Abstract
Total pancreatectomy with islet autotransplantation (TPIAT) is the treatment of choice to preserve pancreatic endocrine function, alleviate pain, and improve quality of life (QoL) when other strategies are ineffective for chronic pancreatitis (CP) patients. This study utilized pancreatic disease-specific surveys developed by the European Organisation for Research and Treatment of Cancer (EORTC) to conduct a comprehensive, single-center examination of a large cohort of patients to gain understanding of QoL post-TPIAT. Two validated QoL surveys of the EORTC—QLQ-C30 and QLQ-PAN26—were administered in a prospective cohort of CP patients during pre-and post-operative scheduled visits. A total of 116 patients responded to the preoperative survey and were included in this study. The global health scale of QLQ-C30 was significantly improved after TPIAT when compared to baseline with delta scores of 24.26, 20.54, and 26.7 at 1, 2, and 3 years post-TPIAT (p < 0.001). The EORTC-PAN26 revealed significant improvements in symptom scales for pancreatic pain, bloating, digestive symptoms, taste, indigestion, weight loss, body image, and future worries. The comprehensive surveys in such a large cohort expands the QoL criterion in CP patients and indicates significant improvement in QoL post-TPIAT, further validating TPIAT as a treatment option for refractory CP.
Introduction
Chronic pancreatitis (CP) is an irreversible inflammatory and fibrotic disease of the pancreas leading to varying degrees of exocrine and endocrine dysfunction. In severe cases, CP can lead to permanent loss of exocrine and endocrine function [1]. Furthermore, 75% of CP patients experience abdominal pain which can become debilitating [2]. CP patients report recurrent hospitalizations and numerous treatments to relieve pain and restore some semblance of normality in their quality of life (QoL). Initial medical management for CP may include but is not limited to narcotic analgesics, replacement of pancreatic enzymes, and radiological endoscopic procedures [3–5]. Patients with progressive symptoms in which medication and endoscopic intervention fails may be candidates for surgery [6]. Surgical techniques such as Puestow, Frey, Beger, and Whipple procedures are performed to achieve pain relief in CP patients. However, there is no evidence that these procedures lead to stable maintenance of endocrine function [7].
Total pancreatectomy followed by islet autotransplantation (TPIAT) is a preferred technique to preserve endocrine function and alleviate pain when other strategies are ineffective [8]. The first human TPIAT was performed by Dr. David Sutherland at the University of Minnesota in 1977. The rationale for this procedure is by removing the source of pain and disease exacerbations, this will improve a very poor QoL, reduce or eliminate chronic narcotic use, and facilitate return to work and self-care [9].
During the last 30 years, numerous collaborations between various North American centers, including ours, have developed the TPIAT program and documented metabolic outcomes. Many studies have reported achieving the main objective, improvement of QoL, through the SF-36 questionnaire, which evaluates health-related QoL [10–15]. In the current study, we evaluated QoL in patients who received TPIAT for CP at Baylor University Medical Center (Dallas, TX, United States) using the European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire (QLQ)-C30 survey combined with the QLQ-PAN26, designed specifically for patients with pancreatic disease [16].
Patients and Methods
Study Participants
This prospective observational study assessed the patient-oriented outcomes of QoL in CP patients who underwent TPIAT at Baylor University Medical Center. All patients were evaluated by a multidisciplinary team and had multiple indications for TPIAT. Patient eligibility for TPIAT includes intractable pain despite previous medical treatment, detectable endogenous insulin secretion capacity evident by serum C-peptide, and the capacity to consent to the treatment. Pregnant women were not eligible for the surgical procedure. We obtained consent for the intervention and study enrollment from all participants after they had been adequately informed of the risks. This study was conducted after approval of the institutional review board of Baylor Scott and White Research Institute (IRB#009-271).
Data Collection
Patients were asked to answer two QoL surveys of the EORTC—QLQ-C30 and QLQ-PAN26—before TPIAT and completed the survey during follow-up or by mail at 1, 2, and 3 years after transplantation. The EORTC QLQ-C30 and QLQ-PAN26 instruments were selected because they have been validated and used in trials to evaluate other pancreatic procedures [17–19]. The QLQ-C30 consists of 30 questions. The first section examines functioning: physical functioning, role functioning, emotional functioning, cognitive functioning, and social functioning in addition to a single item of global health. The second section addresses nine symptoms: nausea and vomiting, pain, fatigue, insomnia, loss of appetite, constipation, diarrhea, dyspnea, and financial difficulties. The QLQ-PAN26 assesses functioning and pancreatic-specific symptoms. It has 26 questions that evaluate pancreatic pain, bloating, digestive symptoms, taste, indigestion, flatulence, weight loss, dry mouth, hepatic symptoms, altered bowel habit, body image, trouble with side effects, future worries, and planning of activities in addition to healthcare satisfaction and sexuality [17–19]. All scales range from 0 to 100. QLQ-C30 high scores indicate healthier status or improved QoL for global health. High scores on the symptom scales correlate with a poor QoL. High scores on the QLQ-PAN26 indicate a poor QoL, except for healthcare satisfaction and sexuality.
The patients completed these surveys during their regularly scheduled follow-up appointments in electronic or hard copy format. We confirmed the validity of the electronic format. Surveys were completed via telephone or mail for 2 and 3 years post-TPIAT if the patients were unable to attend their clinic visits. Subjects with reduced ability to understand the questionnaires were excluded from the study. Details on the patient participation in these surveys is shown in Figure 1.
FIGURE 1
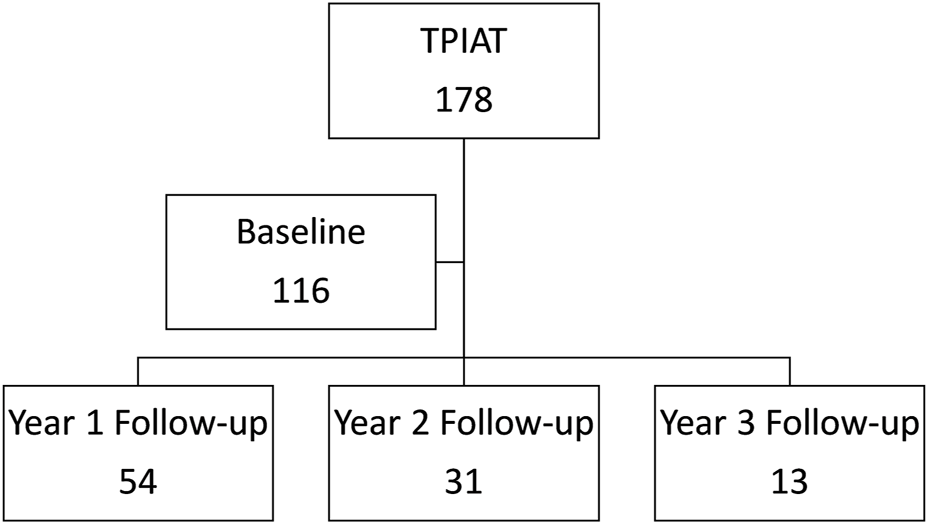
Consortium diagram of patient participation in the two surveys from baseline to 3 years follow-up.
