- 1Department of Nephrology, Vall d’Hebron University Hospital, Barcelona, Spain
- 2Centro de Referencia en Enfermedad Glomerular Compleja del Sistema Nacional de Salud (CSUR), Vall d’Hebron University Hospital, Barcelona, Spain
- 3Department of Nephrology, San Carlos Clinical University Hospital, Madrid, Spain
- 4Centro de Referencia en Enfermedad Glomerular Compleja del Sistema Nacional de Salud (CSUR), San Carlos Clinical University Hospital, Madrid, Spain
- 5Department of Pathology, Vall d’Hebron University Hospital, Barcelona, Spain
Dear Editors,
Targeted-release-formulation of budesonide (TRF-budesonide) has demonstrated promising results in terms of proteinuria and renal function in patients with immunoglobulin A nephropathy (IgAN) (1). Regarding its well tolerance, enteric budesonide may become the first step of immunosuppressive treatment of IgAN, although real world clinical practice publications are lacking (2-4). We evaluated the effect of budesonide in our cohort of patients affected by IgAN. We included all patients, either transplanted kidney or native, which were diagnosed of IgAN and were treated with enteric budesonide in our center from December 2017 to January 2022. At baseline clinical and analytical parameters were collected during the next 3, 6, 12, and 24 months. We also assessed the occurrence of budesonide-related adverse events.
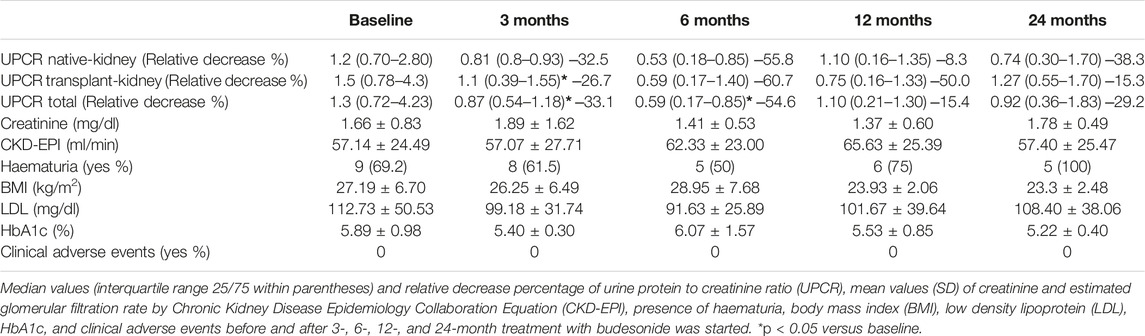
A total of 14 patients were included in the study. Nine of the patients had IgAN in their native kidneys (7 males) and 5 were transplanted (5 males), age of 46 ± 17.21 years. The relative decrease of proteinuria was of 33.1% and 54.6% after 3 and 6 months of treatment with budesonide, respectively (p < 0.05) (Table 1). Evaluating native and transplant kidney separately, proteinuria in transplant kidney also significantly decreased (26.7%) after 3 months of treatment (Table 1). These results are in line with previous literature (2–6). There is increasing evidence about the role of gut-associated lymphoid tissue and complexes with Gd-IgA1 deposition in IgAN pathogenesis (7). The first study that evaluated TRF-budesonide published a significant albuminuria reduction of 40% in 16 patients with IgAN after 2 months of treatment (2). Afterwards, the phase 2b clinical trial NEFIGAN demonstrated significant proteinuria reduction (21%–27%) in 199 patients with IgAN after 9 months of treatment with TRF-budesonide (5). This latest trial justified to carry out the phase 3 trial NEFIGARD, where TRF-budesonide significantly reduced UPCR by 27% of 199 patients (6). There is only another retrospective study that evaluated the effect of TRF-budesonide in native kidneys IgAN with significant proteinuria reduction (3). This constant effect of local budesonide in proteinuria reduction is quite remarkable, as proteinuria is considered as the main sign of disease progression in IgAN (7) and a surrogate marker of kidney outcome in IgAN (8).
To our knowledge, there is only a case report published that described a successful post-transplant IgAN treated with TRF-budesonide (4). As 58% of IgAN recurs post-transplant (4,9) and 20%–40% progress to end-stage chronic kidney disease (9,10), TRF-budesonide could be a promising effective treatment in these patients. None of the patients experimented any adverse event. HbA1c, LDL and body mass index, whose increment could be considered as adverse events of steroid therapy, remained stable (Table 1). NEFIGAN and NEFIGARD trials corroborate this well tolerance (5,6). Local steroid therapy, like enteric budesonide, provides the immunosuppressive result directly in the IgAN origin and avoids serious side effects usually present in systemic steroid treatment.
Our results support that TRF-budesonide causes significant proteinuria reduction and maintain eGFR stable without adverse events in IgAN. Remarkably, the effect of local steroid treatment in transplant kidneys should also be analyzed in proper designed randomized clinical trials. Targeting intestinal mucosal immune system seems to be a good therapeutic strategy of IgAN treatment which will probably replace systemic steroids.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by (AG) 252/2018. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
MS, IA-P, and FM collaborated on the original idea and study design. IA-P, ML-M, MS, SB, NR, MA, FM, OB, IT, CG-C, AV, MB, and NT contributed to the inclusion of patients in the cohort. ML-M and MS collaborated on the statistical analysis and wrote the paper. All authors have read and agreed to the published version of the manuscript.
