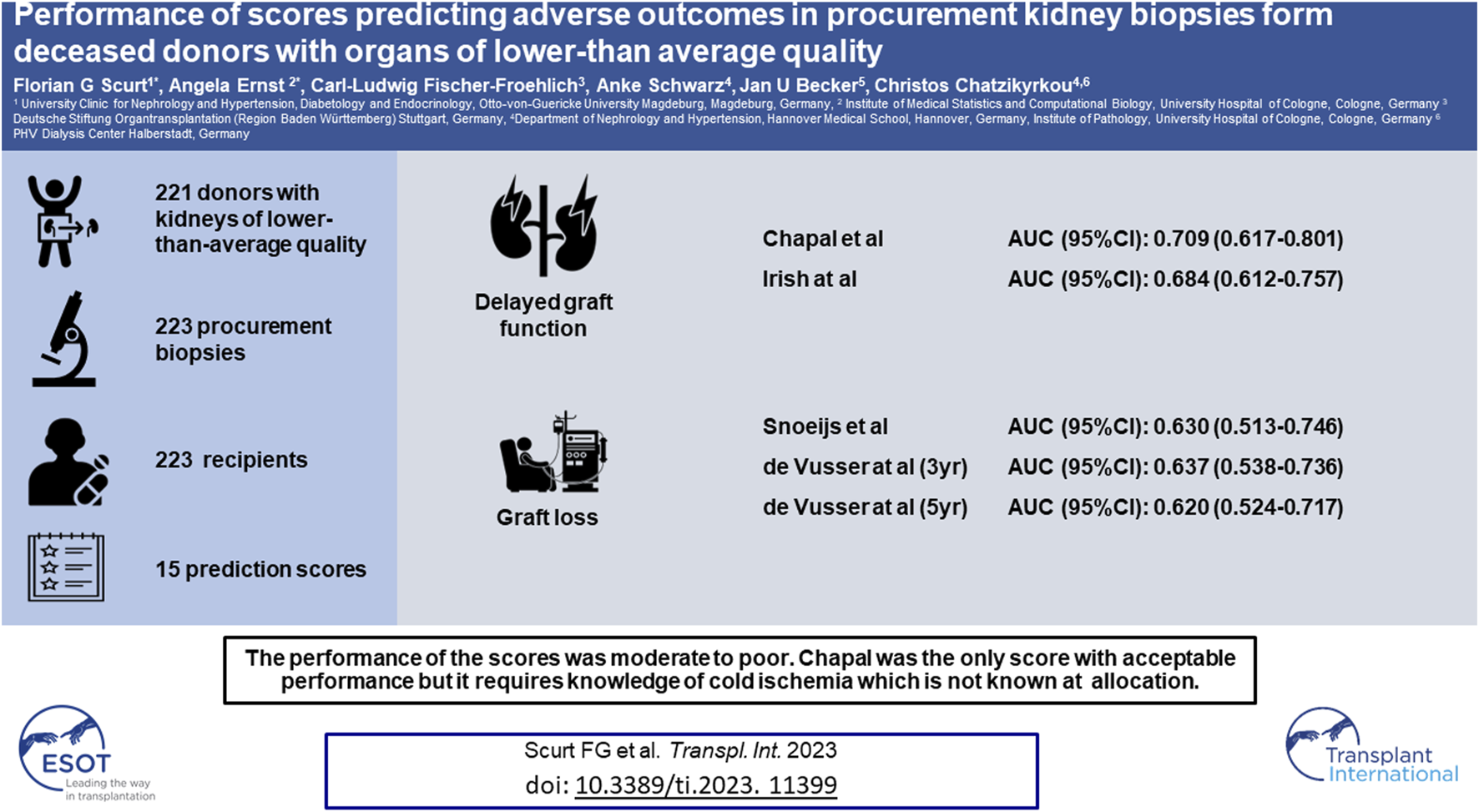
Abstract
Several scores have been devised for providing a prognosis of outcomes after kidney transplantation. This study is a comprehensive test of these scores in a cohort of deceased donors with kidneys of lower-than-average quality and procurement biopsies. In total, 15 scores were tested on a retrospective cohort consisting of 221 donors, 223 procurement biopsies, and 223 recipient records for performance on delayed graft function, graft function, or death-censored graft loss. The best-performing score for DGF was the purely clinical Chapal score (AUC 0.709), followed by the Irish score (AUC 0.684); for graft function, the Nyberg score; and for transplant loss, the Snoeijs score (AUC 0.630) and the Leuven scores (AUCs 0.637 and 0.620). The only score with an acceptable performance was the Chapal score. Its disadvantage is that knowledge of the cold ischemia time is required, which is not known at allocation. None of the other scores performed acceptably. The scores fared better in discarded kidneys than in transplanted kidneys. Our study shows an unmet need for practical prognostic scores useful at the time of a decision about discarding or accepting deceased donor kidneys of lower-than-average quality in the Eurotransplant consortium.
Graphical Abstract
Introduction
For most patients with end-stage kidney disease, kidney transplantation is the best available treatment with better survival, quality of life and lower use of healthcare resources [1–3]. Despite the increasing use of living donation [4, 5], most patients on dialysis still have to wait on a deceased donor kidney transplant (DDK). Today, transplant physicians are facing the dilemma of how to best use the scarce pool of increasingly older DDKs while avoiding the risk of a poor outcome for the recipients which can be associated with delayed graft function (DGF), premature transplant loss or even endanger their lives [1, 3].
Several purely clinical [6–13], combined clinicohistological [14–16], or purely histological scores [17–20] have been devised for quality assessment of DDKs; the Nyberg score, is for practical purposes best considered clinical, as it does not requires histopathology [9]. The scores with a histology component have been developed on preimplantation but not the clinically decisive procurement biopsies from unselected cohorts, reflecting the full spectrum of DDK quality, including those with the lowest risk. Some of these scores have been internally [9, 14, 16] or externally validated in the publications of subsequent scores from other authors or in separate studies. A recent publication has tested four scores [6–8, 12] for their performance in the prognostication of DGF in a large Dutch cohort of unselected preimplantation biopsies [21]. An earlier study from the United Kingdom evaluated the performance of four scores [9, 11, 22, 23] regarding mid-term transplant function [24], two of which have been updated since [7, 9]. A recent study from the United States (US) validated three scores [9, 25, 26] on a single-centre cohort of donors with kidneys of lower quality for the prognostic performance regarding two-year-transplant survival [27]. Similarly, in another study [28], four scores, including that proposed by Banff [16, 19, 25, 29] failed to predict graft survival and early graft function. The scores and their validation studies have helped to better understand and address the causes of DGF and premature transplant failure. However, these scores have never been validated regarding their usefulness for the decision about acceptance or discard of a DDK on a set of procurement biopsies, taken to assess organ quality before allocation. This is particularly important in view of recent data showing that procurement biopsies lead to discard of organs suitable of transplantation [30].
Primary aim of this study is to conduct the overdue comprehensive test of a variety of scores (listed in Table 1) for their performance on various end points, such as delayed graft function, graft function, or death-censored graft loss on a retrospective cohort of procurement biopsies specifically commissioned for DDK quality assessment by the Deutsche Stiftung Organtransplantation (DSO; German Foundation for Organ Transplantation), operating within the Eurotransplant consortium. As a secondary aim, we examined whether purely clinical scores perform as well as scores including a histopathology component. Lastly, we wanted to test their performance on the considerable proportion of the discarded kidneys in our cohort.
TABLE 1
| Score | Donor | Transplant procedure | Donor kidney | Recipient | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | Type | Cause of death | Hypertension | DM | Kidney function | Age | Ethnicity | Weight | HCV | CIT | WIT | DKT | MM | CMV match | Induction therapy with ATG | Global GS | Banff ci | Banff ct | Banff cv | Banff cg | Banff i | Banff ah | Banff mm | Renal artery plaque | Age | Weight | Body mass index | Previous Tx | DM | Dialysis duration | PRA |
| Balaz 15 | C + P | x | x | x | x | ||||||||||||||||||||||||||
| Chapal6 | C | x | x | x | x | x | |||||||||||||||||||||||||
| Irish7 | C | x | x | x | x | x | x | x | x | x | |||||||||||||||||||||
| Jeldres8 | C | x | x | x | x | x | x | ||||||||||||||||||||||||
| Schold11 | C | x | x | x | x | x | x | x | x | ||||||||||||||||||||||
| Navarro 17 | P | x | x | x | x | x | |||||||||||||||||||||||||
| Port10 | C | x | x | x | x | ||||||||||||||||||||||||||
| Rao26 | C | x | x | x | x | x | x | x | x | x | x | ||||||||||||||||||||
| Snoeijs 20 | P | x | x | x | x | x | x | ||||||||||||||||||||||||
| de Vusser16 | C + P | x | x | x | |||||||||||||||||||||||||||
| Remuzzi19 | P | x | x | x | x | ||||||||||||||||||||||||||
| Anglicheau 14 | C + P | x | x | x | |||||||||||||||||||||||||||
| Nyberg9 | C | x | x | x | x | x | x | ||||||||||||||||||||||||
| Ortiz 18 | P | x | x | x | x | x | x | x | |||||||||||||||||||||||
| Foucher13 | C | x | x | x | x | ||||||||||||||||||||||||||
Parameters used in the previously published scores for the quality assessment of DDKs.
Abbreviations: ah, arteriolar hyalinosis; ATG, anti-thymocyte globulin; BMI, body mass index; CIT, cold ischemia time; ci, interstitial fibrosis; cg, glomerular basement membrane splitting; CMV, cytomegalovirus; ct, tubular atrophy; cv, arterial intimal fibrosis; DDK, deceased donor kidney; DKT, double kidney transplantation; DM, diabetes mellitus; EPTS, Estimated post transplant survival score; HCV, hepatitis C virus; GS, glomerulosclerosis; i, interstitial infiltrates; KDRI, kidney donor risk index; MM, miss matches, mm mesangial matrix; PRA, panel-reactive antibodies; WIT, warm ischemia time.
The score designation and the reference are given in the first column; the type of score as in purely clinical (C), combined clinical and pathological (C + P) or solely pathological (P) is given in the second column. Subsequent columns list the parameters used in the respective scores. The parameters are organized as relating to the donor, to the transplant procedure, to the transplant itself or to the recipient. Note that although renal artery plaque as used in the Nyberg score is a pathological finding, it is not typically assessed by a pathologist (pathological and clinic-pathological scores are in italics; the numbers correspond to the references in the manuscript).
Materials and Methods
Biopsies, Reporting, Donor, and Recipient Data
We extracted data from the “DSO Region Nord” and from the German transplant centers of kidneys allocated, between 1 January 2003, and 31 March 2012. The collection of recipient follow-up data was completed in December 2015. Data were analyzed between 1 January 2018, and 31 May 2020. Only adult recipients of deceased donor kidneys of lower quality were included. Recipients with dual kidney- and combined kidney transplantation were excluded. Our cohort consisted exclusively of brain death donors since donation after cardiac death is not allowed in Germany.