All clinical data were recorded for each patient in a prospectively maintained database. Preoperative and postoperative clinical data in this study included levels of hemoglobin A1c, serum C-peptide, exogenous insulin requirement, pain score based on visual analog scale (VAS), and morphine equivalent dose. The VAS for pain ranged from 0 (no pain) to 10 (most severe pain). The daily dose of opioids was converted into morphine milligram equivalents (MME).
TPIAT Procedure
All patients underwent total pancreatectomy with the surgical technique described previously [20–23], with or without splenectomy based on surgeon decision. The distal common bile duct was removed, and the pancreatic blood supply was preserved during surgery as long as possible to minimize islet cell ischemia. On the back table, the spleen (if removed) and duodenum were detached from the pancreas, the pancreatic duct was cannulated, and the pancreas was placed in a container with cold preservation solution. Subsequently, the pancreas was transferred to a current good tissue practice (cGTP) facility for islet isolation processing.
Liberase MTF with Thermolysin MTF (Roche, Basel, Switzerland) or Collagenase NB with neutral proteases (SERVA Electrophoresis GMbH, Heidelberg, Germany) was infused into the pancreatic duct for digestion. Islets were isolated by the modified Ricordi method, which has been previously described [24, 25]. When the tissue volume (mL) exceeded 0.25 times body weight (kg), islets were purified with a COBE 2991 cell processor (Caridian BCT Inc., Lakewood, CO) using a density-adjusted iodixanol-based continuous density gradient. Endotoxin testing, gram staining, and bacterial and fungal cultures were performed on the final products as indicators of sterility. Isolated islets were infused into the portal vein via the superior mesenteric vein with heparin (70 unit/kg body weight) while the patient was under general anesthesia. The portal vein pressure was regularly monitored during the islet infusion.
Statistical Analysis
Data were presented as numbers and percentages for binary and categorical variables or as median and interquartile range (IQR) or as mean with standard deviations (SD) for continuous variables. The primary outcomes in this study were independent trends over time of the various scales and items of the EORTC QLQ-C30 and QLQ-PAN26. The surveys were administered at four time points: baseline, 1, 2, and 3 years. Raw scores measured at baseline and at years 1 and 2 were analyzed in longitudinal repeated measures analyses.
Generalized least square models without random effects, fitted by restricted maximum likelihood (REML) were used to examine if there was a differential effect across time (baseline to follow-up) in score measurements. The analysis focused on longitudinal single group analyses, where a single homogeneous population was followed over time. To account for the correlation in repeated measurements on the same subject, using various correlation structures with constant variance were considered. The correlation structure was selected based on AIC [29].
Due to the small sample size of respondents, scores measured at year 3 were not considered in longitudinal analyses. Additionally, a generalized least square model without random effects with restricted maximum likelihood (REML) was used instead of ordinary maximum likelihood estimation (MLE) [26, 27].
The constant variance assumption was checked using typical residual plots. The univariate normality assumption was checked using typical Q-Q plots on residuals. For checking the correlation pattern, variograms based on estimating correlations of all possible pairs of residuals at different time points were used [26–30].
Delta, defined as a difference over time from baseline, was assessed to estimate clinical significance in the EORTC questionnaires, according to recommendations by Osoba, where a difference in health-related QoL score of 10 points or more is regarded as clinically significant [31, 32]. The radar charts depict patients’ scores for the EORTC QLQ-C30 and EORTC QLQ-PAN26 scales for each domain as observed marginal means at different time points. Each domain is represented on separate axes (scaled from 0 to 100). All statistical analyses were conducted using R Statistical Software (version 4.1.2; R Foundation for Statistical Computing, Vienna, Austria). For the longitudinal analyses, the gls function from the nlme: Linear and Non-linear Mixed Effects Models, R package version 3.1-162, and the rms: Regression Modeling Strategies, R package version 6.7-0, developed by Harrell [28] were used.
Results
Participant Characteristics
Between 31 March 2011, and 1 April 2021, 178 consecutive patients underwent TPIAT at our center. Among that group, 116 patients agreed to answer the two QoL surveys before transplant (65% participation rate) and were included in this study. The demographic and clinical characteristics of the study participants are presented in Table 1. Participants’ median age at TPIAT was 41.1 (30.4–49.0) years, 35% were male, and the median body mass index was 26.3 (21.5–29.8) kg/m2. To better understand our patient cohort disease progression, we looked at prior pancreatic interventions. Prior endoscopic stent management failed for 82 patients (71%). 18 patients (16%) had previous pancreatic surgery before TPIAT. Within this cohort, the median duration of diagnosed pancreatitis was 5.0 (3.0, 10.0) years. Post-TPIAT data revealed a median transplanted islet equivalent dose was 5.1 (2.9–7.2) × 103 IEQ/kg patient body weight. The median follow-up was 78.8 months (range 9.4–125.5 months) and at 1, 2, and 3 year follow-up, 2, 6, and 9 patients had died, respectively.
TABLE 1
| Characteristics | Overall (n = 116) |
|---|---|
| Age (years): median (IQR) | 41.1 (30.4, 49.0) |
| Male: n (%) | 41 (35%) |
| Body mass index (kg/m2): median (IQR) | 26.3 (21.5, 29.8) |
| Epidemiology: n (%) | |
| Alcoholic | 9 (7.8%) |
| Autoimmune | 7 (6.0%) |
| Hereditary | 19 (16%) |
| Idiopathic | 55 (47%) |
| Other | 26 (22%) |
| Pancreatic duct stent insertion or EST: n (%) | 82 (71%) |
| Past history of pancreas operation: n (%) | 18 (16%) |
| Duration of symptoms (years): median (IQR) | 5.0 (3.0, 10.0) |
| Dose (×103 IEQ/kg patient body weight): median (IQR) | 5.07 (2.93, 7.15) |
Characteristics of study participants.
EST, endoscopic sphincterotomy; IEQ, islet equivalent. IQR values are in parentheses and italicized.
