Abstract
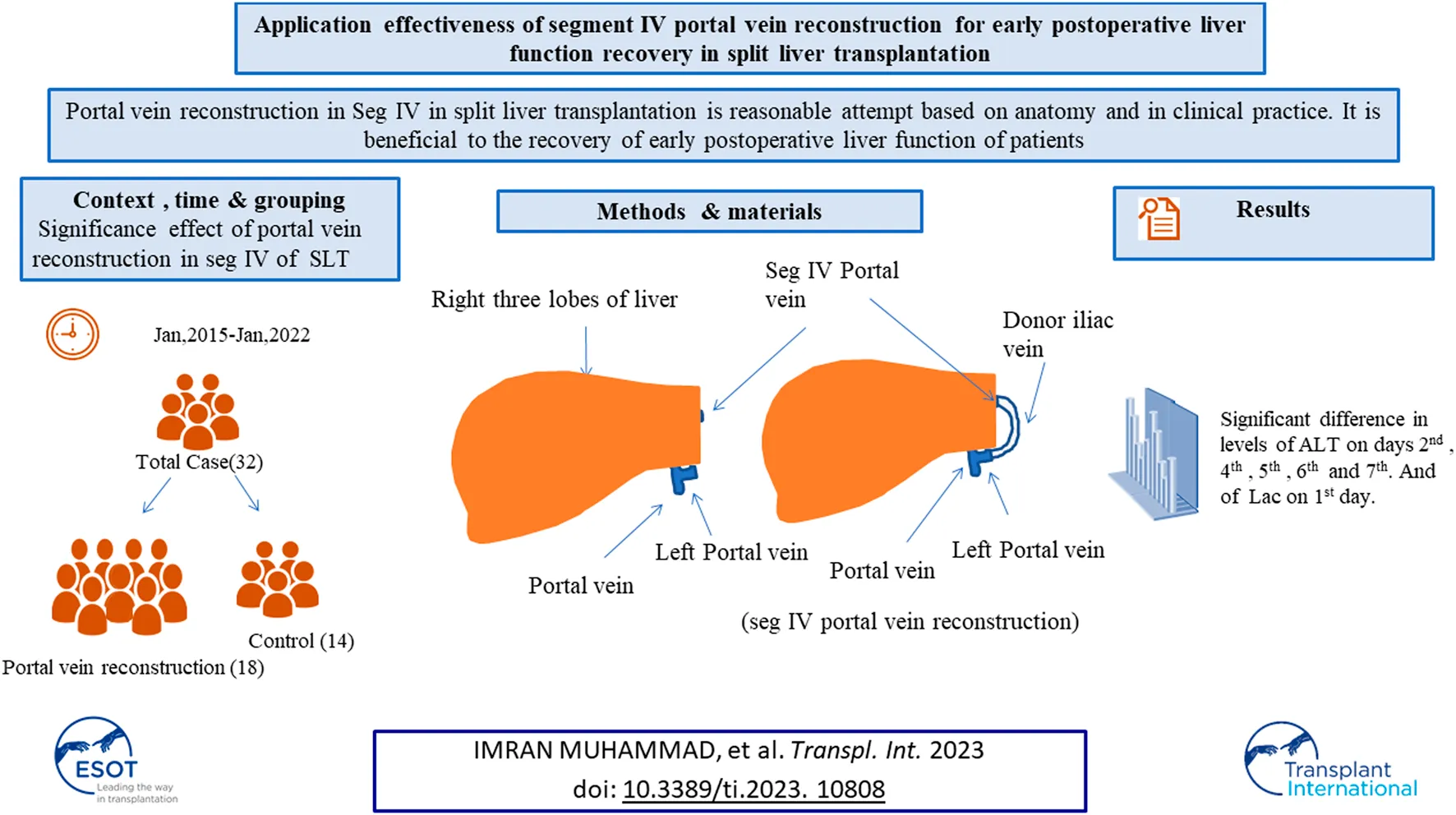
The objective of this study was to investigate the significance of portal vein reconstruction in segment IV of the liver on early postoperative liver function recovery in split liver transplantation. The clinical data of patients of right trilobe split liver transplantation in our center were analyzed and divided into two groups, including a group without portal vein reconstruction and a group with portal vein reconstruction. Clinical data of alanine aminotransferase (ALT), aspartate transaminase (AST), albumin (ALB), creatinine (Cr), total bilirubin (TB), alkaline phosphatase (ALP), gamma-glutamyl Transferase (GGT), lactic acid (Lac), and international normalized ratio (INR) levels were analyzed. The technique of segment IV portal vein reconstruction is beneficial to the early postoperative recovery of liver function. Statistically, there was no significant effect of portal vein reconstruction in the IV segment of the liver on the recovery of liver function within 1 week after split liver transplantation. There was no significant difference in survival rate between the control group and reconstruction group over the 6 months follow-up period after surgery.
Introduction
Since the first liver transplant was performed, with the continuous advancement of surgical techniques, extensive development, and clinical application of various new immunosuppressants, liver transplantation has become the most effective means of treating various end-stage liver diseases (1). Successful transplantation of a reduced volume liver to children, and right/left hemi-split liver transplantation performed on two adults have also been documented in the literature (2, 3). Expanding the source of donor livers has always been a major problem to be solved. According to statistics, the development of split liver transplantation can increase the number of donor livers, so it has become an important way for experts in the field of liver transplantation to solve the shortage of donor livers (4). In split liver transplantation, one donor liver is transplanted to two recipients, thereby expanding the source of donor livers.