The allocation was under the auspices of Eurotransplant, an international non-profit organization responsible for the coordination and distribution of organs for transplantation between residents of eight European countries.1
The following donor data were collected: age, sex, weight, height, body mass index (BMI), length of hospital stay, cardiopulmonary resuscitation, cardiovascular comorbidities, history of smoking, cause of brain death, use of vasopressors, hemodynamic parameters such as, blood pressure and central venous pressure, creatinine at admission, peak creatinine and creatinine at organ recovery, diuresis volume 24 h and at the last hour before recovery, and urine dipstick test at recovery. Recipients’ records were searched for medical history, immunologic risk, peritransplant data, and outcome.
The biopsies were evaluated at the Institute of Pathology in parallel to the transport of the DDK and the preparation for transplantation. Procurement biopsies were not performed in all kidneys but only in that deemed to be of lower quality to increase their chance of acceptance. The results were reported after rapid paraffin-embedding on multiple hematoxylin-eosin and periodic-acid-Schiff-stained sections within 4 h. The DSO oversaw DDK management after notification. The decision about use or discard of the DDK was then made by the transplant physician in the receiving centre. The first assessment was done by the pathologist on duty and included information on representativeness of biopsy, number of glomeruli and arteries, percentage of tubular atrophy, and grading of acute tubular injury. The recommendation was usually suitable/not suitable or partially suitable. The histopathological scores reported below were provided in a second, blinded reading by an experienced nephropathologist. A flowchart of the study is given in Figure 1.
FIGURE 1
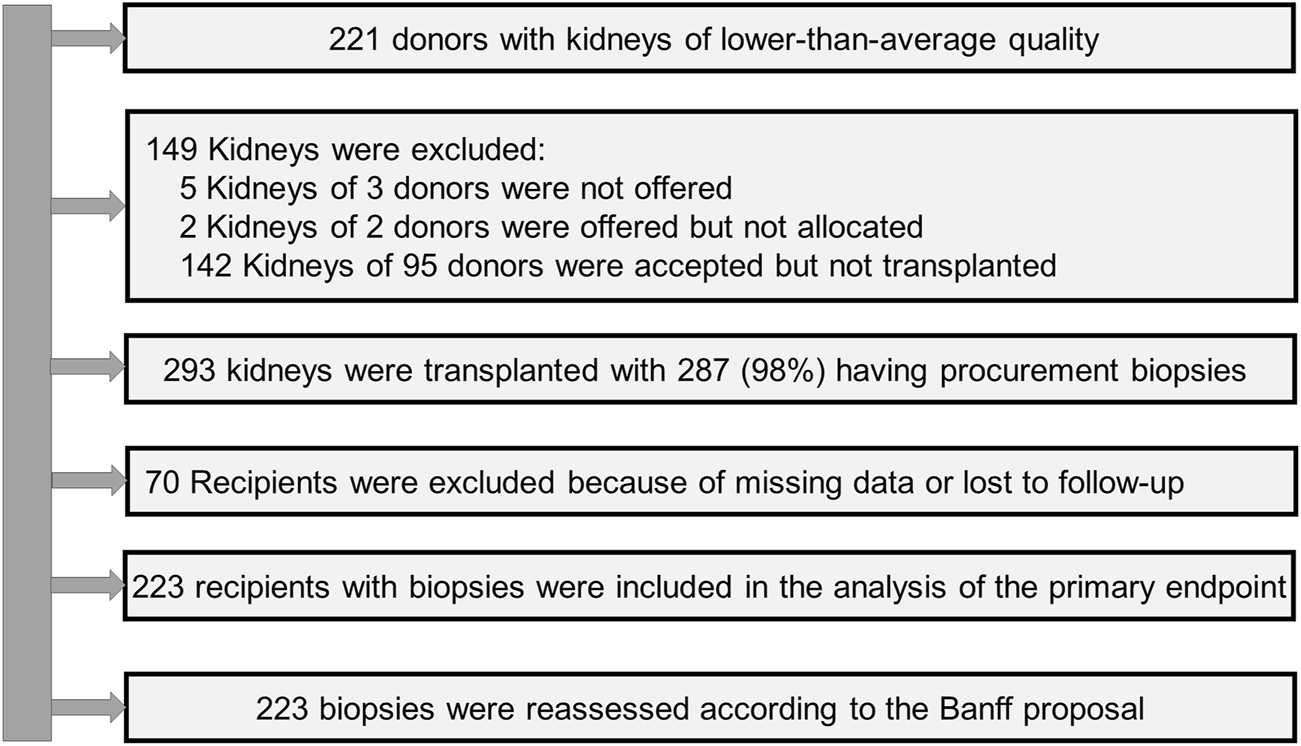
Flow chart of the study (DGF, delayed graft function; m, months; PNF, primary non-function; yr, year).
Histopathological parameters included type of biopsy (needle or wedge), total number of glomeruli, ratio of globally sclerosed glomeruli, number of arteries (media ≥2 smooth muscle cell layers), presence of focal and segmental glomerulosclerosis (FSGS), Banff Lesion Scores i, t, v, g, ptc, ci, ct, cv, cg, ah, arteriolar fibrosis scored as absent, mild, moderate and severe, cortical tubular hypertrophy, epithelial cell flattening, brush border loss, vacuolisation and luminal detritus scored as 0 (absent), 1 (<25%), 2 (<50%) and 3 (≥50%), tubular nuclear loss scored as 0 (absent), 1 (1 quadrant), 2 (two quadrants), 3 (3 quadrants of the most affected tubular cross-section), pyelonephritis and thrombotic microangiopathy. The Banff meeting report 2011, the Banff consensus criteria for preimplantation biopsies, the german recommendations for procurement biopsies [29, 31, 32] and classification systems for glomerular diseases [33, 34], as well as scoring systems for calcification [35] and acute tubular injury [36, 37] were also considered. A summary of all histopathological parameters is provided in Supplementary Material.
Definitions
The definition of lower organ quality depended not on strict criteria but was based on clinical judgment considering the macroscopic appearance of the organ in combination with donor’s clinical data. The macroscopic appraisal was done on the “back table,” after removal of the perinephric fat and the clean dissection of the vessels from the surrounding tissues. It included organ quality as well as perfusion quality, both of which were rated as good, medium, or poor; likewise, atherosclerosis was characterized as no, mild, or severe. The decision was usually felt after discussion of each case between the senior surgeon of the harvesting team and the physician of the recipient’s center. Senior surgeons were accredited by the DSO and had many years of experience in the transplant field.
Extended criteria donors (ECD) were classified as previously reported [38]. eGFR was calculated by means of the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. Admission, highest, lowest, and terminal eGFR were respectively estimated by using the first, the lowest, the highest and the last serum creatinine prior to organ recovery [39]. Primary non-function (PNF) was defined as the permanent lack of graft function from the time of transplantation [40] and delayed graft function (DGF) as the need for dialysis in the first week [41].
Scores
An overview of the parameters included in the respective scores is given in Table 1. Kidney Donor Profile and Risk Index (KDP, KDRI) were calculated according to the Organ Procurement and Transplantation Network (OPTN)2 and estimated post transplant survival (EPTS) score by the web calculator provided by OPTN (EPTS calculator—OPTN).3
Outcome Measures
The following outcomes were analyzed: PNF, DGF, graft function at 3 months, one- and 3 years, death censored graft failure and patient death at one, three and 5 years. All survival times were censored at the last date a patient was known to be alive. eGFR results were presented as 10 mL/min per 1.73 m2 for ease of interpretation.
Statistics
Continuous variables were described as mean ± standard deviation (SD) and central trends between groups compared by Mann-Whitney-U-tests. Fisher’s exact- and χ2-tests were used to compare distributions of categorical variables, respectively. To estimate how well a risk-score discriminates the different endpoints, the area underneath the receiver operating characteristics curve (AUC) was calculated. AUCs range from 0% to 100%, with 0% suggesting perfect inaccuracy, 100% perfect accuracy, 50% suggesting no discrimination and 50%–70% suggesting poor discrimination, 70%–80% suggesting acceptable and 80%–90% excellent and finally 90% suggesting outstanding performance [42, 43]. A p-value below 0.05 was considered significant in all comparisons in two-sided tests; however, in this retrospective observational study, p-values can only be considered descriptive. Statistical analysis was performed with the use of SPSS software, v24 (IBM Corp, Armonk, NY, United States) and IBM SPSS Statistics Essentials for R.
Ethical Permission
All organ transplants were performed according the Declaration of Istanbul [44]; no transplants from prisoners were used. The study was conducted in accordance with the Declarations of Helsinki and approved by the local ethical review board of Hannover Medical School (No. 1519-2012).
Results
Donors’ and Recipients’ Characteristics
From 442 kidneys recovered from 221 donors, 149 were discarded. In 287 (98%) of the 293 transplanted kidneys the tissue blocks were found. Follow-up data were available from 223 recipients (Figure 1). The KDRI was 1.48 and 107 (63.3%) were ECD. The average age was 61 years and 54% were males. Only 13% of donors had diabetes and 30% cardiovascular disease. The prevalence of hepatitis B and C was low (6.5% and 1.2%). Cerebrovascular accident was the most common cause of brain death (60%). The serum creatinine at recovery was 149 μmol/L. Approximately 50% of donors experienced acute kidney injury (AKI) (Table 2). The accepted kidneys showed macroscopically a good perfusion and organ quality at all, except for atherosclerosis which was severe in 46.5% of them. Biopsies were performed in 80% and the majority were needle biopsies with a representative number of glomeruli and arteries. Mean and minimal (<5%) global glomerulosclerosis were 10.4% and 50% respectively, whereas the majority of acute and chronic tubular, interstitial, and vascular Banff lesion scores were of low grade. On the contrary, acute tubular injury was, as expected, more severe (Table 3). The average age of recipients was 61 years. They showed a low immunologic risk profile, a cold ischemia time (13.8 h) which was at the lower range of that reported for Eurotransplant [45] and a high EPTS score (Table 4). PNF occurred in 26 (11.7%) and DGF in 109 (48.9%) patients. We observed 49 graft losses during a median follow-up of 43.8 months (IQR 19–68 months). Patient and death-censored graft survival at 1, 3, and 5 years after kidney transplantation were respectively 90.6% and 91.1%, and 86.1% and 82.9% and 83% and 81.6% (Table 5).
