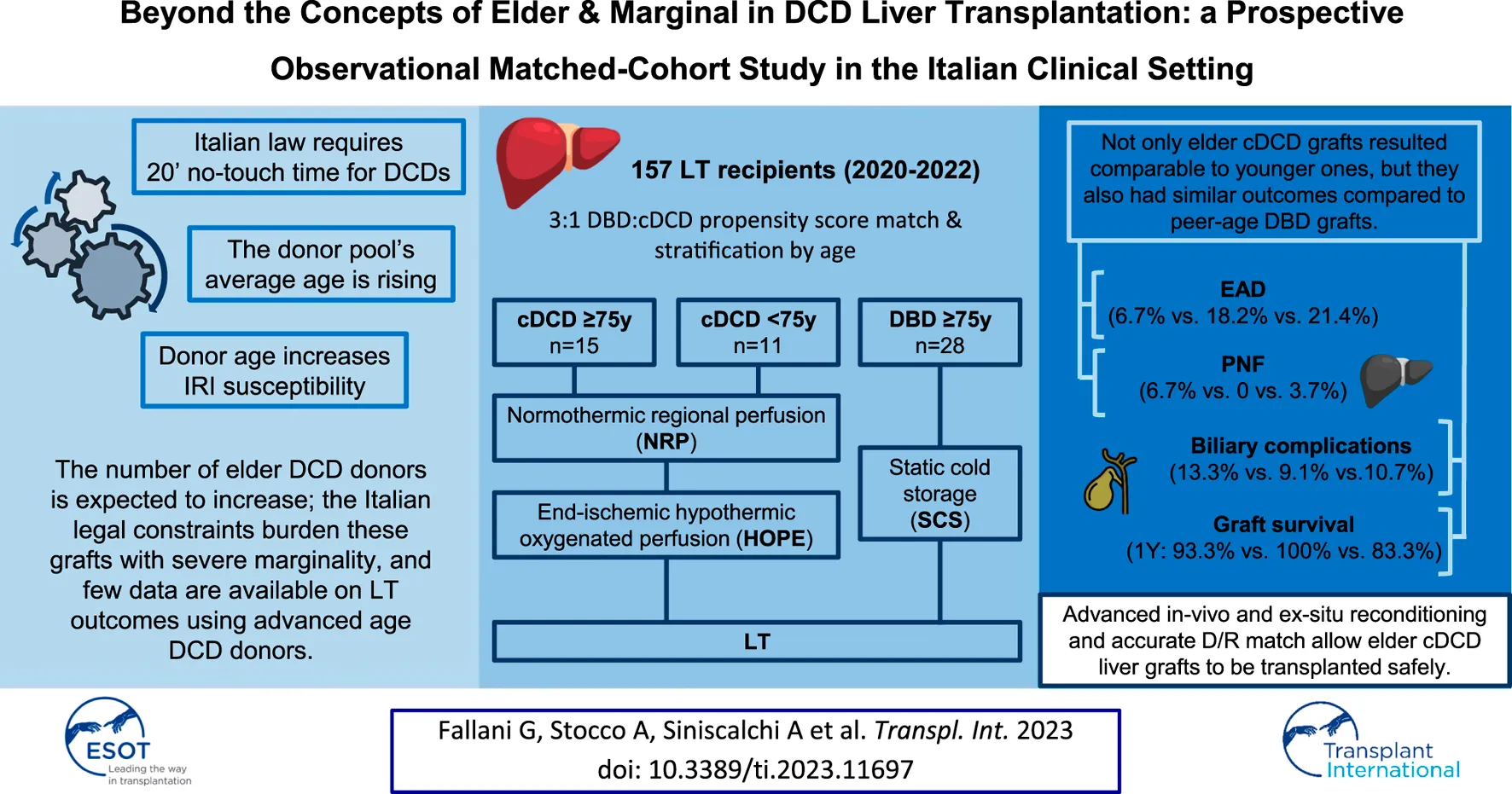
Abstract
Donation after circulatory determination of death (DCD) is a valuable strategy to increase the availability of grafts for liver transplantation (LT). As the average age of populations rises, the donor pool is likely to be affected by a potential increase in DCD donor age in the near future. We conducted a prospective cohort study to evaluate post-transplantation outcomes in recipients of grafts from elderly DCD donors compared with younger DCD donors, and elderly donors after brainstem determination of death (DBD). From August 2020 to May 2022, consecutive recipients of deceased donor liver-only transplants were enrolled in the study. DCD recipients were propensity score matched 1:3 to DBD recipients. One-hundred fifty-seven patients were included, 26 of whom (16.6%) were transplanted with a DCD liver graft. After propensity score matching and stratification, three groups were obtained: 15 recipients of DCD donors ≥75 years, 11 recipients of DCD donors <75 years, and 28 recipients of DBD donors ≥75 years. Short-term outcomes, as well as 12 months graft survival rates (93.3%, 100%, and 89.3% respectively), were comparable among the groups. LT involving grafts retrieved from very elderly DCD donors was feasible and safe in an experienced high-volume center, with outcomes comparable to LTs from younger DCD donors and age-matched DBD donors.
Introduction
Liver transplantation (LT) is considered the treatment of choice for patients with end-stage liver disease. The inclusion of extended criteria donors (ECDs) and donors after circulatory determination of death (DCD) is growing in the attempt to address the critical gap between donors and recipients. DCD donors are a valuable source of grafts, even if concerns have been raised about potentially impaired outcomes related to prolonged warm ischemia time (WIT). Nevertheless, several recent studies have described acceptable results after transplantation involving those donors [1, 2].
According to Italian law [3, 4], death can only be declared after 20 min of lack of any cardiac electrical activity. A strategy of in-situ normothermic regional perfusion (NRP) [5]—aimed to interrupt the prolonged ischemia and to maintain a near-physiologic environment during retrieval [6]—is considered mandatory in the Italian scenario. Moreover, to further mitigate ischemia-reperfusion injury (IRI), most Italian transplant centers also implement end-ischemic hypothermic oxygenated perfusion (HOPE) on DCD liver grafts [7].