There is no statistically significant difference in the graft and recipient survival rates at 1 year for those who have had whole liver transplantation and those who have undergone adult split liver transplantation (5, 6). In experienced transplantation institutions, split liver transplantation has a similar impact to whole liver transplantation, and its survival rate is comparable (7–9). The selection of donors and recipients is critical to the success of split liver transplantation. The ideal donor for splitting is someone who is young, has normal liver enzymes, hemodynamically stable, has no history of liver illness, and has a brief hospital stay (10, 11). Different donor splitting criteria have been suggested in previous studies, and they differ across nations and transplant institutions (12, 13).
A team disclosed two separate in situ split techniques for the fabrication of split grafts acceptable for two adult patients. In order to enhance the arterial supply to segment IV, they retain the common portal vein and the common hepatic duct with the right graft and the celiac axis with the left graft (14, 15). Another group released an evaluation, this time they described distinct anatomic situations following dissection of the portal subdivisions to segment IV, exposing the left hilar plate beneath the left portal vein, and surgery of the biliary ducts from segments II and III for traditional split liver transplantation (16).
In our center, the donor iliac blood vessels are used to bridge the partial segment IV portal vein branches that have been severed, thereby preserving the portal vein blood supply of the segment IV liver, ensuring functional liver volume, and improving the transplant rate of split liver transplantation. Our center’s exploration of portal vein reconstruction in segment IV liver for split liver transplantation is a reasonable attempt based on anatomy, and it is beneficial in clinical practice to the recovery of early postoperative liver function of patients.
Patients and Methods
This was a single center study, and after the necessary approval, the medical records of all patients who underwent split liver transplant were obtained. During the study from January 2015 to January 2022, a total of 32 cases of split liver transplant were obtained in which right trefoil hepatic portal vein reconstruction was carried out in 18 cases, and non-reconstruction of hepatic portal vein segment IV made up 14 cases and the general information of patients is shown in Table 1. In split liver transplantation, blood vessel splitting and distribution are the key to the success or failure of the operation. The choice of middle hepatic vein during left and right liver splitting is determined according to the situation of the two recipients before operation. Our center has made a series of improvements to the invivo split liver transplantation technology, especially the intraoperative vascular reconstruction. This mainly includes reconstruction of the segment IV portal vein after left lateral lobe and right trilobe splitting, left and right half liver splitting, reconstruction of middle hepatic vein after splitting, and formation of posterior vena cava and portal vein after splitting. The vascular materials required for reconstruction mainly come from donor iliac vessels and all of above discussed procedures are shown in Figures 1A–D.
TABLE 1
| Control | Reconstruction | t/x2 | p-value | |
|---|---|---|---|---|
| Male | 5 | 10 | — | — |
| Female | 9 | 8 | — | — |
| Total | 14 | 18 | — | — |
| Age (Mean)Yrs | 50.0 ± 12.48 | 50.66 ± 14.45 | −0.667 | 0.8918 |
| Height | 162.57 ± 9.13 | 167.22 ± 7.90 | 0.1333 | −4.651 |
| Weight | 57.28 ± 13.04 | 64.27 ± 9.55 | −6.992* | 0.0901 |
| BMI | 21.38 ± 3.25 | 22.93 ± 2.52 | −1.548 | 0.1458 |
| MELD Score (Points)† | 16.42 ± 8.38 | 10.84 ± 6.62 | 5.584** | 0.0475 |
| Intraoperative conditions | ||||
| Weight of graft | 1207.28 ± 267.69 | 1238.55 ± 198.14 | 31.270 | 0.7066 |
| ALT/g | 0.472 ± 0.276 | 0.413 ± 0.242 | −0.059 | 0.5227 |
| AST/g | 0.714 ± 0.421 | 0.723 ± 0.354 | 0.009 | 0.9481 |
| Operation time (min) | 677.85 ± 122.65 | 603.611 ± 85.40 | 74.246* | 0.0546 |
| Anhepatic time (min) | 52.21 ± 13.26 | 54.333 ± 16.60 | −2.119 | 0.7071 |
| Cold ischemia (min) | 300.14 ± 21.46 | 302.50 ± 47.15 | −2.357 | 0.8878 |
| Blood transfusion RBC(U) | 12.64 ± 5.57 | 9.05 ± 3.83 | 3.587* | 0.0575 |
| Postoperative recovery | ||||
| ICU (days) | 4.78 ± 1.57 | 4.66 ± 1.64 | 0.119 | 0.8376 |
| Postoperative hospital stay | 36.28 ± 19.18 | 32.38 ± 7.88 | 3.897 | 0.4394 |
General information of control and reconstruction group.