TABLE 2
| Characteristic | Value |
|---|---|
| Donor characteristics (No. of donors = 169) | |
| Age, y | 60.8 ± 16.2 |
| Sex, n (%) | |
| Female | 77 (45.6) |
| Male | 92 (54.4) |
| BMI, kg/m2 | 27.4 ± 5.8 |
| Diabetes mellitus, n (%) | 22 (13.0) |
| Hypertension, n (%) | 96 (56.8) |
| Cardiovascular disease, n (%) | 49 (29.2) |
| Smoker, n (%) | 46 (27.2) |
| Hepatitis B Virus positive, n (%) | 11 (6.5) |
| Hepatitis C Virus positive, n (%) | 2 (1.2) |
| Cytomegalovirus positive, n (%) | 110 (65.1) |
| Cerebrovascular accident (CVA), n (%) | 101 (59.8) |
| Extended Criteria Donors (ECD), n (%) | 107 (63.3%) |
| Kidney Donor Risk Index (KDRI) | 1.48 ± 0.51 |
| KDRI Grading, n (%) | |
| KDRI Grade I (0–20) | 11 (6.5) |
| KDRI Grade II (21–40) | 17 (10.1%) |
| KDRI Grade III (41–60) | 29 (17.2) |
| KDRI Grade IV (61–80) | 27 (16.0) |
| KDRI Grade V (81–100) | 85 (50.3) |
| Donor ICU data | |
| Time ICU until confirmed brain death, h | 118.1 ± 126.6 |
| Time confirmed brain death until cross-clamp, h | 13.2 ± 14.8 |
| CPR at ICU stay, n (%) | 30 (17.8) |
| Transfusion at ICU stay, n (%) | 18 (10.7) |
| Units of RBC, n (%) | 3.06 ± 8.13 |
| Units of FFP, n (%) | 3.28 ± 9.28 |
| Volume expander at ICU stay, n (%) | 25 (14.8) |
| Diuretics at ICU stay, n (%) | 22 (13.1) |
| Antidiuretics at ICU stay, n (%) | 57 (33.9) |
| Antibiotics at ICU stay, n (%) | 90 (53.3) |
| AKI, n (%) | 79 (46.7) |
| RIFLE criteria, n (%) | |
| No AKI | 90 (53.3) |
| Risk | 46 (27.2) |
| Injury | 13 (7.7) |
| Failure | 20 (11.8) |
| Serum creatinine, µmol/L | |
| At admission | 111 ± 88 |
| Minimum | 101 ± 73 |
| Peak | 161 ± 129 |
| Last | 149 ± 119 |
| Last blood and urine values before cross clamp | |
| Hemoglobin, g/dL | 17.5 ± 3.4 |
| White cell count, per cubic millimeter | 13.58 ± 5.052 |
| Platelet count, per cubic millimeter | 176,868 ± 97,578 |
| International normalized ratio | 1.27 ± 0.51 |
| Activated partial thromboplastin time, sec | 39.4 ± 16.6 |
| Aspartate transaminase, IU/L | 123.4 ± 293.4 |
| Alanine transaminase, IU/L | 109.2 ± 357.4 |
| Alkaline phosphatase, IU/L | 98.3 ± 51.2 |
| Lactate dehydrogenase, IU/L | 456.9 ± 500.6 |
| Total bilirubin, mg/dL | 16.7 ± 16.6 |
| C-reactive protein, mg/L | 187.8 ± 191.6 |
| Urine protein dipstick, % (neg/1+/2+) | 64.1/29.9/6.0 |
| Urine volume last 24 h, mL/kg | 42.3 ± 32.4 |
| Urine volume last hour, mL/kg | 2.44 ± 5.93 |
| Donor data at cross-clamp period | |
| Time incision until cross-clamp, min | 52.6 ± 31.0 |
| Time cross-clamp until ectomy right | 43.7 ± 16.6 |
| Time cross-clamp until ectomy left | 49.0 ± 17.9 |
| Catecholamines, n (%) | 130 (76.9) |
| Mean Arterial Blood pressure, mmHg | 97.2 ± 15.8 |
| Pulse,/min | 96.4 ± 25.6 |
| Central venous pressure, mmHg | 9.74 ± 3.69 |
| Temperature, °C | 36.61 ± 1.17 |
Demographic data and ICU monitoring parameters of the donors.
Continuous parameters are given as mean ± standard deviation, numerical and ordinal parameters as count and percentage.
Abbreviations: AKI, acute kidney injury; BMI, body mass index; DDK, deceased donor kidney; DGF, delayed graft function; FFP, fresh frozen plasma; HBsAG, hepatitis B virus surface antigen; ICU, intensive care unit; IU, international units; RBC, red blood cells.
TABLE 3
| Characteristics | Transplanted kidneys (n = 223) |
|---|---|
| Macroscopic parametersa | |
| Perfusions quality, % (good/medium/bad) | 94.6/3.1/2.2 |
| Organ quality, % (good/medium/bad) | 74.0/24.2/1.8 |
| Atherosclerosis, % (no/mild/severe) | 38.4/15.2/46.5 |
| Organ localization, % (right kidney/left kidney) | 48.4/51.6 |
| Histopathological parameters | |
| Biopsy performed, n (%) | 179 (80.3) |
| Art of biopsy, % (Needle/Wedge) | 82.1/17.9 |
| Renal cortex proportion of total parenchyma, % | 66.1 ± 34.2 |
| Glomeruli, n | 36.2 ± 69.0 |
| Arteries, n | 8.1 ± 15.2 |
| Global glomerulosclerosis, % of total glomeruli | 10.4 ± 15.0 |
| Global glomerulosclerosis < 5, % | 50.3 |
| Any FSGS, % of biopsies | 2.5 |
| Banff Lesion Scores (0/1/2/3), % | |
| Interstitial inflammation (i) | 84.2/14.6/1.2/0.0 |
| Tubulitis (t) | 88.9/11.1/0.0/0.0 |
| Intimal arteritis (v) | 99.4/0.6/0.0/0.0 |
| Glomerulitis (g) | 86.0/12.3/1.1/0.6 |
| Peritubular capillaritis (ptc) | 100.0/0.0/0.0/0.0 |
| Interstitial fibrosis (ci) | 80.1/18.1/1.2/0.6 |
| Tubular atrophy (ct) | 61.4/36.8/1.2/0.6 |
| Vascular fibrous Intimal thickening (cv) | 41.5/33.9/21.1/3.5 |
| Glomerular basement membrane splitting (cg) | 97.1/2.9/0.0/0.0 |
| Mesangial matrix expansion (mm) | 81.9/14.0/1.8/2.3 |
| Arteriolar hyalinosis (ah) | 35.1/38.6/22.8/3.5 |
| Interstitial fibrosis and tubular atrophy, % (0–10/10–25/25–50/>50) | 70.4/14.3/13.9/0.9 |
| Arteriolar wall fibrosis, % (no/mild/moderate/severe) | 54.4/35.1/9.4/1.2 |
| RPS diabetic nephropathy class ≥1, % | 5.2 |
| Thrombotic microangiopathy, % | 6.4 |
| Nephrocalcinosis, % (no/mild/moderate to severe) | 88.9/4.7/6.4 |
| Tubular hypertrophy, % | 19.3 |
| Epithelial cell flattening (0/1/2/3), % | 3.5/40.4/32.7/23.4 |
| Brush border membrane defect (0/1/2/3), % | 1.2/26.9/46.8/25.1 |
| Vacuolization (0/1/2/3), % | 7.0/22.8/22.2/48.0 |
| Loss of nuclear staining (0/1/2/3), % | 1.8/27.5/38.0/32.7 |
| Cellular detritus (0/1/2/3), % | 15.8/40.9/23.4/19.9 |
| Pyelonephritis, % | 8.2 |
Macroscopic and histopathological parameters.
Continuous parameters are given as mean ± standard deviation, numerical and ordinal parameters as count and percentage.
Abbreviations: DDK, deceased donor kidney; FSGS, focal and segmental glomerulosclerosis; RPS, renal pathology society.
Of note, macroscopic parameters listed in this table were determined by the harvesting surgeon, and not by a pathologist while the histopathological parameters were determined retrospectively by an experienced nephropathologist.
TABLE 4
| Recipients with follow-up data (n = 223) | |
|---|---|
| Recipients’ parameters | |
| Age, y | 61.0 ± 13.5 |
| Sex, n (%) | |
| Female | 75 (33.6) |
| Male | 148 (66.4) |
| BMI, kg/m2 | 25.5 ± 4.4 |
| Diabetes mellitus, n (%) | 59 (26.5) |
| Hypertension, n (%) | 191 (85.7) |
| Cardiovascular disease, n (%) | 96 (43.0) |
| HBsAg positive, n (%) | 49 (22.0) |
| Hepatitis C Virus positive, n (%) | 6 (2.7) |
| Cytomegalovirus positive, n (%) | 148 (66.4) |
| Dialysis vintage, months | 166.9 ± 79.2 |
| Prior organ transplant, n (%) | 24 (10.8) |
| Estimated Post Transplant Survival (EPTS) | 2.66 ± 0.62 |
| Estimated Post Transplant Survival (EPTS) Groups, n (%) | |
| Group 1: 0%–20% | 18 (8.1) |
| Group 2: 21%–40% | 14 (6.3) |
| Group 3: 41%–60% | 29 (13.0) |
| Group 4: 61%–80% | 30 (13.5) |
| Group 5: 81%–100% | 131 (58.7) |
| Transplant baseline parameters | |
| HLA-A mismatch (0/1/2), % | 14.3/56.1/29.6 |
| HLA-B mismatch (0/1/2), % | 8.1/48.9/43.0 |
| HLA-DR mismatch (0/1/2), % | 14.3/55.2/30.5 |
| Negative PRA at transplantation, n (%) | 200 (89.7) |
| Average PRA at transplantation, % | 2.4 ± 9.7 |
| Historic Peak of PRA, % | 7.5 ± 20.8 |
| Origin of donor kidney (right/left/both), % | 50.7/48.0/1.3 |
| Cold ischemia time, h | 13.8 ± 5.0 |
| Warm ischemia time, min | 40.6 ± 14.3 |
| Maintenance therapy | |
| Calcineurin inhibitors, % (Cyclosporin/Tacrolimus/other) | 74.8/24.5/0.7 |
| Anti-metabolites, % (Azathioprine/Mycophenolate/other) | 0.7/84.1/15.2 |
| mTOR inhibitors, % | 4.2 |
| Steroids, % | 91.0 |
Clinical parameters of recipients.
Continuous parameters are given as mean ± standard deviation, numerical and ordinal parameters as count and percentage.
Abbreviations: b, both; BMI, body mass index; HBsAg, hepatitis b virus surface antigen; HLA, human leukocyte antigen; PRA, panel reactive antibodies.
