- 1Department of Pathology, John Sealy School of Medicine, University of Texas Medical Branch at Galveston, Galveston, TX, United States
- 2Department of Pathology, Medical University of Vienna, Vienna, Austria
- 3Nephrology Center, Ludwig Maximilian University of Munich, Munich, Germany
- 4Department of Pathology, Sahlgrenska University Hospital, Gothenburg, Sweden
- 5Department of Pathology and Genomic Medicine, The Houston Methodist Hospital, Houston, TX, United States
- 6Laboratory Medicine Program, Toronto General Hospital, University Health Network (UHN), Toronto, ON, Canada
- 7Department of Pathology and Laboratory Medicine, Weill Cornell Medicine, New York, NY, United States
- 8Arkana Laboratories, Little Rock, AR, United States
- 9Department of Pathology and Laboratory Medicine, Emory University, Atlanta, GA, United States
- 10Department of Pathology, Université de Sorbonne, Assistance Publique—Hôpitaux de Paris, Hôpital Tenon, Paris, France
- 11New York Medical College, Valhalla, NY, United States
- 12Department of Pathology and Immunology, Baylor College of Medicine, Houston, TX, United States
- 13Department of Pathology and Clinical Bioinformatics, Erasmus University Center Rotterdam, Rotterdam, Netherlands
- 14Institute of Experimental Medicine and Systems Biology, RWTH Aachen University, Aachen, Germany
- 15Departamento de Patologia Geral, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil
- 16Department of Pathology, Koç University School of Medicine, Istanbul, Türkiye
- 17Department of Anatomical Pathology, NSW Health Pathology, Callaghan, NSW, Australia
- 18School of Medicine and Public Health, College of Health, Medicine and Wellbeing, The University of Newcastle, Callaghan, NSW, Australia
- 19Department of Pathology, School of Medicine, University of Zagreb, Zagreb, Croatia
- 20Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN, United States
- 21AeskuLab Pathology and Department of Pathology, Charles University, Prague, Czechia
- 22Department of Pathology and Laboratory Medicine, King Fahad Specialist Hospital-Dammam, Dammam, Saudi Arabia
- 23Department of Pathology, Istanbul Faculty of Medicine, Istanbul University, Istanbul, Türkiye
- 24Department of Pathology, Necker-Enfants Malades Hospital, Université de Paris Cité, Paris, France
- 25Department of Pathology, Maisonneuve-Rosemont Hospital, University of Montreal, Montreal, QC, Canada
- 26Kidney Pancreas Transplantation, Instituto de Nefrología-Nephrology, Buenos Aires, Argentina
- 27Department of Pathology, Isfahan Kidney Diseases Research Center, Isfahan University of Medical Sciences, Isfahan, Iran
- 28Urology Research Center, Sina Hospital, Tehran University of Medical Sciences, Tehran, Iran
The Thrombotic Microangiopathy Banff Working Group (TMA-BWG) was formed in 2015 to survey current practices and develop minimum diagnostic criteria (MDC) for renal transplant TMA (Tx-TMA). To generate consensus among pathologists and nephrologists, the TMA BWG designed a 3-Phase study. Phase I of the study is presented here. Using the Delphi methodology, 23 panelists with >3 years of diagnostic experience with Tx-TMA pathology listed their MDC suggesting light, immunofluorescence, and electron microscopy lesions, clinical and laboratory information, and differential diagnoses. Nine rounds (R) of consensus resulted in MDC validated during two Rs using online evaluation of whole slide digital images of 37 biopsies (28 TMA, 9 non-TMA). Starting with 338 criteria the process resulted in 24 criteria and 8 differential diagnoses including 18 pathologic, 2 clinical, and 4 laboratory criteria. Results show that 3/4 of the panelists agreed on the diagnosis of 3/4 of cases. The process also allowed definition refinement for 4 light and 4 electron microscopy lesions. For the first time in Banff classification, the Delphi methodology was used to generate consensus. The study shows that Delphi is a democratic and cost-effective method allowing rapid consensus generation among numerous physicians dealing with large number of criteria in transplantation.
Introduction
Transplantation is a relatively young and undoubtedly challenging science. In 1991, to address the main questions of organ transplantation a group of 20 experts composed of transplant clinicians/surgeons/pathologists gathered in Banff/Canada to build the Banff classification on allograft pathology [1]. Since then and for the past 30 years, experts have met every 2 years at Banff meetings, and generated many guidelines thankfully used by the Transplantation community. The Banff Working Group (BWG) for Thrombotic Microangiopathy (TMA) was formed in 2015 under the auspices of the Banff Foundation for Allograft Pathology to standardize criteria for diagnosing and classifying renal transplant TMA (Tx-TMA) [2]. In January 2016, a survey was circulated among the BWG participants regarding Tx-TMA. The results presented at the 2017 Banff conference, revealed considerable heterogeneity among nephropathologists regarding the criteria used for Tx-TMA diagnosis [3]. Therefore, standardization of diagnostic criteria deemed necessary. To achieve this goal, three phases were designed: Phase I (consensus among nephropathologists), Phase II (consensus among nephrologists), and Phase III (consensus of the consensus groups). The Delphi method of consensus generation was chosen to be used for the first time in Banff classification. Delphi is a structured process in which a panel of experts (the panelists) reaches consensus through iterative surveys with controlled feedback from the facilitator [4–7]. The panelists remain anonymous during surveys to ensure that their interactions remain devoid of biases that are usually introduced by group dynamics [4, 8, 9]. In addition, in contrast to other techniques like the nominal group technique or the NIH’s consensus conference, as the Delphi method does not require the physical presence of the participants in an actual meeting [10, 11], all interactions are designed to be online. The current work represents Phase I or the pathology phase of the study. Phase II, representing consensus among nephrologists, has already started and its results will be reported in the future. Phase I generated two interconnected papers that are being presented here. To omit redundancy, the results obtained from applying the Delphi method to transplantation, specifically to the diagnosis of Tx-TMA are reported in the current paper; in paper 2, published in the same issue [12], the pathology criteria themselves are being discussed in terms of their importance in the diagnosis of Tx-TMA in the practice of transplantation pathology.
