Dear Editors,
We and others have shown that kidney transplant recipients (KTR) exhibit a reduced immune response with a seroconversion (SC) rate <50% after a regular 2-dose mRNA SARS-CoV-2 vaccination regimen (1, 2). Very limited data on a heterologous 3-dose vaccination with the vector vaccine Ad26COVS1 are available in this patient group. In the only published trial, a 3-dose homologous vaccination protocol was compared with a heterologous one in KTR without SC after a 2-dose mRNA vaccination (3). The third dose increased the antibody response and was well tolerated. However, still less than 50% of the initial non-responders developed SC 4 weeks after either a third mRNA vaccine (35%) or the vector vaccine Ad26COVS1 (42%) (3).
Herein, we provide additional data on the humoral response in 142 Austrian KTR (mean age 60.6 years, 59.9% male, median transplantation vintage 109 months) after double mRNA and triple heterologous vaccination (Figure 1). Patients provided written informed consent, and the study was conducted in compliance with the Helsinki Declaration of 1975, as revised in 2013. Out of 122 patients with follow-up, 76 patients being vaccinated with two doses of a mRNA vaccine (75% mRNA-1273, 25% BNT162b2) received a third dose of Ad26COVS1, administered on average 109 days (range 109.0–145.0 days) after the second dose. SC was determined on average 47 days (range 35.5–61.0 days) after the third vaccination by quantifying anti-SARS-CoV-2 spike IgG antibodies (LIAISON® SARS-CoV-2-TrimericS IgG chemiluminescent immunoassay, Diasorin S.p.A., Saluggia, Italy; cut-off value for seroconversion: ≥33.8 BAU/mL). After double mRNA vaccination the SC rate was 48%. Following heterologous triple vaccination an additional 54% of the initial non-responders achieved SC. Altogether, 97 out of 122 KTR (80%) achieved SC after either double mRNA vaccination or the heterologous triple vaccination. Forty-eight of the 142 KTR showed high-level SC after double mRNA vaccination. Twenty patients developed low antibody concentrations (arbitrary threshold <350 BAU/mL). After a third heterologous dose all these 20 patients significantly boosted their humoral response (1391.9 (SD 687.2) vs. 144.8 (SD 94.6) BAU/mL, p < 0.001). Non-responders after heterologous triple vaccination were significantly older (65.5 vs. 59.4 years; p = 0.033), were more often treated with prednisolone or belatacept (88% vs. 46.4%, 28% vs. 2.1%; p < 0.001 for both) and had a shorter median transplantation vintage (66.0 vs. 141.7 months; p < 0.001). They showed a trend of lower mean eGFR (48.1 vs. 55.5 ml/min/1.73 m2; p = 0.058) and being treated more often with mycophenolic acid (84% vs. 64%; p = 0.090). Higher mycophenolic acid doses did not correlate with inferior antibody response (p = 0.299). As a limitation, our study lacks cellular immune response and neutralizing antibody data. But anti-spike IgG antibodies are highly correlated with neutralizing antibodies, and a level >264 BAU/mL (95% CI: 108, 806) has been found to be associated with 80% vaccine efficacy against primary symptomatic Covid-19, although limited to the B.1.177 and B.1.1.7 SARS-CoV-2 variant (4). Fifty-three percent (40/76) of our patients with a third heterologous dose achieved this threshold, 62% of those with SC after initial non-response (18/29). Whether this threshold indicates the same vaccine efficacy against the now dominant SARS-CoV-2 Omicron variant is unknown. The longer transplantation vintage (9.0 vs. 4.6 years) and extended interval between second and third dose (109 vs. 80 days) in our cohort compared to the study by Reindl-Schwaighofer et al. (3) might be responsible for the higher seroconversion rate in our heterologous prime-boost vaccinees, as both factors significantly influence the vaccination response (1, 5). Nevertheless, due to our study design we cannot recommend one vaccine platform as superior over the other for booster vaccination in KTR, a clinically relevant question addressed by others (3). It remains to be proven whether a heterologous prime-boost regimen combining mRNA and vector vaccine improves the neutralizing humoral response against the now dominant SARS-CoV-2 Omicron variant in KTR as has been shown in the general population (6) or enhances the variant-specific cellular immune response (7) which might translate into better clinical outcomes.
