Dear Editors,
Ravulizumab is a long-acting C5-complement monoclonal antibody developed through targeted modifications of eculizumab to significantly extend the half-life of the drug with comparable affinity and specificity to eculizumab (approx. 52 days vs. approx. 11 days) [1]. The efficacy and safety of ravulizumab in patients with aHUS treated with or without complement inhibitors has been adequately studied in adults [2] and pediatric patients [3] and recently led to the approval of the drug by the European Medicines Agency and the US Food and Drug Administration (Ultomiris® SmPC). During 26 weeks of treatment, ravulizumab provided rapid and effective complement inhibition with no unexpected safety issues.
In renal transplant patients, there has been only a single report of ravulizumab use. Ravulizumab was successfully administered in the case of a living kidney donation in a patient with aHUS over the reported treatment period of 6 months [4].
Here we report the results of a young woman who was successfully switched from chronic aHUS treatment with eculizumab to ravulizumab after kidney transplantation.
Back in 2013, we published on the long-term eculizumab treatment of a kidney transplant patient who had a relapse of her aHUS shortly after a living kidney donation [5]. The cause of the aHUS relapse was an MCP mutation and, as was determined in a later analysis, also a factor H mutation. Recurrent aHUS attributable to both complement factor mutations requires lifelong anti-C5 treatment due to high risk [6]. Our patient had been treated with eculizumab administered every 14 days for more than 10 years. As shown in Figure 1, the complete available laboratory data of creatinine, hemoglobin, and platelets show a very stable course of the patient. Remarkably, only one episode of fever occurred during the entire observation period, the cause of which remained unclear. However, the patient achieved restitutio ad integrum with short-term inpatient treatment with piperacillin/tazobactam. Because eculizumab has been shown to be effective after renal transplantation for treatment of aHUS and because ravulizumab is a modified version of eculizumab, we expected comparable efficacy and safety of both products [7]. Immunosuppressive therapy consisted of tacrolimus (target through 4–6 ng/ml), low dose mycophenolate mofetil, and prednisolone. At the time of conversion, our 39-year-old patient (body weight 70 kg, BMI 19.6 kg/m2) had a serum creatinine of 1.66 mg/dl (eGFR 39 ml/min/1.73 m2 (CKD-EPI formula)), hemoglobin concentration of 11.6 g/dl, and platelet count of 359 × 103/mm3). After 22 months of therapy with ravulizumab 3,300 mg every 8 weeks following an induction therapy with additional administration of 3,300 mg 2 weeks after the first infusion according to the prescribing information, serum creatinine [1.63 mg/dl (eGFR 39 ml/min/1.73 m2)], hemoglobine (12.6 g/dl), and platelet count (367 × 103/mm3) were stable over time (Figure 1). However, 14 months after conversion, SARS-CoV-2 infection was diagnosed out-of-hospital between two infusion appointments without our knowledge. The patient, who had been vaccinated three times had severe illness lasting 10 days, but without respiratory distress or graft failure. The patient’s migraine was not changed by the switch to ravulizumab.
FIGURE 1
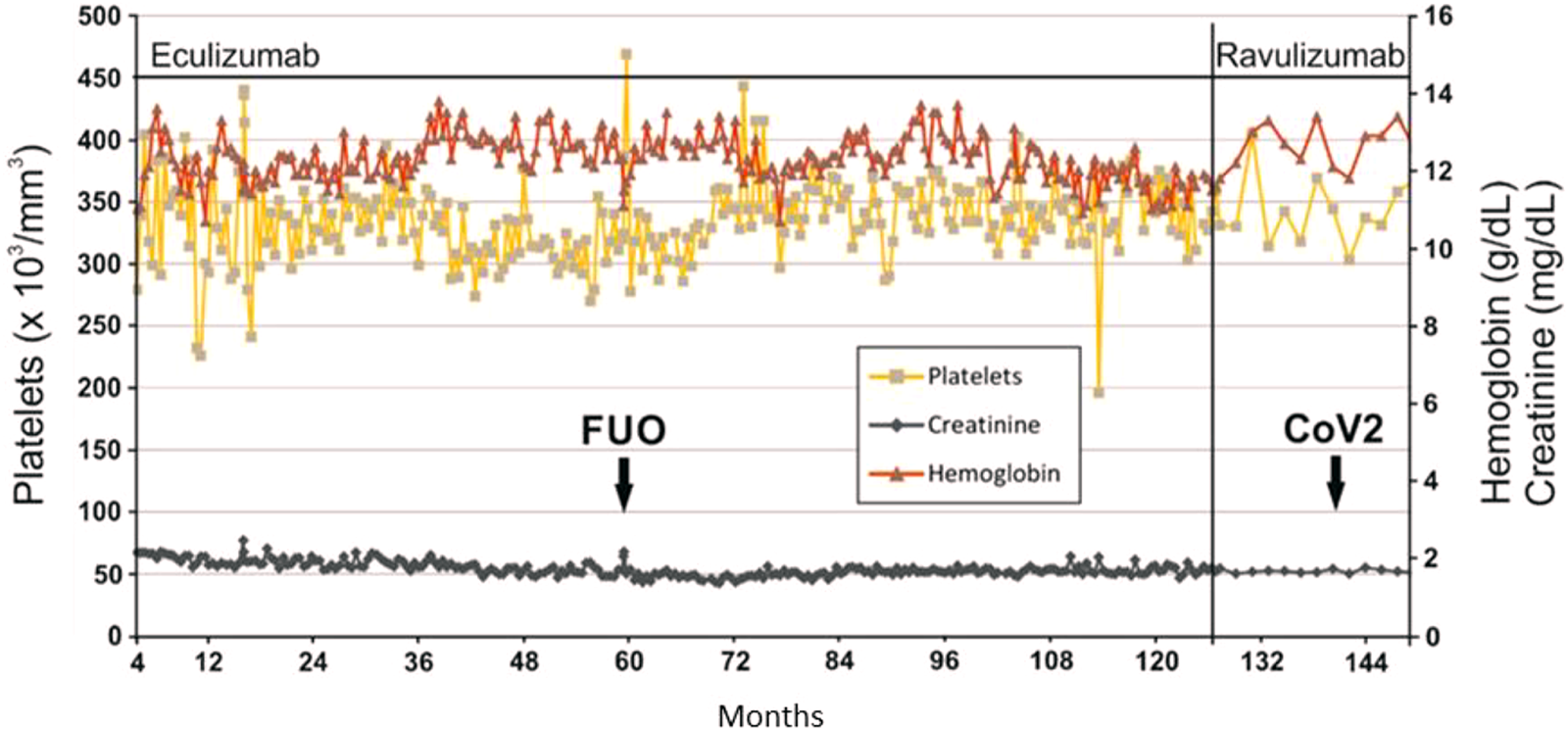
Laboratory values under complement inhibition with eculizumab or ravulizumab. FUO, fever of unknown origin; CoV2, SARS CoV2 infection.