Metabolic Outcomes and Pain Control Status
12.1% of patients had diabetes before TPIAT, and 78%, 73%, and 71% were insulin-dependent at 1, 2, and 3 years after TPIAT, respectively. Glycemic outcomes pre- and post-TPIAT are outlined in Table 2. Daily morphine requirements and pain scores significantly decreased over time after TPIAT (p < 0.001) (Figure 2). There was notable decrease in mean MME dose with 118 (±137) mg before TPIAT and 44 (±93), 42 (±68), and 35 (±65) mg at years 1, 2, and 3, respectively. Pain scores evaluated with VAS also decreased after TPIAT: 5.7 (±2.1) at baseline, 2.2 (±2.9) at year 1, 2.1 (±2.8) at year 2, and 1.9 (±2.6) at year 3.
TABLE 2
| Variables | Baseline (n = 116) | Follow-up | ||
|---|---|---|---|---|
| Year 1 (n = 79) | Year 2 (n = 40) | Year 3 (n = 27) | ||
| Endocrine outcomes | ||||
| Hemoglobin A1c (%) | 6.0 (1.1) | 7.3 (2.0) | 7.3 (2.4) | 7.0 (1.4) |
| Serum C-peptide (ng/dL) | 1.8 (1.3) | 1.2 (1.2) | 1.4 (1.5) | 1.1 (1.3) |
| Fasting blood glucose (mg/dL) | 102 (29) | 152 (94) | 151 (65) | 124 (54) |
| Exogenous insulin amount (unit/day) | 2.2 (8.0) | 14.7 (15.0) | 15.5 (15.9) | 14.4 (17.5) |
| Pain control | ||||
| Pain scorea | 5.7 (2.1) | 2.2 (2.9) | 2.1 (2.8) | 1.9 (2.6) |
| Morphine equivalent dose (mg/day) | 118 (137) | 44 (93) | 42 (68) | 35 (65) |
Metabolic and pain outcomes at baseline and after total pancreatectomy followed by islet autotransplantation.
Evaluated with the visual analog scale, ranging from 0 (no pain) to 10 (the most severe pain). SD values are in parentheses and italicized.
FIGURE 2
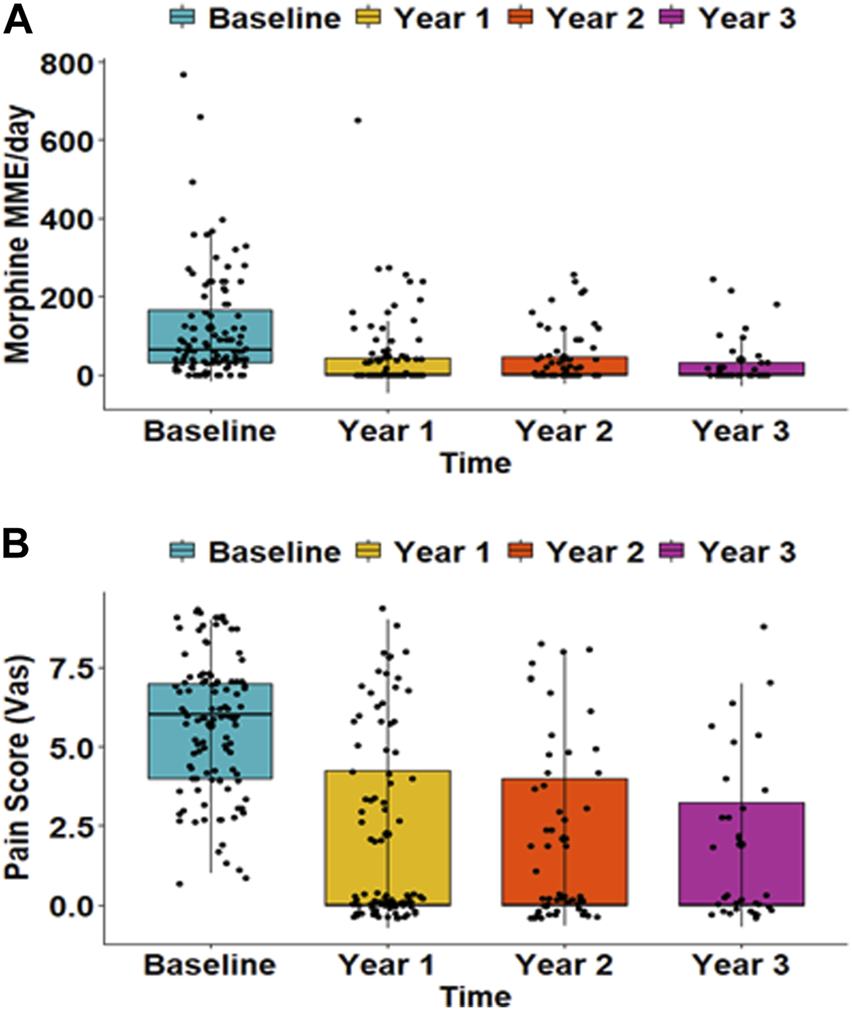
(A) Morphine equivalent dose and (B) pain scores at baseline and at 1, 2, and 3 years after total pancreatectomy followed by islet autotransplantation. The morphine dose is presented as daily milligrams morphine equivalent (MME). Pain scores were evaluated with a visual analog scale (VAS) that ranged from 0 (no pain) to 10 (the most severe). Both daily morphine dose and pain score were significantly reduced over time (p < 0.001).
EORTC QLQ-30 and QLQ-PAN26 Surveys
Initially, 116 patients responded to the preoperative survey and respondents decreased at yearly follow-up. 54 patients completed the survey at year 1, 31 patients at year 2, and 13 patients at year 3. Radar charts visually display each domain of the EORTC QLQ-C30 functional scales and symptom scales of EORTC QLQ-C30 and EORTC QLQ-PAN26 (Figure 3). We displayed domains with a statistically significant trends over time as indicated by generalized least square models for repeated measures.