The constant increase in the average age of the general population also implies a subsequent change in the demography of the organ donor pool. This phenomenon is most likely to affect the pool of DCD donors too, and data on LT from advanced-age DCD donors are emerging in the literature [8–10].
Even if donor age impacts the outcomes of LT [11–13], the acceptance of older DBD donor grafts could be effective in selected recipients [14]. Thus, this study aims to compare the outcomes of LTs involving elder DCD donors with both younger DCD donors and elder DBD donors in the specific context of an experienced Italian transplant center.
Methods
We conducted a single center prospective observational cohort study including consecutive recipients of liver-only transplantation of controlled DCD (cDCD) donors [15, 16] from August 2020 to May 2022. Based on pre-transplant donor and recipient characteristics, cDCD liver recipients underwent propensity score matching (PSM) with recipients of liver-only transplantation from DBD donors in a 1:3 DCD:DBD ratio. The study population was stratified by age to compare outcomes of grafts from younger cDCD donors (<75 years-old) and elder cDCD donors (≥75 years-old), with elder DBD donors (≥75 years-old).
Informed consent was obtained from all the recipients. The study was approved by the Institutional Review Board (Comitato Etico—Area Vasta Emilia Centro, CE-AVEC, protocol no. 895/2021/Oss/AOUBo).
Donor Management and Procurement
The technique of abdominal organ procurement in cDCD donors in Italy has been previously described in detail [17], and the timeline of events is reported in Supplementary Figure S1.
After circulatory arrest and no-touch time, NRP is initiated, targeting lactate clearance, pH normalization, normocapnia, and avoidance of hyperoxemia; hemoglobin concentration is maintained above 8 g/dL and hyperglicemia is corrected to facilitate organ resuscitation. Since NRP starts every 30′, blood gas analysis and liver enzyme measurement are performed throughout extracorporeal perfusion. NRP is maintained for at least 60–90 min to assess the organ functional recovery; the criteria of viability include fWIT<60 min, pH normalization and stability, progressive lactate decrease, and SGOT/SGPT decrease (usually evident after 30 min of NRP). When the metabolic and perfusion profiles of the donors are considered optimal, the surgical procedure is initiated: first, liver (and kidney) biopsies are obtained, then the warm dissection phase of the procurement is performed. In our practice, liver biopsy is a cornerstone to assess organ viability, as the presence of extensive lobular necrosis represents a contraindication to further proceed with retrieval.
NRP ends with in-situ cold preservation (ISP): once the organs are retrieved, they are put in static cold storage (SCS) to be transported to the transplant center where bench surgery is performed, and HOPE is implemented until implantation.
Conversely, liver grafts from DBD donors were retrieved with standard technique and preserved with SCS until implantation.
Outcome Definitions and Measures
For DCD donors, total WIT (tWIT) was defined as the timeframe occurring between WLST and NRP initiation, while functional WIT (fWIT) was defined as the timeframe between hypotension (systolic arterial pressure below 50 mmHg) or desaturation (peripheral oxygen saturation below 70%)—whichever occurring first—and NRP initiation. Cold preservation time (CPT) was defined as the interval from aortic cross-clamping/ISP and portal reperfusion upon LT, thus including both SCS and HOPE duration (Figure 1).
FIGURE 1
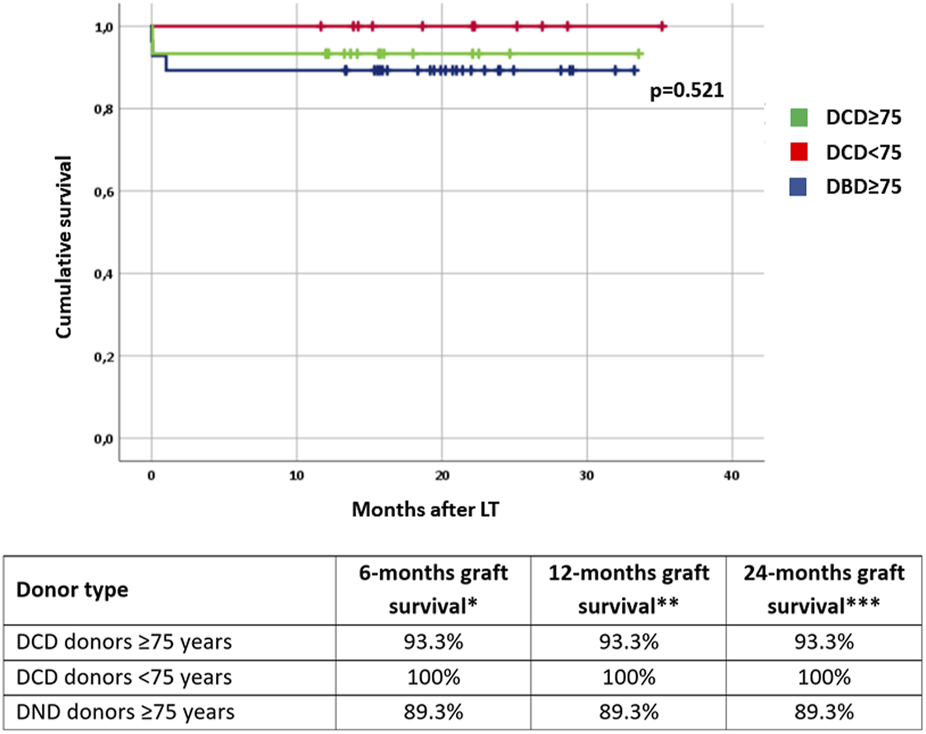
Graft survival rates stratified by donor type and age. DCD: donation after circulatory determination of death; DBD, donation after brainstem death. * number of patients at risk: 54; ** number of patients at risk: 54; *** number of patients at risk: 14.