We present this case report because ravulizumab therapy offers improvement in health-related quality of life and greater cost-effectiveness compared with eculizumab therapy because of the longer interval between infusions [8]. The presented case demonstrates that switching C5 inhibition to ravulizumab is safe and effective in renal transplant patients with genetic aHUS, even after decades of therapy with eculizumab. It should be noted that meningococcal vaccination or prophylaxis must be continue with ravulizumab administration (Ultomiris∗ SmPC). Because ravulizumab-based therapy offers significant health-related quality of life and cost-effectiveness benefits, it may be the therapy of choice for these patients.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
This case sudy was approved by the local ethics committee (Ethik Kommission der Ärztekammer Westfalen-Lippe und der Medizinischen Fakultät der Westfälischen Wilhelms-Universität). It was performed in accordance with the current transplantation guidelines and the 1964 Declaration of Helsinki. Written informed consent was obtained from the patient for publication of this case report.
Author contributions
UJ and SR collected the data and wrote the letter, UA and HP revised it.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
The APC was funded by the Open Access Publication Fund of University of Münster.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
Sheridan D Yu Z-X Zhang Y Patel R Sun F Lasaro MA et al Design and Preclinical Characterization of ALXN1210: A Novel Anti-C5 Antibody with Extended Duration of Action. PloS one (2018) 13:e0195909. 10.1371/journal.pone.0195909
2.
Barbour T Scully M Ariceta G Cataland S Garlo K Heyne N et al Long-Term Efficacy and Safety of the Long-Acting Complement C5 Inhibitor Ravulizumab for the Treatment of Atypical Hemolytic Uremic Syndrome in Adults. Kidney Int Rep (2021) 6:1603–13. 10.1016/j.ekir.2021.03.884
3.
Tanaka K Adams B Aris AM Fujita N Ogawa M Ortiz S et al The Long-Acting C5 Inhibitor, Ravulizumab, Is Efficacious and Safe in Pediatric Patients with Atypical Hemolytic Uremic Syndrome Previously Treated with Eculizumab. Pediatr Nephrol (2021) 36:889–98. 10.1007/s00467-020-04774-2
4.
Schmidt T Gödel M Mahmud M Fischer L Huber TB Kluger MA et al Ravulizumab in Preemptive Living Donor Kidney Transplantation in Hereditary Atypical Hemolytic Uremic Syndrome. Transpl Direct (2022) 8:e1289. 10.1097/TXD.0000000000001289
5.
Reuter S Heitplatz B Pavenstädt H Suwelack B . Successful Long-Term Treatment of TMA with Eculizumab in a Transplanted Patient with Atypical Hemolytic Uremic Syndrome Due to MCP Mutation. Transplantation (2013) 96:e74–6. 10.1097/01.TP.0000435705.63428.1f
6.
Krishnappa V Gupta M Elrifai M Moftakhar B Ensley MJ Vachharajani TJ et al Atypical Hemolytic Uremic Syndrome: A Meta-Analysis of Case Reports Confirms the Prevalence of Genetic Mutations and the Shift of Treatment Regimens. Ther Apher Dial (2018) 22:178–88. 10.1111/1744-9987.12641
7.
Zimmerhackl LB Hofer J Cortina G Mark W Wurzner R Jungraithmayr TC et al Prophylactic Eculizumab after Renal Transplantation in Atypical Hemolytic-Uremic Syndrome. N Engl J Med (2010) 362:1746–8. 10.1056/NEJMc1001060
8.
O'Connell T Buessing M Johnson S Tu L Thomas SK Tomazos I . Cost-Utility Analysis of Ravulizumab Compared with Eculizumab in Adult Patients with Paroxysmal Nocturnal Hemoglobinuria. PharmacoEconomics (2020) 38:981–94. 10.1007/s40273-020-00929-z
Summary
Keywords
kideny transplantation, ravulizumab, eculizumab, atypical hemolytic uremic syndrome, C5 inhibition
Citation
Jehn U, Altuner U, Pavenstädt H and Reuter S (2022) First Report on Successful Conversion of Long-Term Treatment of Recurrent Atypical Hemolytic Uremic Syndrome With Eculizumab to Ravulizumab in a Renal Transplant Patient. Transpl Int 35:10846. doi: 10.3389/ti.2022.10846
Received
19 August 2022
Accepted
22 September 2022
Published
03 October 2022
Volume
35 - 2022
Updates
Copyright
© 2022 Jehn, Altuner, Pavenstädt and Reuter.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ulrich Jehn, ulrich.jehn@ukmuenster.de
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.