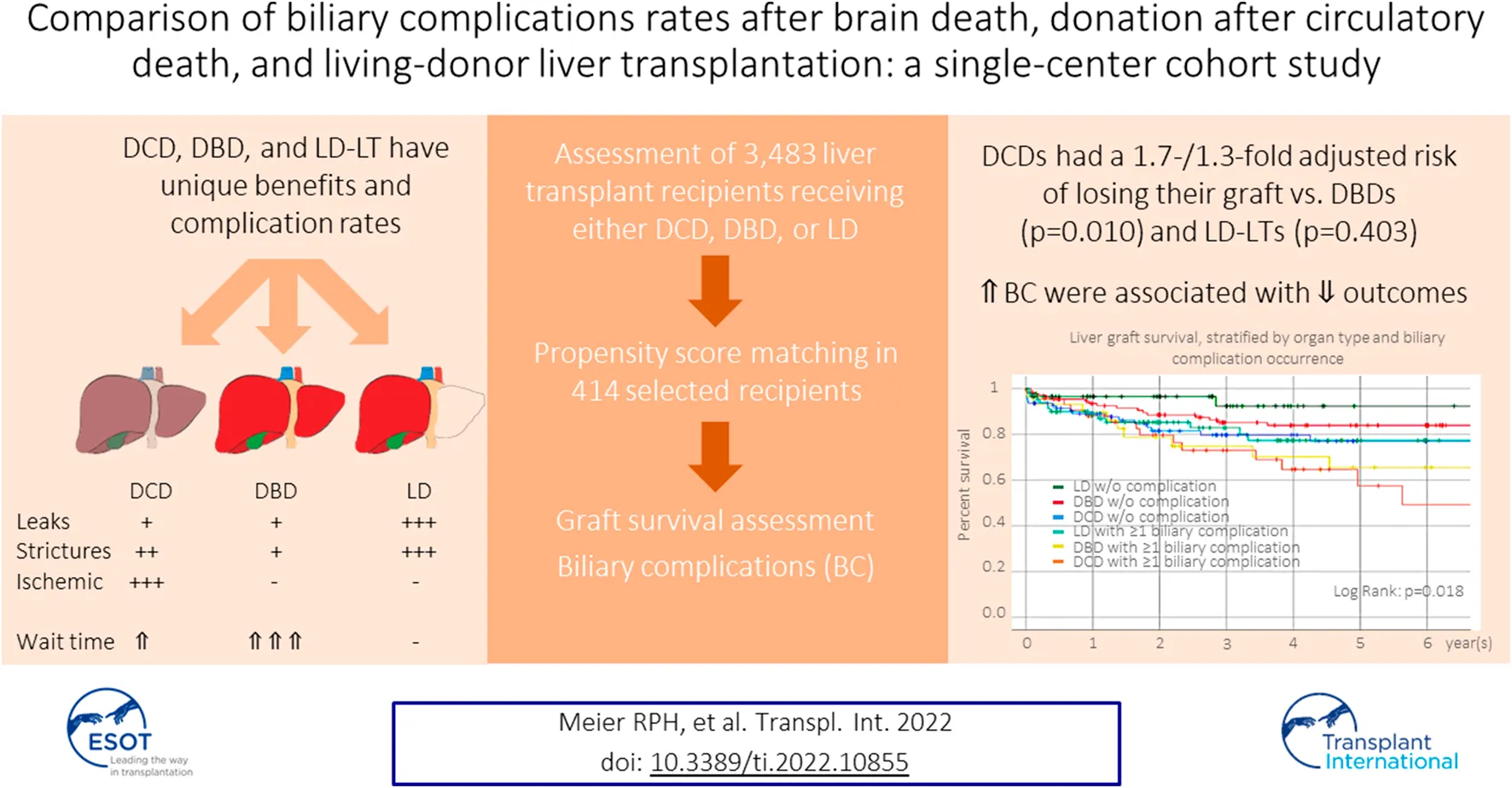
Abstract
Donation-after-circulatory-death (DCD), donation-after-brain-death (DBD), and living-donation (LD) are the three possible options for liver transplantation (LT), each with unique benefits and complication rates. We aimed to compare DCD-, DBD-, and LD-LT-specific graft survival and biliary complications (BC). We collected data on 138 DCD-, 3,027 DBD- and 318 LD-LTs adult recipients from a single center and analyzed patient/graft survival. BC (leak and anastomotic/non-anastomotic stricture (AS/NAS)) were analyzed in a subset of 414 patients. One-/five-year graft survival were 88.6%/70.0% for DCD-LT, 92.6%/79.9% for DBD-LT, and, 91.7%/82.9% for LD-LT. DCD-LTs had a 1.7-/1.3-fold adjusted risk of losing their graft compared to DBD-LT and LD-LT, respectively (p < 0.010/0.403). Bile leaks were present in 10.1% (DCD-LTs), 7.2% (DBD-LTs), and 36.2% (LD-LTs) (ORs, DBD/LD vs. DCD: 0.7/4.2, p = 0.402/<0.001). AS developed in 28.3% DCD-LTs, 18.1% DBD-LTs, and 43.5% LD-LTs (ORs, DBD/LD vs. DCD: 0.5/1.8, p = 0.018/0.006). NAS was present in 15.2% DCD-LTs, 1.4% DBDs-LT, and 4.3% LD-LTs (ORs, DBD/LD vs. DCD: 0.1/0.3, p = 0.001/0.005). LTs w/o BC had better liver graft survival compared to any other groups with BC. DCD-LT and LD-LT had excellent graft survival despite significantly higher BC rates compared to DBD-LT. DCD-LT represents a valid alternative whose importance should increase further with machine/perfusion systems.
Introduction
In regions with a high average Model for End-stage Liver Disease (MELD) score at transplant, organs from donation after brain death (DBD) donors often go to the sicker patients with high MELD scores, and so for patients with liver cancer and/or a low MELD score, organs from donation-after-circulatory-death (DCD) donors and living donors (LD) [1, 2] represent alternatives for liver transplantation (LT). DCD donors are increasingly used for LT in an effort to address organ scarcity and to decrease waiting-list mortality [3]. It is well recognized that DCD livers expose the recipient to increased risk from the inevitably longer donor warm ischemia time (dWIT). Aside from primary nonfunction [4], the most feared complication, and one of the main reasons for graft loss, is ischemic cholangiopathy (IC), defined as the appearance of intrahepatic non-anastomotic biliary strictures (NAS), which occurs in 10%–50% of cases [5–9]. The increasing use of normothermic preservation machines (NMP) might significantly modify these complication rates [10]. However, to date, NMP is not broadly available, and many US centers still avoid DCDs or apply very strict donor selection criteria [9]. In this regard, we and others have developed scores to select donors/recipients in order to optimize outcomes with a special emphasis on minimizing biliary complications [11–14]. Known risk factors for IC are donor age (>40 years) [6, 15], prolonged cold ischemic time (CIT) (>8 h) [6], prolonged dWIT (>20 min), low venous oxygen saturation (SvO2 ≤ 60) [15], and donor liver extraction time [8, 13]. Besides IC, other relevant ischemic complications include anastomotic biliary strictures (AS) and bile leaks which were previously shown to range between 10% and 15% in DCD cases, and not be significantly different from DBD rates [6]. Just as the use of DCD grafts has increased in recent years, so has the use of LD-LT in order to further increase organ availability [16]. The outcomes are overall excellent [17], however, a higher risk of biliary complication is present as well with anastomotic biliary stenosis and leak ranging from 10% to 35% in different series [16, 18–21]. The difficulties encountered by patients experiencing recurrent biliary issues added to the minimal, but a non-null, risk to the living donor [22] and variable access to LD, warrants a thorough assessment and selection of both donor and recipient by the transplant team.
For a given patient with all three options, the choice might be difficult to make since each modality has unique benefits, risks, and potential complications. We sought to compare biliary complications and graft survival between DCD-, DBD-, and LD-LT at a single center, with the intention to provide more data for guiding the decision between these three possible options for transplantation.