*** , ** and * shows the level of significance at 1%, 5% and 10%, respectively.
FIGURE 1
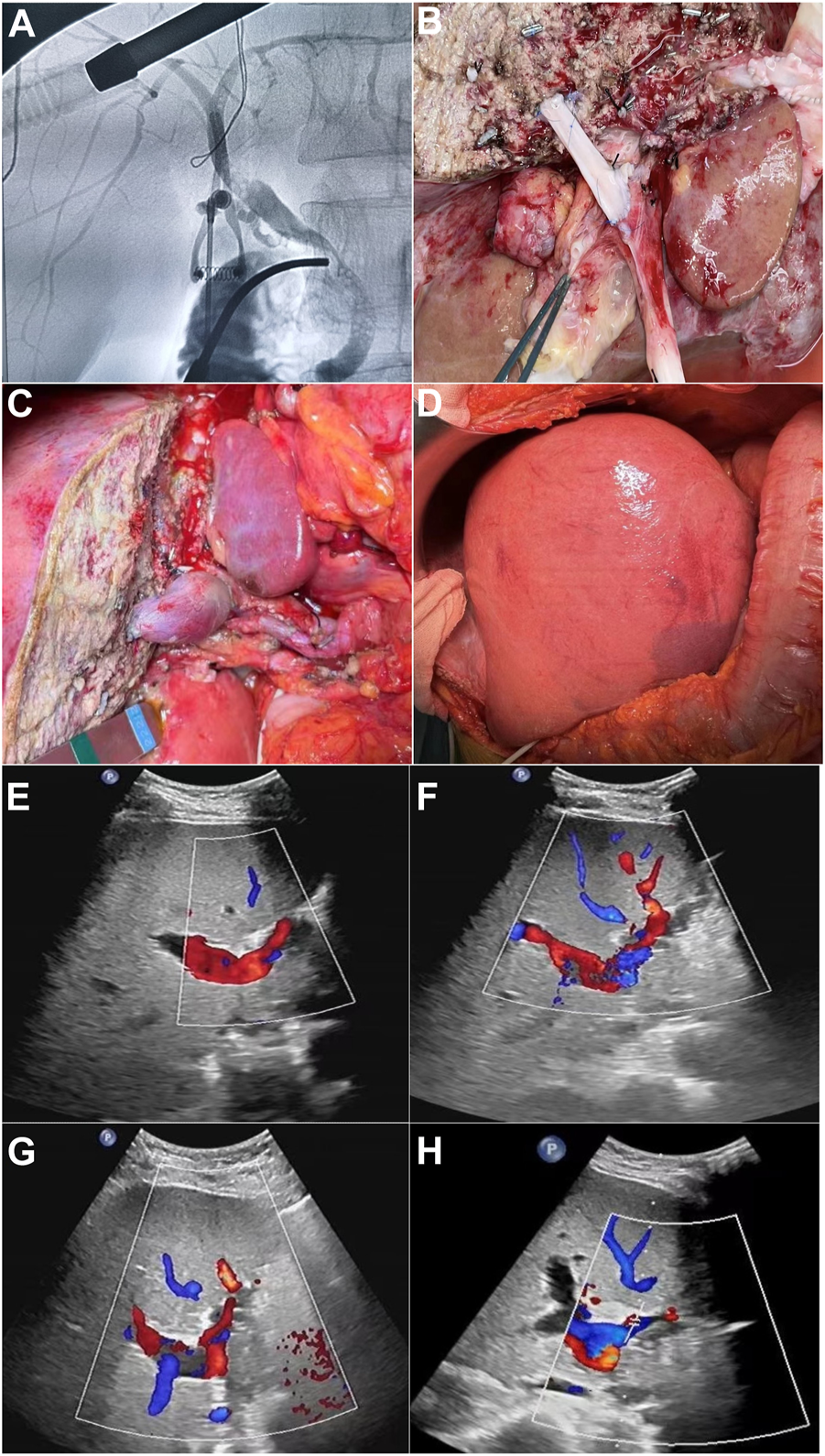
(A) Pre-splitting cholangiography of liver parenchyma. (B) Segment IV portal vein reconstruction before blood flow. (C) Segment IV portal vein reconstruction after blood flow. (D) After surgery/portal vein reconstruction, right three lobes. (E–H) Postoperative ultrasound images of segment IV portal vein bridging vessels of days 1, 3, 5, and 7, respectively.
Donor and Recipient Criteria
Recipients received right hemihepatic and right trilobular liver transplantation in our center if they met the following inclusion criteria: 1) Indications for liver transplantation with no contraindications; 2) preoperative Model for end-stage liver disease (MELD) score <30 points(17); 3) Graft weight/recipient weight (GRWR) ≥ 1.2% in adult recipients and 2%–4% in pediatric recipients (18); 4) No history of multiple abdominal surgeries and the donor also needed to have met the Milan recommendation criteria (19). The donor selection criteria included: 1) age <55 years; 2) hemodynamically stable, no need for high dose escalation maintenance with antidepressants (dopamine ≤5 mg/kg∙min, dobutamine ≤10 mg/kg∙min, no epinephrine or norepinephrine); 3) Intensive care unit (ICU) Hospitalization days <5 days; 4) aspartate transaminase (AST) and alanine aminotransferase (ALT) values lower than two times the normal value; 5) no fatty liver manifestations under the naked eye, if liver biopsy is performed, fatty infiltration moisture <20%; 6) Serum sodium <155 mmol/L. All donor livers which were cardiac-death organ donations were signed by immediate family members, and organ donation consent was given. All recipients signed the patient’s informed consent approved by the hospital ethics committee, in line with medical ethics regulations.