Materials and Methods
Figure 1 illustrates the process of Delphi applied to this study. The pathological aspects of the material and methods are presented in paper 2 [12].
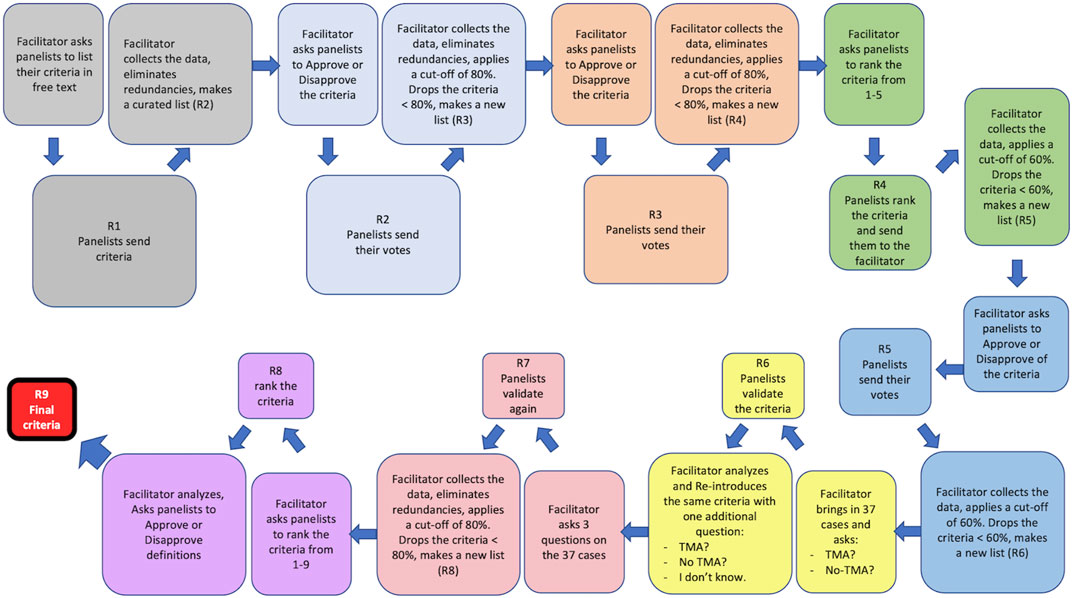
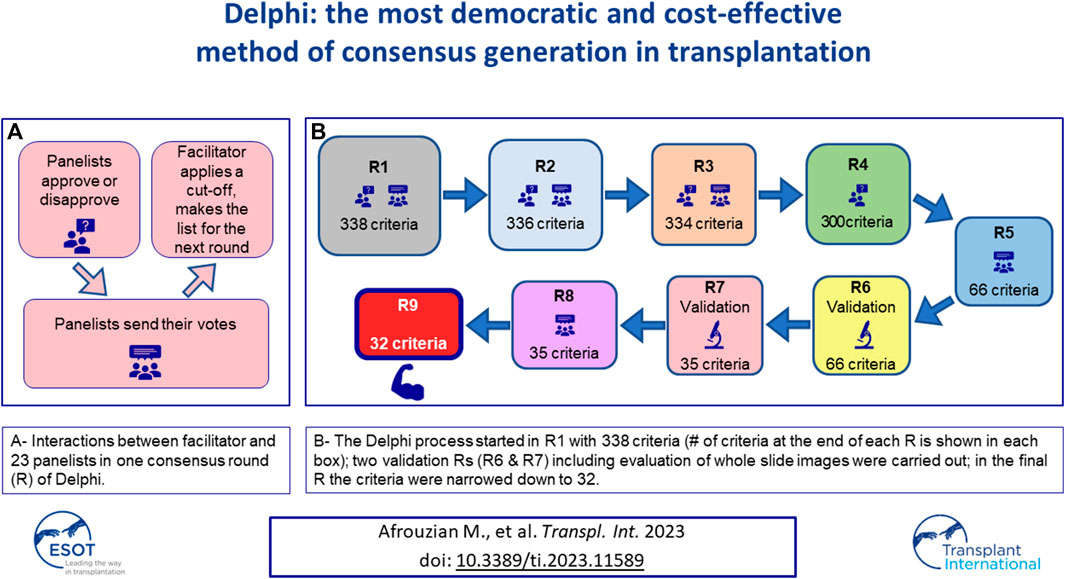
FIGURE 1. The Delphi process applied to this study. Nine rounds of survey (R1–R9) were designed. At the beginning of each round or R, the facilitator presented the panelists with the results (criteria) obtained from the previous R and asked them to either approve/disapprove of the listed criteria or to rank them. The panelists individually responded to this call and sent their votes to the facilitator who would collect the responses, eliminate redundancies, and apply a cut-off (80% or 60%) to that R. The results of the cut-off application were then shared with the panelists. A new list composed of all criteria that were above the cut-off was made by the facilitator and presented in the next R to the panelists. R6 and R7 were two rounds during which the criteria obtained from R5 were validated against 37 real-life cases by the panelists. R9 was a control round during which the integrity of the entire Delphi process was assessed. R9 was used to fine tune the definitions of the lesions that the panelists had difficulty with, during the validation R and was therefore called the Definition R.