Methods
Study Design and Patients
Approval was obtained by the Institutional Review Board of the University. Donor and recipient data were extracted from the UNOS database and included all consecutive adult liver transplants performed at the University Medical Center between 1989 and 2019 (n = 3,483), which included 138 DCD, 318 LD, and 3,027 DBD (Table 1). 138 DCD-LTs were compared to 138 DBD-LTs (selected using a propensity score matching technique), and 138 randomly selected LD-LTs. Ischemia times were defined as previously described [13]. Donor and recipient selection and procedures were performed as previously described [13, 23, 24]. DCD grafts were procured using the super-rapid technique with local modifications [25]. Ischemic cholangiopathy was defined by the presence of intrahepatic, non-anastomotic biliary strictures (NAS) and dilatations occurring in the absence of ductopenic rejection or recurrent primary sclerosing cholangitis. When suspected (increased alkaline phosphatase and bilirubin), NAS was diagnosed on endoscopic retrograde cholangiopancreatography (ERCP) and/or Magnetic Resonance Imaging (MRI). One DBD recipient developed secondary NAS after a hepatic artery thrombosis. The occurrence of AS and biliary leaks were collected from patients’ chart reviews. The median follow-up was 6 years (min-max, 0–29 years) for the entire cohort (n = 3,483) and 3 years (min-max, 0–27 years) for the 1:1 control cohort (n = 414). In the entire cohort (n = 3,483), MELD had 1% missing data, recipient BMI and CIT had 6% missing data, and all the other variables had no missing data. In the 1:1 matched control cohort (n = 414), CIT and dWIT had 1% missing data, and all the other variables had no missing data.
TABLE 1
| Characteristics | DCD LT (n = 138) | DBD LT (n = 3,027) | LD LT (n = 318) | P-valuea | P-valueb |
|---|---|---|---|---|---|
| Recipient | |||||
| Age at transplant, years | 57.5 ± 9.0 | 53.3 ± 10.7 | 53.9 ± 11.1 | <0.001 | <0.001 |
| Gender (%) | |||||
| Male | 103 (74.6) | 1,942 (64.2) | 158 (49.7) | 0.012 | <0.001 |
| Female | 35 (25.4) | 1,085 (35.8) | 160 (50.3) | ||
| Pretransplant BMI, kg/m2 | 27.9 ± 5.9 | 27.3 ± 5.9 | 26.2 ± 4.6 | 0.236 | 0.003 |
| Ethnicity (%) | |||||
| American Indian | 2 (1.4) | 33 (1.1) | 2 (0.6) | 0.428 | 0.652 |
| Asian | 18 (13.0) | 491 (16.2) | 27 (8.5) | ||
| Black | 4 (2.9) | 180 (5.9) | 11 (3.5) | ||
| Native Hawaiian | 1 (0.7) | 30 (1.0) | 2 (0.6) | ||
| Hispanic | 38 (27.5) | 658 (21.7) | 81 (25.5) | ||
| Multiracial | 0 (0.0) | 16 (0.5) | 1 (0.3) | ||
| White | 75 (54.3) | 1,619 (53.5) | 194 (61.0) | ||
| Etiology | |||||
| A1AT | 1 (0.7) | 13 (0.4) | 2 (0.6) | <0.001 | <0.001 |
| Auto-immune | 4 (2.9) | 81 (2.7) | 13 (4.1) | ||
| Amyloidosis | 1 (0.7) | 0 (0.0) | 0 (0.0) | ||
| Biliary atresia | 1 (0.7) | 2 (0.1) | 3 (0.9) | ||
| Cholangiocarcinoma | 2 (1.4) | 4 (0.1) | 0 (0.0) | ||
| Cryptogenic | 3 (2.2) | 205 (6.8) | 23 (7.2) | ||
| EtOH | 29 (21.0) | 363 (12.0) | 41 (12.9) | ||
| HBV | 12 (8.7) | 324 (10.7) | 24 (7.5) | ||
| HCV | 68 (49.3) | 874 (28.9) | 84 (26.4) | ||
| NASH | 9 (6.5) | 132 (4.4) | 25 (7.9) | ||
| Other | 0 (0.0) | 780 (25.8) | 39 (12.3) | ||
| PBC | 2 (1.4) | 112 (3.7) | 28 (8.8) | ||
| PSC | 3 (2.2) | 121 (4.0) | 36 (11.3) | ||
| Wilson | 3 (2.2) | 16 (0.5) | 0 (0.0) | ||
| HCC | |||||
| Presence | 40 (29.0) | 586 (19.4) | 50 (15.7) | 0.005 | 0.001 |
| Absence | 98 (71.0) | 2,441 (80.6) | 268 (84.3) | ||
| Median MELD, IRQ | 23 (12–32) | 38 (31–40) | 18 (13–26) | <0.001 | 0.008 |
| Era | |||||
| 1989–2000 | 0 (0.0) | 912 (30.1) | 10 (3.1) | <0.001 | <0.001 |
| 2001–2010 | 26 (18.8) | 1,038 (34.3) | 128 (40.3) | ||
| 2011–2018 | 112 (81.2) | 1,077 (35.6) | 180 (56.6) | ||
| Donor factors | |||||
| Age, years | 31.7 ± 10.3 | 39.5 ± 16.7 | 36.5 ± 10.8 | <0.001 | <0.001 |
| Gender (%) | |||||
| Male | 92 (66.7) | 1,803 (59.6) | 163 (51.3) | 0.096 | 0.002 |
| Female | 46 (33.3) | 1,224 (40.4) | 155 (48.7) | ||
| BMI, kg/m2 | 25.5 ± 5.2 | 26.5 ± 6.1 | 25.8 ± 4.4 | 0.041 | 0.557 |
| Ethnicity (%) | |||||
| American Indian | 0 (0.0) | 19 (0.6) | 0 (0.0) | 0.132 | 0.070 |
| Asian | 4 (2.9) | 226 (7.5) | 28 (8.8) | ||
| Black | 11 (8.0) | 234 (7.7) | 11 (3.5) | ||
| Hispanic | 34 (24.6) | 677 (22.4) | 69 (21.7) | ||
| Multiracial | 4 (2.9) | 30 (1.0) | 6 (1.9) | ||
| Native Hawaiian | 0 (0.0) | 27 (0.9) | 1 (0.3) | ||
| Unknown | 0 (0.0) | 9 (0.3) | 0 (0.0) | ||
| White | 85 (61.6) | 1,805 (59.6) | 203 (63.8) | ||
| Cause of death | |||||
| Anoxia | 73 (52.9) | 579 (19.1) | NA | <0.001 | NA |
| Cerebrovascular | 17 (12.3) | 1,213 (40.1) | |||
| CNS tumor | 0 (0.0) | 8 (0.3) | |||
| Head trauma | 41 (29.7) | 1,061 (35.1) | |||
| Not reported | 0 (0.0) | 9 (0.3) | |||
| Other | 7 (5.1) | 157 (5.2) | |||
| Cold ischemic time, hours | 7.7 ± 2.6 | 9.0 ± 3.9 | 2.4 ± 2.6 | <0.001 | <0.001 |
| Donor warm ischemia time, minutes | 20 ± 6 | NA | NA | NA | NA |
| Donor hepatectomy time, minutes | 41 ± 16 | NA | NA | NA | NA |
Recipient and donor baseline characteristics of donation after cardiac death, donation after brainstem death, and living donor liver transplantation.
Data are presented as mean ± standard deviation or n (%), unless specified otherwise.
DCD, donation after cardiac death; DBD, donation after brainstem death; LD, living donor; LT, liver transplantation; BMI, body mass index, EtOH, ethanol use; HBV, hepatitis B virus; HCV, hepatitis C virus; NASH, nonalcoholic steatohepatitis; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis; A1AT, alpha-1 antitrypsin; MELD, Model For End-Stage Liver Disease; CNS, central nervous system; IRQ, interquartile range.
DCD versus DBD.
DCD versus LD. Student t-test for continuous variables, X2 test for binary or categorical variables (global p-value).
Patient Selection, Organ Allocation, and Operation
Patients diagnosed with end-stage liver disease were evaluated for candidacy by a multidisciplinary team and placed on the transplant waiting list [24]. Before 2002, the United Network for Organ Sharing (UNOS) criteria were used to determine priority (no DCD-LT was performed during this time). From 2002 to present, the MELD allocation system has been used [26]. Organ selection and LT were performed as previously described [24]. All liver grafts were perfused with University of Wisconsin solution (hepatic artery and portal vein). LT was performed as previously described [24], typically utilizing the piggyback technique and duct-duct biliary anastomosis.
Statistical Analysis
Continuous variables were expressed as means, and standard deviations (SD) and categorical variables were expressed as counts and percentages. Comparison between groups was performed using the Student’s t-test for continuous variables and the chi-squared test for binary or categorical variables. Propensity score matching for each patient was generated using a multivariable binary logistic regression model. DCD patients were matched 1:1 with DBD patients using recipient age, sex, and pretransplant BMI as well as donor age, sex, BMI, and cold ischemia time as a covariate with a caliper of 0.01. Due to the lower number of LD cases and the limited value of selecting one specific matching variable over another, 138 LD-LT recipients were randomly selected for comparison. To ensure that random matching was appropriate, we performed a sensitivity analysis using optimal full propensity score matching restricted to observations that had propensity scores in the extended common support region (0.06–0.75). Acceptable balance was defined by a maximum of 0.1 for the absolute value of standardized difference and by values within the 0.5–2 range for variance ratio. Survival analyses were performed using the Kaplan–Meier method and the log-rank test. Uni-/multivariate Cox proportional-hazard regression was used to compute hazard ratios (HR). We used IBM SPSS Statistics version 26 and SAS version 9.4 for all computations (IBM Corp. Armonk, NY). Ninety-five percent confidence intervals (95%CI) were reported, and an exact two-sided p-value <0.05 was considered statistically significant.