TABLE 5
| Recipients with follow-up data (n = 223) | |
|---|---|
| Primary non function, % | 26 (11.7) |
| Delayed graft function, % | 109 (48.9) |
| Patient survival at 1 year, % | 202 (90.6) |
| Patient survival at 3 years, % | 192 (86.1) |
| Patient survival at 5 years, % | 185 (83.0) |
| Death-censored graft survival at 1 year, % | 163 (91.1) |
| Death-censored graft survival at 3 years, % | 141 (82.9) (nmissing = 6) |
| Death-censored graft survival at 5 years, % | 133 (81.6) (nmissing = 6) |
| Kidney function at 3 months (creatinine), µmol/L | 188.3 ± 77.9 (nmissing = 1) |
| Kidney function at 3 months (eGFR), mL/min/1.73 m2 | 34.6 ± 14.7 (nmissing = 1) |
| Kidney function at 1 year (creatinine), µmol/L | 166.9 ± 52.9 (nmissing = 1) |
| Kidney function at 1 year (eGFR), mL/min/1.73 m2 | 37.4 ± 13.6 (nmissing = 1) |
| Kidney function at 3 years (creatinine), µmol/L | 165.8 ± 59.8 (nmissing = 61) |
| Kidney function at 3 years (eGFR), mL/min/1.73m2 | 38.4 ± 15.2 (nmissing = 61) |
| Rejections overall | 0.65 ± 1.05 (nmissing = 93) |
| Without Rejections (%) | 57.7 |
Outcome data of recipients.
Continuous parameters are given as mean ± standard deviation, numerical and ordinal parameters as count and percentage.
Donor and Organ Related Differences Between Discards and Transplantations
149 of the 442 available kidneys were discarded (33%). 45 were recovered from donors whose contralateral kidney was transplanted and 104 from donors whose both kidneys were discarded (Figure 2).
FIGURE 2
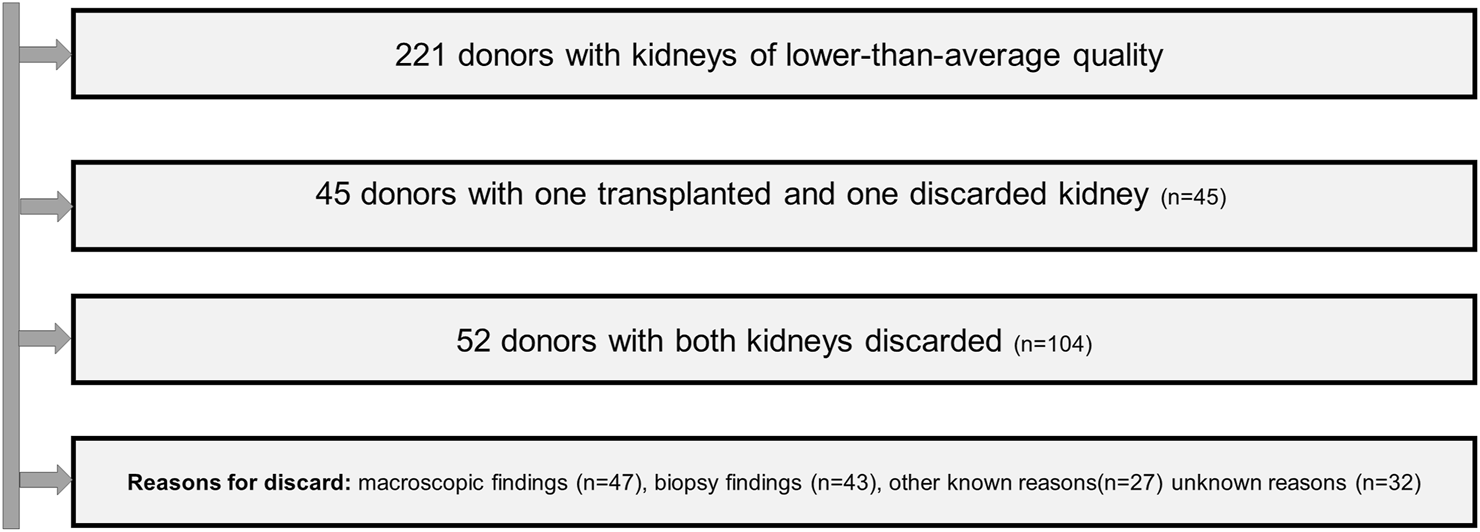
Flow chard of the handling of discarded organs.
Except for the higher prevalence of hepatitis C and the longer duration of brain death, there were no differences in the baseline characteristics between donors of transplanted and discarded kidneys (Table 6).
TABLE 6
| Transplanted kidneys (n = 293) | Discarded kidneys (n = 149) | p-value | |
|---|---|---|---|
| Donor characteristics | |||
| No of Donors | 169 | 97 | |
| Age, y | 60.8 ± 16.2 | 61.4 ± 15.2 | 0.999 |
| Sex, n (%) | |||
| Female | 77 (45.6) | 38 (39.6) | 0.345 |
| Male | 92 (54.4) | 58 (60.4) | |
| BMI, kg/m2 | 27.4 ± 5.8 | 27.3 ± 5.4 | 0.874 |
| Diabetes mellitus, n (%) | 22 (13.0) | 14 (14.6) | 0.721 |
| Hypertension, n (%) | 96 (56.8) | 56 (58.3) | 0.809 |
| Cardiovascular disease, n (%) | 49 (29.2) | 30 (31.6) | 0.682 |
| Smoker, n (%) | 46 (27.2) | 27 (28.1) | 0.874 |
| Hepatitis B Virus positive, n (%) | 11 (6.5) | 7 (7.3) | 0.808 |
| Hepatitis C Virus positive, n (%) | 2 (1.2) | 6 (6.3) | 0.021 |
| Cytomegalovirus positive, n (%) | 110 (65.1) | 65 (67.5) | 0.665 |
| Cerebrovascular accident (CVA), n (%) | 101 (59.8) | 50 (52.1) | 0.225 |
| Kidney Donor Risk Index (KDRI) | 1.48 | 1.52 | 0.788 |
| Time confirmed brain death until cross-clamp, h | 13.2 ± 14.8 | 15.8 ± 17.1 | 0.032 |
| AKI, n (%), Creatinine first, max | 61 (36.1) | 32 (33.3) | 0.651 |
| AKI, n (%), Creatinine min, max | 79 (46.7) | 40 (41.7) | 0.424 |
| Last serum creatinine, mg/dL | 1.68 ± 1.35 | 1.80 ± 1.40 | 0.775 |
| Last creatinine kinase | 849 ± 1,046 | 1,608 ± 8,345 | 0.435 |
| Last Sodium, mmol/L | 148.0 ± 9.4 | 147.6 ± 7.9 | 0.847 |
| Blood pressure, mmHg | |||
| Systolic | 126.1 ± 22.1 | 126.1 ± 25.0 | 0.918 |
| Diastolic | 68.0 ± 12.3 | 68.3 ± 14.9 | 0.870 |
| Mean arterial | 97.2 ± 15.8 | 97.3 ± 18.3 | 0.932 |
| Pulse/min | 96.4 ± 25.6 | 97.2 ± 24.1 | 0.889 |
| Central venous pressure, mmHg | 9.74 ± 3.69 | 9.5 ± 3.7 | 0.803 |
| Central venous pressure—PEEP, mmHg | 4.94 ± 4.60 | 4.7 ± 4.5 | 0.568 |
| Temperature, °C | 36.61 ± 1.17 | 36.7 ± 1.2 | 0.631 |
| PaO2/FiO2 Ratio | 252.1 ± 108.8 | 266.6 ± 108.7 | 0.425 |
| Last urine test strip, % (neg/+/++) | |||
| Protein | 64.1/29.9/6.0 | 63.8/33.0/3.2 | 0.570 |
| Leukocytes | 56.8/27.7/15.5 | 53.6/34.5/11.9 | 0.491 |
| Red blood cells | 36.8/40.1/23.0 | 29.4/40.0/30.6 | 0.351 |
| Nitrite | 81.3/18.7/0.0 | 85.1/14.9/0.0 | 0.458 |
| Urine volume last 24 h, mL | 3,347 ± 2,272 | 3,372 ± 2,053 | 0.657 |
| Urine volume last 24 h, mL/kg | 42.3 ± 32.4 | 43.075 ± 29.4 | 0.533 |
| Urine volume last hour, mL | 194 ± 499 | 196 ± 288.6 | 0.236 |
| Urine volume last hour, mL/kg | 2.44 ± 5.93 | 2.53 ± 3.87 | 0.247 |
Comparison of baseline characteristics between donors with transplanted and discarded kidneys.
Continuous variables are presented as mean ± standard deviation.
Abbreviations: AKI, acute kidney injury; BMI, body mass index; CPR, cardiopulmonary resuscitation; DGF: delayed graft function; dl, deciliter; g, gram; h, hours; IU, international units; kg, kilogram; L, liter; mL, milliliter; min, minutes; mmHg, Millimeter of mercury; mmol, millimole; m2, square meter; sec; seconds; y, years; µg, microgram.
Bold values represent statistically significant parameters.
One kidney from one donor with missing data about transplantation status.
The discarded kidneys were of lower macroscopic organ quality (deemed to be bad in 9.2% vs. 1.7%, p < 0.001) and showed more chronic glomerular (FSGS: 9.4% vs. 2.1%, p = 0.013; cg3: 2% vs. 0%, p = 0.03), more severe acute tubular cell (cellular detritus score 3: 33% vs. 21%, p = 0.036), more chronic tubulointerstitial (ci, ct, IFTA p < 0.001) and more chronic macrovascular injury (cv ≥ 1: 75% vs. 62%, p = 0.02). There were no differences in the percentage of glomerulosclerosis at all (11% vs. 10%, p = 0.305) or other tubular cell injury features. Lastly, findings of thrombotic microangiopathy (TMA) were more often observed (14.9% vs. 5.6%, p = 0.001) (Table 7).