Steering Committee and Panelists
A steering committee composed of two nephropathologists (MA, HL) performed literature review, identified areas of difficulty in Tx-TMA diagnosis and defined the terms “experts or panelists” by introducing inclusion and exclusion criteria, as required by Delphi [8]. Panelist was defined as a nephropathologist who had reported or published on Tx-TMA biopsies in the past 3 years (2012–2015). The steering committee members as well as the facilitator (MA) were excluded from the expert panel to avoid bias. Twenty-three nephropathologists from five continents met the above criterion and qualified as panelists.
Design of the Delphi Rounds
To develop a core set of histopathological lesions (hereafter called “criteria”) a total of 10 rounds R) of survey (R0, R1 … R9) were launched at different points of the study, which spanned over a total of 5 years. Detailed information about each R and statistical analysis are provided below.
R0: The panelists were asked to send in free text their questions and areas of difficulty or ambiguity in the diagnosis of Tx-TMA. The panelists’ responses were shared with them at the end of R0. This survey was inserted based on the critique of the Delphi method by Keeney et al. and Diamond et al. [9, 10].
R1: The facilitator created a curated list of the criteria/opinions of the panelists and categorized them into positive (+) and negative (−) criteria. A positive criterion was defined as a criterion that, when present, would help the panelist make the diagnosis of TMA. A negative criterion was, by definition, a criterion which, when present, would help the panelist in ruling out the diagnosis of TMA. Based on the list obtained, 4 classes and 12 categories were formed: As shown in Supplementary Figure S1, the Pathology Class included six categories: Light microscopy positive (LM+); Light microscopy negative (LM−); Immunofluorescence microscopy positive (IF+); Immunofluorescence microscopy negative (IF-); Electron microscopy positive (EM+); and Electron microscopy negative (−). The Clinical Class comprised two categories: Clinical positive (Clin+); and Clinical negative (Clin−). The Laboratory Class included two categories: Laboratory positive (Lab+); and Laboratory negative (Lab−). Genetic criteria (Gen) were composed of tests that would help confirm the diagnosis of TMA. As some panelists had suggested a number of differential diagnoses, the facilitator also created a separate class for Differential Diagnosis Class (#D). Of note, TMA is a lesion with many mimickers. At the same time, different conditions may cause TMA. Therefore, the category of differential diagnosis included both mimickers and conditions that could cause TMA. After data collection and elimination of redundancies by the facilitator, the results were communicated to the panelists.
R2: Panelists were asked to either approve or disapprove of the results obtained from R1. Responses were collected, a cut-off of 80% called 80% agreement level (80%AL) was established by the facilitator: those criteria approved by 80% or more panelists were retained and the remaining criteria were held as potential candidates in the list that would be circulated in the next R. Results of R2 were shared with the panelists. In other words, an 80%AL would be, by definition, the level at which 80% of the participants would reach an agreement on a criterion. It is worth noting that according to the Delphi literature, the decision regarding the cut-off for each R, is totally arbitrary and can be changed from one R to another [6, 10, 13].
R3: Panelists were asked to approve or disapprove of the criteria including the differential diagnoses. A reasonable deadline was set, after which, the panelists’ R3 responses were collected. At this point, the facilitator eliminated redundancies, unified those criteria/opinions that were close in terms of meaning, and included in the same line terminologies that described the same phenomenon. These actions were taken to near opinions that were similar or at least not contradictory. The cut-off for this R was chosen to be 80% therefore, criteria approved by 80% or more panelists were retained and shared with the panelists. The remaining criteria approved by less than 80% of the panelists, were still shared with the panelists for the sake of transparency, however, were not included in the list circulated in the next R.
R4: A curated list of criteria was presented to the panelists who were asked to rank the criteria. The ranking was performed on a Likert-scale from 1 to 5 with anchors on 1 (highly suggestive of TMA), 2 (moderately suggestive of TMA), 3 (mildly suggestive of TMA), 4 (rather less favorable for diagnosis of TMA) and 5 (non-specific for diagnosis of TMA). After receiving all panelists’ responses, the mean rank for each criterion was calculated at one-decimal numbers. To make sure that no important criteria are dropped for the next R, the cut-off for this R was set at 60%. Criteria with mean ranks between 1 and 2.9 were considered being above the 60% cut-off and therefore were retained for the next R, while those with mean ranks between 3 and 5 were considered below the 60% cut-off and eliminated. Any criterion below the cut-off was also presented to the panelists at the end of R4 but dropped from the next R’s list. Based on the application by Jones et al [14], the facilitator provided feedback to panelists regarding all positive and negative criteria and the differential diagnoses.
R5: A curated list of criteria was presented to the panelists. To further narrow down the criteria, the panelists were asked to repeat the ranking of the criteria obtained from R4, using the scale of 1–5, with 1 being the most diagnostic criteria and 5 being the least favorable criteria. Responses were collected by the facilitator. To make sure that no important criteria are dropped for the next R, the cut-off for this R was set at 60% (as in R4); Mean ranks were calculated, and results shared with the panelists.
R6: This R was the first validation R. At this point, 37 cases collected and scanned by the facilitator were shared with the panelists who were asked to label the cases as either “TMA” or “No TMA.” Additionally, the panelists were asked to indicate which criterion on the list was used to make their diagnosis. For each biopsy the panelists ought to provide a mandatory comment about the case in free text, providing suggestions and criticizing the adequacy of the case or, the process. After receipt of all responses, facilitator and statistician analyzed R6 responses. Supplementary Table S1 reflects a snapshot of R6’s process. Comments not fitting in the “yes” or “no” responses were counted in a separate line called “N/A”. Based on the commentaries, it became clear that the R6 clearly needed to be re-designed, as some panelists were undecided regarding the diagnosis of some cases and could not decide if those cases were TMA or not. Therefore, the facilitator did not establish any cut-off for R6 and did not share the results of R6 with the panelists. To re-design the validation R, a third choice of “equivocal” (meaning I do not know) was added by the facilitator to the other two choices of “TMA” and “No TMA” and a new validation R called R7 was launched.