Results
Patient Characteristics
During the study period, 3,483 liver transplants were performed, including 138 DCD, 3,027 DBD and 318 LD (Figure 1; Table 1). Compared to DBD, DCD recipients were significantly older and more likely to be males. The top two indications in DCD-LTs were cirrhosis from alcohol (EtOH) use and hepatitis C virus (HCV), and more recipients had hepatocellular carcinoma (HCC) compared to DBD-LTs. The median (interquartile range (IRQ)) MELD in DCD recipients was 23 (12–32) versus 38 (31–40) in DBD recipients (p < 0.001). DCD donors and LD were younger compared to DBD donors. Other baseline differences are shown in Table 1.
FIGURE 1
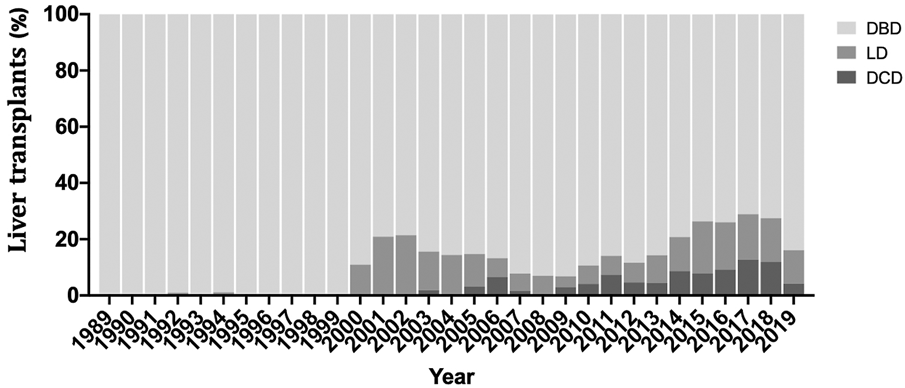
Percentage of recipient receiving a donation after cardiac death (DCD), donation after brainstem death (DBD), or living donor liver transplantation (LD) over the study period.
Graft Survival
Univariate Cox proportional-hazards regression identified several recipient and donor factors associated with graft loss (Table 2). After adjustment for variables with p-value<0.1 in the univariate model or key variable of interest (graft type), the multivariate Cox regression model identified older recipient age and Asian race, the presence of cholangiocarcinoma, era, the use of a DCD graft (compared to a DBD graft), a graft from a Native Hawaiian donor, older donor age, and increased CIT as independent risk factors for graft loss. CIT was not different between Native Hawaiian donors and non-Native Hawaiian donors, 9.7 h vs. 8.6 h, p = 0.132. Recipients receiving DCD grafts were 1.7 times more likely to lose their graft compared to DBD grafts, p = 0.010, and 1.3- times more compared LD grafts, p = 0.410. Protective factors against graft loss included Asian recipient ethnicity and recent transplantation era. We represented the distribution of groups within the different era (Supplementary Figure S2A) and confirmed the improvement of outcomes, overall and for DCD-LT, DBD-LT, and LD-LT independently (Supplementary Figures S2B,C). We confirmed that Graft survival at 1- and 5-year were 88.6% and 70.0% for DCD-LT, 92.6% and 79.9% for DBD-LT, and, 91.7% and 82.9% for LD-LT. Kaplan-Meier graft survival curves are shown in Figure 2.
TABLE 2
| Variables | Univariate analysisa | Multivariate analysisb | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Recipient factors | ||||||
| Age at transplant, years | 1.0 | 1.0–1.0 | 0.029 | 1.0 | 1.0–1.0 | 0.002 |
| Gender, male | 1.0 | 0.9–1.2 | 0.323 | NA | NA | NA |
| Pretransplant BMI, kg/m2 | 1.0 | 1.0–1.0 | 0.109 | NA | NA | NA |
| Race/Ethnicity | ||||||
| American Indian | 1.3 | 0.8–2.2 | 0.368 | NA | NA | NA |
| Asian | 0.8 | 0.7–1.0 | 0.014 | 0.8 | 0.7–1.0 | 0.013 |
| African American | 1.2 | 0.9–1.5 | 0.126 | NA | NA | NA |
| Native Hawaiian | 0.5 | 0.2–1.2 | 0.123 | NA | NA | NA |
| Hispanic | 0.9 | 0.8–1.0 | 0.073 | 0.9 | 0.7–1.0 | 0.128 |
| Multiracial | 0.6 | 0.2–1.6 | 0.288 | NA | NA | NA |
| Etiology | ||||||
| Auto-immune | 0.8 | 0.5–1.1 | 0.099 | 0.8 | 0.6–1.1 | 0.217 |
| Amyloidosis | 0.1 | 0.0 - NR | 0.797 | NA | NA | NA |
| Biliary atresia | 1.8 | 0.4–7.2 | 0.412 | NA | NA | NA |
| Cholangiocarcinoma | 3.8 | 1.4–10.2 | 0.008 | 4.4 | 1.6–11.8 | 0.004 |
| Cryptogenic | 1.1 | 0.9–1.4 | 0.217 | NA | NA | NA |
| EtOH | 1.1 | 0.9–1.3 | 0.499 | NA | NA | NA |
| HBV | 0.9 | 0.7–1.1 | 0.272 | NA | NA | NA |
| HCV | 1.2 | 1.0–1.3 | 0.026 | 1.1 | 1.0–1.3 | 0.071 |
| NASH | 0.8 | 0.5–1.1 | 0.178 | NA | NA | NA |
| PBC | 1.0 | 0.8–1.3 | 0.926 | NA | NA | NA |
| PSC | 1.0 | 0.8–1.3 | 0.873 | NA | NA | NA |
| Wilson | 0.1 | 0.2–1.0 | 0.049 | 0.0 | 0.0 - NA | 0.862 |
| A1AT | 0.4 | 0.1–1.5 | 0.173 | NA | NA | NA |
| Other/unknown | 0.9 | 0.8–1.1 | 0.253 | NA | NA | NA |
| HCC | 1.1 | 0.9–1.3 | 0.331 | NA | NA | NA |
| MELD | 1.0 | 1.0–1.0 | 0.037 | 1.0 | 1.0–1.0 | 0.945 |
| Era | ||||||
| 1990–2000 | NA | 1 [Reference] | NA | NA | NA | NA |
| 2001–2010 | 0.7 | 0.6–0.8 | <0.001 | 0.7 | 0.6–0.8 | <0.001 |
| 2011–2018 | 0.6 | 0.5–0.8 | <0.001 | 0.6 | 0.5–0.7 | <0.001 |
| Donor factors | ||||||
| Donor type | ||||||
| DCD | NA | 1 [Reference] | NA | NA | NA | NA |
| DBD | 0.8 | 0.6–1.2 | 0.250 | 0.6 | 0.4–0.9 | 0.010 |
| LD | 0.7 | 0.5–1.1 | 0.153 | 0.8 | 0.5–1.4 | 0.403 |
| Age, years | 1.0 | 1.0–1.0 | <0.001 | 1.0 | 1.0–1.0 | <0.001 |
| Gender, male | 0.9 | 0.8–1.1 | 0.304 | NA | NA | NA |
| BMI, kg/m2 | 1.0 | 1.0–1.0 | 0.737 | NA | NA | NA |
| Race/Ethnicity | ||||||
| American Indian | 0.7 | 0.3–2.0 | 0.537 | NA | NA | NA |
| Asian | 1.0 | 0.8–1.2 | 0.794 | NA | NA | NA |
| African American | 1.2 | 0.9–1.4 | 0.185 | NA | NA | NA |
| Hispanic | 1.0 | 0.8–1.1 | 0.571 | NA | NA | NA |
| Multiracial | 0.6 | 0.3–1.4 | 0.217 | NA | NA | NA |
| Native Hawaiian | 1.6 | 1.0–2.7 | 0.073 | 2.2 | 1.3–3.6 | 0.004 |
| Unknown | 0.8 | 0.3–2.1 | 0.631 | NA | NA | NA |
| White | 1.0 | 0.9–1.1 | 0.991 | NA | NA | NA |
| Cause of death | ||||||
| Anoxia | 0.8 | 0.8–0.9 | 0.010 | 0.9 | 0.7–1.1 | 0.332 |
| Cerebrovascular | 1.2 | 1.1–1.3 | 0.004 | 1.0 | 0.8–1.1 | 0.580 |
| Head trauma | 1.0 | 0.8–1.1 | 0.434 | NA | NA | NA |
| CNS tumor | 1.2 | 0.4–3.1 | 0.758 | NA | NA | NA |
| Other | 1.1 | 0.9–1.3 | 0.566 | NA | NA | NA |
| Not reported | 0.9 | 0.7–1.1 | 0.291 | NA | NA | NA |
| Cold ischemic time, hours | 1.0 | 1.0–1.0 | 0.015 | 1.0 | 1.0–1.0 | 0.026 |
Estimated hazard ratios for liver graft survival using a uni-/multivariate Cox proportional hazard model.
