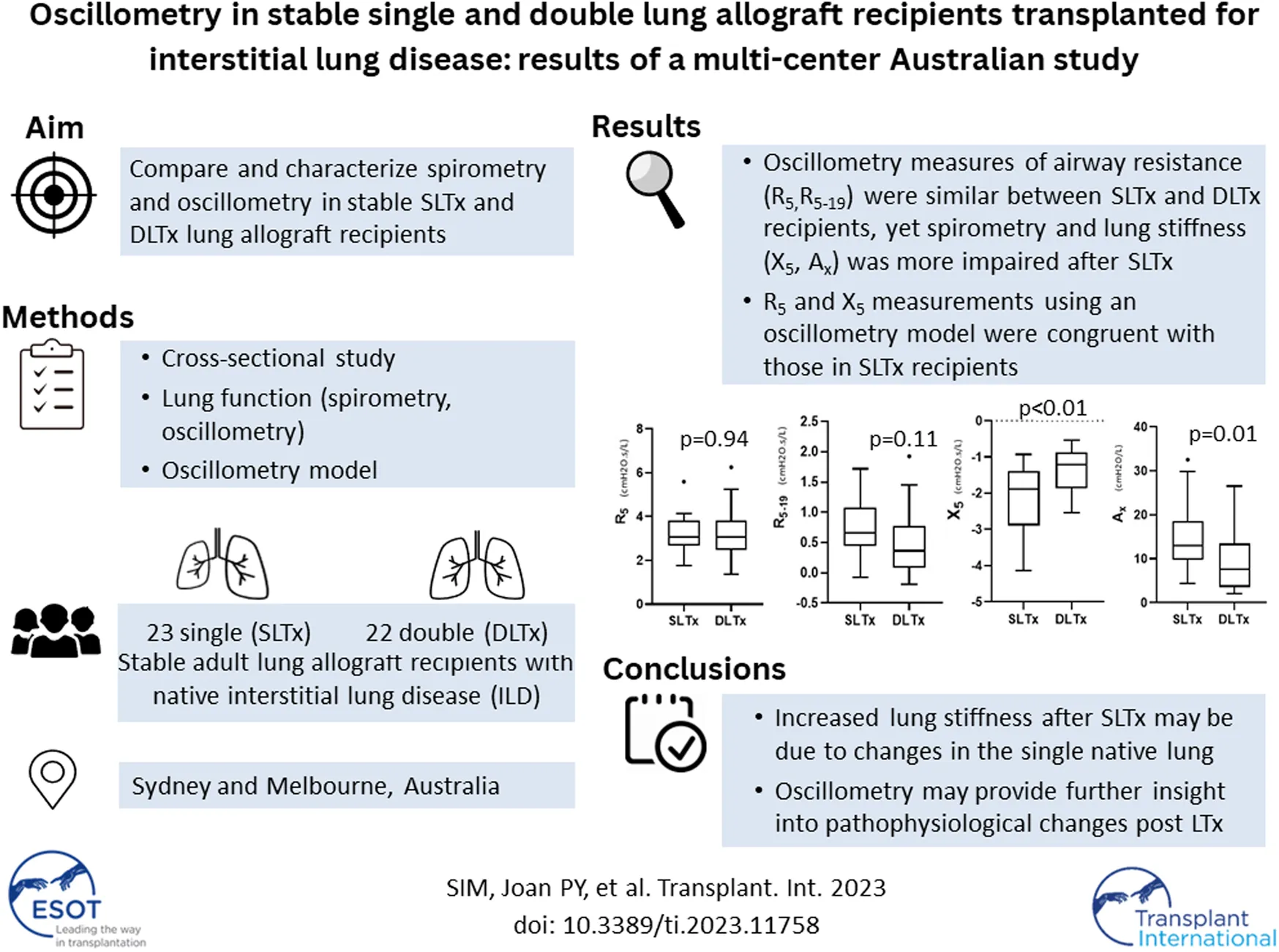
Abstract
Peak spirometry after single lung transplantation (SLTx) for interstitial lung disease (ILD) is lower than after double lung transplantation (DLTx), however the pathophysiologic mechanisms are unclear. We aim to assess respiratory mechanics in SLTx and DLTx for ILD using oscillometry. Spirometry and oscillometry (tremoflo® C-100) were performed in stable SLTx and DLTx recipients in a multi-center study. Resistance (R5, R5–19) and reactance (X5) were compared between LTx recipient groups, matched by age and gender. A model of respiratory impedance using ILD and DLTx data was performed. In total, 45 stable LTx recipients were recruited (SLTx n = 23, DLTx n = 22; males: 87.0% vs. 77.3%; median age 63.0 vs. 63.0 years). Spirometry was significantly lower after SLTx compared with DLTx: %-predicted mean (SD) FEV1 [70.0 (14.5) vs. 93.5 (26.0)%]; FVC [70.5 (16.8) vs. 90.7 (12.8)%], p < 0.01. R5 and R5–19 were similar between groups (p = 0.94 and p = 0.11, respectively) yet X5 was significantly worse after SLTx: median (IQR) X5 [−1.88 (−2.89 to −1.39) vs. −1.22 (−1.87 to −0.86)] cmH2O.s/L], p < 0.01. R5 and X5 measurements from the model were congruent with measurements in SLTx recipients. The similarities in resistance, yet differences in spirometry and reactance between both transplant groups suggest the important contribution of elastic properties to the pathophysiology. Oscillometry may provide further insight into the physiological changes occurring post-LTx.