R7: In R7 panelists validated the criteria against the same 37 cases. During this R the panelists were asked to label each case as either “TMA,” “No TMA,” or “Equivocal.” Like R6, the panelists were asked to indicate which criteria on the list were used to make the final diagnosis on each case and enter their opinion in free text. After receipt of all responses, the facilitator and the statistician analyzed the responses. The cut-off for R7 was set at 80% i.e., a new analysis calculated the 80%AL for each of the 37 cases, and on each criterion. Criteria with <80% agreement were dropped for the next R. For clarification, the authors provide an example on criterion 1A here: in R7, the number of panelists who used criterion 1A for ANY of the 37 cases was counted. If out of 23 panelists, 19 or more (≥82.65%) used criterion 1A in at least 1 case, it was considered that criterion 1A was “used by more than 80% of the panelists” and therefore should be kept in the list for the next R. Results of R7 were shared with the panelists.
R8: The panelists were challenged in this R with the criteria obtained from R7 and asked to rank the criteria from 1 to n (1 being the most favorable criterion and n being the least favorable criterion), depending on the number of criteria in each category. Mean ranks of the criteria obtained from this R were calculated and shared with the panelists. This list contained the final criteria for diagnosis of Tx-TMA. It should be emphasized that R8 was originally planned to produce major and minor criteria by taking in to account panelists’ ranking. However, after examination of the results, the facilitator decided that future validation studies are needed to develop the concept of major/minor criteria.
R9: This R is usually used as a “control R” to assess the internal integrity of the process. The facilitator decided to use R9 to generate consensus on the definition of some lesions, that appeared to be morphologically problematic for some of the panelists during the previous Rs. Therefore, a consensus was needed regarding their definition. For example, the lesion “mesangiolysis”, an important diagnostic tool, did not receive sufficient vote in one of the rounds and was eliminated. The facilitator had to modify the cut-off for that round to keep this lesion as a criterion on the list. Therefore, R9 was called the definition R during which panelists were asked to define some terms used for a few light and electron microscopy criteria. All panelists had to provide in text format their own definition on these selected lesions. These definitions were then curated with elimination of redundancies, assembled in sentences by the steering committee, and shared with the panelists.
Percentage Agreement (%A) and Percentage Agreement Levels (%AL)
Two terms were used to reflect the agreement between the panelists. The first term, %A, showed the agreement amongst the panelists concerning a diagnosis or criterion. The second term, %AL, reflected %A falling into a cut-off of agreement. For example, a 100%AL was the level on which 97%–100% of the panelists agreed on the same diagnosis on X number of cases. A 100%AL was therefore interpreted as ‘total agreement”. By the same token, a %AL was considered: poor if in the range of 0–40; fair if between 41 and 60; good if between 61 and 80; excellent if between 81 and 96 and total if between 97 and 100.
Statistical Analysis
A detailed explanation of the statistical analysis is rendered below.
In R0 and R1 no statistical analysis was performed.
In R2 and R3, we calculated the approval percentage for each criterion based on the following formula:
In R4 and R5, we calculated the percentage of ranking based on the following formula:
In R6 and R7, we calculated the %A for each criterion based on the following formula:
To assess the relative importance of the criteria, in R8, we calculated the percentage of favorable ranking based on the following formula:
All statistical modeling were performed using SAS, version 9.4 (SAS, Inc., Cary, NC). Some figures were drawn using the open source data visualization tool RAWGraphs [15].
Results
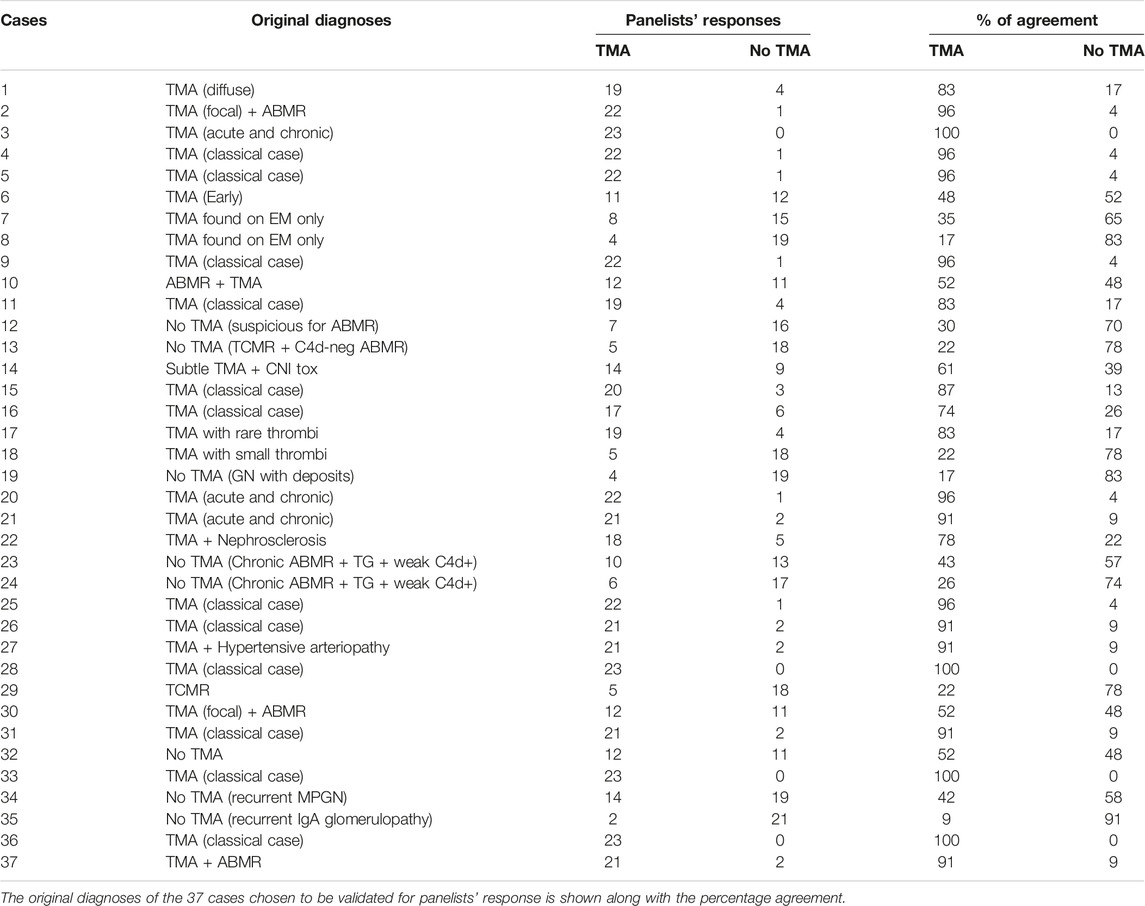
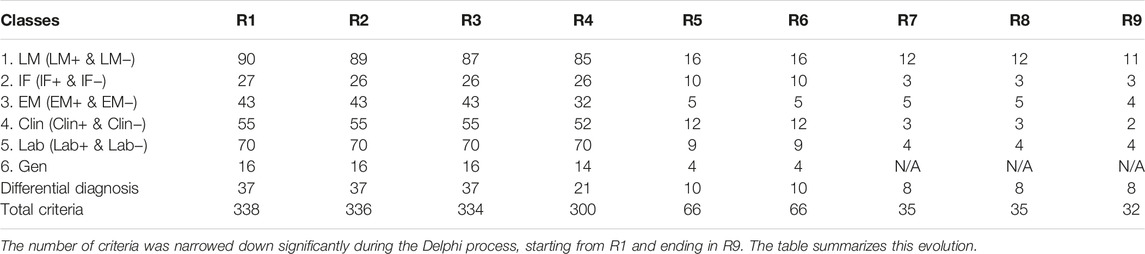
Table 1 lists the original diagnoses of the 37 cases that were chosen to be validated (for panelists’ response, see below). The project started with 338 items/criteria obtained at the end of R1. Table 2 summarizes the evolution of the criteria from R1 to R9. By the end of R5, the facilitator was able to narrow down the items to 66 which included 56 criteria and 8 differential diagnoses. A list of the items entering R6 is provided in Supplementary Table S1. At the end of R7 the items were narrowed down to 35 including 27 criteria and 8 differential diagnoses. In R8, the number of items remained at 35. After R9, the facilitator eliminated three negative criteria that were expressed as “there is no criterion to help ruling out TMA.” These were eliminated because they could not be counted as criteria. Therefore, at the end of R9 the study ended up with 32 items including 24 criteria and 8 differential diagnoses. A detailed list of criteria and discussion about each criterion is outside the scope of this manuscript and will be published in the future.
Supplementary Table S2 lists the number of the final criteria classified in each of the 12 categories which included 18 Pathological criteria (16 positive or 2 negative including 11 LM+, 1 IF+, 2 IF−, 4 EM + criteria); 2 Clinical criteria (2 Clin + criteria); 4 Laboratory criteria (including 4 Lab+ criteria). The 2 Lab- criteria were dropped because of insufficient votes (<20%). The process generated eight differential diagnoses entertained during the two validation Rs. Defining of eight criteria including 4 LM+ and 4 EM+ criteria emerged as a necessity at the end of R8. The panelists achieved this task during R9 which also served as a control R for the entire Delphi process.
Agreement Among Panelists
The facilitator observed the panelists’ performance looking at multiple agreement levels and at different points of the study. At the end of R6, the first validation R, %AL was assessed at 50%, 60%, 70%, 80%, 90%, and 100% levels (shown in Figure 2). The results show that at 70%AL (middle bar), consensus was reached on 28/37 (76%) of cases. This means that almost three-quarters of the panelists agreed on three-quarters of the cases.
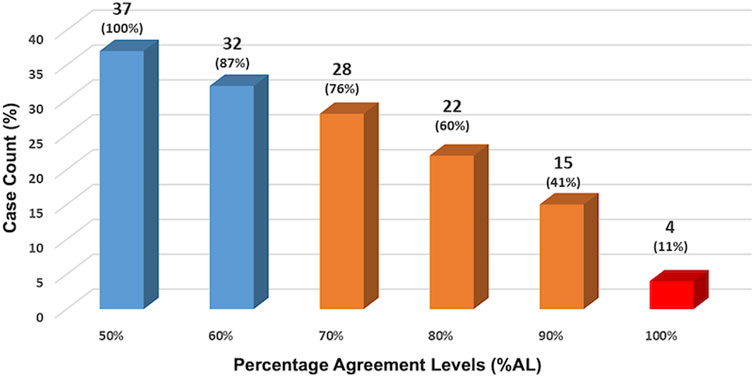
FIGURE 2. Panelists’ performance assessed at the end of R6. The facilitator observed the panelists’ performance looking at multiple agreement levels. At the end of R6, the first validation R, %AL was assessed at 50%, 60%, 70%, 80%, 90% and 100% levels. The results show that at 70%AL (middle bar), consensus was reached on 28/37 (76%) of cases. This means that almost three-quarters of the panelists agreed on three-quarters of the cases.
A deeper look at the %AL at the end of the study is shown in Table 3 which shows the cumulative agreement levels among panelists and reveals that: 1- Total agreement (97–100%AL) was achieved in 4 cases (10.81% of cases; 2- Excellent agreement (81–100%AL) in 20/37 cases (54.05%); Good agreement (61–100%AL) in 31/37 cases (83.78%) and Fair agreement (41–100%AL) in all 37 cases (100%).