FIGURE 3
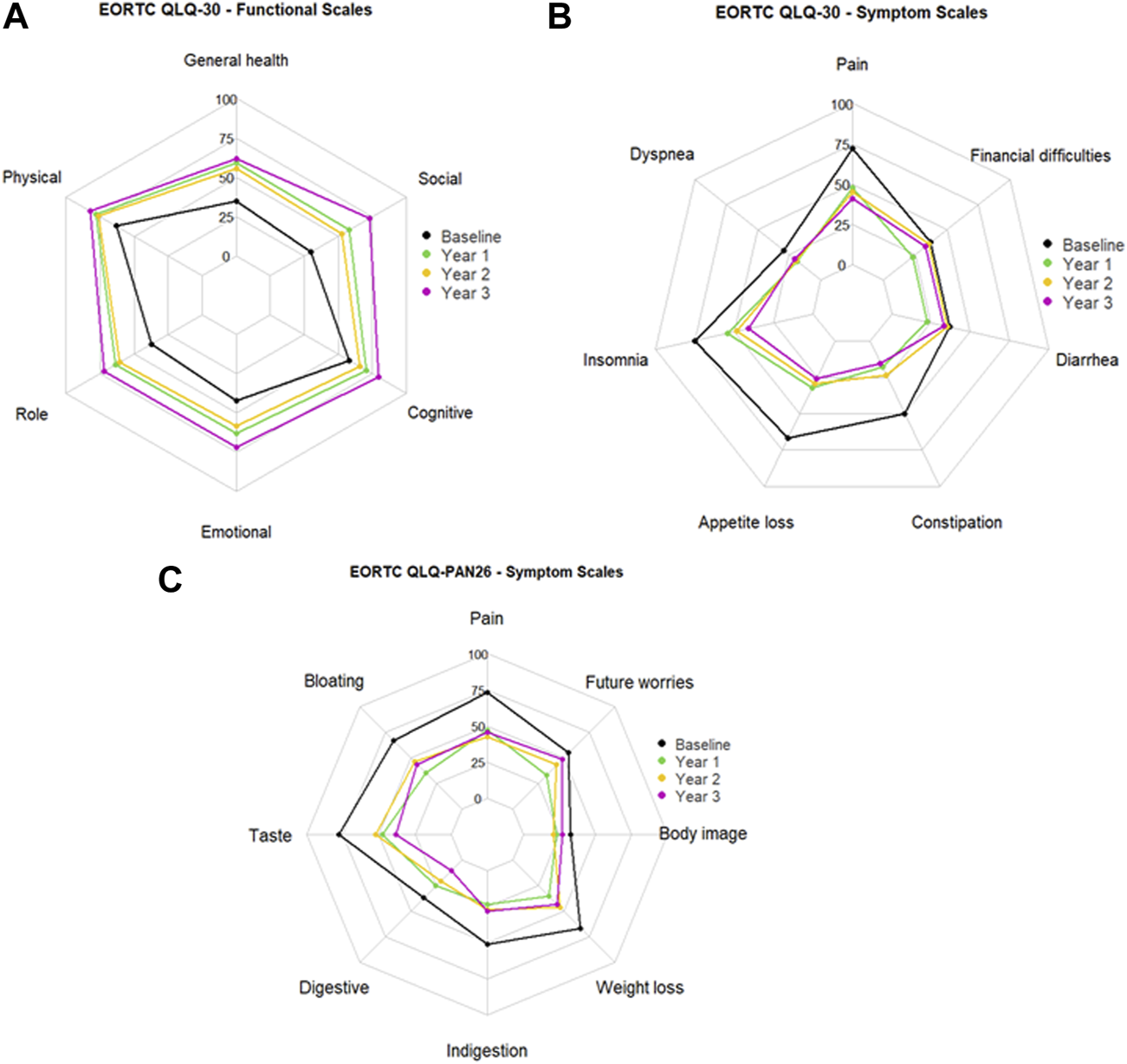
Mean scores at baseline and 1, 2, and 3 years after total pancreatectomy followed by islet autotransplantation for (A) EORTC QLQ-C30 functioning scale, (B) EORTC QLQ-C30 symptom scale, and (C) EORTC QLQ-PAN26 symptom scale.
EORTC QLQ-30 survey functional scores increase with improved QoL after 1, 2, and 3 years (Figure 3A). In our patient cohort, the generalized least square models for repeated measures of functioning scales demonstrated that global health QoL, physical functioning, role functioning, emotional functioning, cognitive functioning, and social functioning significantly increased over 2 years post-TPIAT (p < 0.001, <0.001, <0.001, <0.001, = 0.007, and <0.001, respectively). Delta mean scores outlined in Table 3 indicate the change from baseline (pre-TPIAT) to each follow-up year. In each functional scale domain of EORTC QLQ-C30 the score was ≥10 points, indicating a clinically relevant improvement from baseline.
TABLE 3
| Domain | Baseline (n = 116) | Follow-up | Delta | p-value | ||||
|---|---|---|---|---|---|---|---|---|
| 1 year (n = 54) | 2 years (n = 31) | 3 years (n = 13) | Delta 1 year | Delta 2 years | Delta 3 years | |||
| Global health | 34.84 (23.79) | 59.10 (27.82) | 55.38 (27.01) | 61.54 (26.69) | 24.26 | 20.54 | 26.7 | <0.001 |
| Functional scales | ||||||||
| Physical functioning | 62.82 (24.14) | 78.27 (22.92) | 76.13 (21.83) | 82.05 (24.70) | 15.45 | 13.31 | 19.23 | <0.001 |
| Role functioning | 37.21 (30.72) | 63.58 (36.78) | 60.75 (38.38) | 71.79 (32.19) | 26.37 | 23.54 | 34.58 | <0.001 |
| Emotional functioning | 42.17 (26.36) | 62.81 (31.75) | 58.06 (30.99) | 71.79 (20.84) | 20.64 | 15.89 | 29.62 | <0.001 |
| Cognitive functioning | 55.26 (29.94) | 70.37 (31.67) | 65.59 (27.87) | 79.49 (18.20) | 15.11 | 10.33 | 24.23 | 0.007 |
| Social functioning | 30.32 (32.65) | 58.33 (36.02) | 52.69 (36.03) | 73.08 (33.71) | 28.01 | 22.37 | 42.76 | <0.001 |
| Symptom scales | ||||||||
| Fatigue | 72.41 (26.39) | 47.59 (31.61) | 45.16 (24.83) | 41.03 (20.98) | −24.82 | −27.25 | −31.38 | <0.001 |
| Nausea and vomiting | 57.90 (33.08) | 28.70 (31.79) | 35.48 (35.42) | 24.36 (29.36) | −29.2 | −22.42 | −33.54 | <0.001 |
| Pain | 72.41 (26.39) | 47.59 (31.61) | 45.16 (24.83) | 41.03 (20.98) | −24.82 | −27.25 | −31.38 | <0.001 |
| Dyspnea | 29.02 (31.25) | 18.52 (27.22) | 19.35 (25.49) | 20.51 (28.99) | −10.5 | −9.67 | −8.51 | 0.0697 |
| Insomnia | 75.00 (30.10) | 54.32 (36.22) | 48.39 (32.02) | 41.03 (33.76) | −20.68 | −26.61 | −33.97 | <0.001 |
| Appetite loss | 66.95 (34.19) | 32.10 (35.44) | 29.03 (31.90) | 25.64 (33.76) | −34.85 | −37.92 | −41.31 | <0.001 |
| Constipation | 50.00 (37.94) | 17.90 (26.47) | 23.66 (27.48) | 15.38 (22.01) | −32.1 | −26.34 | −34.62 | <0.001 |
| Diarrhea | 37.07 (35.10) | 22.84 (31.61) | 35.48 (38.43) | 33.33 (38.49) | −14.23 | −1.59 | −3.74 | 0.061 |
| Financial difficulties | 37.07 (35.10) | 22.84 (31.61) | 35.48 (38.43) | 33.33 (38.49) | −14.23 | −1.59 | −3.74 | 0.061 |
Responses on the EORTC QLQ-C30 survey before and after total pancreatectomy followed by islet autotransplantation.