Conflict of Interest
ML-M received support for attending meetings and/or travel from Sanofi and AstraZeneca. SB received consulting fees and payment for lectures, presentations, speakers, bureaus, manuscript writing or educational events, participation on a data safety monitoring board or advisory board from AstraZeneca and Mundipharma. CG-C has received travel and congress fees support from AstraZeneca, Esteve, Novo Nordisk, Boehringer Ingelheim Lilly, Astellas, Otsuka, Novartis and Baxter, and has given scientific lectures and participated in advisory boards organized by AstraZeneca, Boehringer Ingelheim Lilly, Mundipharma and Novo Nordisk. AV received grants or contracts from Instituto Carlos III (ISCIII) and Fundación Alfonso Martín Escudero, support for attending meetings and/or travel from Mundipharma, Sanofi, and Novo Nordisk. NR Received grants from the participation on a data safety monitoring board or advisory board from Alexion. MS received grants or contracts from Boehringer, ISCIII, and Marató TV3; honoraria for lectures from NovoNordisk, Jansen, Boehringer, Mundipharma, AstraZeneca, Ingelheim Lilly, Vifor, ICU Medical, Fresenius, and Travere Therapeutics; support for attending meetings from Travere; participation on a data safety, monitoring board or advisory board from NovoNordisk, Jansen, Boehringer, Mundipharma, AstraZeneca, Ingelheim Lilly, Vifor, ICU Medical, Bayer, GE Healthcare, and Travere Therapeutics. MS has the following leadership or fiduaciary roles: SEC Board member, SEN board member, Ex ERA board member, Ex-ASN Board News, Ex-ERA-EDTA SAB, Ex-Council member ERA, Elected EIC CKJ.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. McGrogan, A, Franssen, CF, and de Vries, CS. The Incidence of Primary Glomerulonephritis Worldwide: a Systematic Review of the Literature. Nephrol Dial Transpl (2011) 26(2):414–30. doi:10.1093/ndt/gfq665
2. Smerud, HK, Bárány, P, Lindström, K, Fernström, A, Sandell, A, Pahlsson, P, et al. New Treatment for IgA Nephropathy: Enteric Budesonide Targeted to the Ileocecal Region Ameliorates Proteinuria. Nephrol Dial Transpl (2011) 26:3237–42. doi:10.1093/ndt/gfr052
3. Ismail, G, Obrisca, B, Jurubita, R, Andronesi, A, Sorohan, B, Vornicu, A, et al. Budesonide versus Systemic Corticosteroids in IgA Nephropathy. A Retrospective, Propensity-Matched Comparison. Medicine (2020) 99(26):e21000. doi:10.1097/MD.0000000000021000
4. Lingaraj, U, Aralapuram, K, Chikkanayakanhalli, S, Vishwanathan, A, and Vankalkunti, M. Successful Treatment of a Patient with Posttransplant IgA Nephropathy with Targeted Release Formulation of Budesonide. Saudi J Kidney Dis Transpl (2020) 31(2):521–3. doi:10.4103/1319-2442.284029
5. Fellström, BC, Barratt, J, Cook, H, Coppo, R, Feehaly, J, de Fitjter, JW, et al. Targeted-release Budesonide versus Placebo in Patients with IgA Nephropathy (NEFIGAN): a Double-Blind, Randomised, Placebo-Controlled Phase 2b Trial. Lancet (2017) 389(10084):2117–27. doi:10.1016/S0140-6736(17)30550-0
6. Barratt, J, Stone, A, and Kristensen, K. Pos-830 Nefecon for the Treatment of Iga Nephropathy in Patients at Risk of Progressing to End-Stage Renal Disease: the Nefigard Phase 3 Trial Results. Kidney Int Rep (2021) 6:S361–S362. doi:10.1016/j.ekir.2021.03.868
7.Kidney Disease: Improving Global Outcomes KDIGO Glomerular Diseases Work Group. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int (2021) 100:S1–S276. doi:10.1016/j.kint.2021.05.021
8. Reich, HN, Troyanov, S, and Scholey, JW. Cattran D, for the Toronto Glomerulonephritis Registry: Remission of Proteinuria Improves Prognosis in IgA Nephropathy. J Am Soc Nephrol (2007) 18:3177–83. doi:10.1681/ASN.2007050526
9. Maixnerova, D, Hruba, P, Neprasova, M, Bednarova, K, Slatinska, J, Suchanek, M, et al. Outcome of 313 Czech Patients with IgA Nephropathy after Renal Transplantation. Front Immunol (2021) 12:726215. doi:10.3389/fimmu.2021.726215
Keywords: IgA nephropathy, IgAN recurrence in transplant kidney, proteinuria reduction, TRF-budesonide, CKD progression
Citation: Lopez-Martinez M, Torres I, Bermejo S, Moreso F, Garcia-Carro C, Vergara A, Ramos N, Perello M, Gabaldon A, Azancot MA, Bolufer M, Toapanta N, Bestard O, Agraz-Pamplona I and Soler MJ (2022) Enteric Budesonide in Transplant and Native IgA Nephropathy: Real-World Clinical Practice. Transpl Int 35:10693. doi: 10.3389/ti.2022.10693
Received: 07 June 2022; Accepted: 04 October 2022;
Published: 14 October 2022.
Copyright © 2022 Lopez-Martinez, Torres, Bermejo, Moreso, Garcia-Carro, Vergara, Ramos, Perello, Gabaldon, Azancot, Bolufer, Toapanta, Bestard, Agraz-Pamplona and Soler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesc Moreso, fjmoreso@vhebron.net; Maria Jose Soler, mjsoler01@gmail.com
†These authors share senior authorship
 Marina Lopez-Martinez1,2
Marina Lopez-Martinez1,2 Francesc Moreso
Francesc Moreso Oriol Bestard
Oriol Bestard Irene Agraz-Pamplona
Irene Agraz-Pamplona Maria Jose Soler
Maria Jose Soler