Discussion
Delphi and Consensus
The term consensus is clarified in Delphi and defined as “general agreement, but not necessarily unanimity,” and includes the process to resolve objections by interested parties. A process would be considered a consensus, if all comments have been fairly considered, each objector has been advised of the disposition of his or her objection(s) and the reasons why, and the consensus body members have been given an opportunity to change their votes after reviewing the comments [16]. Delphi is a structured process of consensus generation in an iterative fashion through repeated anonymous surveys with controlled feedbacks given by the facilitator [6]. In Delphi a panel of experts (the panelists) can reach consensus through multiple online interactions, that would prevent introduction of bias from group dynamics. In contrast to other techniques like the nominal group technique or the NIH’s consensus conference, the Delphi method does not require the physical presence of the participants in an actual meeting [11].
Comparing the NIH Type of Consensus Generation With Delphi
To compare the NIH type of consensus with the Delphi method, and why the Delphi method is preferred in some situations, a point-by-point description of both methods is presented below.
The NIH Type of Consensus Generation
The reader of the current paper is most probably familiar with the rules of the usual NIH type consensus generation. In this type of consensus: 1. Opinions/questions/criteria are usually pre-designed by a steering committee composed of the most experienced members of the group at the beginning of the process; 2. The literature has already covered some information about the incidence and/or definitions of the criteria/lesions and all panelists are on the same page; 3. Communications are in person or through online video-conferencing, therefore, the identity and opinions of the panelists, the most experienced, the less famous, the loudest and the silent, the most and least popular members of the group are known by all participants, introducing “human interaction bias” into the process. Therefore, “discussions” in this consensus model are performed by directly addressing one or multiple panelists and accepting or not an argument, in situ and within the group; 4. The criteria or the pathology cases brought to the consensus are the “subjects” of the study. This means that in the NIH model, the study is expected to validate the criteria with a significant number of cases, report p-values and inter-correlation coefficients (ICC), which evaluate criteria performance when put to test.
The Delphi Method of Consensus Generation
Delphi, however, has a fundamentally different approach to the panelists and the criteria. In Delphi: 1. Questions or criteria are not set in advance by the steering committee and the entire group of panelists set the tone by expressing their own opinions/questions/criteria at the beginning of Delphi; 2. Definitions of the criteria/lesions are not known at the start of the process as no one knows which lesions are going to reach the finish line. For example, this study started with 338 criteria and lesions suggested by the panelists. It is obvious that the steering committee could not possibly define all the 338 criteria at the beginning of the study, as this would introduce an external bias. Hence, such interventions from the steering committee or the facilitator are strictly prohibited during Delphi, allowing a democratic process devoid of any peer pressure, interference, and bullying. All 338 criteria had to enter R1, and those reaching the finish line by R8 were the result of a vigorous election process; 3. In Delphi, the “subjects” are the panelists, not the criteria nor the validated cases, therefore, p-values and ICCs are not expected to be generated; 4. All opinions are expressed anonymously, not only to eliminate peer pressure but also to allow a different type of “discussion.” To expand on this notion, it suffices to mention that in Delphi, the cognitive exercise starts with the first Rs when each panelist faces the list of criteria voted by other panelists, permitting self-reflection on personal knowledge, opinion, and experience. Later, in-mid process, after multiple Rs of voting and elimination of the criteria that have not received enough vote, a cognitive connection is automatically established between this panelist and the rest of the group creating a collective mind ready to validate the final list.
Results show that 3/4 of the panelists agreed on the diagnosis of 3/4 of cases. Comparing these results to the results of similar studies that used the NIH-type of consensus, one can draw the following conclusion regarding the quality of consensus: our results are comparable to other studies even though different methodologies and statistical analyses were used. For example, Liapis et al. reported that consensus was reached among about 75% of the pathologists who agreed on 75% of cases when scoring the number of glomeruli present in implantation (donor) biopsies. The consensus was below 75% when scoring was performed on glomerulosclerosis and other parameters such as number of arteries, and tubular atrophy (Data obtained from table 2, ICC results, Liapis H et al, AJT 2017) [17]. Although the quality of consensus appears to be similar in both studies, it is worth mentioning that the present study generated consensus on criteria and on diagnoses, not just on a single factor such as number of glomeruli. Therefore, a much complex consensus process was applied to our Delphi-based study.
Performance of the Panelists
When analyzing panelists’ performance, the results are encouraging: a “good” level of agreement, was obtained on 31 cases, and consensus was reached among 70% of panelists on 28/37 (76%) of cases, basically implying that about three-quarters of the panelists agreed on three-quarters of the cases. This result shows that the Delphi process was able to generate an acceptable level of consensus among our panelists.
Novelty
The use of Delphi as a consensus building method started in the last decade of the 20th century and, therefore, has been used by some disciplines for years. However, its introduction to the world of pathology is recent [18, 19], moreover, it has never been used in Banff classification. Furthermore, the novelty of our study is in the integration of a classical histopathology workup into the Delphi process, including interpretation of digital whole slide images accompanied by clinical history and laboratory data. This approach which is a modification of the usual Delphi method can be used in medicine, especially in transplantation pathology, where criteria generated during multiple consensus rounds could be validated against real-life cases. This modified Delphi method is, therefore, adapted to the needs of the pathology consensus process.
As in other methods, Delphi is partly an exercise to educate a group of participants to think and re-think about their definitions/cutoffs, adapting alternative terminologies in the process (in this case histopathologic criteria) and running the risk of less than 100% agreement. The latter, however, is not unexpected in an observational discipline, like histopathology, thus agreement cutoffs have to be introduced and are generally valid. Finally, the world has changed since the initiation of consensus building on allograft pathology and the creation of the Banff classification. Pandemic-related travel and contact restrictions, financial constraints, and global warming concerns—also related to academic air travel—all advocate for a revision of old practices. In this perspective, the Delphi methodology represents a great solution for consensus building in general and the Banff Classification operating through consensus in particular.