Complications were defined as any event deviating from the expected postoperative course that does not imply failure to cure [18]. For each patient, the postoperative complications were graded according to the Clavien-Dindo classification [18] and summarized through the Comprehensive Complication Index (CCI®) [19]; major complications were defined as Clavien-Dindo grade ≥3A.
Post-reperfusion syndrome (PRS) was defined according to Aggarwal et al. [20] Primary non-function (PNF) of the graft was defined according to the Organ Procurement and Transplantation Network (OPTN) criteria [21], while early allograft dysfunction (EAD) was defined according to the criteria proposed by Olthoff et al. [22] Severe acute kidney injury was defined according to the Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guideline [23].
Statistical Analysis
Qualitative variables were reported as absolute values and percentages, whereas quantitative variables were reported as median values and IQR or mean ± SD as appropriate. Univariate analysis was performed using Pearson’s chi-squared or Fisher’s exact test for categorical variables, depending on the sample size, and with Student’s t-test or Mann-Whitney U test for continuous variables, depending on their distribution.
PSM was performed through logistic regression analysis to adjust for clinically confounding factors between groups, including donor age, recipient age at the time of transplantation, MELD score, and hepatocellular carcinoma (HCC) as an indication for LT. DCD and DBD recipients were matched in a 1:3 ratio with the closest estimated propensity. Survival curves were plotted with the Kaplan-Meier estimators and compared through the log-rank test.
Differences of p < 0.05 were considered significant.
All the statistical analyses were performed using IBM SPSS, version 26.0 (IBM Corporation—Armonk, NY).
Results
From August 2020 to May 2022, 183 liver grafts were offered for transplantation to the Department of Hepatobiliary Surgery and Transplantation of Policlinico Sant’Orsola in Bologna. Of these, 30 (16.4%) came from DCD donors: four were declined, with an acceptance rate of 86.7%. Two livers were discarded due to a combination of prolonged fWIT, and poor liver and metabolic parameters during NRP (as per our aforementioned criteria). The remaining two livers were refused as malignancy was detected during the retrieval procedure. The remaining 153 liver grafts offered for transplantation (83.6%) came from DBD donors. Of these, 12 were discarded for marginality upon senior transplant surgeon judgment, with an acceptance rate of 92.2%. Other 10 liver grafts were allocated for multiorgan transplantation or re-transplantation and thus excluded from the study.
The final population included in the study consisted of 157 recipients, 131 of which (83.4%) were transplanted with grafts from DBD donors, and 26 (16.6%) with grafts from cDCD donors.
Donor and Recipient Pre- and Post-operative Characteristics
Compared with DBD donors, DCDs had higher median age (75 years vs. 63 years, p = 0.018); notably, most DCD donors were 75 years or older. Recipients of DCD liver grafts had lower MELD scores (10 vs. 15, p < 0.001), and were more often transplanted due to HCC (69.2% vs. 41.2%, p = 0.009). Full demographic and clinical characteristics of recipients and donors are summarized in Table 1.
TABLE 1
| Variables | Before PSM | After PSM | ||||
|---|---|---|---|---|---|---|
| DCD-LT (n = 26) | DBD-LT (n = 131) | p | DCD-LT (n = 26) | DBD-LT (n = 78) | p | |
| Recipient age in years, median [IQR] | 61 [56–64] | 58 [53–64] | 0.214 | 61 [56–64] | 59 [54–65] | 0.435 |
| Recipient BMI in kg/m2, median [IQR] | 23.8 [22.3–29.2] | 26 [23.1–28.1] | 0.340 | 23.8 [22.3–29.2] | 25.5 [22.8–28.1] | 0.538 |
| Indication for LT | ||||||
| Hepatocellular carcinoma, n (%) | 18 (69.2) | 54 (41.2) | 0.009 | 18 (69.2) | 41 (52.6) | 0.137 |
| Virus-related cirrhosis, n (%) | 10 (38.5) | 52 (39.7) | 0.906 | 10 (38.5) | 31 (39.7) | 0.908 |
| Alcohol-relates cirrhosis, n (%) | 8 (30.8) | 52 (39.7) | 0.392 | 8 (30.8) | 31 (39.7) | 0.413 |
| NAFLD, n (%) | 3 (11.5) | 24 (18.3) | 0.572 | 3 (11.5) | 11 (14.1) | 1 |
| Cholestatic liver disease, n (%) | 3 (11.5) | 17 (13) | 1 | 3 (11.5) | 12 (15.4) | 0.756 |
| Acute liver failure, n (%) | 0 | 6 (4.6) | 0.590 | 0 | 2 (2.6) | 1 |
| Other, n (%) | 4 (15.4) | 27 (20.6) | 0.541 | 4 (15.4) | 15 (19.2) | 0.766 |
| Previous abdominal surgery, n (%) | 17 (65.4) | 76 (58.5) | 0.511 | 17 (65.4) | 51 (66.2) | 0.937 |
| Previous liver resection, n (%) | 5 (19.2) | 10 (7.6) | 0.077 | 5 (19.2) | 8 (10.3) | 0.303 |
| TIPSS, n (%) | 3 (11.5) | 12 (7.9) | 0.463 | 3 (11.5) | 3 (3.8) | 0.163 |
| Portal thrombosis, n (%) | 4 (15.4) | 24 (18.3) | 1 | 4 (15.4) | 12 (15.4) | 1 |
| Platelet count *103/µL, median [IQR] | 103 [63–161] | 72 [46–133] | 0.140 | 103 [63–161] | 96 [61–154] | 1 |
| MELD at transplant, median [IQR] | 10 [8–14] | 15 [10–24] | <0.001 | 10 [8–14] | 12 [9–15] | 0.297 |
| Recipient comorbidities | ||||||
| Diabetes mellitus, n (%) | 10 (40) | 39 (29.8) | 0.321 | 10 (40) | 22 (28.2) | 0.267 |
| Cardiovascular disease, n (%) | 3 (11.5) | 28 (21.4) | 0.250 | 3 (11.5) | 16 (20.5) | 0.390 |
| Respiratory disease, n (%) | 6 (23.1) | 26 (19.8) | 0.709 | 6 (23.1) | 15 (19.2) | 0.672 |
| Renal disease, n (%) | 3 (11.5) | 18 (13.7) | 1 | 3 (11.5) | 7 (9) | 0.708 |
| Donor age in years, median [IQR] | 75 [64–78] | 63 [50–75] | 0.018 | 75 [64–78] | 69 [56–79] | 0.367 |
| Donor BMI in kg/m2, median [IQR] | 26.2 [23.1–29.8] | 25.7[23.8–27.8] | 0.797 | 26.2 [23.1–29.8] | 25.9 [24.2–29.1] | 0.901 |
Baseline preoperative recipients’ characteristics and donor characteristics before and after propensity score matching.