TABLE 7
| Transplanted kidneys (n = 293) | Discarded kidneys (n = 149) | p-value | |
|---|---|---|---|
| Macroscopic characteristics | |||
| Perfusions quality, (good/medium/bad), % | 93.5/4.4/2.0 | 92.2/7.1/0.7 | 0.310 |
| Organ quality (good/medium/bad) % | 73.4/24.9/1.7 | 61.0/29.8/9.2 | <0.001 |
| Atherosclerosis (No/Mild/Severe), % | 38.2/16.8/45.0 | 36.4/14.5/49.1 | 0.864 |
| Histopathological characteristics | |||
| Glomerulosclerosis, % | 11.3 ± 17.2 | 10.1 ± 12.8 | 0.305 |
| FSGS, % | 2.1 | 9.4 | 0.013 |
| Banff Lesion Scores (0/1/2/3), % | |||
| Interstitial inflammation (i) | 82.3/15.7/2.0/0.0 | 86.1/9.9/2.0/2.0 | 0.130 |
| Tubulitis (t) | 88.9/11.1/0.0/0.0 | 85.1/13.9/0.0/1.0 | 0.288 |
| Intimal arteritis (v) | 99.0/1.0/0.0/0.0 | 96.0/4.0/0.0/0.0 | 0.085 |
| Glomerulitis (g) | 86.4/12.1/1.0/0.5 | 88.1/7.9/1.0/3.0 | 0.244 |
| Peritubular capillaritis (ptc) | 100.0/0.0/0.0/0.0 | 100.0/0.0/0.0/0.0 | >0.999 |
| Interstitial fibrosis (ci) | 78.8/19.2/1.5/0.5 | 61.4/25.7/7.9/5.0 | <0.001 |
| Tubular atrophy (ct) | 60.6/37.4/1.5/0.5 | 33.7/53.5/7.9/5.0 | <0.001 |
| Interstitial fibrosis and tubular atrophy (IFTA), % (0–10/10–25/25–50/>50) | 73.4/12.3/14.0/0.3 | 54.7/18.9/23.0/3.4 | <0.001 |
| Interstitial fibrosis and tubular atrophy (IFTA), % MW (±SD) | 3.10 ± 6.80 | 7.39 ± 13.09 | <0.001 |
| Vascular fibrous Intimal thickening (cv) | 38.4/37.9/20.2/3.5 | 24.8/43.6/26.7/5.0 | 0.120 |
| cv ≥ 1 | 61.6 | 75.2 | 0.018 |
| GBM double contours (cg) (0/1/2/3) | 97.0/3.0/0.0/0.0 | 98.0/0.0/0.0/2.0 | 0.030 |
| Mesangial matrix expansion (mm) | 82.8/12.1/2.0/3.0 | 86.1/5.0/1.0/7.9 | 0.058 |
| Arteriolar hyalinosis (ah) | 33.3/38.9/22.7/5.1 | 29.7/49.5/13.9/6.9 | 0.163 |
| Interstitial fibrosis and tubular atrophy (IFTA) | 3.10 ± 6.80 | 7.39 ± 13.09 | <0.001 |
| Thrombotic microangiopathy, % | 5.6 | 14.9 | 0.007 |
| Nephrocalcinosis (No/Mild Moderate/Severe), % | 89.4/4.0/6.6/0.0 | 88.1/7.9/4.0/0.0 | 0.260 |
| Tubular hypertrophy, % | 18.7 | 27.7 | 0.073 |
| Epithelial cell flattening (0/1/2/3), % | 4.0/39.4/32.8/23.7 | 7.9/33.7/32.7/25.7 | 0.461 |
| Brush border membrane defect (0/1/2/3), % | 1.0/25.8/46.5/26.8 | 2.0/18.8/43.6/35.6 | 0.291 |
| Vacuolization (0/1/2/3), % | 7.6/22.2/21.2/49.0 | 4.0/24.8/20.8/50.5 | 0.660 |
| Loss of nuclear staining (0/1/2/3), % | 2.5/28.3/37.9/31.3 | 0.0/22.8/44.6/32.7 | 0.251 |
| Cellular detritus (0/1/2/3), % | 16.2/40.9/22.2/20.7 | 7.9/33.7/25.7/32.7 | 0.036 |
Comparison of macroscopic and histological characteristics between transplanted and discarded kidneys.
Continuous variables are presented as mean ± standard deviation. MW, mean value; SD, standard deviation.
Bold values represent statistically significant parameters.
The following categories of reasons for discard were recorded: 1) Macroscopic organ damage, such as renal capsule fissure, cortical hemorrhage, large infarcts, large renal cysts, heavy aortic patch and/or renal artery atherosclerosis and mottled appearance after reperfusion. 2) findings of procurement biopsies. 3) concerns about a transmissible donor infection, 4) extrarenal malignancy known or detected during procurement or tumor of the contralateral kidney; 5) denial of the transplant center to finally accept the offer 6) non transplantability of the recipient.
47 kidneys were discarded due to macroscopic findings, 43 due to the results of biopsy and 27 due to one of the reasons belonging to categories 3 to 6. Unfortunately, for nearly every fifth discarded kidney (32/149, 21.5%) the exact reason remained unknown.
Score Performance in Transplanted Kidneys
The performance of the scores is shown in Table 8. Depending on missing data, up to 103 (46%) out of the 223 DDKs had to be excluded for the analysis of the endpoints.
TABLE 8
| Publication/Score | Endpoints | Performance in original publication | Performance in our cohort |
|---|---|---|---|
| Delayed graft function | |||
| 15 Balaz et al. (n = 171) | DGF | AUC (95% CI)CIV Score: 0.659 (0.606–0.710) | AUC (95% CI)CIV Score: 0.506 (0.417–0.595) |
| AUC (95% CI)CIV Score + donor age + cause of death: 0.694 (0.642–0.743) | AUC (95% CI)CIV Score + donor age + cause of death: 0.490 (0.401–0.579) | ||
| 6Chapal et al. (n = 131) | DGF | AUC (95% CI): 0.73 (0.68–0.77) | AUC (95% CI): 0.709 (0.617–0.801) |
| a 7Irish et al. (n = 223) | DGF | AUC 0.704 | AUC (95% CI): 0.684 (0.612–0.757) |
| 8Jeldres et al. (n = 223) | DGF | AUC: 0.743 | AUC (95% CI): 0.503 (0.423–0.582) |
| 11Schold et al. (n = 222) | DGF | Rate of DGF | Rate of DGF |
| Donor Grade I: 16.7% | Donor Grade I: 42.9% | ||
| Donor Grade II: 23.1% | Donor Grade II: 70.0% | ||
| Donor Grade III: 30.3% | Donor Grade III: 68.6% | ||
| Donor Grade IV: 39.2% | Donor Grade IV: 61.2% | ||
| Donor Grade V: 46.3% | Donor Grade V: 53.2% | ||
| AUC (95% CI): NA | AUC (95% CI): 0.451 (0.373–0.530) | ||
| Graft survival | |||
| 17 Navarro et al. (n = 223) | 5 years graft survival | HR (95% CI)Full Score: NA | HR (95% CI)Full Score: 1.501 (1.143–1.972) |
| HR (95% CI)Score >5 vs. ≤5: 6.95 (1.57–30) | HR (95% CI)Score >5 vs. ≤5: 1.994 (0.975–4.079) | ||
| AUC (95% CI)Full Score: NA | AUC (95% CI)Full Score: 0.617 (0.513–0.722) | ||
| AUC (95% CI)Score >5 vs. ≤5: NA | AUC (95% CI)Score >5 vs. ≤5: 0.567 (0.462–0.673) | ||
| 19Port et al. (n = 223) | 1 and 3 years graft survival | 1 year graft survival for RR < 1.7/≥1.7: 90.6/84.5% | 1 year graft survival for RR <1.7/≥1.7: 91.0/80.7% |
| AUC (95% CI)1 year: NA | AUC (95% CI)1 year: 0.662 (0.369–0.955) | ||
| 3 years graft survival for RR <1.7/≥1.7: 79.4/68.0% | 3 years graft survival for RR <1.7/≥1.7: 87.5/75.8% | ||
| AUC (95% CI)3 years: NA | AUC (95% CI)3 years: 0.603 (0.515–0.692) | ||
| 26Rao et al. (n = 223) | 1, 3, and 5 years graft survival | AUC (95% CI)1 year: NA | AUC (95% CI)1 year: 0.699 (0.459–0.939) |
| AUC (95% CI)3 years: NA | AUC (95% CI)3 years: 0.557 (0.456–0.658) | ||
| AUC (95% CI)5 years: NA | AUC (95% CI)5 years: 0.576 (0.474–0.679) | ||
| 5 years graft survival KDRI quintile 1: 82% | 5 years graft survival KDRI quintile 1: 80.6% | ||
| 5 years graft survival for KDRI quintile 2: 79% | 5 years graft survival for KDRI quintile 2: 73% | ||
| 5 years graft survival for KDRI quintile 3: NA | 5 years graft survival for KDRI quintile 3: 79% | ||
| 5 years graft survival for KDRI quintile 4: NA | 5 years graft survival for KDRI quintile 4: 76% | ||
| 5 years graft survival for KDRI quintile 5: 63% | 5 years graft survival for KDRI quintile 5: 68% | ||
| 20 Snoeijs et al. (n = 171) | 5 years graft survival | AUC: 0.74 | AUC (95% CI): 0.630 (0.513–0.746) |
| 16 Vusser et al. (n = 223) | 3 years graft survival | AUC (Historic cohort): 0.65 | AUC (95% CI): 0.637 (0.538–0.736) |
| AUC (Validation cohort): 0.70 | |||
| 16 Vusser et al. (n = 223) | 5 years graft survival | AUC (Historic cohort): 0.67 | AUC (95% CI): 0.620 (0.524–0.717) |
| AUC (Validation cohort): 0.81 | |||
| 19 Remuzzi et al. (n = 223) | 3 years graft survival | AUC: N/A | AUC (95% CI): 0.605 (0.501–0.709) |
| Graft function | |||
| 14 Anglicheau et al. (n = 223) | 1 year graft function | AUC eGFR < 25 mL/min at 1 year: 0.84 | AUC (95% CI) eGFR < 25 mL/min at 1 year: 0.649 (0.540–0.758) |
| 9Nyberg et al. (n = 223) | 1 year graft function | Mean creatinine clearance | Mean creatinine clearance |
| Kidney Grade A: 61.1 mL/min | Kidney Grade A: 51.5 mL/min | ||
| Kidney Grade B: 51.8 mL/min | Kidney Grade B: 42.7 mL/min | ||
| Kidney Grade C: 42.6 mL/min | Kidney Grade C: 35.7 mL/min | ||
| Kidney Grade D: 33.7 mL/min | Kidney Grade D: 34.8 mL/min | ||
| 18 Ortiz et al. (n = 171) | 1 and 2 years graft function | Kendall’s tau1 year: 0.277 (p = 0.0006) | Kendall’s tau1 year: 0.157 (p = 0.026) |
| Kendall’s tau2 years: 0.286 (p = 0.0005) | Kendall’s tau2 years: NA | ||
| Patient survival | |||
| 13Foucher et al. (n = 120) | Patient Survival | AUC: 0.69 | AUC (95% CI): 0.642 (0.548–0.736) |
Previously published scores for the quality assessment of DDKs tested in this study including the endpoints they were designed for and their performance in the original publication.
Abbreviations: aHR, adjusted hazard ratio; AUC, area under the receiver operating characteristic curve; CADI, chronic allograft damage index; CI, confidence interval; ECD, expanded criteria donor; eGFR, estimated glomerular filtration rate; HR, hazard ratio; KDRI, kidney donor risk index; NA, not available; RR, relative risk; SCR, standard criteria donor.
Pathological and combined clinical and pathological scores are in italics; the numbers correspond to the references in the revised manuscript.
The Irish score was applied without considering the parameter history of transition, which was not available in the majority of recipients.