In conclusion, the Delphi methodology is a method of consensus generation that has not been used in Transplantation. For the first time in Banff classification, and in the Phase I of the study, Delphi was used by the TMA-BWG to generate consensus on MDC for TMA in renal allograft biopsies. We adapted the Delphi methodology to the needs of consensus building in pathology by using digital imaging during validation Rs. Delphi proved to be a highly efficient method of consensus generation among pathologists. The novelty of the study is in its anonymous yet democratic approach, online implementation, low cost, and ability to reach many participants from around the globe.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
MA designed the study and acted as facilitator for Delphi. MA and HL conceived the study and selected cases for validation. HL supervised the study. NK, VB, LT, CA-C, HR, JA, AF, DB, PC, MC-vG, SdA, DE, MF, DG, EH, NM, YO, MR, VR, HS, and DT participated as panelists, suggested the initial 338 criteria, participated in Delphi consensus rounds and performed two validation rounds on 37 cases. EH, VB, LT, CA-C, JA, NK, MC-vG, SdA, and SS provided crucial contribution by submitting cases. LC performed data collection and participated in data analysis. SS contributed to the development of the initial framework. MA, NK, and HL reviewed the results of R9 and wrote the manuscript. VB, LT, CA-C, HR, JA, and AF critically reviewed, commented on, and edited the manuscript. MA and NK acquired funding for the research. All authors contributed to the article and approved the submitted version.
Funding
The authors declare that this study received funding from Alexion Pharmaceuticals (grant # 100288) and the Banff Foundation on Allograft Pathology (for publication fees). The funders were not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to acknowledge the support of Dr. Reza Alaghehbandan for statistical analysis of R5 data, and Dr. Michael Mengel for helpful discussions and suggestions.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/ti.2023.11589/full#supplementary-material
Supplementary Figure S1 | Classification of the initial suggested criteria. The panellists were initially asked to suggest their criteria for diagnosis of Tx-TMA. After 338 opinions/ criteria were collected, the facilitator classified them into four classes including Pathologic, Laboratory and clinical criteria and differential diagnoses. Each of the classes were further divided into positive and negative categories, for example the pathologic criteria were divided into LM+, LM−, IF+, IF−, EM+ and EM− categories.
Supplementary Table S1 | A snapshot of R6’s process. After receipt of all responses, facilitator and statistician analyzed R6 responses. This is a snapshot from the excel sheet that showed the result of the analysis on a few cases (labelled as KB1, KB2, …etc.). In the left column, the validation questions are shown including question 1 (Is this a case of TMA or No TMA?), and question 2 (Please mark the criteria—listed below—which helped you in your final diagnosis).
Supplementary Table S2 | Number of final criteria obtained at the end of the study. The final criteria were classified in to 12 categories which included 18 Pathological criteria (16 positive or 2 negative including 11 LM+, 1 IF+, 2 IF−, 4 EM+ criteria); 2 Clinical criteria (2 Clin+ criteria); 4 Laboratory criteria (including 4 Lab+ criteria). The 2 Lab− criteria were dropped because of insufficient votes (<20%). The genetic category remained without any criteria due to lack of sufficient information. The process generated eight differential and the definition of eight criteria including 4 LM+ and 4 EM+ criteria was refined through consensus in R9.
Abbreviations
ABMR, antibody-mediated rejection; (a)HUS, (atypical) hemolytic uremic syndrome; BWG, Banff Working Group; Clin, clinical data; CNI, calcineurin inhibitor; EM, electron microscopy; #D, differential diagnosis; DIC, disseminated intravascular coagulation; EC, endothelial cells; Gen, genetic criterion; GN, glomerulonephritis; HTN, hypertension; ICC, intraclass correlation; IF/IHC, immunofluorescence microscopy/immunohistochemistry; IgAN, IgA nephropathy; Lab, laboratory; LDH, lactate dehydrogenase; LM, light microscopy; MPGN, membranoproliferative GN; MDC, minimum diagnostic criteria; NT-ABMR, non-TMA-associated antibody-mediated rejection; PI GN, post-infectious glomerulonephritis; R, round; SLE, systemic lupus erythematosus; TTP, thrombotic thrombocytopenic purpura; TMA, thrombotic microangiopathy; Tx-TMA, transplant TMA; %A, percentage agreement; %AL, percentage agreement level.