DCD-LT, donation after circulatory determination of death liver transplantation; DBD-LT, donation after brainstem death liver transplantation; IQR, interquartile range; BMI, body mass index; NAFLD, nonalcoholic fatty liver disease; TIPSS, transjugular intrahepatic portosystemic shunt; MELD, model for end-stage liver disease.
Bold values highlight statistical significance.
After PSM, the population resulted in 26 DCD donors and 78 DBD donors; the subsequent analysis demonstrated the comparability of baseline characteristics (Table 1).
DCD grafts had a median tWIT of 45 min and a median fWIT of 40 min, with a median timeframe of 6 min from death declaration to NRP initiation. NRP had a median duration of 209 min and end-ischemic HOPE had a median duration of 105 min. No significant differences have been observed between elder and younger DCD donors in terms of tWIT, fWIT, NRP duration, metabolic and functional parameters during NRP (pH, lactates, SGOT, SGPT), bioptic findings, or HOPE duration. These results are summarized in Supplementary Table S1. Grafts from DCD donors underwent shorter CPT (345 vs. 388 min, p = 0.010) and shorter duration of transplant (430 vs. 470 min, p = 0.040).
The analysis of postoperative data showed that recipients of livers from DCD and DBD donors had comparable results in terms of surgical complications, length of hospital stay, and graft function (Table 2).
TABLE 2
| Variables | DCD-LT (n = 26) | DBD-LT (n = 78) | p |
|---|---|---|---|
| Cold preservation time in minutes, median [IQR] | 345 [314–393] | 388 [344–473] | 0.010 |
| Reperfusion syndrome, n (%) | 2 (7.7) | 2 (2.7) | 0.260 |
| Transplant duration in minutes, median [IQR] | 430 [381–493] | 470 [429–523] | 0.040 |
| ICU stay in days, median [IQR] | 4 [3–5] | 3 [2–5] | 0.116 |
| Peak SGOT (POD 1–7) in U/L, median [IQR] | 347 [259–1,026] | 566 [289–1,361] | 0.363 |
| Peak SGPT (POD 1–7) in U/L, median [IQR] | 495 [168–854] | 519 [272–1,057] | 0.416 |
| Post-operative infectious complications, n (%) | 7 (26.9) | 20 (25.6) | 0.896 |
| Severe acute kidney injury, n (%) | 1 (4) | 4 (5.1) | 1 |
| Respiratory failure, n (%) | 0 | 2 (2.6) | 1 |
| Post-operative haemorrhage, n (%) | 0 | 1 (1.3) | 1 |
| Reintervention, n (%) | 1 (4) | 8 (10.3) | 0.546 |
| Hepatic artery thrombosis, n (%) | 0 | 1 (1.3) | 1 |
| 12 months biliary complications, n (%) | 3 (11.5) | 7 (9.1) | 0.710 |
| 30 days acute cellular rejection, n (%) | 0 | 3 (3.9) | 0.570 |
| 90 days mortality, n (%) | 0 | 2 (2.6) | 1 |
| Early allograft dysfunction, n (%) | 3 (11.5) | 17 (21.8) | 0.250 |
| Primary graft non function, n (%) | 1 (4) | 3 (3.9) | 1 |
| Re-transplantation, n (%) | 1 (4) | 5 (5.3) | 0.678 |
| Major complications, n (%) | 4 (15.4) | 16 (20.5) | 0.566 |
| Comprehensive Complication Index®, 75th percentile | 29.6 | 30.8 | 0.487 |
| Hospital stay in days, median [IQR] | 15 [13–23] | 15 [11–23] | 0.919 |
Procedural and outcome data after propensity score matching.
DCD-LT, donation after circulatory determination of death liver transplantation; DBD-LT, donation after neurological determination of death liver transplantation; IQR, interquartile range; NRP, normothermic regional perfusion; HOPE, hypothermic oxygenated perfusion; ICU, intensive care unit; SGOT, serum glutamic oxaloacetic transaminase; SGPT, serum glutamic pyruvic transaminase; POD, postoperative day.
Bold values highlight statistical significance.
Post-Transplant Outcomes According to Donor Type and Donor Age
After stratification by age, three subgroups were identified: 15 recipients of DCD donors ≥75 years, 11 recipients of DCD donors <75 years, and 28 recipients of DBD donors ≥75 years. No significant differences were evident in terms of CPT, surgical complications, length of hospital stay, and graft function. One recipient of an elder DCD donor experienced PNF and was successfully retransplanted. Amongst the elder DBD recipients two have been retransplanted due to PNF in one case and hepatic artery thrombosis in the other; another recipient of an elder DBD donor died a few hours after LT due to massive myocardial infarction. Altogether, six patients developed biliary complications during the follow-up period (with comparable rates between groups), all consisting of anastomotic strictures with successful endoscopic management. The results are summarized in Table 3. Patients were followed up for a median of 19 months [IQR: 14–24 months] without any significant difference in terms of graft survival for the three subgroups (Figure 1).