Chapal and Irish had the best predictability for DGF with an AUC of 0.709 and 0.684, respectively, whereas Jeldres had an AUC of 0.503, Balaz of 0.506/0.490, and Schold of 0.451. For the prognostication of graft survival, the best-performing scores were of Rao and Port for 1 year with a significant AUC of 0.699 and 0.662, followed by de Vusser for 3 years, Snoeijs and de Vusser for 5 years with respective AUCs of 0.637, 0.630 and 0.620. Regarding graft function the trend was similar. Here, Navaro was acceptable, whereas the performance of Anglicheau poor (AUC 0.649) and the significance of Ortiz marginal (Kendall’s tau 1 year 0.157, p = 0.026). The predictive power of the EPTS score was poor (AUC 0.642).
Score Performance in Discarded Kidneys
In another approach we tested the scores for the prediction of discards (Table 9). The best results for the comparison between bilateral discard and bilateral transplantation (column A vs. column C of Table 9) showed Balaz (1.80 vs. 1.11, p = 0.034), Snoeijs (4.55 vs. 3.12, p = 0.028), Remuzzi (p = 0.013) and Ortiz (4.36 vs. 2.83, p = 0.029). For the comparison between unilateral discard and bilateral transplantation (column B vs. column C of Table 9), Balaz <1 (p = 0.030), Navaro (p = 0.010) and Remuzzi (p = 0.011) came out to be significant.
TABLE 9
| Score | Both kidneys were discarded (nkindeys = 104) | One kidney was transplanted, one kidney was discarded (nkindeys = 90) | Both kidneys were transplanted (nkindeys = 248) | Overall | p-value | p-value |
|---|---|---|---|---|---|---|
| p-value | A vs. C | B vs. C | ||||
| 15 CIV Score (Balaz et al.) | 1.80 ± 1.42 | 1.33 ± 1.07 | 1.11 ± 1.08 | <0.001 | 0.034 | 0.233 |
| 15 CIV Score (Balaz et al.), (<1), % | 15.9 | 23.4 | 34.1 | 0.012 | 0.382 | 0.030 |
| 15 Composite CIV Score (Balaz et al.), %(0/1/2/3) | 4.3/31.9/49.3/14.5 | 9.4/25.0/46.9/18.8 | 4.2/30.5/52.7/12.6 | 0.583 | 0.533 | 0.804 |
| 6DGFS scoring system (Chapal et al.), Value | — | −0.1440 ± 0.7896 | −0.1201 ± 0.7989 | 0.924 | — | 0.924 |
| 6DGFS scoring system (Chapal et al.), % (Low risk/medium risk/high risk) | — | 36.4/54.5/9.1 | 33.3/61.7/5.0 | — | 0.808 | |
| 7DGF risk calculator (Irish et al.), Points | — | 210.6 ± 18.9 | 223.6 ± 28.6 | 0.092 | — | 0.092 |
| 7DGF risk calculator (Irish et al.), Probability of DGF (%) | — | 19.9 ± 17.3 | 24.1 ± 20.0 | 0.303 | — | 0.303 |
| 8Jeldres scoring system (Jeldres et al.), Points | — | 137.9 ± 31.2 | 131.3 ± 35.2 | 0.358 | — | 0.358 |
| 8Jeldres scoring system (Jeldres et al.), Probability of DGF (%) | — | 48.5 ± 20.1 | 44.6 ± 21.6 | 0.370 | — | 0.370 |
| 11Schold Risk Index | — | 1.05 ± 0.32 | 0.95 ± 0.35 | 0.190 | — | 0.190 |
| 11Schold Grade I-V | — | 0.0/3.8/23.1/26.9/46.2 | 3.6/9.7/23.0/30.3/33.2 | — | 0.554 | |
| 17 Navarro Score (≤3/4-5/6-7/>7) | 59.6/13.5/10.6/16.3 | 62.2/22.2/6.7/8.9 | 69.8/15.3/10.1/4.8 | 0.011 | 0.165 | 0.010 |
| 17 Navarro Score > 5, % | 26.9 | 15.6 | 14.9 | 0.022 | 0.080 | 0.947 |
| 19Port | 1.96 ± 0.52 | 1.97 ± 0.51 | 1.96 ± 0.47 | 0.991 | 0.909 | 274 |
| 26Rao | — | 1.33 ± 0.31 | 1.17 ± 0.43 | 0.054 | — | 0.106 |
| 20 Snoeijs | 4.55 ± 3.47 | 3.36 ± 2.61 | 3.12 ± 2.39 | 0.001 | 0.028 | 0.387 |
| 16 Vusser (3 years prediction) | 66.5 ± 16.8 | 64.0 ± 17.4 | 62.6 ± 17.7 | 0.158 | 0.315 | 0.185 |
| 16 Vusser (5 years prediction) | 63.2 ± 14.8 | 62.3 ± 16.0 | 61.2 ± 16.7 | 0.581 | 0.693 | 0.240 |
| 19 Remuzzi Score (pirani) | 2.41 ± 2.76 | 1.90 ± 1.94 | 1.55 ± 1.84 | 0.002 | 0.141 | 0.011 |
| 19 Remuzzi Grading (Score 1-3/4-6/7-12) (pirani) 1-3: for single transplantation, 4-6: for dual transplantation | 67.3/24.0/8.7 | 83.3/15.6/1.1 | 85.1/12.9/2.0 | 0.001 | 0.013 | 0.715 |
| 14 Anglicheau (GS−/CP−; GS−/CP+; GS+/CP−; GS+/CP+) | 29.8/46.2/2.9/21.2 | 16.7/64.4/1.1/17.8 | 20.6/52.8/6.0/20.6 | 0.060 | 0.058 | 0.500 |
| 9Nyberg Score | — | 26.1 ± 7.1 | 24.1 ± 9.0 | 0.261 | — | 0.261 |
| 9Nyberg Grading (A/B/C/D) | — | 0.0/25.0/25.0/50.0 | 9.1/23.4/28.9/38.6 | 0.318 | ||
| 18 Ortiz | 4.36 ± 2.91 | 3.34 ± 2.35 | 2.83 ± 1.98 | <0.001 | 0.029 | 0.148 |
| 13Foucher | — | 9.56 ± 2.90 | 8.39 ± 2.04 | 0.236 | — | 0.240 |
Performance of the investigated scores for the prediction of discards vs. transplantation.
CIV, chronic interstitial and vascular score according to the Banff classification; composite CIV Score: CIV score considering also clinical parameters (donor age >51 years, anoxic donor brain injury).
A, B and C refer to the first (bilateral discard), second (unilateral discard) and third (bilateral transplantation) column of the table.
Pathological and combined clinical and pathological scores are in italics, the numbers correspond to the references of the manuscript.
Bold values represent statistically significant parameters.
Discussion
Primary aim of this retrospective study was to test the performance of scores previously devised for quality assessment of a DDK of lower quality for their value in supporting the decision about discard or acceptance. The rather dismal clinical outcome in our cohort with 48.9% and 15.8% of recipients respectively developing DGF or losing their graft within the first year shows that it was indeed a formidable real-life challenge for the scores.
For DGF we found an acceptable discrimination with an AUC of 0.709 for the Chapal score. The Irish score could have even performed better if we would have been able to provide the missing recipient parameter of “previous blood transfusion.” Moreover, the applicability of the purely clinical and thus economical Irish score is limited because it requires the cold and warm ischemia time, both unknow at the time of allocation. Conversely, the Chapal score required donor- and recipient parameters, which, except for the cold ischemia time, are easily to obtain. The score of Chapal showed a lower AUC than that reported in the initial publication [6]. This may be explained by the higher incidence of DGF in our cohort (48.9% vs. 25.4% reported by Chapal).
Similarly poor results were seen for the Anglicheau and Ortiz scores to predict graft function. Their poor performance may be explained by the higher age of our recipients, compared with those in the cohorts of Anglicheau and Ortiz (61.0 vs. 50.6 vs. 48 years), as well as the higher ratio of our donors with hypertension (56.8% vs. 30.8%) and their higher creatinine levels before organ removal (149 vs. 101 μmol/L) compared with those in the cohort of Anglicheau. However, the better performing score of Nyberg, requires cold ischemia time, a parameter not known at the time of allocation.
None of the scores for graft survival reached an acceptable performance. The pathological scores of Navarro and Snoejjs and the clinicopathological of de Vusser outperformed the solely clinical Rao and Port’s scoring systems. This suggests that there are aspects of donor organ quality that cannot be reliably determined from clinical data alone. Inclusion of pathologic data could allow for better assessment of overall organ quality, particularly in kidneys of lower-than-average quality and explain the better performance of the scores with histopathology. Still, this was not sufficient to push AUC into the acceptable range. The score of Navarro [17] has been adopted by the Spanish Society of Nephrology [46]. Here, kidneys with a score <8 are proposed for single transplantation. The very poor results obtained by Navarro et al in their study transplanting kidneys with a score 6–7 were not confirmed later by others [47].
In summary, the majority of the scores are not suitable for procurement biopsies because they include information, which is not available during procurement. Beyond that, the scores were developed after examination of paraffin embedded renal tissue, a procedure that is time consuming and not practical in the limited time setting of allocation. The only exception is the Remuzzi score, which was based on frozen sections. However, in our experience frozen sections are often difficult to evaluate due to inappropriate handling during transport [31].
Procurement may also lead to needless discards if the histopathologic evaluation is conducted by general pathologists and not by nephropathologists. The failure of pretransplant biopsies to predict graft outcomes was highlighted in an older metaanalysis of 47 studies testing 15 scores [48]. In a recent paper, more than half of kidneys discarded in US would have been suitable for transplant in France, where procurement biopsies are rarely performed [49]. Furthermore, their usefulness has been questioned due to low reproducibility and poor predictive power [50], albeit there are centers proposing punch- instead of wedge or needle- biopsies as a means to improve standardization, sample adequacy and reproducibility [51]. At all, scores based on preimplantation biopsies can be implemented to predict graft function but their applicability to decide on transplantation or discard has probably been overestimated [52].
Strengths of our study were the comprehensive evaluation exclusively of procurement biopsies by an experienced nephropathologist according to the most recent Banff criteria [29] and the validation of the most known scores for the endpoints for which they have been developed.