FIGURE 1
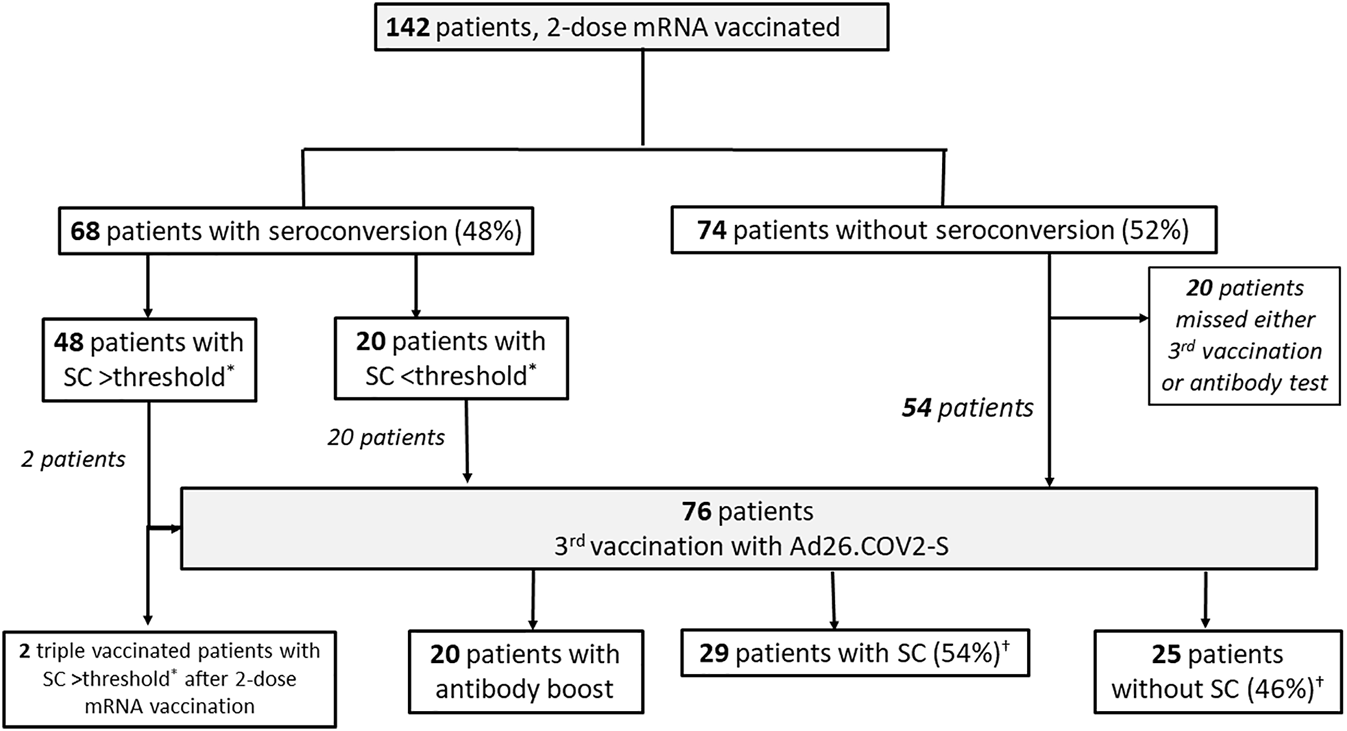
Patient Flow Chart. *Seroconversion (SC) >threshold defined by anti-SARS-CoV-2 spike protein IgG antibodies >350 BAU/mL (LIASION© anti-SARS-CoV-2 TrimericS IgG chemiluminescent immunoassay, Diasorin S.p.A., Saluggia, Italy; cut off value for seroconversion: ≥33.8 BAU/mL). †% of patients (n = 54) without initial seroconversion after two mRNA doses.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Patients provided written informed consent, and the study was conducted in compliance with the Helsinki Declaration of 1975, as revised in 2013.
Author contributions
JS, KL, and EZ designed the study. JS, TD, AA-N, and HS-M collected data. JS and EZ analyzed data and wrote the first draft of the manuscript. All authors reviewed the manuscript and approved the submitted version of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
Giannella M Pierrotti LC Helanterä I Manuel O . SARS‐CoV‐2 Vaccination in Solid‐organ Transplant Recipients: What the Clinician Needs to Know. Transpl Int (2021) 34(10):1776–88. 10.1111/tri.14029
2.
Schimpf J Sprenger-Mähr H Davidovic T Lhotta K Zitt E . Humoral Response in SARS-CoV-2 Convalescent Compared to Vaccinated Kidney Transplant Patients. Transpl Int (2022) 35:10060. 10.3389/ti.2021.10060
3.
Reindl-Schwaighofer R Heinzel A Mayrdorfer M Jabbour R Hofbauer TM Merrelaar A et al Comparison of SARS-CoV-2 Antibody Response 4 Weeks after Homologous vs Heterologous Third Vaccine Dose in Kidney Transplant Recipients. JAMA Intern Med (2022) 182:165. 10.1001/jamainternmed.2021.7372
4.
Feng S Phillips DJ White T Sayal H Aley PK Bibi S et al Correlates of protection against Symptomatic and Asymptomatic SARS-CoV-2 Infection. Nat Med (2021) 27(11):2032–40. 10.1038/s41591-021-01540-1
5.
Amirthalingam G Bernal JL Andrews NJ Whitaker H Gower C Stowe J et al Serological Responses and Vaccine Effectiveness for Extended COVID-19 Vaccine Schedules in England. Nat Commun (2021) 12(1):7217. 10.1038/s41467-021-27410-5
6.
Cheng SMS Mok CKP Leung YWY Ng SS Chan KCK Ko FW et al Neutralizing Antibodies against the SARS-CoV-2 Omicron Variant BA.1 Following Homologous and Heterologous CoronaVac or BNT162b2 Vaccination. Nat Med (2022) 1:1. 10.1038/s41591-022-01704-7
7.
Atmar RL Lyke KE Deming ME Jackson LA Branche AR El Sahly HM et al Homologous and Heterologous Covid-19 Booster Vaccinations. N Engl J Med (2022) 1:1. 10.1056/NEJMoa2116414
Summary
Keywords
Covid-19, Sars-CoV-2, kidney transplantation, Ad26COVS1, heterologous vaccination
Citation
Schimpf J, Davidovic T, Abbassi-Nik A, Sprenger-Mähr H, Lhotta K and Zitt E (2022) Enhanced SARS-CoV-2 Antibody Response After a Third Heterologous Vector Vaccine Ad26COVS1 Dose in mRNA Vaccine-Primed Kidney Transplant Recipients. Transpl Int 35:10357. doi: 10.3389/ti.2022.10357
Received
13 January 2022
Accepted
03 March 2022
Published
22 March 2022
Volume
35 - 2022
Updates
Copyright
© 2022 Schimpf, Davidovic, Abbassi-Nik, Sprenger-Mähr, Lhotta and Zitt.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Emanuel Zitt, emanuel.zitt@lkhf.at
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.