SD values are in parentheses and italicized.
Lower symptom scores in the EORTC QLQ-C30 indicate better QoL (Figure 3B). The generalized least square model of the symptom scales revealed that fatigue, nausea and vomiting, pain, insomnia, appetite loss, and constipation were significantly reduced post-TPIAT (p < 0.001, <0.001, <0.001, <0.001, = 0.001, and <0.001, respectively). Moreover, the corresponding delta scores showed changes of ≥20 points, indicating that the reduction in these symptoms was also clinically meaningful (Table 3).
EORTC QLQ-PAN26 surveyed symptom scales pre- and post- TPIAT in which lower scores indicate better QoL (Figure 3C). Again, the generalized least square model demonstrated that pancreatic pain, bloating, digestive symptoms, taste, indigestion, weight loss, body image, and future worries had a statistically significant trend of reduction over time (p < 0.001, <0.001, <0.001, = 0.009, = 0.001, <0.001, = 0.003, and = 0.009, respectively). The corresponding delta scores indicated clinically meaningful reductions in symptoms in all domains except flatulence, hepatic symptoms, and trouble with side effects (Table 4). Functional scales in QLQ-PAN26 related to satisfaction with healthcare and sexuality were also significantly ameliorated after TPIAT (p = 0.004 and <0.001, respectively).
TABLE 4
| Domain | Baseline (n = 116) | Follow-up | Delta | p-value | ||||
|---|---|---|---|---|---|---|---|---|
| 1 year (n = 54) | 2 years (n = 31) | 3 years (n = 13) | Delta 1 year | Delta 2 years | Delta 3 years | |||
| Symptom scales | ||||||||
| Pancreatic pain | 73.41 (24.95) | 47.07 (30.98) | 42.74 (23.25) | 45.51 (26.27) | −26.34 | −30.67 | −27.90 | <0.001 |
| Bloating | 66.67 (30.46) | 35.19 (32.64) | 46.24 (32.97) | 43.59 (31.58) | −31.48 | −20.43 | −23.08 | <0.001 |
| Digestive symptoms | 77.97 (28.66) | 47.53 (33.71) | 52.15 (34.09) | 38.46 (32.19) | −30.44 | −25.82 | −39.51 | <0.001 |
| Taste | 37.07 (33.99) | 25.31 (33.61) | 20.43 (28.12) | 10.26 (21.01) | −11.76 | −16.64 | −26.81 | 0.009 |
| Indigestion | 50.86 (36.11) | 23.46 (27.19) | 26.88 (29.08) | 28.21 (35.61) | −27.40 | −23.98 | −22.65 | <0.001 |
| Flatulence | 47.41 (34.09) | 48.15 (37.01) | 50.54 (38.37) | 64.10 (34.59) | 0.74 | 3.13 | 16.69 | −0.868 |
| Weight loss | 66.67 (30.46) | 35.19 (32.64) | 46.24 (32.97) | 43.59 (31.58) | −31.48 | −20.43 | −23.08 | <0.001 |
| Dry mouth | 42.53 (34.78) | 30.25 (30.56) | 38.71 (39.53) | 20.51 (25.60) | −12.28 | −3.82 | −22.02 | 0.057 |
| Hepatic symptoms | 17.98 (19.35) | 16.98 (23.01) | 17.20 (22.56) | 17.95 (19.79) | −1.00 | −0.78 | −0.03 | −0.9524 |
| Altered bowel habit | 37.36 (30.03) | 41.67 (28.18) | 47.31 (35.25) | 50.00 (34.02) | 4.31 | 9.95 | 12.64 | 0.2427 |
| Body image | 33.05 (22.84) | 22.84 (24.29) | 20.97 (18.24) | 26.92 (31.58) | −10.21 | −12.08 | −6.13 | 0.004 |
| Troubled with side effects | 7.76 (22.14) | 9.26 (20.90) | 8.60 (22.72) | 15.38 (32.25) | 1.50 | 0.84 | 7.62 | −0.914 |
| Future worries | 54.89 (37.37) | 33.33 (31.72) | 43.01 (30.05) | 48.72 (39.94) | −21.56 | −11.88 | −6.17 | <0.001 |
| Planning of activities | 56.03 (34.50) | 41.36 (32.33) | 48.39 (34.25) | 51.28 (32.25) | −14.67 | −7.64 | −4.75 | 0.03 |
| Functional scale | ||||||||
| Satisfaction with healthcare | 18.25 (23.97) | 29.94 (31.62) | 22.58 (23.78) | 30.77 (28.74) | 11.69 | 4.33 | 12.52 | 0.004 |
| Sexuality | 78.26 (24.50) | 51.23 (33.93) | 57.53 (30.98) | 44.87 (32.90) | −27.03 | −20.73 | −33.39 | <0.001 |
Responses on the EORTC QLQ-PAN26 survey before and after total pancreatectomy followed by islet autotransplantation.
SD values are in parentheses and italicized.
To safeguard against potential bias in study findings, an analysis was conducted to examine whether study patients who completed at least a baseline questionnaire and excluded patients that never participated in the study, displayed distinct characteristics worth exploring (Table 5). Moreover, a comparison between patients who responded at baseline only with participants who responses at follow-up surveys was conducted follow-up and shown in Table 6. Pearson’s Chi-squared test; Wilcoxon rank sum test; Fisher’s exact test were used for group comparisons. Both study participants and excluded patients exhibited similar characteristics unlikely to lead to potential bias with a strong impact on the study findings. The results showed that participants who did not return follow-up surveys had a significantly higher body mass index (BMI) (p = 0.036) with a median of 27.7, (IQR 23.6–32.0), compared to participants with follow-up measures, who had a median of 25.2 (IQR: 20.7–28.9).