Limitations should also be recognized. First, the definition of DGF as need for dialysis within the first week after transplantation, an endpoint that may be influenced by various clinical factors (such as heart failure, hyperkalemia, etc.) is not uniformly accepted. Furthermore, we excluded PNF, because it has a different pathogenesis [40] and was not tested as outcome parameter in the scores. The extraordinarily high incidence of PNF and DGF was probably due to bias by indication; our cohort was highly selective since biopsies were performed only in those donors whose organs were supposed to be of lower quality. Another reason was the higher incidence of donors with AKI an acknowledged risk factor for both outcomes [53]. Second, the scores have been constructed on preimplantation biopsies, which are in terms of prognostication completely different from procurement biopsies due to the accrued damage during cold preservation and transport as well as the reperfusion injury after implantation. Third, the number of missing data implies that each score was tested on different or partially overlapping sub-cohorts. However, this problem is unavoidable, since the data required for the calculation of all scores, are not routinely collected in the ET database nor at the DSO or the transplant centers. A registry with data of all sources (DSO, ET, transplant centers) is not available. Fourth, the test cohort dates back approximately 10 years. However, most of the evidence base of kidney transplantation relies on data collected before 2010 and the follow-up period of our study should not have changed considerably in the decade before and after 2010.4Fifth, the indications for procurement biopsies relied not on objective criteria since they were performed on case-by- case basis and not according to a standardized protocol. For example, the macroscopic assessment of the recovered organs was quite subjective. However, it can be of value if performed in a more structured way by experienced surgeons [54]. Finally, an inherent, unavoidable drawback of all similar studies is the unknown performance of the certainly non-randomly discarded DDKs. Despite all these limitations, this is the only study examining the performance of these scores on the dataset for which they are most usefully from a clinical point of view: procurement biopsies for the decision of DDK transplantation or discard. We found that, that none of the tested scores should allow a confident, evidence-based decision about acceptance or discard of a DDK based on prognosis of the different endpoints within the ET context. Probably, clinical parameters not included in that scores, such as donor’s AKI or donor’s creatinine metrics are more important for short term outcomes [53, 55].
Here, some conclusions can be drawn: First, organs from donors with AKI should not be accepted for recipients at high risk for DGF or these recipients may be preferentially treated with an immunosuppression protocol based on belatacept [56]. Second, the recipient should return timely to dialysis to avoid losing it waitlist points if an early graft failure is expected. Finally, we must always keep in mind that especially for the elderly patients, rejection of organs leads in the end to an increase in mortality due to the longer waiting list time [57].
Regarding the second aim, we could indeed show that for the endpoint death censored graft survival histological [17, 20], or clinicopathological [16] scores performed marginally better than purely clinical ones. But even if the AUCs were slightly better their overall performance was moderate to poor. While for some DDKs donor and recipient parameters might be entirely sufficient for a prognosis, for some donor/recipient matches histopathology might add valuable information. We are currently investigating such an approach with a facultative histopathology component including only reproducible parameters independently from each other associated with prognosis.
As to the testing of the scores in the discarded kidneys, we found that scores with a histological component were better than the solely clinical. However, an inherent bias cannot be excluded since the histologic evaluation of an offered organ is often the principal reason of its discard. Here, we can only postulate that histological assessment is warranted in kidneys supposed to be unsuitable for transplantation. Probably, the most important finding was that many of the discarded kidneys could have been successfully transplanted.
Conclusion
Procurement biopsies are often used during allocation to increase the possibility of acceptance of kidneys of lower quality. However, the available prognostic scores perform at best only moderately. Though none of the scores could reach an acceptable discriminatory power, those based on histopathologic criteria performed slightly better than the more practical solely clinical ones. Our findings are based on data from the Eurotransplant region but can also be applied to other Multinational or National Transplant Organizations or -even more- be valuable for individual decisions in transplant centers.
Statements
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
All organ transplants were performed according the Declaration of Istanbul; no transplants from prisoners were used. The study was conducted in accordance with the Declarations of Helsinki and approved by the local ethical review board of Hannover Medical School (No. 1519-2012).
Author contributions
JB and CC conceived the study and wrote the manuscript; JB re-evaluated the biopsies; CC was responsible for the acquisition of recipient and donor data; C-LF provided donor data. FS created the tables; FS and AE performed the statistical analysis; AS provided intellectual input, reviewed, and edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
JB and AE received funding by the DFG (BE-3801). JB is supported by the European Rare Kidney Disease Network (ERKNet), funded by the European Commission.
Acknowledgments
We would thank the following German transplant centers who provided data for the present analysis. Transplantationszentrum Uniklinik RWTH Aachen; Transplantation Berlin-Charité-Universität Berlin; Transplantationszentrum Bochum; Transplantationszentrum-Universitätsklinikum Bonn; Dr. Frans Zantvoort, Klinikum Bremen Mitte; Transplantationszentrum Uniklinik Düsseldorf; Transplantationszentrum-Uniklinikum Essen; Prof. Dr. med. Martina Koch, Universitätsklinikum Hamburg-Eppendorf; Transplantationschirurgie-Uniklinik Innsbruck; Transplantationszentrum-Uniklinik Köln; PD Dr. med. Antje Habicht, Transplantationszentrum München der LMU-LMU Klinikum; Prof. Dr. med. Bernhard Banas, Universitäres Transplantationszentrum Regensburg; PD Dr. med. Martin Nitschke, Universitätsklinikum Schleswig-Holstein; Dr. Peter Weithofer, Transplantationszentrum Süd-Niedersachsen.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/ti.2023.11399/full#supplementary-material
Footnotes
2.^ https://optn.transplant.hrsa.gov/media/1512/guide_to_calculating_interpreting_kdpi.pdf
References
1.
Laupacis A Keown P Pus N Krueger H Ferguson B Wong C et al A Study of the Quality of Life and Cost-Utility of Renal Transplantation. Kidney Int (1996) 50:235–42. 10.1038/ki.1996.307
2.
Tonelli M Wiebe N Knoll G Bello A Browne S Jadhav D et al Systematic Review: Kidney Transplantation Compared With Dialysis in Clinically Relevant Outcomes. Am J Transpl (2011) 11:2093–109. 10.1111/j.1600-6143.2011.03686.x
3.
Wolfe RA Ashby VB Milford EL Ojo AO Ettenger RE Agodoa LY et al Comparison of Mortality in All Patients on Dialysis, Patients on Dialysis Awaiting Transplantation, and Recipients of a First Cadaveric Transplant. N Engl J Med (1999) 341:1725–30. 10.1056/NEJM199912023412303
4.
Chatzikyrkou C Scurt FG Clajus C Roumeliotis S Mertens PR Haller H et al Predictors of Outcomes of Living Kidney Donation: Impact of Sex, Age and Preexistent Hypertension. Transpl Proc (2019) 51:396–404. 10.1016/j.transproceed.2019.01.015
5.
Scurt FG Ewert L Mertens PR Haller H Schmidt BMW Chatzikyrkou C . Clinical Outcomes After ABO-Incompatible Renal Transplantation: A Systematic Review and Meta-Analysis. Lancet (2019) 393:2059–72. 10.1016/S0140-6736(18)32091-9
6.
Chapal M Le Borgne F Legendre C Kreis H Mourad G Garrigue V et al A Useful Scoring System for the Prediction and Management of Delayed Graft Function Following Kidney Transplantation From Cadaveric Donors. Kidney Int (2014) 86:1130–9. 10.1038/ki.2014.188
7.
Irish WD Ilsley JN Schnitzler MA Feng S Brennan DC . A Risk Prediction Model for Delayed Graft Function in the Current Era of Deceased Donor Renal Transplantation. Am J Transpl (2010) 10:2279–86. 10.1111/j.1600-6143.2010.03179.x
8.
Jeldres C Cardinal H Duclos A Shariat SF Suardi N Capitanio U et al Prediction of Delayed Graft Function After Renal Transplantation. Can Urol Assoc J (2009) 3:377–82. 10.5489/cuaj.1147
9.
Nyberg SL Matas AJ Kremers WK Thostenson JD Larson TS Prieto M et al Improved Scoring System to Assess Adult Donors for Cadaver Renal Transplantation. Am J Transpl (2003) 3:715–21. 10.1034/j.1600-6143.2003.00111.x
10.
Port FK Bragg-Gresham JL Metzger RA Dykstra DM Gillespie BW Young EW et al Donor Characteristics Associated With Reduced Graft Survival: An Approach to Expanding the Pool of Kidney Donors. Transplantation (2002) 74:1281–6. 10.1097/00007890-200211150-00014
11.
Schold JD Kaplan B Baliga RS Meier-Kriesche HU . The Broad Spectrum of Quality in Deceased Donor Kidneys. Am J Transpl (2005) 5:757–65. 10.1111/j.1600-6143.2005.00770.x
12.
Zaza G Ferraro PM Tessari G Sandrini S Scolari MP Capelli I et al Predictive Model for Delayed Graft Function Based on Easily Available Pre-Renal Transplant Variables. Intern Emerg Med (2015) 10:135–41. 10.1007/s11739-014-1119-y
13.
Foucher Y Daguin P Akl A Kessler M Ladrière M Legendre C et al A Clinical Scoring System Highly Predictive of Long-Term Kidney Graft Survival. Kidney Int (2010) 78:1288–94. 10.1038/ki.2010.232
14.
Anglicheau D Loupy A Lefaucheur C Pessione F Létourneau I Côté I et al A Simple Clinico-Histopathological Composite Scoring System Is Highly Predictive of Graft Outcomes in Marginal Donors. Am J Transpl (2008) 8:2325–34. 10.1111/j.1600-6143.2008.02394.x
15.
Balaz P Rokosny S Wohlfahrtova M Wohlfahrt P Bartonova A Pokorna E et al Identification of Expanded-Criteria Donor Kidney Grafts at Lower Risk of Delayed Graft Function. Transplantation (2013) 96:633–8. 10.1097/TP.0b013e31829d9225
16.
De Vusser K Lerut E Kuypers D Vanrenterghem Y Jochmans I Monbaliu D et al The Predictive Value of Kidney Allograft Baseline Biopsies for Long-Term Graft Survival. J Am Soc Nephrol (2013) 24:1913–23. 10.1681/ASN.2012111081
17.
Navarro MD López-Andréu M Rodríguez-Benot A Ortega-Salas R Morales MLA López-Rubio F et al Significance of Preimplantation Analysis of Kidney Biopsies From Expanded Criteria Donors in Long-Term Outcome. Transplantation (2011) 91:432–9. 10.1097/TP.0b013e318204bdd7
18.
Ortiz F Paavonen T Törnroth T Koskinen P Finne P Salmela K et al Predictors of Renal Allograft Histologic Damage Progression. J Am Soc Nephrol (2005) 16:817–24. 10.1681/ASN.2004060475
19.
Remuzzi G Cravedi P Perna A Dimitrov BD Turturro M Locatelli G et al Long-Term Outcome of Renal Transplantation From Older Donors. N Engl J Med (2006) 354:343–52. 10.1056/NEJMoa052891
20.
Snoeijs MG Buurman WA Christiaans MH van Hooff JP Goldschmeding R van Suylen RJ et al Histological Assessment of Preimplantation Biopsies May Improve Selection of Kidneys From Old Donors After Cardiac Death. Am J Transpl (2008) 8:1844–51. 10.1111/j.1600-6143.2008.02318.x
21.
Kers J Peters-Sengers H Heemskerk MBA Berger SP Betjes MGH van Zuilen AD et al Prediction Models for Delayed Graft Function: External Validation on the Dutch Prospective Renal Transplantation Registry. Nephrol Dial Transpl (2018) 33:1259–68. 10.1093/ndt/gfy019
22.