Grouping and Observation Metrics
Of 32 patients, those who did not undergo portal vein reconstruction were included in the control group, consisting of 14 cases in total, and the 18 patients who underwent portal vein reconstruction were included in the reconstruction group. From the first to seventh days after the operation, ALT, AST, albumin (ALB), creatinine (Cr), total bilirubin (TB), alkaline phosphatase (ALP), gamma-glutamyl Transferase (GGT), lactic acid (Lac), and international normalized ratio (INR) level data were collected to analyze the significance of portal vein reconstruction of donor liver segment IV in patients with early postoperative liver function recovery.
Statistical Methods
STATA statistical software was used for data processing, normally distributed measurement data were expressed as mean ± standard deviation, and t-test was used for independent samples. The reconstruction group was compared with the control group. Data with a p-value of less than 0.05 were considered statistically significant.
Results
Comparison of Postoperative Data of Recipients in the Reconstruction and Control Groups
There was a statistically significant difference between the control and reconstruction groups in the levels of ALT on days 2, 4, 5, 6, and 7 after surgery, and in the levels of Lac on day 1 after surgery, but there was no statistically significant difference in AST, ALB, Cr, TB, ALP, GGT, and INR as determined by a comparison of data which is shown in Table 2.
TABLE 2
| Control | Value | Reconstruction | Value | t/x2 | p-value | |
|---|---|---|---|---|---|---|
| Postoperative ALT comparison (U/L) | ||||||
| ALT1 | 14 | 942.50 ± 574.05 | 18 | 741.94 ± 235.13 | 200.556 | 0.1875 |
| ALT2 | 14 | 759.42 ± 502.56 | 18 | 546.83 ± 149.02 | 212.595* | 0.0980 |
| ALT3 | 14 | 495.42 ± 292.19 | 18 | 374.88 ± 161.88 | 120.540 | 0.1478 |
| ALT4 | 14 | 353.69 ± 196.94 | 18 | 240.88 ± 79.73 | 112.803** | 0.0434 |
| ALT5 | 14 | 231.92 ± 128.51 | 18 | 145.33 ± 73.50 | 86.595** | 0.0226 |
| ALT6 | 14 | 186.41 ± 101.71 | 18 | 106.16 ± 66.38 | 80.250** | 0.0136 |
| ALT7 | 14 | 117.46 ± 50.02 | 18 | 80.83 ± 55.75 | 36.628* | 0.0785 |
| Postoperative Lac comparison (mmol/L) | ||||||
| Lac1 | 14 | 2.00 ± 1.36 | 18 | 3.17 ± 1.21 | −1.163** | 0.0194 |
| Lac2 | 14 | 1.32 ± 0.55 | 18 | 1.40 ± 0.56 | −0.071 | 0.7257 |
| Lac3 | 14 | 1.26 ± 0.47 | 18 | 1.18 ± 0.59 | 0.078 | 0.6979 |
| Lac4 | 14 | 1.26 ± 0.57 | 18 | 1.27 ± 0.55 | −0.013 | 0.9513 |
| Lac5 | 14 | 1.41 ± 0.76 | 18 | 1.31 ± 0.54 | 0.104 | 0.8152 |
| Lac6 | 14 | 0.92 ± 0.36 | 18 | 1.28 ± 0.58 | −0.358 | 0.3908 |
| Lac7 | 14 | 1.40 ± 0.65 | 18 | 1.11 ± 0.71 | 0.286 | 0.4891 |
| Postoperative AST comparison (U/L) | ||||||
| AST1 | 14 | 1125.07 ± 1075.57 | 18 | 885.33 ± 405.62 | 239.738 | 0.3899 |
| AST2 | 14 | 549.57 ± 374.29 | 18 | 443.33 ± 218.22 | 106.238 | 0.3221 |
| AST3 | 14 | 241.42 ± 157.11 | 18 | 224.77 ± 128.40 | 16.651 | 0.7436 |
| AST4 | 14 | 146.76 ± 93.67 | 18 | 126.33 ± 79.69 | 20.436 | 0.5206 |
| AST5 | 14 | 100.28 ± 90.25 | 18 | 95.44 ± 84.26 | 4.841 | 0.8768 |
| AST6 | 14 | 91.58 ± 80.57 | 18 | 69.16 ± 46.03 | 22.417 | 0.2820 |
| AST7 | 14 | 71.69 ± 60.55 | 18 | 63.77 ± 34.10 | 7.915 | 0.6554 |