TABLE 3
| Variables | DCD≥75 [group 1] (n = 15) | DCD<75 [group 2] (n = 11) | DBD≥75 [group 3] (n = 28) | p (1 vs. 2) | p (1 vs. 3) |
|---|---|---|---|---|---|
| Cold preservation time in minutes, median [IQR] | 335 [300–390] | 350 [320–400] | 360 [340–405] | 0.336 | 0.097 |
| Reperfusion syndrome, n (%) | 2 (13.3) | 0 | 1 (3.6) | 0.492 | 0.275 |
| Transplant duration in minutes, median [IQR] | 425 [383–486] | 480 [360–505] | 469 [419–503] | 0.568 | 0.221 |
| ICU stay in days, median [IQR] | 3 [3–5] | 4 [3–5] | 3 [2–5] | 0.576 | 0.467 |
| Peak SGOT (POD 1–7) in U/L, median [IQR] | 429 [298–1,010] | 305 [253–1,399] | 478 [248–1,218] | 0.494 | 0.904 |
| Peak SGPT (POD 1–7) in U/L, median [IQR] | 501 [159–825] | 292 [176–1,572] | 555 [210–1,007] | 0.913 | 0.775 |
| Post-operative infectious complications, n (%) | 5 (33.3) | 2 (18.2) | 9 (32.1) | 0.679 | 0.938 |
| Severe acute kidney injury, n (%) | 0 | 1 (9.1) | 0 | 0.440 | — |
| Respiratory failure, n (%) | 0 | 0 | 0 | — | — |
| Post-operative haemorrhage, n (%) | 0 | 0 | 0 | — | — |
| Reintervention, n (%) | 1 (6.7) | 0 | 3 (10.7) | 0.874 | 0.909 |
| Hepatic artery thrombosis, n (%) | 0 | 0 | 1 (3.7) | — | 1 |
| 12 months biliary complications, n (%) | 2 (13.3) | 1 (9.1) | 3 (10.7) | 0.774 | 0.807 |
| 30 days acute cellular rejection, n (%) | 0 | 0 | 2 (7.4) | — | 0.530 |
| 90 days mortality, n (%) | 0 | 0 | 1 (3.7) | — | 1 |
| Early allograft dysfunction, n (%) | 1 (6.7) | 2 (18.2) | 6 (21.4) | 0.556 | 0.391 |
| Primary graft non function, n (%) | 1 (6.7) | 0 | 1 (3.7) | 0.874 | 1 |
| Re-transplantation, n (%) | 1 (6.7) | 0 | 2 (7.4) | 0.874 | 0.956 |
| Major complications, n (%) | 3 (20) | 1 (9.1) | 6 (21.4) | 0.614 | 1 |
| Comprehensive Complication Index®, 75th percentile | 29.6 | 29.6 | 30.5 | 0.979 | 0.612 |
| Hospital stay in days, median [IQR] | 15 [13–23] | 13 [12–23] | 17 [13–24] | 0.465 | 0.798 |
Procedural and outcome data after propensity score matching and stratification by donor type and age.
DCD-LT, donation after circulatory determination of death liver transplantation; DBD-LT, donation after neurological determination of death liver transplantation; IQR, interquartile range; NRP, normothermic regional perfusion; HOPE, hypothermic oxygenated perfusion; ICU, intensive care unit; SGOT, serum glutamic oxaloacetic transaminase; SGPT, serum glutamic pyruvic transaminase; POD, postoperative day.
Discussion
The average age of donors in Italy is continuously increasing, with the median age rising from 57.7 in 2012 to 60.9 years in 2021. The number of donors over the age of 80 is also increasing, representing—to date—a consistent portion of the donor pool (13.5% in 2021). In parallel, the mean age of cDCD donors increased to 67 years, with 6% of donations coming from octogenarians. In the hypothesis of a steady trend, a progressive increase in elderly cDCD donors is expected, possibly representing an additional opportunity for transplant candidates, especially for those at greater risk of drop-out or death while on the waiting list.
We reported the first Italian data involving a relatively large number of elder DCD donors; in particular, as far as we know, the 75 years age cut-off that we considered is higher than any other previously published and outreaches the eldest age reported in many reports on elderly DCD donors [8–10].
This study aimed to investigate whether utilizing very elderly DCD donors allows them to achieve comparable outcomes to younger DCD donors and peer-age DBD donors.
Since DCD in the Italian scenario entails severe ischemic burden on liver grafts, these donors have long been considered “marginal”. Nevertheless, recent literature demonstrated a progressive alignment of post-transplant outcomes to those of DBD donors in terms of graft function and graft and recipient survival [24–26]. These findings may suggest that an incremental volume and expertise of the transplant team play a significant role in contributing to the success of DCD liver transplantation. Furthermore, some reports showed that accepting DCD donors or elderly donors, despite their perceived marginality, can significantly improve the survival of selected recipients [27–29].
Our results appear to be consistent with this recent evidence. Although recipients of DCD liver grafts are characterized by a lower MELD score (10 vs. 15, p < 0.001), and a higher prevalence of HCC as a primary indication to transplant (69.2% vs. 41.2%, p = 0.009), post-operative outcome results were comparable after adequate minimization of existing biases.