DCD, Donation after cardiac death; DBD, donation after brainstem death; LD, living donor; LT, liver transplantation; BMI, body mass index, EtOH, ethanol use; HBV, hepatitis B virus; HCV, hepatitis C virus; NASH, nonalcoholic steatohepatitis, PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis; A1AT, alpha-1 antitrypsin; MELD, Model For End-Stage Liver Disease; CNS, central nervous system; BMI, body mass index; CI, confidence interval; HR, hazard ratio; NR, not reported (values superior to 106); DCD, donation after cardiac death; DBD, donation after brainstem death (DBD); LD, living donor.
Univariate Cox proportional-hazards regression model.
Multivariate Cox regression model. Only those variables with p < 0.1 or of key clinical interest (graft type) in the univariate analysis were entered in the multivariate analysis.
FIGURE 2
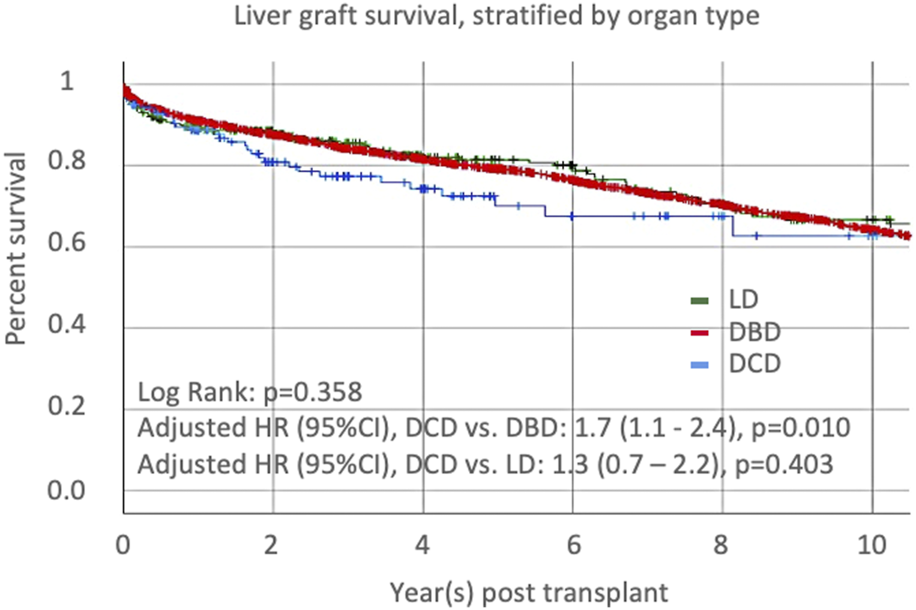
Liver graft survival, stratified by organ type: donation after cardiac death (DCD), donation after brainstem death (DBD), or living donor liver transplantation (LD).
Outcomes of donor after cardiac death liver transplant recipients compared to paired donation after brain death and living donor recipients.
Three groups of 138 LT recipients were constituted based on graft donation type (Table 3). Propensity matching allowed correction for most of the baseline variables between DCD and DBD donor/recipient characteristics. The etiology of liver disease, MELD score, HCC status, and era remained significantly different between groups. Out of 318 LD-LT recipients, 138 were randomly selected to be compared to DCDs. The sensitivity analysis included 265 LD-LT recipients. The baseline differences between the whole dataset and either the randomly matched or the propensity score-matched group remained unchanged. The differences in organ survival curves between the three donor types were mostly unchanged compared to the whole dataset (Supplementary Figure S1). Overall, eighteen LT recipients (4.3%, 18/414) had arterial complications (thrombosis and stenosis; no difference between groups). Eighteen patients (4.3%) were retransplanted, and there was no significant difference in retransplant rate between groups.
TABLE 3
| Characteristics | DCD LT (n = 138) | DBD LT (n = 138) | LD LT (n = 138) | P-valuea | P-valueb |
|---|---|---|---|---|---|
| Recipient | |||||
| Age at tx. years | 57.5 ± 9.0 | 57.8 ± 10.6 | 55.0 ± 11.2 | 0.797 | 0.041 |
| (min–max) | (22–75) | (18–72) | (18–75) | ||
| Gender (%) | |||||
| Male | 103 (74.6) | 98 (71.0) | 67 (48.6) | 0.499 | <0.001 |
| Female | 35 (25.4) | 40 (29.0) | 71 (51.4) | ||
| Pretransplant BMI. kg/m2 | 27.9 ± 5.9 | 27.4 ± 5.6 | 25.8 ± 4.3 | 0.478 | 0.001 |
| Race/Ethnicity (%) | |||||
| White | 75 (54.3) | 64 (46.4) | 78 (56.5) | 0.053 | 0.583 |
| African American | 4 (2.9) | 15 (10.9) | 6 (4.3) | ||
| Hispanic | 38 (27.5) | 29 (21.0) | 41 (29.7) | ||
| Asian | 18 (13.0) | 27 (19.6) | 12 (8.7) | ||
| Hawaii | 1 (0.7) | 2 (1.4) | 1 (0.7) | ||
| American Indian | 2 (1.4) | 1 (0.7) | 0 (0.0) | ||
| Etiology | |||||
| A1AT | 1 (0.7) | 1 (0.7) | 2 (1.4) | <0.001 | <0.001 |
| Auto-immune | 4 (2.9) | 0 (0.0) | 7 (5.1) | ||
| Amyloidosis | 1 (0.7) | 0 (0.0) | 0 (0.0) | ||
| Biliary atresia | 1 (0.7) | 0 (0.0) | 2 (1.4) | ||
| Cholangiocarcinoma | 2 (1.4) | 2 (1.4) | 0 (0.0) | ||
| Cryptogenic | 3 (2.2) | 8 (5.8) | 6 (4.3) | ||
| EtOH | 29 (21.0) | 4 (2.9) | 23 (16.7) | ||
| HBV | 12 (8.7) | 18 (13.0) | 11 (8.0) | ||
| HCV | 68 (49.3) | 76 (55.1) | 31 (22.5) | ||
| NASH | 9 (6.5) | 5 (3.6) | 19 (13.8) | ||
| Other | 0 (0.0) | 3 (2.2) | 12 (8.7) | ||
| PBC | 2 (1.4) | 0 (0.0) | 11 (8.0) | ||
| PSC | 3 (2.2) | 17 (12.3) | 14 (10.1) | ||
| Wilson | 3 (2.2) | 4 (2.9) | 0 (0.0) | ||
| HCC | |||||
| Presence | 40 (29.0) | 60 (43.5) | 27 (19.6) | 0.012 | 0.068 |
| Absence | 98 (71.0) | 78 (56.5) | 111 (80.4) | ||
| MELD | 22.8 ± 11.1 | 34.2 ± 5.9 | 18.6 ± 7.5 | <0.001 | <0.001 |
| Era | |||||
| 1990–2000 | 0 (0.0) | 19 (13.8) | 0 (0.0) | <0.001 | <0.001 |
| 2001–2010 | 26 (18.8) | 31 (22.5) | 1 (0.7) | ||
| 2011–2018 | 112 (81.2) | 88 (63.8) | 137 (99.3) | ||
| Donor factors | |||||
| Age, years | 31.7 ± 10.3 | 31.5 ± 13.5 | 35.7 ± 10.5 | 0.912 | 0.001 |
| Gender (%) | |||||
| Male | 92 (66.7) | 87 (63.0) | 64 (46.4) | 0.528 | 0.001 |
| Female | 46 (33.3) | 51 (37.0) | 74 (53.6) | ||
| BMI, kg/m2 | 25.5 ± 5.2 | 25.9 ± 7.1 | 25.6 ± 4.0 | 0.533 | 0.849 |
| Race/Ethnicity (%) | |||||
| American Indian | 0 (0.0) | 2 (1.4) | 0 (0.0) | 0.293 | 0.157 |
| Asian | 4 (2.9) | 8 (5.8) | 12 (8.7) | ||
| African American | 11 (8.0) | 11 (8.0) | 5 (3.6) | ||
| Hispanic | 34 (24.6) | 43 (31.2) | 39 (28.3) | ||
| Multiracial | 4 (2.9) | 2 (1.4) | 4 (2.9) | ||
| Hawaii | 0 (0.0) | 1 (0.7) | 1 (0.7) | ||
| White | 85 (61.6) | 71 (51.4) | 77 (55.8) | ||
| Cause of death | |||||
| Anoxia | 73 (52.9) | 40 (29.0) | NA | <0.001 | NA |
| Cerebrovascular | 17 (12.3) | 35 (25.4) | |||
| Head trauma | 41 (29.7) | 62 (44.9) | |||
| Not reported | 7 (5.1) | 1 (0.7) | |||
| Cold ischemic time, hours | 7.7 ± 2.6 | 7.6 ± 3.5 | 2.1 ± 1.5 | 0.680 | <0.001 |
Recipient and donor baseline characteristics of donation after cardiac death donation, and matched/paired control recipients receiving a graft after brainstem death and living donor.