| Postoperative ALB comparison (U/L) | ||||||
| ALB1 | 14 | 40.22 ± 6.45 | 18 | 42.87 ± 6.09 | −2.655 | 0.2616 |
| ALB2 | 14 | 41.44 ± 4.59 | 18 | 41.22 ± 5.52 | 0.218 | 0.9091 |
| ALB3 | 14 | 38.30 ± 8.16 | 18 | 39.27 ± 5.74 | −0.972 | 0.7064 |
| ALB4 | 14 | 38.83 ± 5.29 | 18 | 39.43 ± 5.82 | −0.606 | 0.7842 |
| ALB5 | 14 | 37.96 ± 6.40 | 18 | 40.11 ± 6.23 | −2.142 | 0.3738 |
| ALB6 | 14 | 35.49 ± 8.07 | 18 | 38.55 ± 5.28 | −3.065 | 0.2211 |
| ALB7 | 14 | 36.25 ± 8.72 | 18 | 37.16 ± 5.30 | −0.907 | 0.7321 |
| Postoperative ALP comparison (U/L) | ||||||
| ALP1 | 14 | 90.54 ± 48.89 | 18 | 97.938 ± 53.24 | −7.392 | 0.7308 |
| ALP 2 | 14 | 143.09 ± 109.84 | 18 | 102.688 ± 57.20 | 40.403 | 0.3828 |
| ALP 3 | 14 | 153.18 ± 133.19 | 18 | 101.063 ± 44.78 | 52.119 | 0.2289 |
| ALP 4 | 14 | 177.30 ± 176.76 | 18 | 100.063 ± 39.37 | 77.238 | 0.1408 |
| ALP 5 | 14 | 151.63 ± 121.61 | 18 | 100.267 ± 35.31 | 51.370 | 0.2310 |
| ALP 6 | 14 | 138.55 ± 100.92 | 18 | 107.533 ± 44.02 | 31.022 | 0.3223 |
| ALP 7 | 14 | 121.63 ± 70.58 | 18 | 110.333 ± 44.27 | 11.303 | 0.6507 |
| Postoperative GGT comparison (U/L) | ||||||
| GGT1 | 14 | 108.25 ± 138.24 | 18 | 53.063 ± 34.46 | 55.188 | 0.1655 |
| GGT 2 | 14 | 84.18 ± 48.12 | 18 | 60.125 ± 34.69 | 24.057 | 0.1839 |
| GGT 3 | 14 | 94.72 ± 48.91 | 18 | 65.563 ± 33.07 | 29.165 | 0.1263 |
| GGT 4 | 14 | 114.50 ± 61.18 | 18 | 76.063 ± 37.58 | 38.438 | 0.1339 |
| GGT 5 | 14 | 112.45 ± 50.36 | 18 | 87.933 ± 44.86 | 24.521 | 0.2637 |
| GGT 6 | 14 | 113.33 ± 47.13 | 18 | 97.267 ± 49.50 | 16.067 | 0.4285 |
| GGT 7 | 14 | 102.36 ± 36.74 | 18 | 106.733 ± 58.78 | −4.370 | 0.8287 |
| Postoperative Cr comparison (mmol/L) | ||||||
| Cr1 | 14 | 93.91 ± 31.28 | 18 | 79.82 ± 27.80 | 14.082 | 0.1885 |
| Cr2 | 14 | 80.55 ± 25.96 | 18 | 81.95 ± 26.62 | −1.398 | 0.8826 |
| Cr3 | 14 | 75.54 ± 22.73 | 18 | 71.16 ± 26.61 | 4.380 | 0.6266 |
| Cr4 | 14 | 74.80 ± 15.50 | 18 | 65.99 ± 21.76 | 8.807 | 0.2102 |
| Cr5 | 14 | 75.27 ± 25.70 | 18 | 62.44 ± 20.17 | 12.829 | 0.1238 |
| Cr6 | 14 | 64.50 ± 17.63 | 18 | 63.66 ± 22.56 | 0.835 | 0.9198 |
| Cr7 | 14 | 56.67 ± 19.03 | 18 | 61.05 ± 24.24 | −4.381 | 0.6166 |
| Postoperative TB comparison (µmol/L) | ||||||
| TB1 | 14 | 76.20 ± 63.89 | 18 | 71.81 ± 35.09 | 4.390 | 0.8058 |
| TB2 | 14 | 74.72 ± 48.48 | 18 | 70.76 ± 43.01 | 3.960 | 0.8086 |
| TB3 | 14 | 65.38 ± 52.76 | 18 | 63.77 ± 40.03 | 1.619 | 0.9219 |
| TB4 | 14 | 52.16 ± 37.59 | 18 | 73.14 ± 70.8 | −20.978 | 0.3419 |
| TB5 | 14 | 57.71 ± 37.32 | 18 | 62.53 ± 53.42 | −4.815 | 0.7763 |
| TB6 | 14 | 61.39 ± 37.96 | 18 | 52.81 ± 33.83 | 8.579 | 0.5393 |
| TB7 | 14 | 57.25 ± 41.97 | 18 | 46.810 ± 25.21 | 10.440 | 0.4126 |
| Postoperative INR comparison | ||||||
| INR1 | 14 | 1.60 ± 0.39 | 18 | 1.53 ± 0.24 | 0.067 | 0.5587 |
| INR 2 | 14 | 1.44 ± 0.24 | 18 | 1.40 ± 0.17 | 0.045 | 0.5509 |
| INR 3 | 14 | 1.36 ± 0.24 | 18 | 1.29 ± 0.20 | 0.070 | 0.3877 |
| INR 4 | 14 | 1.34 ± 0.43 | 18 | 1.30 ± 0.21 | 0.042 | 0.7248 |
| INR 5 | 14 | 1.30 ± 0.34 | 18 | 1.27 ± 0.21 | 0.033 | 0.7394 |
| INR 6 | 14 | 1.29 ± 0.32 | 18 | 1.25 ± 0.22 | 0.035 | 0.7176 |
| INR 7 | 14 | 1.26 ± 0.29 | 18 | 1.24 ± 0.24 | 0.022 | 0.8169 |
Postoperative data comparison.