TABLE 5
| Characteristics | Study participants (n = 116) | Excluded participants (n = 62) | p-value |
|---|---|---|---|
| Age (years): median (IQR) | 41.1 (30.4, 49.0) | 39.2 (28.6, 49.6) | 0.680 |
| Male: n (%) | 41 (35%) | 25 (40%) | 0.510 |
| Body mass index (kg/m2): median (IQR) | 26.3 (21.5, 29.8) | 25.1 (21.6, 29.6) | 0.620 |
| Epidemiology: n (%) | 0.087 | ||
| Alcoholic | 9 (7.8%) | 3 (4.8%) | |
| Autoimmune | 7 (6.0%) | 1 (1.6%) | |
| Hereditary | 19 (16.4%) | 21 (33.9%) | |
| Idiopathic | 55 (47.4%) | 23 (37.1%) | |
| Other | 26 (22.4%) | 14 (22.6%) | |
| Pancreatic duct stent insertion or EST: n (%) | 82 (71%) | 42 (69%) | 0.820 |
| Past history of pancreas operation: n (%) | 15 (13%) | 10 (16%) | 0.560 |
| Duration of symptoms (years): median (IQR) | 5.0 (4.0, 8.0) | 5.0 (3.0, 10.0) | 0.820 |
| Dose (×103 IEQ/kg patient body weight): median (IQR) | 5.07 (2.93, 7.15) | 4.47 (2.88, 6.12) | 0.440 |
Characteristics of study participants and non-participants.
EST, endoscopic sphincterotomy; IEQ, islet equivalent. IQR values are in parentheses and italicized.
TABLE 6
| Characteristics | With follow-up (n = 55) | No follow-up (n = 61) | p-value |
|---|---|---|---|
| Age (years): median (IQR) | 37.7 (30.8, 48.4) | 42.7 (30.4, 49.0) | 0.580 |
| Male: n (%) | 18 (33%) | 23 (38%) | 0.420 |
| Body mass index (kg/m2): median (IQR) | 27.8 (23.6, 32.0) | 25.5 (20.7, 28.9) | 0.036 |
| Epidemiology: n (%) | 0.087 | ||
| Alcoholic | 1 (1.8%) | 8 (13%) | |
| Autoimmune | 2 (3.6%) | 5 (8.2%) | |
| Hereditary | 12 (22%) | 7 (11%) | |
| Idiopathic | 26 (47%) | 29 (48%) | |
| Other | 14 (25%) | 12 (25%) | |
| Pancreatic duct stent insertion or EST: n (%) | 39 (71%) | 43 (70%) | 0.960 |
| Past history of pancreas operation: n (%) | 11 (20%) | 7 (11%) | 0.210 |
| Duration of symptoms (years): median (IQR) | 5.0 (4.0, 10.0) | 5.0 (3.0, 10.0) | 0.380 |
| Dose (×103 IEQ/kg patient body weight): median (IQR) | 5.43 (3.76, 6.95) | 4.63 (2.74, 7.34) | 0.440 |
Characteristics of study participants by follow-up group.
EST, endoscopic sphincterotomy; IEQ, islet equivalent. IQR values are in parentheses and italicized.
Discussion
In North America, multiple centers perform TPIAT and have demonstrated an improvement in health-related QoL in patients with CP and recurrent acute pancreatitis. The objective means used to ascertain an improvement in QoL were the SF-36 and SF-12 questionnaires [10–15, 33]. These studies evaluated QoL by reporting scores for body pain, mental composite, physical composite, and social functioning, and results showed persistent improvement for up to 5–10 years follow-up [10, 13]. By implementing pancreatic disease-specific surveys EORTC QLQ-30 and QLQ-PAN16, we were able to gain a better understanding of the QoL of our TPIAT patients by including more specific criterion in the surveys. These surveys were originally used to evaluate patients with pancreatic cancer and were validated in 2005 for evaluation of pancreatic surgery for CP [16, 18, 34, 35]. EORTC QLQ-C30 global general health scores increased by delta score of 26.70 (77% increase) which further validates that TPIAT improves patient general health.
Management of pain is a major objective of TPIAT and thus another critical metric for success. Nonetheless, pancreatic pain can be misinterpreted or reported generically through the use of the VAS score and the Body Pain scale in the SF-36. We utilized surveys with more specific measures to more accurately present statistical and clinical evidence of persistent pain reduction for up to 3 years. Pancreatic pain evaluated with the QLQ-PAN26 revealed clinical and statistical improvement with a delta score of 27.90. It is noteworthy that 71% of patients had a previous pancreatic duct stent inserted and 16% had a previous pancreas operation with neither resolving pain maintenance. The survey showed an average percent reduction in morphine dose of approximately 37%, 35%, and 30% at 1, 2, and 3 years, respectively, with mean daily MME decreasing from 118 (±137) mg at baseline to 35 (±65) mg at 3 years. Our results are consistent with the international consensus guideline, where opioid doses were reduced by 71%, 69%, and 67% at 1, 2, and 5 years [36]. Our study supports improved pain management and metabolic functioning even in patients with a history of pancreatic surgery.
In addition to the pain reduction of TPIAT, we observed a significant reduction of other symptoms of CP including nausea, vomiting, weight loss, and digestive disturbances. Our study found no statistical variation in diarrhea symptoms, but there was an overall decreasing trend. This is similar to Crosby et al. report in which more than 60% of patients still reported diarrhea after TPIAT, adding that enzyme non-adherence was not a major contributor [37]. Our surveys showed no improvement in flatulence and altered bowel habits. These symptoms may be related to exocrine insufficiency, intestinal resection with reconstruction in TPIAT, and new intestinal motility after the reduction of narcotic drugs [38, 39]. As the TPIAT procedure involves infusion of pancreatic islets into the portal vein of the liver, it was important for us to document any changes in hepatic symptoms which were not present in our cohort.
A limitation of this study was reduced sample size in follow-up years as patient participation waned. We have observed reports of similar instances in other centers [10, 13, 28, 40, 41]. However, we were able to evaluate the same patient sample over time and have statistically significant data that allows us to highlight new aspects of QoL in TPIAT.
In conclusion, we found that pancreatic disease-specific surveys allow us to gain a deeper understanding of patient QoL post-TPIAT for CP. We observed significant improvements in QoL after TPIAT, and expanded our knowledge in the functional and symptom scales for CP patients up to 3 years post-transplant. Our study strongly supports the benefits of TPIAT as a treatment option for refractory CP.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Baylor Scott & White Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MC and BN contributed to study concept and design. MC and GS performed the statistical analysis. MC, GS, and BN drafted the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported in part by an internal grant from the Baylor University Medical Center.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
CP, chronic pancreatitis; EORTC, European organisation for research and treatment of cancer; MME, morphine milligram equivalents; QoL, quality of life; SD, standard deviation; TPIAT, total pancreatectomy followed by islet autotransplantation; VAS, visual analog scale.