Data are presented as mean ± standard deviation or n (%).
DCD, donation after cardiac death; DBD, donation after brainstem death; LD, living donor; LT, liver transplantation; BMI, body mass index, EtOH, ethanol use; HBV, hepatitis B virus; HCV, hepatitis C virus; NASH, nonalcoholic steatohepatitis; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis; A1AT, alpha-1 antitrypsin; MELD, Model For End-Stage Liver Disease; CNS, central nervous system.
DCD versus DBD.
DCD versus LD. Student t-test for continuous variables, X2 test for binary or categorical variables (global p-value).
Biliary Complications
We studied the occurrence of anastomotic and non-anastomotic stricture and bile leak in the 414 adult recipients selected, as noted above. Anastomotic biliary strictures occurred in 28.3% of DCD recipients. Compared to DCD-LT, DBD recipients had fewer anastomotic strictures (18.1%), and LD recipients had more anastomotic strictures (43.5%) (Table 4; Figure 3A). Non-anastomotic biliary strictures developed in 15.2% of DCD recipients versus 1.4% of DBD recipients and 4.3% of LD recipients (Table 4; Figure 3B). NASs were observed much sooner after transplant in DCDs (median: 59 days) compared to DBDs (median: 409 days) or LDs (median: 172 days). Bile leak was observed in 10.1% of DCD recipients versus 7.2% of DBD recipients and 36.2% of LD recipients (Table 4; Figure 3C). Bile leaks usually occur between two to four weeks post-transplant. Patients who had leaks (regardless of the donor group) had more than a 4-time risk of developing AS and a three-time risk of developing NAS [HR (95%CI), 4.4 (3.1–6.4), p < 0.001 and HR (95%CI), 3.5 (1.7–7.4), p = 0.001), respectively]. Graft survival in the three groups (n = 414) was further stratified by organ type and biliary complication occurrence (none versus any) (Figure 3D). LD-LTs free of any biliary complications had the best graft survival, whereas DCD-LTs with ≥1 biliary complication (presence of a bile leak and/or AS and/or NAS) had the worst graft survival (global p = 0.018) (Figure 3D). Among the 21 DCD-LT patients with non-anastomotic strictures, six died (contraindication to retransplantation), two were retransplanted, three remain stent dependent, and notably, half (n = 10) are ultimately stent-free. LT recipients with NAS had worse graft survival compared to the NAS-free patients (p < 0.05). Patients with NAS had a median (IRQ) of 7 (5–10) ERCPs. We searched potential risk factors for any biliary complications in the matched/paired cohort (n = 414) (Table 5). After multivariate adjustment, the use of DBD grafts/donors with head trauma were found to be a protective factor against the occurrence of biliary complication(s). Higher donor BMI was associated with more biliary complications.
TABLE 4
| Characteristics | DCD LT (n = 138) | DBD LT (n = 138) | LD LT (n = 138) | Odds ratio P-valuea | Odds ratio P-valueb |
|---|---|---|---|---|---|
| Anastomotic biliary stricture | 39 (28.3) | 25 (18.1) | 60 (43.5) | 0.5 (0.3–0.9) | 1.8 (1.2–2.6) |
| Time to stricture, d (min–max) | 87.7 (0.0–2,191.5) | 98.6 (0.0–5,113.5) | 54.8 (0.0–1,826.3) | 0.018 | 0.006 |
| Non-anastomotic biliary stricture | 21 (15.2) | 2 (1.4) | 6 (4.3) | 0.1 (0.0–0.4) | 0.3 (0.1–0.7) |
| Time to stricture, d (min–max) | 59.0 (24.0–551.0) | 409.0 (53.0–765.0) | 172.0 (18.0–722.0) | 0.001 | 0.005 |
| Bile leak (%) | 14 (10.1) | 10 (7.2) | 50 (36.2) | 0.7 (0.3–1.6) | 4.2 (2.3–7.7) |
| Time to bile leak, d (min–max) | 27.5 (1.0–334.0) | 16.5 (6.0–199.0) | 17.5 (1.0–169.0) | 0.402 | <0.001 |
Biliary complications in liver transplant recipients after receiving a liver from a cardiac death donor, a brainstem death donor, or living donor.
Data are presented as median (minimum–maximum) or n (%).
DCD, donation after cardiac death; DBD, donation after brainstem death; LD, living donor; LT, liver transplantation.
DBD versus DCD.
LD versus DCD. Student t-test for continuous variables, X2 test for binary variables.
FIGURE 3
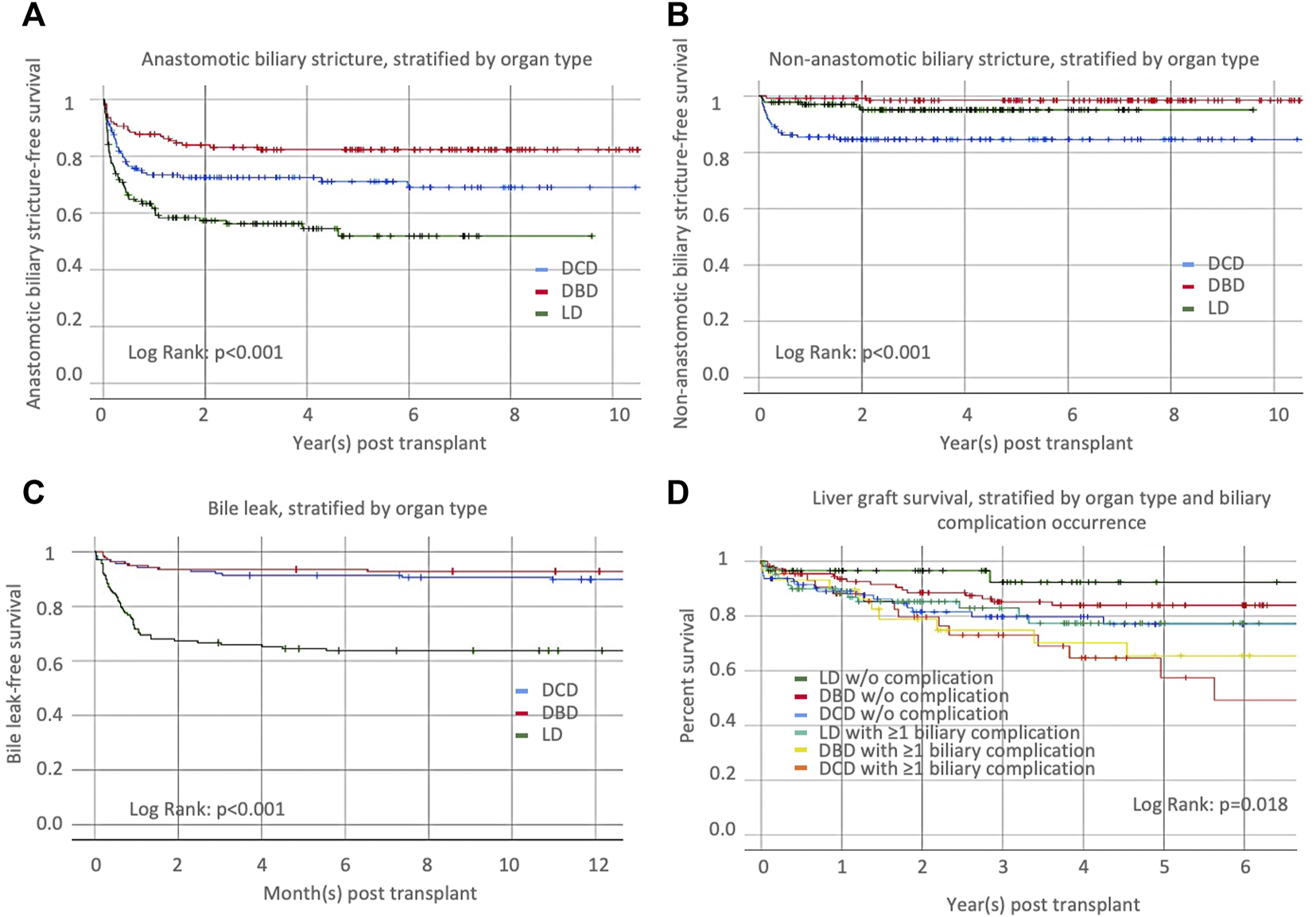
Occurrence of (A) anastomotic biliary stricture, (B) non-anastomotic biliary stricture, and (C) bile leaks, stratified by organ type [donation after cardiac death (DCD), donation after brainstem death (DBD), or living donor liver transplantation (LD)]. (D) Liver graft survival, stratified by organ type and biliary complication occurrence.