*** , ** and * shows the level of significance at 1%, 5% and 10%, respectively.
Significance of Portal Vein Reconstruction of Donor Liver Segment IV on Early Postoperative Liver Function Recovery
Judging from the recovery of various indicators of the recipients in the two groups after surgery, the recovery of liver function in the reconstruction group was significantly better than that in the control group. No serious bleeding, biliary fistula, or other complications occurred in the two groups of recipients after operation. In the control group, 14 recipients did not undergo segment IV hepatic portal vein reconstruction, and the segment IV liver was insufficiently perfused. Ultrasonography indicated that segment IV liver atrophy and necrosis occurred earlier, and early postoperative liver function recovery was poor. The postoperative ultrasound of the 18 recipients in the reconstruction group showed blood flow through the reconstructed vessels of the portal vein in the recipients within 1 week after surgery, the speed of IV segment liver atrophy was significantly slower than that of the control group, and the postoperative liver function recovered faster. It can be seen that intraoperative reconstruction of the portal vein of the donor liver segment IV can effectively reduce the damage of hepatocytes, preserve more functional liver tissue, and promote the early postoperative liver function recovery of patients, thereby improving the prognosis of patients and restoring blood recirculation, which is shown in Figures 1E–H.
Survival Analysis
We compared the 6-month data to calculate the survival rate between these two groups.
The 6-month rate in the Control group and Reconstruction group was [85.7% vs. 94.4%] with an overall 90.6% rate of survival. There was no significant difference in survival rate between these two groups is shown in the Figure 2.
FIGURE 2
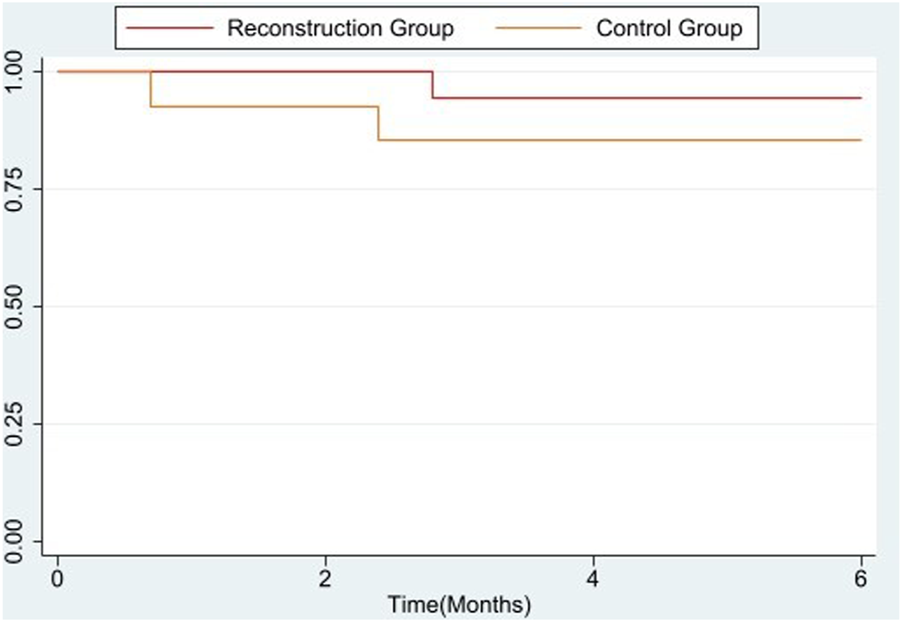
Survival rate analysis of 6 months between control and reconstruction group.
Discussion
The development of split liver transplant was prompted by a lack of organs and rising morbidity on waiting lists. The gap between organ supply and recipient demand has never been wider than it is today. This has rekindled interest in expanding the use of traditional adult/pediatric split liver transplant and adult/adult split liver transplant. At centers that routinely use these techniques, split liver transplant applied to pediatric recipients offers good results, with considerable decreases in pediatric wait times, wait-list morbidity, and living-donation utilization, according to a decade of experience with left lateral segment grafts (20, 21). Split liver transplantation, as the most difficult liver transplantation technology, comprehensively embodies this feature from the preoperative evaluation of the general conditions of donors and recipients, to the distribution of blood vessels including hepatic artery, portal vein, inferior vena cava, and hepatic vein and biliary tract. The splitting of liver parenchyma, the acquisition of donor liver, and the fine individual management after operation represents the forefront of the development of precision medicine (22). With the accumulation of clinical experience, especially the deepening of the research on the local anatomical structure of the liver, and the continuous summary and exchange of the experience of multi center split liver transplantation, the effect of split liver transplantation has been significantly improved (23).
The recipient selection of donation after brainstem death (DBD) orthotopic split liver transplantation is also key to the success of the transplantation, especially when the recipient is a double adult split liver transplantation. The matching degree between the graft size and the recipient needs to be carefully evaluated before operation to prevent the possibility of small for size syndrome or large for size syndrome due to the mismatch between the donor and the recipient. The GRWR standard of DBD orthotopic split liver transplantation should be appropriately increased compared with living donor liver transplantation, and it is recommended to be greater than 1.0%–1.2% (24). The GRWR of adult recipients of DBD in situ split liver transplantation in our center was controlled at more than 1.0%, and there was no obvious small liver syndrome after operation. We believe that the hyperoxia environment of the transplanted liver is beneficial to the regeneration of liver cells.