Introduction
Lung transplantation (LTx) is an established intervention for patients with advanced interstitial lung disease (ILD) refractory to medical therapy [1]. LTx improves survival in patients with ILD [2] and outcomes depend on donor and recipient factors, choice of procedure and post-operative progress [3]. Single lung transplantation (SLTx) has been the predominant procedure used in patients with ILD, however, double lung transplantation (DLTx) is increasingly used [4]. Survival after LTx is limited by acute and chronic allograft dysfunction and subsequent failure, however there is conflicting data comparing outcomes post-SLTx versus DLTx [1,5,6].
Chronic allograft dysfunction is usually detected on spirometric surveillance [7] and defined as a persistent decline in the forced expiratory volume in one second (FEV1), from the best achieved post-operative FEV1 [8]. Studies have consistently demonstrated that FEV1 and forced vital capacity (FVC) are significantly lower in patients post-SLTx compared to post-DLTx during both short- and long-term follow up [9–11]. Lower spirometry post-SLTx may be attributed to disease progression in the contralateral native lung [9]. However, spirometry alone provides limited insight into the mechanisms contributing to the complex physiological differences between SLTx and DLTx. Furthermore, spirometry may be confounded and therefore produce variable results in SLTx recipients due to possible allograft compression during the forced breathing maneuver [12].
Oscillometry is a non-invasive lung function test performed during quiet tidal breathing that measures the respiratory mechanics of the chest wall, lung and airways [13]. During oscillometry measurement, pressure oscillations, usually of frequencies between 5 and 19 Hertz (Hz), are superimposed at the mouth [14]. The measured pressure and airflow changes are used to calculate impedance—comprised of resistance (Rrs), a measure of airway calibre; and reactance (Xrs) representing the elastic (compliance) components. Oscillometry has predominantly been used in obstructive respiratory diseases with a paucity of studies in patients with ILD. Studies have demonstrated increased Rrs and decreased Xrs in those with ILD [15, 16] compared to healthy controls [17] and people with mild-moderate COPD [18]. Conversely, other studies have demonstrated that Rrs in ILD, specifically interstitial pulmonary fibrosis, is normal yet Xrs is decreased [19, 20], likely reflecting reduced lung compliance from lung fibrosis [19]. Despite its increasing use in tertiary centers, including six in Sydney thus far, studies assessing oscillometry measurements post-LTx remain limited. One study identified physiological changes, increased R5–19 and reactance area (Ax) and decreased X5, in biopsy-proven acute cellular rejection post-DLTx that were undetectable by spirometry [21]. Mathematical models have also been used to calculate impedance using various airway and lung tissue models to describe respiratory mechanics in different disease states [22]. However, none has examined oscillometry measurements in patients with ILD following LTx. Thus, combining our existing knowledge of oscillometry in other disease states and the lack of understanding in our study’s patient population, oscillometry may provide further useful pathophysiological insights in patients with ILD following LTx.
We hypothesized that in patients with ILD who have undergone SLTx, resistance (Rrs) would be increased, reactance (Xrs) decreased, and Ax increased compared to those post-DLTx. Thus, the aim of this study was to characterize resistance (R5 and R5–19) and reactance (X5) and Ax in stable recipients and evaluate the relationship between spirometry and oscillometry results following SLTx and DLTx for ILD.
Materials and Methods
A cross-sectional study of adult LTx recipients performed for patients with ILD was undertaken at two Australia centers (Sydney and Melbourne), between January-2020 and May-2021. Patients attending routine clinic appointments were approached and consented to participate in the study. The study was initiated just prior to the COVID-19 pandemic which limited data collection. ILD was defined by a consensus clinical, physiological and radiological diagnosis. Donor and recipient matching and surgical techniques were performed as per standard clinical practice [23, 24]. Patients underwent unilateral or bilateral thoracotomy for SLTx and DLTx, respectively. For ILD recipients, lung donors for DLTx are selected based on the predicted total lung capacity (TLC), usually being between the recipients actual measured TLC and their predicted TLC. Lung donors for SLTx are typically larger than that of the recipients (i.e., oversized).