References
1. Solez, K, Axelsen, RA, Benediktsson, H, Burdick, JF, Cohen, AH, Colvin, RB, et al. International Standardization of Criteria for the Histologic Diagnosis of Renal Allograft Rejection: The Banff Working Classification of Kidney Transplant Pathology. Kidney Int (1993) 44:411–22. doi:10.1038/ki.1993.259
2. Loupy, A, Haas, M, Solez, K, Racusen, L, Glotz, D, Seron, D, et al. The Banff 2015 Kidney Meeting Report: Current Challenges in Rejection Classification and Prospects for Adopting Molecular Pathology. Am J Transpl (2017) 17:28–41. doi:10.1111/ajt.14107
3. Haas, M, Loupy, A, Lefaucheur, C, Roufosse, C, Glotz, D, Seron, D, et al. The Banff 2017 Kidney Meeting Report: Revised Diagnostic Criteria for Chronic Active T Cell-Mediated Rejection, Antibody-Mediated Rejection, and Prospects for Integrative Endpoints for Next-Generation Clinical Trials. Am J Transpl (2018) 18:293–307. doi:10.1111/ajt.14625
4. Green, B, Jones, M, Hughes, D, and Williams, A. Applying the Delphi Technique in a Study of GPs’ Information Requirements. Health Soc Care Community (1999) 7:198–205. doi:10.1046/j.1365-2524.1999.00176.x
5. Arce, JM, Hernando, L, Ortiz, A, Díaz, M, Polo, M, Lombardo, M, et al. Designing a Method to Assess and Improve the Quality of Healthcare in Nephrology by Means of the Delphi Technique. Nefrologia (2014) 34:158–74. doi:10.3265/Nefrologia.pre2013.Dec.12286
6. Humphrey-Murto, S, Varpio, L, Wood, TJ, Gonsalves, C, Ufholz, LA, Mascioli, K, et al. The Use of the Delphi and Other Consensus Group Methods in Medical Education Research: A Review. Acad Med (2017) 92:1491–8. doi:10.1097/ACM.0000000000001812
7. Freedman, BI, Burke, W, Divers, J, Eberhard, L, Gadegbeku, CA, Gbadegesin, R, et al. Diagnosis, Education, and Care of Patients With APOL1-Associated Nephropathy: A Delphi Consensus and Systematic Review. J Am Soc Nephrol (2021) 32:1765–78. doi:10.1681/ASN.2020101399
8. Dalkey, N. An Experimental Study of Group Opinion: The Delphi Method. Futures (1969) 1:408–26. doi:10.1016/s0016-3287(69)80025-x
9. Keeney, S, Hasson, F, and McKenna, HP. A Critical Review of the Delphi Technique as a Research Methodology for Nursing. Int J Nurs Stud (2001) 38:195–200. doi:10.1016/s0020-7489(00)00044-4
10. Diamond, IR, Grant, RC, Feldman, BM, Pencharz, PB, Ling, SC, Moore, AM, et al. Defining Consensus: A Systematic Review Recommends Methodologic Criteria for Reporting of Delphi Studies. J Clin Epidemiol (2014) 67:401–9. doi:10.1016/j.jclinepi.2013.12.002
11. Rosenfeld, RM, Nnacheta, LC, and Corrigan, MD. Clinical Consensus Statement Development Manual. Otolaryngol Head Neck Surg (2015) 153:S1–S14. doi:10.1177/0194599815601394
12. Afrouzian, M, Kozakowski, N, Liapis, H, Broecker, V, Truong, L, Avila-Casado, C, et al. Thrombotic Microangiopathy in the Renal Allograft: Results of the TMA Banff Working Group Consensus on Pathologic Diagnostic Criteria. Transpl Int (2023). doi:10.3389/ti.2023.11590
13. Hasson, F, Keeney, S, and McKenna, H. Research Guidelines for the Delphi Survey Technique. J Adv Nurs (2000) 32:1008–15. doi:10.1046/j.1365-2648.2000.01567.x
14. Jones, J, and Hunter, D. Consensus Methods for Medical and Health Services Research. BMJ (1995) 311:376–80. doi:10.1136/bmj.311.7001.376
15. Mauri, M, Elli, T, Caviglia, G, Uboldi, G, and Azzi, M. RAWGraphs: A Visualisation Platform to Create Open Outputs. In: Proceedings of the 12th Biannual Conference on Italian SIGCHI Chapter. Cagliari, Italy: Association for Computing Machinery (2017). Article 28.
16. Burstin, H. Brokerage Ref #2 Office of Management and Budget: Circular A-119 Revised (1998). Available at: https://www.whitehouse.gov/omb/circulars_a119/ (Accessed April 13, 2022).
17. Liapis, H, Gaut, JP, Klein, C, Bagnasco, S, Kraus, E, Farris, AB, et al. Banff Histopathological Consensus Criteria for Preimplantation Kidney Biopsies. Am J Transpl (2017) 17:140–50. doi:10.1111/ajt.13929
18. Dufraing, K, van Krieken, JH, De Hertogh, G, Hoefler, G, Oniscu, A, Kuhlmann, TP, et al. Neoplastic Cell Percentage Estimation in Tissue Samples for Molecular Oncology: Recommendations From a Modified Delphi Study. Histopathology (2019) 75:312–9. doi:10.1111/his.13891
Keywords: Delphi, Banff, thrombotic microangiopathy, kidney, transplantation
Citation: Afrouzian M, Kozakowski N, Liapis H, Broecker V, Truong L, Avila-Casado C, Regele H, Seshan S, Ambruzs JM, Farris AB, Buob D, Chander PN, Cheraghvandi L, Clahsen-van Groningen MC, de Almeida Araujo S, Ertoy Baydar D, Formby M, Galesic Ljubanovic D, Herrera Hernandez L, Honsova E, Mohamed N, Ozluk Y, Rabant M, Royal V, Stevenson HL, Toniolo MF and Taheri D (2023) Delphi: A Democratic and Cost-Effective Method of Consensus Generation in Transplantation. Transpl Int 36:11589. doi: 10.3389/ti.2023.11589
Received: 18 May 2023; Accepted: 09 August 2023;
Published: 23 August 2023.
Copyright © 2023 Afrouzian, Kozakowski, Liapis, Broecker, Truong, Avila-Casado, Regele, Seshan, Ambruzs, Farris, Buob, Chander, Cheraghvandi, Clahsen-van Groningen, de Almeida Araujo, Ertoy Baydar, Formby, Galesic Ljubanovic, Herrera Hernandez, Honsova, Mohamed, Ozluk, Rabant, Royal, Stevenson, Toniolo and Taheri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marjan Afrouzian, bWFhZnJvdXpAdXRtYi5lZHU=
 Marjan Afrouzian
Marjan Afrouzian Nicolas Kozakowski
Nicolas Kozakowski Helen Liapis3
Helen Liapis3 Heinz Regele
Heinz Regele Surya Seshan
Surya Seshan Alton Brad Farris
Alton Brad Farris Yasemin Ozluk
Yasemin Ozluk Marion Rabant
Marion Rabant