Despite the requirement for PSM, it is important to mention that the different utilization of DCD and DBD liver grafts in our case series reflects the contemporary trends from medical literature and the higher individual transplant benefit for patients at high risk of drop-out from the waiting list [27–29]. Consistently with this evidence, we usually match DCD donors with recipients who, despite stable liver function, have a high-risk of drop-out from the waiting list due to either oncological risk (e.g., recurrent or down-staged HCC) or infectious risk (e.g., recurrent cholangitis); donor age has little influence in our allocation algorithm, given that DCD donors are preferably accepted for patients with stable liver function, which can more easily tolerate the increased ischemic burden. As a result, outcomes of LT for both DCD and DBD donors not only appeared comparable after PSM, but they also showed similar results after stratification by age and type of donation. Specifically, recipients of very elderly DCD donors had homogenous results in terms of CPT, surgical complications, hospitalization, and graft function, compared to recipients of same-age DBD and younger DCD donors. Moreover, the rate of biliary complications was acceptable in all subgroups despite donors being at high risk, conversely to the previously reported data [30–32]. In our opinion, this resulted from the extensive use of HOPE for DCD grafts pre-conditioning, and from shorter CPT (345 min vs. 388 min, p = 0.010).
Considering the strict Italian legislation, the tWIT liver grafts are exposed to are strongly conditioned by the requirement of a 20-minute-long standoff period before NRP initiation. The outcomes of DCD liver transplantation observed in our cohort, besides being consistent with DBD transplantation, are also the result of meticulous donor management, coupling advanced strategies of in-situ and ex-situ perfusion strategies, and accurate, tailored donor-recipient matching. The improvement in donor and recipient management to minimize tWIT and CPT, starting with routine use of end-ischemic HOPE for DCD liver grafts, showed promising results in preventing and mitigating ischemic insults, and related post-transplant ischemia-reperfusion injury, ultimately leading to satisfactory results [33–36].
We can also assume that the strict Italian legislation [3, 4] aimed to overguarantee the respect of the so-called “dead-donor-rule”—played a major role in forcing transplant teams to pursue accurate management of both DCD donors and grafts in order to overcome the imposed procedural limitations.
Limitations
This study has some limitations. First, the need for case-matching, as well as the limited number of patients in the derived subgroups, affected our ability to draw definite conclusions. Nevertheless, the relatively limited size of the study group reflects a hopefully initial experience with very elderly DCD donors. The short duration of the study implied a limited follow-up for the patients with a late enrolment, although a minimum twelve-month follow-up was provided for all the included recipients. Finally, in this study, we compared grafts from elderly cDCD donors—exposed to extensive reconditioning through NRP and end-ischemic HOPE—with ECD liver grafts (at least according to donor age) from DBD donors, which have been implanted without advanced perfusion strategies. This approach might be considered a bias, as DBD grafts can benefit from HOPE too [35, 36], but it reflects the state of the art in DCD liver transplantation in Italy. In fact, under Italian legal and procedural circumstances, this approach enabled the safe transplant of organs potentially carrying a relevant ischemic burden. Moreover, its extensive use also achieved comparable outcomes between cDCD grafts and ECD grafts, with the latter still being transplanted without HOPE in the majority of transplant centers worldwide.
Conclusion
According to the preliminary results of our single center experience, the inclusion of very elderly cDCD donors in liver transplantation programs might provide acceptable outcomes, comparable to those achieved with younger cDCD donors, and with same-age DBD donors. With donor management and graft allocation and reconditioning becoming more and more accurate as further experience and evidence accumulate, the ability of clinicians to achieve optimal utilization and to improve transplantation outcomes for DCD grafts will be enhanced, overcoming existing concerns about these donors’ perceived marginality.
Statements
Data availability statement
The data that support the findings of this study are available upon reasonable request from the corresponding author.
Ethics statement
The studies involving humans were approved by Comitato Etico - Area Vasta Emilia Centro (CE-AVEC). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
Research conception: GF, AlS, MC, MR. Research design: GF, AlS, AnS, LT, MM, MC, MR. Data acquisition: GF, AlS, APS, AA, EP, MA. Data analysis: GF, AlS, MC, MR. Manuscript draft: GF, AlS, EP, MA, MC, MR. Critical revision: GF, AlS, AnS, APS, AA, EP, MA, LT, MM, MC, MR.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/ti.2023.11697/full#supplementary-material
Supplementary Figure S1Timeline of events preceding and following declaration of death with cardiocirculatory criteria Maastricht category 3 controlled donors. Agonal phase and fWIT start with hypotension (mean arterial pressure dropping below 50 mmHg) or desaturation (peripheral oxygen saturation dropping below 50%), whichever occurring first; tWIT and fWIT refer to the potential donor. NRP interrupts ischemic time. CIT refers to the graft; the time the graft is treated with hope is not to be considered functionally ischemic, despite the lack of hematic perfusion, as the liver is oxygenated. Times not to scale. WLST, withdrawal of life sustaining treatments; tWIT, total warm ischemia time; fWIT, functional warm ischemia time (hypoxemic and/or hypotensive); NRP, normothermic regional perfusion; ISP, in situ-preservation; SCS, static cold storage; CPT, cold preservation time; HOPE, hypothermic oxygenated perfusion; LT, liver transplantation.
Abbreviations
aWIT, asystolic Warm Ischemia Time; CCI®, Comprehensive Complication Index; CPT, Cold Preservation Time; DBD, Donors after Brainstem Death; DCD, Donation after Circulatory determination of Death; EAD, Early Allograft Dysfunction; fWIT, functional Warm Ischemia Time; HCC, HepatoCellular Carcinoma; HOPE, Hypothermic Oxygenated PErfusion; IQR, Inter-Quartile Range; IRI, Ischaemia-Reperfusion Injury; ISP, In-Situ Perfusion; LT, Liver Transplant; MAP, Mean Arterial Pressure; MELD, Model for End Stage Liver Disease; NRP, Normothermic Regional Perfusion; OPTN, Organ Procurement and Transplantation Network; PNF, Primary Non Function; PRS, Post Reperfusion Syndrome; PSM, Propensity Score Match; SCS, Static Cold Storage; tWIT, total Warm Ischemia Time; WIT, Warm Ischemia Time.
References
1.