Therefore, the receptor should be emphasized to ensure long-term oxygen inhalation after operation. CT examination involves radiation and may affect the regeneration of liver cells. Therefore, we suggest that abdominal CT examination should be avoided as much as possible in the early stage after operation. Split liver transplantation is complex, the operation time is relatively increased, there are risks such as cross-sectional bile leakage and infection after operation, and the general requirements for the recipient are high because relevant studies show that a high MELD score before operation is an independent risk factor for serious complications after liver transplantation, so care should be taken to select recipients with a MELD score >14 for split liver transplantation (17, 18, 25). The donor liver splitting operation in our center adopts in situ splitting in vivo compared with the traditional in vitro splitting after acquisition, it can significantly reduce the cold ischemia time, dissect the hilar tissue more finely, deal with the liver section more accurately, and reduce the incidence of postoperative complications. It is suggested that the middle hepatic vein should be accurately located by intraoperative ultrasound before splitting, and perfusion can be carried out after splitting when the middle hepatic vein is exposed (26).
Although the vascular reconstruction and repair molding of the left and right liver halves respectively increase the operation time, it ensures that the left and right liver grafts have a complete middle hepatic vein system, the operation method is more reasonable, the necrosis of the graft liver tissue caused by outflow tract obstruction is avoided, and the functional liver volume of the graft is effectively increased. After the blood supply of the donor liver is restored during the operation, the sections of each anastomosis and liver parenchyma are comprehensively checked, and the bleeding points and broken ends with bile leakage are also treated in time. The reconstruction of the artery is flexibly evaluated according to the distribution of the donor hepatic artery and the recipient’s own arterial conditions. If the length of the vessel is not sufficient, the donor iliac vessel can be used for bridging if necessary. In general, T-tube drainage is routinely placed in our center. The advantages of T-tube drainage are as follows: first, the recovery of donor liver function and the occurrence of rejection can be evaluated by observing the amount and color of drained bile in the early stage after operation; The second is that it can fulfil the role of biliary decompression before the recovery of gastrointestinal function, so as to reduce the occurrence of biliary complications such as bile leakage.
In conclusion, split liver transplantation can effectively alleviate the problem of liver shortages. Split liver transplantation can give the same clinical results as whole liver transplantation if the right donors and recipients are chosen and if the surgery is planned and carried out well. With the continuous maturity and progress of split liver transplantation technology, split liver transplantation is expected to become a routine operation in clinical liver transplantation and to become widely used. To sum up, our center’s exploration of portal vein reconstruction in segment IV of the liver in split liver transplantation is a reasonable attempt based on anatomy, which is conducive to the recovery of patient’s early postoperative liver function in clinical practice. However, because this surgical method is in the early exploratory stage, the number of samples included in this study is limited and, due to the differences in the technical level of the operators, the results have certain limitations that need to be further verified by continuing to expand the sample size in a multi-center practice.
Statements
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by ethics committee of organ transplant center and affiliated Hospital of Qingdao University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
IM and FR participated in research design, data collection and writing of paper. FW, XX, and ZL helped in data collection. CJ participated in the research design the writing of the paper.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81670600).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
ALB, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate transaminase; BMI, body mass index; Cr, creatinine; DBD, donation after brainstem death; GGT, gamma-glutamyl transferase; GRWR, graft weight/recipient weight; ICU, intensive care unit; INR, international normalized ratio; Lac, lactic acid; MELD, model for end-stage liver disease; TB, total bilirubin.
References
1.
Starzl TE Groth CG Brettschneider L Penn I Fulginiti VA Moon JB et al Orthotopic Homotransplantation of the Human Liver. Ann Surg (1968) 168(3):392–415. 10.1097/00000658-196809000-00009
2.
Bismuth H Houssin D . Reduced-sized Orthotopic Liver Graft in Hepatic Transplantation in Children. Surgery (1984) 95(3):367–70.
3.
Bismuth H Morino M Castaing D Gillon M Declere AD Saliba F et al Emergency Orthotopic Liver Transplantation in Two Patients Using One Donor Liver. J Br Surg (1989) 76(7):722–4. 10.1002/bjs.1800760723
4.
D Cintorino M Spada S Gruttadauria S Riva A Luca R Volpes et al editors. In Situ split Liver Transplantation for Adult and Pediatric Recipients: an Answer to Organ Shortage. Transplantation Proceedings. Elsevier (2006).
5.
Azoulay D Castaing D Adam R Savier E Delvart V Karam V et al Split-liver Transplantation for Two Adult Recipients: Feasibility and Long-Term Outcomes. Ann Surg (2001) 233(4):565–74. 10.1097/00000658-200104000-00013
6.
Moussaoui D Toso C Nowacka A McLin VA Bednarkiewicz M Andres A et al Early Complications after Liver Transplantation in Children and Adults: Are Split Grafts Equal to Each Other and Equal to Whole Livers? Pediatr Transplant (2017) 21(4):e12908. 10.1111/petr.12908
7.
Renz JF Yersiz H Reichert PR Hisatake GM Farmer DG Emond JC et al Split‐liver Transplantation: a Review. Am J Transplant (2003) 3(11):1323–35. 10.1046/j.1600-6135.2003.00254.x
8.
Zimmerman A Flahive J Hertl M Cosimi A Saidi R . Outcomes of Full-Right-Full-Left Split Liver Transplantation in Adults in the USA: a Propensity-Score Matched Analysis. Int J Organ Transplant Med (2016) 7(2):69–76.
9.
