:1645–57. 10.1097/tp.0000000000001710
5.
Herrero JI Alegre F Quiroga J Pardo F Iñarrairaegui M Sangro B et al Usefulness of a Program of Neoplasia Surveillance in Liver Transplantation. A Preliminary Report. Clin Transpl (2009) 23:532–6. 10.1111/j.1399-0012.2008.00927.x
6.
Melk A Babitsch B Borchert-Mörlins B Claas F Dipchand AI Eifert S et al Equally Interchangeable? How Sex and Gender Affect Transplantation. Transplantation (2019) 103:1094–110. 10.1097/tp.0000000000002655
7.
Lai JC Terrault NA Vittinghoff E Biggins SW . Height Contributes to the Gender Difference in Wait-List Mortality under the MELD-Based Liver Allocation System. Am J Transpl (2010) 10:2658–64. 10.1111/j.1600-6143.2010.03326.x
8.
Serrano T Berenguer M . Gender Disparities in Liver Transplantation. In: RahimiRS, editor. The Critically Ill Cirrhotic Patient. Switzerland: Springer Nature (2020).
9.
Germani G Zeni N Zanetto A Adam R Karam V Belli LS et al Influence of Donor and Recipient Gender on Liver Transplantation Outcomes in Europe. Liver Int (20202020) 40(40):1961–71. 10.1111/liv.14510
10.
Bruns H Lozanovski VJ Schultze D Hillebrand N Hinz U Büchler MW et al Prediction of Postoperative Mortality in Liver Transplantation in the Era of MELD-Based Liver Allocation: a Multivariate Analysis. PLoS One (2014) 9:e98782. 10.1371/journal.pone.0098782
11.
Burra P Martin ED Gitto S Villa E . Influence of Age and Gender before and after Liver Transplantation. Liver Transpl (2013) 19:122–34. 10.1002/lt.23574
12.
Lai JC Verna EC Brown RS Jr O'Leary JG Trotter JF Forman LM et al Hepatitis C Virus-Infected Women Have a Higher Risk of Advanced Fibrosis and Graft Loss after Liver Transplantation Than Men. Hepatology (2011) 54:418–24. 10.1002/hep.24390
13.
Belli LS Burroughs AK Burra P Alberti AB Samonakis D Cammà C et al Liver Transplantation for HCV Cirrhosis: Improved Survival in Recent Years and Increased Severity of Recurrent Disease in Female Recipients: Results of a Long Term Retrospective Study. Liver Transpl (2007) 13:733–40. 10.1002/lt.21093
14.
Belli LS Romagnoli R Nardi A Marianelli T Donato F Corradini SG et al Recipient Female Gender Is a Risk Factor for Graft Loss after Liver Transplantation for Chronic Hepatitis C: Evidence from the Prospective Liver Match Cohort. Dig Liver Dis (2015) 47:689–94. 10.1016/j.dld.2015.04.006
15.
Arora SS Axley P Ahmed Z Satapathy SK Wong R Kuo YF et al Decreasing Frequency and Improved Outcomes of Hepatitis C-Related Liver Transplantation in the Era of Direct-Acting Antivirals - a Retrospective Cohort Study. Transpl Int (2019) 32:854–64. 10.1111/tri.13424
16.
Cuervas-Mons V de la Rosa G Pardo F San Juan F Valdivieso A en representación del Registro Español de Trasplante Hepático. Activity and Results of Liver Transplantation in Spain during 1984-2012. Analysis of the Spanish Liver Transplant Registry. Medicina Clínica (English Edition) (2015) 144:337–47. 10.1016/j.medcle.2015.11.011
17.
Sahlman P Nissinen M Pukkala E Färkkilä M . Incidence, Survival and Cause-specific Mortality in Alcoholic Liver Disease: a Population-Based Cohort Study. Scand J Gastroenterol (2016) 51:961–6. 10.3109/00365521.2016.1157889
18.