Ziogas IA Kakos CD Esagian Smet AL Skarentzos K Alexopoulos SP Shingina A et al Liver Transplant After Donation from Controlled Circulatory Death versus Brain Death: A UNOS Database Analysis and Publication Bias Adjusted Meta-Analysis. Clin Transpl (2022) 36(2):e14521. 10.1111/ctr.14521
2.
Fernández-de la Varga M Del Pozo-Del Valle P Béjar-Serrano S López-Andújar R Berenguer M Prieto M et al Good Post-Transplant Outcomes Using Liver Donors after Circulatory Death when Applying Strict Selection Criteria: A Propensity-Score Matched-Cohort Study. Ann Hepatol (2022) 27(5):100724. 10.1016/j.aohep.2022.100724
3.
Parlamento. Legge 1° aprile 1999, n. 91. Disposizioni in Materia di Prelievi e di Trapianti di Organi e di Tessuti (2023). Available From: www.parlamento.it/parlam/leggi/99091l.htm (Accessed February, 2023).
4.
Comitato Nazionale per la Bioetica. Accertamento della morte secondo il criterio cardiocircolatorio e "donazione controllata": aspetti etici e giuridici. 9 dicembre 2021 (2023). Available From: https://bioetica.governo.it/it/pareri/pareri-e-risposte/accertamento-della-morte-secondo-il-criterio-cardiocircolatorio-e-donazione-controllata-aspetti-etici-e-giuridici/ (Accessed February, 2023).
5.
Conrad SA Broman LM Taccone FS Lorusso R Malfertheiner MV Pappalardo F et al The Extracorporeal Life Support Organization Maastricht Treaty for Nomenclature in Extracorporeal Life Support. A Position Paper of the Extracorporeal Life Support Organization. Am J Respir Crit Care Med (2018) 198(4):447–51. 10.1164/rccm.201710-2130CP
6.
Miñambres E Suberviola B Dominguez-Gil B Rodrigo E Ruiz-San Millan JC Rodríguez-San Juan JC et al Improving the Outcomes of Organs Obtained From Controlled Donation after Circulatory Death Donors Using Abdominal Normothermic Regional Perfusion. Am J Transpl (2017) 17(8):2165–72. 10.1111/ajt.14214
7.
Schlegel A Muller X Kalisvaart M Muellhaupt B Perera MTPR Isaac JR et al Outcomes of DCD Liver Transplantation Using Organs Treated by Hypothermic Oxygenated Perfusion Before Implantation. J Hepatol (2019) 70(1):50–7. 10.1016/j.jhep.2018.10.005
8.
Cascales-Campos PA Ferreras D Alconchel F Febrero B Royo-Villanova M Martínez M et al Controlled Donation After Circulatory Death up to 80 Years for Liver Transplantation: Pushing the Limit Again. Am J Transpl (2020) 20(1):204–12. 10.1111/ajt.15537
9.
Schlegel A Scalera I Perera MTPR Kalisvaart M Mergental H Mirza DF et al Impact of Donor Age in Donation After Circulatory Death Liver Transplantation: Is the Cutoff "60" Still of Relevance? Liver Transpl (2018) 24(3):352–62. 10.1002/lt.24865
10.
Giorgakis E Khorsandi SE Mathur AK Burdine L Jassem W Heaton N . Comparable Graft Survival Is Achievable With the Usage of Donation after Circulatory Death Liver Grafts from Donors at or above 70 Years of Age: A Long-Term UK National Analysis. Am J Transpl (2021) 21(6):2200–10. 10.1111/ajt.16409
11.
Schlegel A Kalisvaart M Scalera I Laing RW Mergental H Mirza DF et al The UK DCD Risk Score: A New Proposal to Define Futility in Donation-After-Circulatory-Death Liver Transplantation. J Hepatol (2018) 68(3):456–64. 10.1016/j.jhep.2017.10.034
12.
Cepeda-Franco C Bernal-Bellido C Barrera-Pulido L Álamo-Martínez JM Ruiz-Matas JH Suárez-Artacho G et al Survival Outcomes in Liver Transplantation With Elderly Donors: Analysis of Andalusian Transplant Register. Transpl Proc (2016) 48(9):2983–6. 10.1016/j.transproceed.2016.09.026
13.
Asrani SK Saracino G Wall A Trotter JF Testa G Hernaez R et al Assessment of Donor Quality and Risk of Graft Failure After Liver Transplantation: The ID2 EAL Score. Am J Transpl (2022) 22:2921–30. 10.1111/ajt.17191
14.
Shimada S Shamaa T Ivanics T Kitajima T Collins K Rizzari M et al Liver Transplant Recipient Characteristics Associated With Worse Post-Transplant Outcomes in Using Elderly Donors. Transpl Int (2022) 35:10489. 10.3389/ti.2022.10489
15.
Kootstra G Daemen JH Oomen AP . Categories of Non-Heart-Beating Donors. Transpl Proc (1995) 27(5):2893–4. PMID: 7482956.
16.
Thuong M Ruiz A Evrard P Kuiper M Boffa C Akhtar MZ et al New Classification of Donation After Circulatory Death Donors Definitions and Terminology. Transpl Int (2016) 29(7):749–59. 10.1111/tri.12776
17.
Circelli A Antonini MV Gamberini E Nanni A Benni M Castioni CA et al EISOR Delivery: Regional Experience with Sharing Equipe, Equipment & Expertise to Increase cDCD Donor Pool in Time of Pandemic. Perfusion (2022):026765912211035. 10.1177/02676591221103535
18.
Dindo D Demartines N Clavien PA . Classification of Surgical Complications: A New Proposal With Evaluation in a Cohort of 6336 Patients and Results of a Survey. Ann Surg (2004) 240(2):205–13. 10.1097/01.sla.0000133083.54934.ae
19.