Chul Yoon K Song S Jwa EK Lee S Man Kim J Kim OK et al Survival Outcomes in Split Compared with Whole Liver Transplantation. Liver Transplant (2018) 24(10):1411–24. 10.1002/lt.25196
10.
Azoulay D Astarcioglu I Bismuth H Castaing D Majno P Adam R et al Split-liver Transplantation. The Paul Brousse Policy. Ann Surg (1996) 224(6):737–46. 10.1097/00000658-199612000-00009
11.
Yersiz H Renz JF Farmer DG Hisatake GM McDiarmid SV Busuttil RW . One Hundred In Situ Split-Liver Transplantations: a Single-center Experience. Ann Surg (2003) 238(4):496–505. 10.1097/01.sla.0000089852.29654.72
12.
Cardillo M De Fazio N Pedotti P De Feo T Fassati LR Mazzaferro V et al Split and Whole Liver Transplantation Outcomes: a Comparative Cohort Study. Liver Transplant (2006) 12(3):402–10. 10.1002/lt.20720
13.
S, Emre V Umman , editors. Split Liver Transplantation: an Overview. Transplantation Proceedings. Elsevier (2011).
14.
Yersiz H Renz JF Hisatake G Reichert PR Feduska NJ Lerner S et al Technical and Logistical Considerations of In Situ Split-Liver Transplantation for Two Adults: Part I. Creation of Left Segment II, III, IV and Right Segment I, V-VIII Grafts. Liver Transplant (2001) 7(12):1077–80. 10.1053/jlts.2001.30384
15.
Yersiz H Renz JF Hisatake G Reichert PR Feduska NJ Jr Lerner S et al Technical and Logistical Considerations of In Situ Split-Liver Transplantation for Two Adults: Part II. Creation of Left Segment I-IV and Right Segment V-VIII Grafts. Liver Transplant (2002) 8(1):78–81. 10.1053/jlts.2002.31036
16.
Broering D Fischer L Rogiers X . Split Liver Transplantation. Hpb (2004) 6(2):76–82. 10.1080/13651820310020774
17.
Nadalin S Schaffer R Fruehauf N . Split‐liver Transplantation in the high‐MELD Adult Patient: Are We Being Too Cautious?Transpl Int (2009) 22(7):702–6. 10.1111/j.1432-2277.2009.00850.x
18.
Hashimoto K Quintini C Aucejo F Fujiki M Diago T Watson M et al Split Liver Transplantation Using Hemiliver Graft in the MELD Era: a Single center Experience in the United States. Am J Transplant (2014) 14(9):2072–80. 10.1111/ajt.12791
19.
Aseni P De Feo TM De Carlis L Valente U Colledan M Cillo U et al A Prospective Policy Development to Increase Split-Liver Transplantation for 2 Adult Recipients: Results of a 12-year Multicenter Collaborative Study. Ann Surg (2014) 259(1):157–65. 10.1097/SLA.0b013e31827da6c9
20.
Azoulay D Samuel D Adam R Savier E Karam V Delvard V et al Paul Brousse Liver Transplantation: the First 1,500 Cases. Clin transplants (2000) 273–80.
21.
Broering DC Mueller L Ganschow R Kim J-S Achilles EG Schäfer H et al Is There Still a Need for Living-Related Liver Transplantation in Children? Ann Surg (2001) 234(6):713–21. 10.1097/00000658-200112000-00002
22.
Hong JC Yersiz H Busuttil RW . Where Are We Today in Split Liver Transplantation?Curr Opin Organ Transplant (2011) 16(3):269–73. 10.1097/MOT.0b013e328346572e
23.
Ge J Lai JC . Split-Liver Allocation: An Underused Opportunity to Expand Access to Liver Transplantation. Liver Transpl (2019) 25(5):690–1. 10.1002/lt.25458
24.
Patil NS Goyal N Pareek S Nayeem M Gupta S . In Situ splitting of the Cadaver Liver for Two Adult Recipients by LDLT Technique. J Clin Exp Hepatol (2017) 7(3):179–83. 10.1016/j.jceh.2017.05.001
25.
Park G-C Hwang S Song G-W Jung D-H Ha T-Y Ahn C-S et al Prognosis of Split Liver Transplantation Compared with Whole Liver Transplantation in Adult Patients: Single-center Results under the Korean MELD Score-Based Allocation Policy. J Korean Med Sci (2020) 35(37):e304. 10.3346/jkms.2020.35.e304
26.
Ghobrial RM Amersi F Busuttil RW . Surgical Advances in Liver Transplantation: Living Related and Split Donors. Clin Liver Dis (2000) 4(3):553–65. 10.1016/s1089-3261(05)70126-4
Summary
Keywords
liver transplantation, donors, split, liver function, portal reconstruction
Citation
Muhammad I, Rehman FUL, Wang F, Xiong X, Lianghao Z and Jinzhen C (2023) Application Effectiveness of Segment IV Portal Vein Reconstruction for Early Postoperative Liver Function Recovery in Split Liver Transplantation. Transpl Int 36:10808. doi: 10.3389/ti.2023.10808
Received
30 July 2022
Accepted
06 April 2023
Published
26 April 2023
Volume
36 - 2023
Updates
Copyright
© 2023 Muhammad, Rehman, Wang, Xiong, Lianghao and Jinzhen.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cai Jinzhen, caijinzhen@qdu.edu.cn
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.