Irish WD McCollum DA Tesi RJ Owen AB Brennan DC Bailly JE et al Nomogram for Predicting the Likelihood of Delayed Graft Function in Adult Cadaveric Renal Transplant Recipients. J Am Soc Nephrol (2003) 14:2967–74. 10.1097/01.asn.0000093254.31868.85
23.
Metzger RA Delmonico FL Feng S Port FK Wynn JJ Merion RM . Expanded Criteria Donors for Kidney Transplantation. Am J Transpl (2003) 3(4):114–25. 10.1034/j.1600-6143.3.s4.11.x
24.
Moore J Ramakrishna S Tan K Cockwell P Eardley K Little MA et al Identification of the Optimal Donor Quality Scoring System and Measure of Early Renal Function in Kidney Transplantation. Transplantation (2009) 87:578–86. 10.1097/TP.0b013e3181949e71
25.
Munivenkatappa RB Schweitzer EJ Papadimitriou JC Drachenberg CB Thom KA Perencevich EN et al The Maryland Aggregate Pathology Index: A Deceased Donor Kidney Biopsy Scoring System for Predicting Graft Failure. Am J Transpl (2008) 8:2316–24. 10.1111/j.1600-6143.2008.02370.x
26.
Rao PS Schaubel DE Guidinger MK Andreoni KA Wolfe RA Merion RM et al A Comprehensive Risk Quantification Score for Deceased Donor Kidneys: The Kidney Donor Risk index. Transplantation (2009) 88:231–6. 10.1097/TP.0b013e3181ac620b
27.
Jackson KR Munivenkatappa RB Wesson RN Garonzik-Wang J Massie A Philosophe B . What's the Score? A Comparison of Deceased Donor Kidney Scoring Systems and Correlation With Graft Outcome. Clin Transpl (2020) 34:e13802. 10.1111/ctr.13802
28.
Chen K Guntur G Stalam T Okonkwo N Drachenberg C Goussous N et al Deceased-Donor Kidney Biopsy Scoring Systems for Predicting Future Graft Function: A Comparative Study. Transpl Proc (2021) 53:906–12. 10.1016/j.transproceed.2020.09.002
29.
Liapis H Gaut JP Klein C Bagnasco S Kraus E Farris AB 3rd et al Banff Histopathological Consensus Criteria for Preimplantation Kidney Biopsies. Am J Transpl (2017) 17:140–50. 10.1111/ajt.13929
30.
Reese PP Aubert O Naesens M Huang E Potluri V Kuypers D et al Assessment of the Utility of Kidney Histology as a Basis for Discarding Organs in the United States: A Comparison of International Transplant Practices and Outcomes. J Am Soc Nephrol (2021) 32:397–409. 10.1681/ASN.2020040464
31.
Pisarski P Schleicher C Hauser I Becker JU . German Recommendations for Pretransplantation Donor Kidney Biopsies. Langenbecks Arch Surg (2016) 401:133–40. 10.1007/s00423-016-1384-5
32.
Mengel M Sis B Haas M Colvin RB Halloran PF Racusen LC et al Banff 2011 Meeting Report: New Concepts in Antibody-Mediated Rejection. Am J Transpl (2012) 12:563–70. 10.1111/j.1600-6143.2011.03926.x
33.
D'Agati VD Fogo AB Bruijn JA Jennette JC . Pathologic Classification of Focal Segmental Glomerulosclerosis: A Working Proposal. Am J Kidney Dis (2004) 43:368–82. 10.1053/j.ajkd.2003.10.024
34.
Tervaert TW Mooyaart AL Amann K Cohen AH Cook HT Drachenberg CB et al Pathologic Classification of Diabetic Nephropathy. J Am Soc Nephrol (2010) 21:556–63. 10.1681/ASN.2010010010
35.
Gwinner W Suppa S Mengel M Hoy L Kreipe HH Haller H et al Early Calcification of Renal Allografts Detected by Protocol Biopsies: Causes and Clinical Implications. Am J Transpl (2005) 5:1934–41. 10.1111/j.1600-6143.2005.00938.x
36.
Gwinner W Hinzmann K Erdbruegger U Scheffner I Broecker V Vaske B et al Acute Tubular Injury in Protocol Biopsies of Renal Grafts: Prevalence, Associated Factors and Effect on Long-Term Function. Am J Transpl (2008) 8:1684–93. 10.1111/j.1600-6143.2008.02293.x
37.
Schumann-Bischoff A Schmitz J Scheffner I Schmitt R Broecker V Haller H et al Distinct Morphological Features of Acute Tubular Injury in Renal Allografts Correlate With Clinical Outcome. Am J Physiol Ren Physiol (2018) 315:F701–F710. 10.1152/ajprenal.00189.2017
38.
Rao PS Ojo A . The Alphabet Soup of Kidney Transplantation: SCD, DCD, ECD--Fundamentals for the Practicing Nephrologist. Clin J Am Soc Nephrol (2009) 4:1827–31. 10.2215/CJN.02270409
39.
Levey AS Stevens LA Schmid CH Zhang YL Castro AF 3rd Feldman HI et al A New Equation to Estimate Glomerular Filtration Rate. Ann Intern Med (2009) 150:604–12. 10.7326/0003-4819-150-9-200905050-00006
40.
Hamed MO Chen Y Pasea L Watson CJ Torpey N Bradley JA et al Early Graft Loss After Kidney Transplantation: Risk Factors and Consequences. Am J Transpl (2015) 15:1632–43. 10.1111/ajt.13162
41.
Siedlecki A Irish W Brennan DC . Delayed Graft Function in the Kidney Transplant. Am J Transpl (2011) 11:2279–96. 10.1111/j.1600-6143.2011.03754.x
42.
Hosmer DW Lemeshow S Sturdivant RX . Applied Logistic Regression. New York, NY, USA: John Wiley and Sons (2000). p. 160–4.
43.
Mandrekar JN . Receiver Operating Characteristic Curve in Diagnostic Test Assessment. J Thorac Oncol (2010) 5:1315–6. 10.1097/JTO.0b013e3181ec173d
44.
Muller E Dominguez-Gil B Martin D . The Declaration of Istanbul on Organ Trafficking and Transplant Tourism (2018 Edition) Introduction. Transplantation (2019) 103:217. 10.1097/TP.0000000000002541
45.
Echterdiek F Latus J Döhler B Schwenger V Süsal C . Influence of Cold Ischemia Time on the Outcome of Kidney Transplants From Donors Aged 70 Years and Above-A Collaborative Transplant Study Report. Transplantation (2021) 105:2461–9. 10.1097/TP.0000000000003629
46.
Serón D Anaya F Marcén R del Moral RG Martul EV Alarcón A et al Guidelines for Indicating, Obtaining, Processing and Evaluating Kidney Biopsies. Nefrologia (2008) 28:385–96.
47.
Azancot MA Moreso F Salcedo M Cantarell C Perello M Torres IB et al The Reproducibility and Predictive Value on Outcome of Renal Biopsies From Expanded Criteria Donors. Kidney Int (2014) 85:1161–8. 10.1038/ki.2013.461
48.
Wang CJ Wetmore JB Crary GS Kasiske BL . The Donor Kidney Biopsy and Its Implications in Predicting Graft Outcomes: A Systematic Review. Am J Transpl (2015) 15:1903–14. 10.1111/ajt.13213
49.
Aubert O Reese PP Audry B Bouatou Y Raynaud M Viglietti D et al Disparities in Acceptance of Deceased Donor Kidneys Between the United States and France and Estimated Effects of Increased US Acceptance. JAMA Intern Med (2019) 179:1365–74. 10.1001/jamainternmed.2019.2322
50.
Carpenter D Husain SA Brennan C Batal I Hall IE Santoriello D et al Procurement Biopsies in the Evaluation of Deceased Donor Kidneys. Clin J Am Soc Nephrol (2018) 13:1876–85. 10.2215/CJN.04150418
51.
Bago-Horvath Z Kozakowski N Soleiman A Bodingbauer M Mühlbacher F Regele H . The Cutting (W)Edge--Comparative Evaluation of Renal Baseline Biopsies Obtained by Two Different Methods. Nephrol Dial Transpl (2012) 27:3241–8. 10.1093/ndt/gfs066
52.
Pérez-Sáez MJ Montero N Redondo-Pachón D Crespo M Pascual J . Strategies for an Expanded Use of Kidneys From Elderly Donors. Transplantation (2017) 101:727–45. 10.1097/TP.0000000000001635
53.
Scurt FG Ernst A Wassermann T Hammoud B Mertens PR Schwarz A et al Short-Term Outcomes After Transplantation of Deceased Donor Kidneys With Acute Kidney Injury: A Retrospective Analysis of a Multicenter Cohort of Marginal Donor Kidneys With Post-Explantation Biopsies. Int Urol Nephrol (2023) 55:115–27. 10.1007/s11255-022-03277-3
54.
Miret Alomar E Moreso F Toapanta N Lorente D Triquell M Pont T et al Surgeon Preimplantation Macroscopic Graft Appraisal Improves Risk Stratification of Deceased Kidney Donors: A Prospective Study. Minerva Urol Nephrol (2022) 74:615–24. 10.23736/S2724-6051.21.04345-7
55.
Scurt FG Ernst A Hammoud B Wassermann T Mertens PR Schwarz A et al Effect of Creatinine Metrics on Outcome After Transplantation of Marginal Donor Kidneys. Nephrology (Carlton) (2022) 27:973–82. 10.1111/nep.14108
56.
Durrbach A Pestana JM Pearson T Vincenti F Garcia VD Campistol J et al A Phase III Study of Belatacept Versus Cyclosporine in Kidney Transplants From Extended Criteria Donors (BENEFIT-EXT Study). Am J Transpl (2010) 10:547–57. 10.1111/j.1600-6143.2010.03016.x
57.
Schold J Srinivas TR Sehgal AR Meier-Kriesche HU . Half of Kidney Transplant Candidates Who Are Older Than 60 Years Now Placed on the Waiting List Will Die Before Receiving a Deceased-Donor Transplant. Clin J Am Soc Nephrol (2009) 4:1239–45. 10.2215/CJN.01280209
Summary
Keywords
kidney transplantation, pathology, transplant loss, marginal donor, procurement biopsies
Citation
Scurt FG, Ernst A, FischerFröhlich C-L, Schwarz A, Becker JU and Chatzikyrkou C (2023) Performance of Scores Predicting Adverse Outcomes in Procurement Kidney Biopsies From Deceased Donors With Organs of Lower-Than-Average Quality. Transpl Int 36:11399. doi: 10.3389/ti.2023.11399
Received
26 March 2023
Accepted
14 September 2023
Published
12 October 2023
Volume
36 - 2023
Updates
Copyright
© 2023 Scurt, Ernst, FischerFröhlich, Schwarz, Becker and Chatzikyrkou.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jan U. Becker, janbecker@gmx.com; Christos Chatzikyrkou, chatziky@gmx.de
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.