Sahlman P Nissinen M Pukkala E Färkkilä M . Cancer Incidence Among Alcoholic Liver Disease Patients in Finland: A Retrospective Registry Study during Years 1996-2013. Int J Cancer (2016) 138:2616–21. 10.1002/ijc.29995
19.
Reddy KR Ellerbe C Schilsky M Stravitz RT Fontana RJ Durkalski V et al Determinants of Outcome Among Patients with Acute Liver Failure Listed for Liver Transplantation in the United States. Liver Transpl (2016) 22:505–15. 10.1002/lt.24347
20.
Escorsell À Mas A de la Mata M . Acute Liver Failure in Spain: Analysis of 267 Cases. Liver Transpl (2007) 13:1389–95. 10.1002/lt.21119
21.
Nephew LD Zia Z Ghabril M Orman E Lammert C Kubal C et al Sex Disparities in Waitlisting and Liver Transplant for Acute Liver Failure. JHEP Rep (2021) 3:100200. 10.1016/j.jhepr.2020.100200
22.
Fishman JA . Infection in Solid-Organ Transplant Recipients. N Engl J Med (2007) 357:2601–14. 10.1056/nejmra064928
23.
Ruggieri A Anticoli S D'Ambrosio A Giordani L Viora M . The Influence of Sex and Gender on Immunity, Infection and Vaccination. Ann Ist Super Sanita (2016) 52:198–204. 10.4415/ANN_16_02_11
24.
Zheng Z Peng F Xu B Zhao J Liu H Peng J et al Risk Factors of Critical & Mortal COVID-19 Cases: A Systematic Literature Review and Meta-Analysis. J Infect (2020) 81:e16–e25. 10.1016/j.jinf.2020.04.021
25.
Angele MK Pratschke S Hubbard WJ Chaudry IH . Gender Differences in Sepsis. Virulence (2014) 5:12–9. 10.4161/viru.26982
26.
Cullaro G Rubin J Mehta N Yao F Verna EC Lai JC . Sex-Based Disparities in Hepatocellular Carcinoma Recurrence after Liver Transplantation. Transplantation (2021) 105:2420–6. Epub ahead of print. 10.1097/TP.0000000000003575
27.
Finkenstedt A Graziadei IW Oberaigner W Hilbe W Nachbaur K Mark W et al Extensive Surveillance Promotes Early Diagnosis and Improved Survival ofDe NovoMalignancies in Liver Transplant Recipients. Am J Transpl (2009) 9:2355–61. 10.1111/j.1600-6143.2009.02766.x
28.
Mathur AK Schaubel DE Gong Q Guidinger MK Merion RM . Sex-based Disparities in Liver Transplant Rates in the United States. Am J Transpl (2011) 11:1435–43. 10.1111/j.1600-6143.2011.03498.x
29.
Moylan CA Brady CW Johnson JL Smith AD Tuttle- Newhall JE Muir AJ . Disparities in Liver Transplantation before and after Introduction of the MELD Score. JAMA (2008) 300:2371–8. 10.1001/jama.2008.720
30.
Darden M Parker G Anderson E Buell JF . Persistent Sex Disparity in Liver Transplantation Rates. Surgery (2021) 169:694–9. 10.1016/j.surg.2020.06.028
Summary
Keywords
liver transplantation, mortality, survival, sex differences, cause of death
Citation
Serrano MT, Sabroso S, Esteban LM, Berenguer M, Fondevila C, Lorente S, Cortés L, Sanchez-Antolin G, Nuño J, De la Rosa G and Salcedo M (2022) Mortality and Causes of Death After Liver Transplantation: Analysis of Sex Differences in a Large Nationwide Cohort. Transpl Int 35:10263. doi: 10.3389/ti.2022.10263
Received
24 November 2021
Accepted
21 March 2022
Published
09 May 2022
Volume
35 - 2022
Updates
Copyright
© 2022 Serrano, Sabroso, Esteban, Berenguer, Fondevila, Lorente, Cortés, Sanchez-Antolin, Nuño, De la Rosa and Salcedo.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: M. Trinidad Serrano, tserrano@salud.argon.es
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.