Slankamenac K Graf R Barkun J Puhan MA Clavien PA . The Comprehensive Complication Index: A Novel Continuous Scale to Measure Surgical Morbidity. Ann Surg (2013) 258(1):1–7. 10.1097/SLA.0b013e318296c732
20.
Aggarwal S Kang Y Freeman JA Fortunato FL Pinsky MR . Postreperfusion Syndrome: Cardiovascular Collapse Following Hepatic Reperfusion During Liver Transplantation. Transpl Proc (1987) 19:54–5.
21.
OPTN. OPTN Policies Effective as of Mar 9 2023 (2023). Available From: https://optn.transplant.hrsa.gov/policies-bylaws/policies/ (Accessed March, 2023).
22.
Olthoff KM Kulik L Samstein B Kaminski M Abecassis M Emond J et al Validation of a Current Definition of Early Allograft Dysfunction in Liver Transplant Recipients and Analysis of Risk Factors. Liver Transpl (2010) 16(8):943–9. 10.1002/lt.22091
23.
KDIGO. 2021 AKI Guideline (2023). Available From: https://kdigo.org/guidelines/acute-kidney-injury/ (Accessed February, 2023).
24.
Wallace D Cowling TE Suddle A Gimson A Rowe I Callaghan C et al National Time Trends in Mortality and Graft Survival Following Liver Transplantation From Circulatory Death or Brainstem Death Donors. Br J Surg (2021) 109(1):79–88. 10.1093/bjs/znab347
25.
Haque O Yuan Q Uygun K Markmann JF . Evolving Utilization of Donation After Circulatory Death Livers in Liver Transplantation: The Day of DCD Has Come. Clin Transpl (2021) 35(3):e14211. 10.1111/ctr.14211
26.
Scalea JR Redfield RR Foley DP . Liver Transplant Outcomes Using Ideal Donation After Circulatory Death Livers Are superior to Using Older Donation after Brain Death Donor Livers. Liver Transpl (2016) 22(9):1197–204. 10.1002/lt.24494
27.
Hobeika MJ Saharia A Mobley CM Menser T Nguyen DT Graviss EA et al Donation After Circulatory Death Liver Transplantation: An In-Depth Analysis and Propensity Score-Matched Comparison. Clin Transpl (2021) 35(6):e14304. 10.1111/ctr.14304
28.
Haugen CE Bowring MG Holscher CM Jackson KR Garonzik-Wang J Cameron AM et al Survival Benefit of Accepting Livers From Deceased Donors over 70 Years Old. Am J Transpl (2019) 19(7):2020–8. 10.1111/ajt.15250
29.
Taylor R Allen E Richards JA Goh MA Neuberger J Collett D et al Survival Advantage for Patients Accepting the Offer of a Circulatory Death Liver Transplant. J Hepatol (2019) 70(5):855–65. 10.1016/j.jhep.2018.12.033
30.
Ravaioli M Grande G Di Gioia P Cucchetti A Cescon M Ercolani G et al Risk Avoidance and Liver Transplantation: A Single-Center Experience in a National Network. Ann Surg (2016) 264(5):778–86. 10.1097/SLA.0000000000001887
31.
Mathur AK Heimbach J Steffick DE Sonnenday CJ Goodrich NP Merion RM . Donation After Cardiac Death Liver Transplantation: Predictors of Outcome. Am J Transpl (2010) 10(11):2512–9. 10.1111/j.1600-6143.2010.03293.x
32.
Croome KP Mathur AK Lee DD Moss AA Rosen CB Heimbach JK et al Outcomes of Donation After Circulatory Death Liver Grafts from Donors 50 Years or Older: A Multicenter Analysis. Transplantation (2018) 102(7):1108–14. 10.1097/TP.0000000000002120
33.
Dondossola D Ravaioli M Lonati C Maroni L Pini A Accardo C et al The Role of Ex Situ Hypothermic Oxygenated Machine Perfusion and Cold Preservation Time in Extended Criteria Donation after Circulatory Death and Donation After Brain Death. Liver Transpl (2021) 27(8):1130–43. 10.1002/lt.26067
34.
Maroni L Musa N Ravaioli M Dondossola DE Germinario G Sulpice L et al Normothermic with or without Hypothermic Oxygenated Perfusion for DCD Before Liver Transplantation: European Multicentric Experience. Clin Transpl (2021) 35(11):e14448. 10.1111/ctr.14448
35.
Ravaioli M De Pace V Angeletti A Comai G Vasuri F Baldassarre M et al Hypothermic Oxygenated New Machine Perfusion System in Liver and Kidney Transplantation of Extended Criteria Donors: First Italian Clinical Trial. Sci Rep (2020) 10(1):6063. 10.1038/s41598-020-62979-9
36.
Ravaioli M Germinario G Dajti G Sessa M Vasuri F Siniscalchi A et al Hypothermic Oxygenated Perfusion in Extended Criteria Donor Liver Transplantation – a Randomized Clinical Trial. Am J Transpl (2022) 22(10):2401–8. 10.1111/ajt.17115
Summary
Keywords
liver transplantation, elderly donors, donation after circulatory determination of death, donation after brainstem death, liver transplantation outcomes
Citation
Fallani G, Stocco A, Siniscalchi A, Antonini MV, Stella AP, Amato A, Prosperi E, Turco L, Morelli MC, Cescon M and Ravaioli M (2023) Beyond the Concepts of Elder and Marginal in DCD Liver Transplantation: A Prospective Observational Matched-Cohort Study in the Italian Clinical Setting. Transpl Int 36:11697. doi: 10.3389/ti.2023.11697
Received
16 June 2023
Accepted
21 August 2023
Published
07 September 2023
Volume
36 - 2023
Updates
Copyright
© 2023 Fallani, Stocco, Siniscalchi, Antonini, Stella, Amato, Prosperi, Turco, Morelli, Cescon and Ravaioli.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matteo Ravaioli, matteo.ravaioli6@unibo.it
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.