References
1.
Conwell DL Lee LS Yadav D Longnecker DS Miller FH Mortele KJ et al American Pancreatic Association Practice Guidelines in Chronic Pancreatitis: Evidence-Based Report on Diagnostic Guidelines. Pancreas (2014) 43(8):1143–62. 10.1097/MPA.0000000000000237
2.
Layer P Yamamoto H Kalthoff L Clain JE Bakken LJ DiMagno EP . The Different Courses of Early- and Late-Onset Idiopathic and Alcoholic Chronic Pancreatitis. Gastroenterology (1994) 107(5):1481–7. 10.1016/0016-5085(94)90553-3
3.
Steer ML Waxman I Freedman S . Chronic Pancreatitis. N Engl J Med (1995) 332(22):1482–90. 10.1056/NEJM199506013322206
4.
Ammann RW . Diagnosis and Management of Chronic Pancreatitis: Current Knowledge. Swiss Med Wkly (2006) 136(11-12):166–74. 10.4414/smw.2006.11182
5.
Choudari CP Nickl NJ Fogel E Lehman GA Sherman S . Hereditary Pancreatitis: Clinical Presentation, ERCP Findings, and Outcome of Endoscopic Therapy. Gastrointest Endosc (2002) 56(1):66–71. 10.1067/mge.2002.125103
6.
Chronic Pancreatitis German Society of Digestive and Metabolic Diseases (DGVS), Hoffmeister A Mayerle J Beglinger C Büchler MW Bufler P et al S3-Consensus Guidelines on Definition, Etiology, Diagnosis and Medical, Endoscopic and Surgical Management of Chronic Pancreatitis German Society of Digestive and Metabolic Diseases (DGVS). Z Gastroenterol (2012) 50(11):1176–224. S3-Leitlinie Chronische Pankreatitis: Definition, Ätiologie, Diagnostik, konservative, interventionell endoskopische und operative Therapie der chronischen Pankreatitis. Leitlinie der Deutschen Gesellschaft für Verdauungs-und Stoffwechselkrankheiten (DGVS). 10.1055/s-0032-1325479
7.
Hart PA Bellin MD Andersen DK Bradley D Cruz-Monserrate Z Forsmark CE et al Type 3c (Pancreatogenic) Diabetes Mellitus Secondary to Chronic Pancreatitis and Pancreatic Cancer. Lancet Gastroenterol Hepatol (2016) 1(3):226–37. 10.1016/S2468-1253(16)30106-6
8.
Arce KM Lin YK Stevens T Walsh RM Hatipoglu BA . Total Pancreatectomy and Islet Cell Autotransplantation: Definitive Treatment for Chronic Pancreatitis. Cleve Clin J Med (2016) 83(6):435–42. 10.3949/ccjm.83a.15056
9.
Najarian JS Sutherland DE Matas AJ Goetz FC . Human Islet Autotransplantation Following Pancreatectomy. Transpl Proc (1979) 11(1):336–40.
10.
Wilson GC Sutton JM Abbott DE Smith MT Lowy AM Matthews JB et al Long-Term Outcomes After Total Pancreatectomy and Islet Cell Autotransplantation: Is it a Durable Operation? Ann Surg (2014) 260(4):659–65. 10.1097/SLA.0000000000000920
11.
Kotagal M Slusher J Ahmad S Aronson LA Brunner J Chima R et al In-Hospital and 90-Day Outcomes After Total Pancreatectomy With Islet Autotransplantation for Pediatric Chronic and Acute Recurrent Pancreatitis. Am J Transpl (2019) 19(4):1187–94. 10.1111/ajt.15150
12.
Bellin MD Abu-El-Haija M Morgan K Adams D Beilman GJ Chinnakotla S et al A Multicenter Study of Total Pancreatectomy With Islet Autotransplantation (TPIAT): POST (Prospective Observational Study of TPIAT). Pancreatology (2018) 18(3):286–90. 10.1016/j.pan.2018.02.001
13.
Bellin MD Beilman GJ Sutherland DE Ali H Petersen A Mongin S et al How Durable Is Total Pancreatectomy and Intraportal Islet Cell Transplantation for Treatment of Chronic Pancreatitis? J Am Coll Surg (2019) 228(4):329–39. 10.1016/j.jamcollsurg.2018.12.019
14.
Sutherland DE Radosevich DM Bellin MD Hering BJ Beilman GJ Dunn TB et al Total Pancreatectomy and Islet Autotransplantation for Chronic Pancreatitis. J Am Coll Surg (2012) 214(4):409–24. 10.1016/j.jamcollsurg.2011.12.040
15.
Wilson GC Sutton JM Smith MT Schmulewitz N Salehi M Choe KA et al Completion Pancreatectomy and Islet Cell Autotransplantation as Salvage Therapy for Patients Failing Previous Operative Interventions for Chronic Pancreatitis. Surgery (2015) 158(4):872–8. 10.1016/j.surg.2015.04.045
16.
Fitzsimmons D Kahl S Butturini G van Wyk M Bornman P Bassi C et al Symptoms and Quality of Life in Chronic Pancreatitis Assessed by Structured Interview and the EORTC QLQ-C30 and QLQ-PAN26. Am J Gastroenterol (2005) 100(4):918–26. 10.1111/j.1572-0241.2005.40859.x
17.
Strate T Taherpour Z Bloechle C Mann O Bruhn JP Schneider C et al Long-Term Follow-Up of a Randomized Trial Comparing the Beger and Frey Procedures for Patients Suffering From Chronic Pancreatitis. Ann Surg (2005) 241(4):591–8. 10.1097/01.sla.0000157268.78543.03
18.
Varghese TK Bell RH Jr . Duodenum-Preserving Head Resection for Chronic Pancreatitis: An Institutional Experience and National Survey of Usage. Surgery (2007) 142(4):588–93. 10.1016/j.surg.2007.08.009
19.
Scholten L Stoop TF Del Chiaro M Busch OR van Eijck C Molenaar IQ et al Systematic Review of Functional Outcome and Quality of Life After Total Pancreatectomy. Br J Surg (2019) 106(13):1735–46. 10.1002/bjs.11296
20.
Shahbazov R Yoshimatsu G Haque WZ Khan OS Saracino G Lawrence MC et al Clinical Effectiveness of a Pylorus-Preserving Procedure on Total Pancreatectomy With Islet Autotransplantation. Am J Surg (2017) 213(6):1065–71. 10.1016/j.amjsurg.2016.09.051
21.
Yoshimatsu G Shahbazov R Saracino G Lawrence MC Kim PT Onaca N et al The Impact of Allogenic Blood Transfusion on the Outcomes of Total Pancreatectomy With Islet Autotransplantation. Am J Surg (2017) 214(5):849–55. 10.1016/j.amjsurg.2017.03.007
22.