TABLE 5
| Variables | Univariate analysisa | Multivariate analysisb | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Recipient factors | ||||||
| Age at transplant, years | 1.0 | 1.0–1.0 | 0.427 | NA | NA | NA |
| Gender, male | 0.9 | 0.6–1.2 | 0.398 | NA | NA | NA |
| Pretransplant BMI, kg/m2 | 1.0 | 1.0–1.0 | 0.960 | NA | NA | NA |
| Race/Ethnicity | ||||||
| American Indian | 1.5 | 0.4–5.9 | 0.602 | NA | NA | NA |
| Asian | 0.6 | 0.4–1.0 | 0.069 | 1.2 | 0.6–2.2 | 0.637 |
| African American | 1.0 | 0.5–1.9 | 0.944 | NA | NA | NA |
| Native Hawaiian | 0.6 | 0.1–4.5 | 0.639 | NA | NA | NA |
| Hispanic | 1.3 | 0.9–1.9 | 0.115 | NA | NA | NA |
| Etiology | ||||||
| Auto-immune | 2.2 | 1.1–4.6 | 0.026 | 1.3 | 0.6–2.9 | 0.437 |
| Amyloidosis | 0.1 | 0.0–NA | 0.650 | NA | NA | NA |
| Biliary atresia | 1.1 | 0.2–7.8 | 0.934 | NA | NA | NA |
| Cholangiocarcinoma | 1.5 | 0.4–6.0 | 0.581 | NA | NA | NA |
| Cryptogenic | 1.1 | 0.5–2.4 | 0.781 | NA | NA | NA |
| EtOH | 1.2 | 0.8–1.8 | 0.486 | NA | NA | NA |
| HBV | 0.4 | 0.2–0.9 | 0.020 | 0.4 | 0.2–1.1 | 0.067 |
| HCV | 0.7 | 0.5–1.0 | 0.079 | 0.9 | 0.6–1.4 | 0.768 |
| NASH | 1.1 | 0.6–1.9 | 0.804 | NA | NA | NA |
| PBC | 2.4 | 1.1–5.0 | 0.027 | 1.4 | 0.6–3.2 | 0.396 |
| PSC | 1.1 | 0.6–1.9 | 0.713 | NA | NA | NA |
| Wilson | 0.7 | 0.2–2.8 | 0.623 | NA | NA | NA |
| A1AT | 2.8 | 0.9–8.7 | 0.082 | 2.7 | 0.8–8.8 | 0.108 |
| Other/unknown | 2.1 | 1.1–3.9 | 0.027 | 1.3 | 0.6–2.6 | 0.477 |
| HCC | 0.7 | 0.5–1.0 | 0.033 | 0.9 | 0.6–1.4 | 0.693 |
| MELD | 1.0 | 1.0–1.0 | <0.001 | 1.0 | 1.0–1.0 | 0.785 |
| Era | ||||||
| 1990–2000 | NA | 1 [Reference] | NA | NA | NA | NA |
| 2001–2010 | 2.7 | 0.8–9.7 | 0.115 | 1.3 | 0.3–5.0 | 0.737 |
| 2011–2018 | 4.0 | 1.2–13.2 | 0.024 | 1.0 | 0.3–3.8 | 0.993 |
| Donor factors | ||||||
| Donor type | ||||||
| DCD | NA | 1 [Reference] | NA | NA | NA | NA |
| DBD | 0.6 | 0.4–0.9 | 0.022 | 0.6 | 0.3–1.0 | 0.049 |
| LD | 2.4 | 1.7–3.5 | <0.001 | 1.7 | 0.9–3.1 | 0.094 |
| Age, years | 1.0 | 1.0–1.0 | 0.002 | 1.0 | 1.0–1.0 | 0.260 |
| Gender, male | 0.7 | 0.5–1.0 | 0.030 | 0.9 | 0.7–1.3 | 0.729 |
| BMI, kg/m2 | 1.0 | 1.0–1.1 | 0.028 | 1.0 | 1.0–1.1 | 0.029 |
| Race/Ethnicity | ||||||
| American Indian | 0.0 | 0.0–388.7 | 0.511 | NA | NA | NA |
| Asian | 1.1 | 0.6–2.1 | 0.731 | NA | NA | NA |
| African American | 1.3 | 0.7–2.3 | 0.437 | NA | NA | NA |
| Hispanic | 1.0 | 0.7–1.4 | 0.994 | NA | NA | NA |
| Multiracial | 0.5 | 0.1–1.9 | 0.299 | NA | NA | NA |
| Native Hawaiian | 1.5 | 0.2–11.1 | 0.664 | NA | NA | NA |
| White | 1.0 | 0.7–1.4 | 0.916 | NA | NA | NA |
| Cause of death | ||||||
| Anoxia | 0.6 | 0.4–0.8 | 0.005 | 0.6 | 0.3–1.1 | 0.094 |
| Cerebrovascular | 1.1 | 0.7–1.7 | 0.659 | NA | NA | NA |
| Head trauma | 0.4 | 0.2–0.6 | <0.001 | 0.5 | 0.3–0.9 | 0.028 |
| Other | 0.6 | 0.1–2.4 | 0.462 | NA | NA | NA |
| Cold ischemic time, hours | 0.9 | 0.9–0.9 | 0.000 | 1.0 | 1.0–1.1 | 0.249 |
Estimated hazard ratios for biliary complication (any versus none) using a uni-/multivariate Cox proportional hazard model.
DCD, donation after cardiac death; DBD, donation after brainstem death; LD, living donor; LT, liver transplantation; BMI, body mass index, EtOH, ethanol use; HBV, hepatitis B virus; HCV, hepatitis C virus; NASH, nonalcoholic steatohepatitis; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis; A1AT, alpha-1 antitrypsin; MELD, Model For End-Stage Liver Disease; CNS, central nervous system; BMI, body mass index; CI, confidence interval; HR, hazard ratio; NR, not reported (values superior to 105); DCD, donation after cardiac death; DBD, donation after brainstem death (DBD); LD, living donor.
Univariate Cox proportional-hazards regression model.
Multivariate Cox regression model. Only those variables with p < 0.1 in the univariate analysis were entered in the multivariate analysis.
Discussion
Liver transplantation using DCD or LD donors is limited to a minority of centers because of the higher rates of ischemic cholangiopathy (DCD-LTs) or biliary complications (DCD and LD-LTs) compared with grafts from DBD donors [7, 11, 14, 23, 27]. Nevertheless, DCD- and LD-LTs often represent the only life-saving option for specific liver recipient candidates in a MELD-based allocation system. For these patients, the benefits of receiving a DCD or LD graft have the potential to increase survival and quality of life compared to staying on the transplant waiting list. Over the last few years, refinement in donor and recipient selection has allowed a significant improvement in outcomes for DCD-LT [11, 12, 14]. However, for many patients, waiting for a DBD, involving an LD, or taking a DCD offer remains a common dilemma. We thus sought to analyze and compare the outcomes and biliary complications of DCDs to DBDs and LDs in a single-center LT recipient population.
We first observed and confirmed the known increased risk of graft loss (HR of 1.7) in recipients receiving DCD livers compared to those receiving DBD grafts, which matches with previously reported risk [28, 29], although the most recent cohort studies suggest this HR was further lowered [30, 31]. The comparison between DCD-LTs and LD-LTs was not significant, possibly due to the lower number of patients in the latter group. Nevertheless, graft survival curves showed that all three categories converged over time, suggesting that DCD is an acceptable alternative when no other organ is available. The graft survival rates for the three categories matches those observed and reported by the Toronto group in a similar analysis [32]. We identified other important predictors of graft loss and patient death in our multivariate analysis, including donor and recipient age, transplantation era, presence of cholangiocarcinoma, and cold ischemia time, considering previously described risk factors [28]. We also highlighted a detrimental effect of donor Hawaiian ethnicity and a protective effect of recipient Asian ethnicity.