Naziruddin B Matsumoto S Noguchi H Takita M Shimoda M Fujita Y et al Improved Pancreatic Islet Isolation Outcome in Autologous Transplantation for Chronic Pancreatitis. Cel Transpl (2012) 21(2-3):553–8. 10.3727/096368911X605475
23.
Shahbazov R Naziruddin B Salam O Saracino G Levy MF Beecherl E et al The Impact of Surgical Complications on the Outcome of Total Pancreatectomy With Islet Autotransplantation. Am J Surg (2020) 219(1):99–105. 10.1016/j.amjsurg.2019.04.007
24.
Ricordi C Lacy PE Finke EH Olack BJ Scharp DW . Automated Method for Isolation of Human Pancreatic Islets. Diabetes (1988) 37(4):413–20. 10.2337/diab.37.4.413
25.
Matsumoto S Noguchi H Naziruddin B Onaca N Jackson A Nobuyo H et al Improvement of Pancreatic Islet Cell Isolation for Transplantation. Proc (Bayl Univ Med Cent) (2007) 20(4):357–62. 10.1080/08998280.2007.11928323
26.
Diggle PJ Heagerty P Liang K-Y Zeger SL . Analysis of Longitudinal Data. Second. Oxford University Press (2002). p. 400.
27.
Pinheiro JC Bates DM . Mixed-Effects Models in S and S-PLUS. New York: Springer (2000). 10.1007/b98882
28.
Harrell FE Jr . Rms: Regression Modeling Strategies. R Package Version 6.7-0 (2023). Available From: https://CRAN.R-project.org/package=rms> (Accessed July 21, 2023).
29.
Keselman HJ Algina J Kowalchuk RK Wolfinger RD . A Comparison of Two Approaches for Selecting Covariance Structures in the Analysis of Repeated Measurements. Comm Stat Sim Comp (1998) 27:591–604. 10.1080/03610919808813497
30.
Pinheiro J Bates D, R Core Team. Nlme: Linear and Nonlinear Mixed Effects Models_. R Package Version 3.1-162 (2023). Available From: https://CRAN.R-Project.org/package=nlme (Accessed July 21, 2023).
31.
Osoba D Rodrigues G Myles J Zee B Pater J . Interpreting the Significance of Changes in Health-Related Quality-Of-Life Scores. J Clin Oncol (1998) 16(1):139–44. 10.1200/JCO.1998.16.1.139
32.
Osoba D Bezjak A Brundage M Zee B Tu D Pater J et al Analysis and Interpretation of Health-Related Quality-Of-Life Data From Clinical Trials: Basic Approach of the National Cancer Institute of Canada Clinical Trials Group. Eur J Cancer (2005) 41(2):280–7. 10.1016/j.ejca.2004.10.017
33.
Walsh RM Saavedra JR Lentz G Guerron AD Scheman J Stevens T et al Improved Quality of Life Following Total Pancreatectomy and Auto-Islet Transplantation for Chronic Pancreatitis. J Gastrointest Surg (2012) 16(8):1469–77. 10.1007/s11605-012-1914-6
34.
McClaine RJ Lowy AM Matthews JB Schmulewitz N Sussman JJ Ingraham AM et al A Comparison of Pancreaticoduodenectomy and Duodenum-Preserving Head Resection for the Treatment of Chronic Pancreatitis. HPB (Oxford) (2009) 11(8):677–83. 10.1111/j.1477-2574.2009.00118.x
35.
Korrel M Roelofs A van Hilst J Busch OR Daams F Festen S et al Long-Term Quality of Life After Minimally Invasive vs Open Distal Pancreatectomy in the LEOPARD Randomized Trial. J Am Coll Surg (2021) 233(6):730–e9. 10.1016/j.jamcollsurg.2021.08.687
36.
Abu-El-Haija M Anazawa T Beilman GJ Besselink MG Del Chiaro M Demir IE et al The Role of Total Pancreatectomy With Islet Autotransplantation in the Treatment of Chronic Pancreatitis: A Report from the International Consensus Guidelines in Chronic Pancreatitis. Pancreatology (2020) 20(4):762–71. 10.1016/j.pan.2020.04.005
37.
Crosby J Bellin MD Radosevich DM Chinnakotla S Dunn TB Pruett TL et al Gastrointestinal Symptoms Before and After Total Pancreatectomy With Islet Autotransplantation: The Role of Pancreatic Enzyme Dosing and Adherence. Pancreas (2015) 44(3):453–8. 10.1097/MPA.0000000000000266
38.
Khansari M Sohrabi M Zamani F . The Useage of Opioids and Their Adverse Effects in Gastrointestinal Practice: A Review. Middle East J Dig Dis (2013) 5(1):5–16.
39.
Mochiki E Asao T Kuwano H . Gastrointestinal Motility After Digestive Surgery. Surg Today (2007) 37(12):1023–32. 10.1007/s00595-007-3525-5
40.
Georgiev G Beltran del Rio M Gruessner A Tiwari M Cercone R Delbridge M et al Patient Quality of Life and Pain Improve After Autologous Islet Transplantation (AIT) for Treatment of Chronic Pancreatitis: 53 Patient Series at the University of Arizona. Pancreatology (2015) 15(1):40–5. 10.1016/j.pan.2014.10.006
41.
Bellin MD Freeman ML Schwarzenberg SJ Dunn TB Beilman GJ Vickers SM et al Quality of Life Improves for Pediatric Patients After Total Pancreatectomy and Islet Autotransplant for Chronic Pancreatitis. Clin Gastroenterol Hepatol (2011) 9(9):793–9. 10.1016/j.cgh.2011.04.024
Summary
Keywords
pancreatitis, islet autograft, quality of life, pain, diabetes mellitus
Citation
Coluzzi M, Takita M, Saracino G, Rub Hakim Mohammed A, Darden CM, Testa G, Beecherl E, Onaca N and Naziruddin B (2023) Improved Quality of Life Among Chronic Pancreatitis Patients Undergoing Total Pancreatectomy With Islet Autotransplantation—Single Center Experience With Large Cohort of Patients. Transpl Int 36:11409. doi: 10.3389/ti.2023.11409
Received
27 March 2023
Accepted
22 August 2023
Published
04 September 2023
Volume
36 - 2023
Updates
Copyright
© 2023 Coluzzi, Takita, Saracino, Rub Hakim Mohammed, Darden, Testa, Beecherl, Onaca and Naziruddin.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bashoo Naziruddin, bashoo.naziruddin@bswhealth.org
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.