The nature and frequency of biliary complications are what differentiate most long-term outcomes in DCD-LT versus DBD-LT or LD-LT. A focused analysis led us to study biliary complications in 414 recipients, including one-third of each donor type. Non-anastomotic biliary stricture developed in 15.2% of DCD recipients, which aligns with what is reported in the literature [7, 8, 11]. This complication was exceptional in DBD-LT or LD-LT in the absence of an arterial supply problem. There was a slight increase in anastomotic biliary strictures and bile leaks in DCD-LT recipients compared to DBD-LT recipients, but the increase was not prohibitive. Living donor recipients had a higher number and completely different pattern of biliary complications compared to the two other groups, as previously reported [32]. They were much more affected with anastomotic biliary strictures (43.5%) and bile leaks (36.2%) compared to DCD-/DBD-LT recipients. It is worth noting that recipients with bile leaks are the group that typically get strictures. Taken together, the type of transplant and the presence of biliary complications had an impact on organ survival. The best 1- and 5-year graft survival were achieved in LD recipients without biliary complication and the worst in DCD recipients with any type of biliary complication. This was further confirmed in a multivariate analysis where DBD grafts/donors with head trauma were the only protective factors against the occurrence of biliary complication(s). It is unclear why higher donor BMI was associated with more biliary complications. This could be a marker of graft quality (steatosis) which could have an impact on the magnitude of ischemia-reperfusion injury and biliary microcirculation damage. However, it is important to note that the magnitude of this association was limited.
The development of NAS negatively affected graft survival; however, 50% of the patients with NAS ultimately kept their graft and remained stent free in the long term.
Overall, given the reported 1- and 5-year graft survival rates and biliary complication rates, it seems that both DCD-LT and LD-LT are viable options when DBD grafts are limited or unavailable. Successful LD selection is well codified, and biliary complication rates vary between different centers [23]. Similarly, DCD donor and recipient selection criteria are center-dependent and may affect survival outcomes and the rate of biliary complications [6–9]. Large discrepancies exist in DCD utilization, the most striking one being the difference between the United States and the United Kingdom: DCD LT currently accounts for about 8% of all deceased donor LTs in the US versus 19% in the United Kingdom [9]. In our center, general DCD selection criteria included donor age younger than 60, an estimated CIT lower than 8 h, dWIT<30 min, and a recipient with a MELD score lower than the average. Several DCD scores [11, 12, 14], including ours [13], have been published to further standardize practices and ensure the best outcomes; however, local constraints (travel distance, local MELD, etc.) and practices can make these scores hard to follow in a global and protocoled manner.
Our study has limitations. We report on a retrospective cohort; thus, information bias and selection bias cannot be totally avoided. It is noteworthy that the number of missing data was low, therefore limiting information bias. Another point is that our study extends over a large period (especially for DBDs and LDs); therefore, we cannot totally exclude bias related to the evolution of surgical technique, donor/recipient selection practices, and recipient management policies. To account for this, we used “era” as an independent study variable in our multivariate analysis. Interestingly, era was significantly associated with graft survival but not with biliary complications. Moreover, the low number of DCDs and LDs and the fact that the evolution of techniques is a continuum prompted us to consider a larger study period. However, to limit its impact, we restricted the matched/paired analysis to the 2003–2019 period. Another limitation is that our conclusions are based on a single-center data analysis and should be confirmed in a multicenter cohort. From a broader perspective, it is also worth noting that the increasing use of machine perfusion devices for DCDs may change the rate and nature of complications in the future [10, 33–36]. Normothermic regional perfusion [37–39] and hypothermic oxygenated perfusion [40] might indeed have an impact on the prevention of biliary complications. To date, it remains to be demonstrated whether the ex situ perfusion technologies will lead to a significant risk modification that is proportional to the costs and logistic difficulties of their use and/or if this risk modification can be achieved through better organ selection. Nevertheless, the choice of proceeding with an LD versus waiting for a DCD or a DBD graft to become available will remain a point of discussion globally and at the individual patient level.
In conclusion, we exposed the differential incidence and effect of biliary complications on the outcomes after liver transplantation using brain-dead donors, donors after circulatory death, and living donors. We demonstrated that LD-LT achieved the best 1-and 5-year graft survival, and DCD-LT achieved excellent graft survival in the absence of biliary complications. DCD-LT is expected to become an equivalent alternative to DBD- and LD-LT given the further reduction of ischemia-reperfusion injury and biliary microcirculation damage offered by machine and regional perfusion systems.
Statements
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Review Board of the University of California, San Francisco. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
RM and GR designed the study. RM, YK, CF, NA, JR, and GR collected the data. RM analyzed the data. RM performed the statistical analysis. RM, YK, DM, CF, NA, JR, and GR interpreted the data and wrote the manuscript. RM and GR had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
Statistical support was provided by the Biostatistics Core, which is funded by the Liver Center P30 DK026743.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/ti.2022.10855/full#supplementary-material
Supplementary Figure S1Liver graft survival, stratified by organ type: donation after cardiac death (DCD), donation after brainstem death (DBD), or living donor liver transplantation (LD) in the matched/paired cohort.
Supplementary Figure S2(A) Relative numbers of liver transplant (%) in the unmatched and matched cohorts. (B) Liver graft survival stratified by era (all liver transplant types). (C) Liver graft survival, stratified by organ type: donation after cardiac death (DCD), donation after brainstem death (DBD), or living donor liver transplantation (LD), and within the different era.
References
1.
Emamaullee J Conrad C Kim M Goldbeck C Kwon Y Singh P et al Assessment of the Global Practice of Living Donor Liver Transplantation. Transpl Int (2021) 34:1914–27. 10.1111/tri.13960
2.
Wanis KN Sarvet A Ruffolo LI Levstik MA Tomiyama K Al-Judaibi BM et al Estimating the Effect of Increasing Utilization of Living Donor Liver Transplantation Using Observational Data. Transpl Int (2021) 34:648–56. 10.1111/tri.13835
3.
Monbaliu D Pirenne J Talbot D . Liver Transplantation Using Donation after Cardiac Death Donors. J Hepatol (2012) 56:474–85. 10.1016/j.jhep.2011.07.004
4.
Passamonti SM Cannavo A Panigada M Trunzo V Bottazzi A Longobardi A et al Donation after Circulatory Death and Liver Transplantation: a Cohort Study. Transpl Int (2021) 34:1271–80. 10.1111/tri.13919
5.
Callaghan CJ Charman SC Muiesan P Powell JJ Gimson AE van der Meulen JH et al Outcomes of Transplantation of Livers from Donation after Circulatory Death Donors in the UK: a Cohort Study. BMJ Open (2013) 3:e003287. 10.1136/bmjopen-2013-003287
6.
Foley DP Fernandez LA Leverson G Anderson M Mezrich J Sollinger HW et al Biliary Complications after Liver Transplantation from Donation after Cardiac Death Donors: an Analysis of Risk Factors and Long-Term Outcomes from a Single center. Ann Surg (2011) 253:817–25. 10.1097/SLA.0b013e3182104784
7.
Jay CL Lyuksemburg V Ladner DP Wang E Caicedo JC Holl JL et al Ischemic Cholangiopathy after Controlled Donation after Cardiac Death Liver Transplantation: a Meta-Analysis. Ann Surg (2011) 253:259–64. 10.1097/SLA.0b013e318204e658
8.
Goussous N Alvarez-Casas J Dawany N Xie W Malik S Gray SH et al Ischemic Cholangiopathy Postdonation after Circulatory Death Liver Transplantation: Donor Hepatectomy Time Matters. Transpl Direct (2022) 8:e1277. 10.1097/M10000000000001277
9.
Kubal C Roll GR Ekser B Muiesan P . Donation after Circulatory Death Liver Transplantation: What Are the Limits for an Acceptable DCD Graft?Int J Surg (2020) 82:36–43. 10.1016/j.ijsu.2020.04.064
10.
Markmann JF Abouljoud MS Ghobrial RM Bhati CS Pelletier SJ Lu AD et al Impact of Portable Normothermic Blood-Based Machine Perfusion on Outcomes of Liver Transplant the OCS Liver PROTECT Randomized Clinical Trial. Jama Surg (2022) 157:189–98. 10.1001/jamasurg.2021.6781
11.
Hong JC Yersiz H Kositamongkol P Xia VW Kaldas FM Petrowsky H et al Liver Transplantation Using Organ Donation after Cardiac Death: a Clinical Predictive index for Graft Failure-free Survival. Arch Surg (2011) 146:1017–23. 10.1001/archsurg.2011.240
12.
Khorsandi SE Giorgakis E Vilca-Melendez H O'Grady J Heneghan M Aluvihare V et al Developing a Donation after Cardiac Death Risk index for Adult and Pediatric Liver Transplantation. World J Transpl (2017) 7:203–12. 10.5500/wjt.v7.i3.203
13.
Meier RPH Kelly Y Yamaguchi S Braun HJ Lunow-Luke T Adelmann D et al Advantages and Limitations of Clinical Scores for Donation after Circulatory Death Liver Transplantation. Front Surg (2021) 8:808733. 10.3389/fsurg.2021.808733
14.
Schlegel A Kalisvaart M Scalera I Laing RW Mergental H Mirza DF et al The UK DCD Risk Score: A New Proposal to Define Futility in Donation-After-Circulatory-Death Liver Transplantation. J Hepatol (2018) 68:456–64. 10.1016/j.jhep.2017.10.034
15.
Coffey JC Wanis KN Monbaliu D Gilbo N Selzner M Vachharajani N et al The Influence of Functional Warm Ischemia Time on DCD Liver Transplant Recipients' Outcomes. Clin Transpl (2017) 31. 10.1111/ctr.13068
16.
Braun HJ Ascher NL Roll GR Roberts JP . Biliary Complications Following Living Donor Hepatectomy. Transpl Rev (Orlando) (2016) 30:247–52. 10.1016/j.trre.2016.07.003
17.
Olthoff KM Smith AR Abecassis M Baker T Emond JC Berg CL et al Defining Long-Term Outcomes with Living Donor Liver Transplantation in North America. Ann Surg (2015) 262:465–75. 10.1097/SLA.0000000000001383
18.
Darius T Rivera J Fusaro F Lai Q de Magnee C Bourdeaux C et al Risk Factors and Surgical Management of Anastomotic Biliary Complications after Pediatric Liver Transplantation. Liver Transpl (2014) 20:893–903. 10.1002/lt.23910
19.
Feier FH Chapchap P Pugliese R da Fonseca EA Carnevale FC Moreira AM et al Diagnosis and Management of Biliary Complications in Pediatric Living Donor Liver Transplant Recipients. Liver Transpl (2014) 20:882–92. 10.1002/lt.23896
20.
Feier FH Seda-Neto J da Fonseca EA Candido HLL Pugliese RS Neiva R et al Analysis of Factors Associated with Biliary Complications in Children after Liver Transplantation. Transplantation (2016) 100:1944–54. 10.1097/Tp.0000000000001298
21.
Sanada Y Katano T Hirata Y Yamada N Okada N Ihara Y et al Biliary Complications Following Pediatric Living Donor Liver Transplantation: Risk Factors, Treatments, and Prognosis. Transplantation (2019) 103:1863–70. 10.1097/Tp.0000000000002572
22.
Lo CM . Complications and Long-Term Outcome of Living Liver Donors: a Survey of 1, 508 Cases in Five Asian Centers. Transplantation (2003) 75:S12–5. 10.1097/01.TP.0000046534.45645.47
23.
Braun HJ Dodge JL Roll GR Freise CE Ascher NL Roberts JP . Impact of Graft Selection on Donor and Recipient Outcomes after Living Donor Liver Transplantation. Transplantation (2016) 100:1244–50. 10.1097/Tp.0000000000001101
24.
Hirose R Yao F Stock P Roberts J Ascher N . Liver Transplantation at UCSF-Aa 20-year Experience. Clin Transpl (2008) 119–25.
25.
Hashimoto K . Liver Graft from Donation after Circulatory Death Donor: Real Practice to Improve Graft Viability. Clin Mol Hepatol (2020) 26:401–10. 10.3350/cmh.2020.0072
26.
Wiesner RH McDiarmid SV Kamath PS Edwards EB Malinchoc M Kremers WK et al MELD and PELD: Application of Survival Models to Liver Allocation. Liver Transpl (2001) 7:567–80. 10.1053/jlts.2001.25879
27.
Selck FW Grossman EB Ratner LE Renz JF . Utilization, Outcomes, and Retransplantation of Liver Allografts from Donation after Cardiac Death: Implications for Further Expansion of the Deceased-Donor Pool. Ann Surg (2008) 248:599–607. 10.1097/SLA.0b013e31818a080e
28.
Feng S Goodrich NP Bragg-Gresham JL Dykstra DM Punch JD DebRoy MA et al Characteristics Associated with Liver Graft Failure: the Concept of a Donor Risk index. Am J Transpl (2006) 6:783–90. 10.1111/j.1600-6143.2006.01242.x
29.
Skaro AI Jay CL Baker TB Wang E Pasricha S Lyuksemburg V et al The Impact of Ischemic Cholangiopathy in Liver Transplantation Using Donors after Cardiac Death: the Untold story. Surgery (2009) 146:543–52. 10.1016/j.surg.2009.06.052
30.
Bohorquez H Seal JB Cohen AJ Kressel A Bugeaud E Bruce DS et al Safety and Outcomes in 100 Consecutive Donation after Circulatory Death Liver Transplants Using a Protocol that Includes Thrombolytic Therapy. Am J Transpl (2017) 17:2155–64. 10.1111/ajt.14261
31.
Croome KP Lee DD Keaveny AP Taner CB . Improving National Results in Liver Transplantation Using Grafts from Donation after Cardiac Death Donors. Transplantation (2016) 100:2640–7. 10.1097/TP.0000000000001483
32.
Kollmann D Sapisochin G Goldaracena N Hansen BE Rajakumar R Selzner N et al Expanding the Donor Pool: Donation after Circulatory Death and Living Liver Donation Do Not Compromise the Results of Liver Transplantation. Liver Transpl (2018) 24:779–89. 10.1002/lt.25068
33.
Nasralla D Coussios CC Mergental H Akhtar MZ Butler AJ Ceresa CDL et al A Randomized Trial of Normothermic Preservation in Liver Transplantation. Nature (2018) 557:50–6. 10.1038/s41586-018-0047-9
34.
Eshmuminov D Becker D Bautista Borrego L Hefti M Schuler MJ Hagedorn C et al An Integrated Perfusion Machine Preserves Injured Human Livers for 1 Week. Nat Biotechnol (2020) 38:189–98. 10.1038/s41587-019-0374-x
35.
Van Rijn R Schurink IJ de Vries Y van den Berg AP Cerisuelo MC Murad SD et al Hypothermic Machine Perfusion in Liver Transplantation - A Randomized Trial. N Engl J Med (2021) 384:1391–401. 10.1056/NEJMoa2031532
36.
Patrono D Zanierato M Vergano M Magaton C Diale E Rizza G et al Normothermic Regional Perfusion and Hypothermic Oxygenated Machine Perfusion for Livers Donated after Controlled Circulatory Death with Prolonged Warm Ischemia Time: A Matched Comparison with Livers from Brain-Dead Donors. Transpl Int (2022) 35:10390. 10.3389/ti.2022.10390
37.
Oniscu GC Watson CJE Wigmore SJ . Redefining Futility in DCD Liver Transplantation in the Era of Novel Perfusion Technologies. J Hepatol (2018) 68:1327–8. 10.1016/j.jhep.2018.02.028
38.
De Beule J Vandendriessche K Pengel LHM Bellini MI Dark JH Hessheimer AJ et al A Systematic Review and Meta-Analyses of Regional Perfusion in Donation after Circulatory Death Solid Organ Transplantation. Transpl Int (2021) 34:2046–60. 10.1111/tri.14121
39.
Hunt F Johnston CJC Coutts L Sherif AE Farwell L Stutchfield BM et al From Haphazard to a Sustainable Normothermic Regional Perfusion Service: A Blueprint for the Introduction of Novel Perfusion Technologies. Transpl Int (2022) 35:10493. 10.3389/ti.2022.10493
40.
Kron P Schlegel A Mancina L Clavien PA Dutkowski P . Hypothermic Oxygenated Perfusion (HOPE) for Fatty Liver Grafts in Rats and Humans. J Hepatol (2017) 68:82–91. 10.1016/j.jhep.2017.08.028
Summary
Keywords
liver transplantation, living donors, donation after brain death, donation after circulatory death, biliary anastomotic stricture, ischemic cholangiopathy, bile leak
Citation
Meier RPH, Kelly Y, Braun H, Maluf D, Freise C, Ascher N, Roberts J and Roll G (2022) Comparison of Biliary Complications Rates After Brain Death, Donation After Circulatory Death, and Living-Donor Liver Transplantation: A Single-Center Cohort Study. Transpl Int 35:10855. doi: 10.3389/ti.2022.10855
Received
22 August 2022
Accepted
23 November 2022
Published
09 December 2022
Volume
35 - 2022
Updates
Copyright
© 2022 Meier, Kelly, Braun, Maluf, Freise, Ascher, Roberts and Roll.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Raphael Pascal Henri Meier, rmeier@som.umaryland.edu
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.