LTx recipients with stable allograft function, defined as concurrent/baseline FEV1 ≥ 90%, were eligible for study enrolment [25]. Baseline FEV1 was defined as the best FEV1 measurement achieved post LTx. Recipients with acute or chronic lung allograft dysfunction were excluded [25] therefore bronchoscopy and transbronchial biopsy data were not included. Selected patient data were also used in Darley et al.’s recent study “Airway oscillometry parameters in baseline lung allograft dysfunction: Associations from a multicenter study,” whose results have no implications on this study [26]. Study participants performed oscillometry followed by spirometry during a single visit (Figure 1). Participants were classified into two groups (SLTx and DLTx) and were matched 1:1 for age and gender. Chest radiographs performed as part of standard clinical care within at least 6 months of the study visit were used as a surrogate measure of lung volumes in the SLTx group.
FIGURE 1
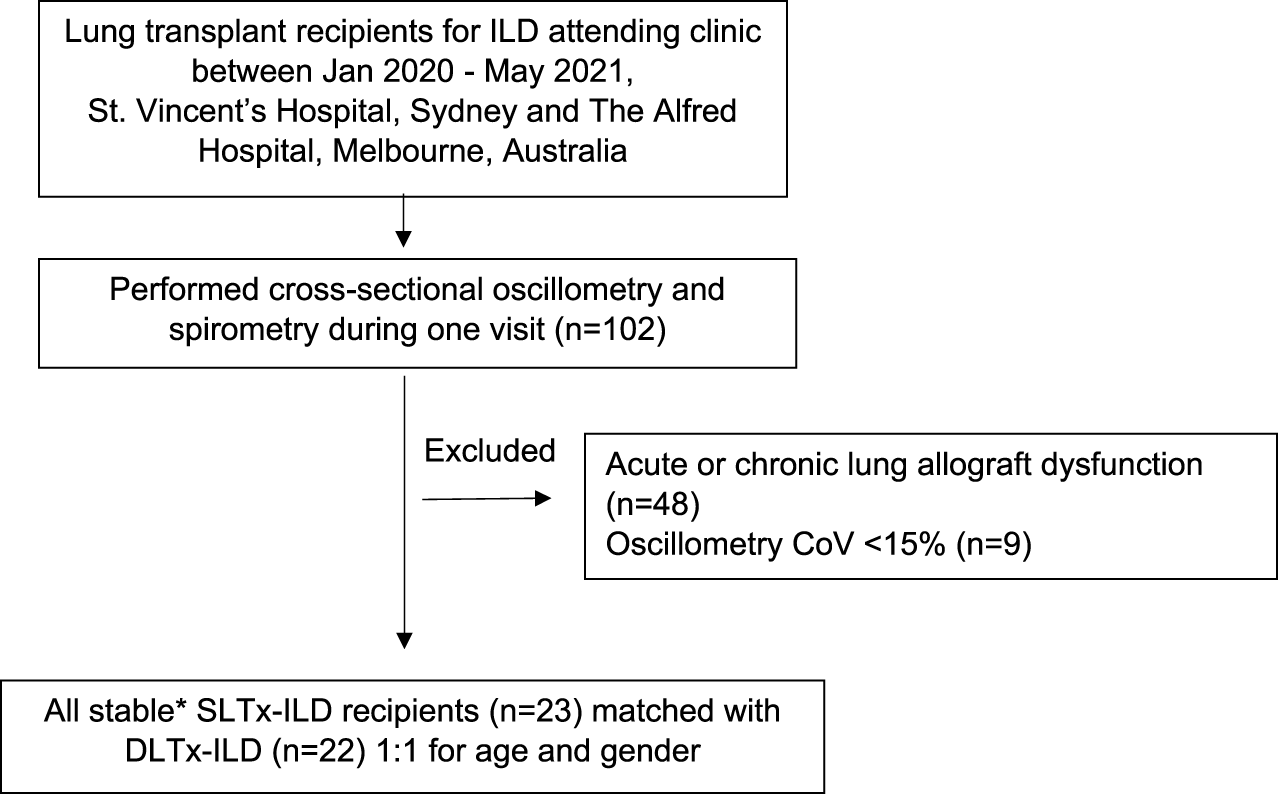
Flow diagram of inclusion and exclusion criteria for participant selection. Definition of abbreviations: ILD, Interstitial Lung Disease; CoV, Coefficient of variation; SLTx-ILD, Single lung transplant for ILD; DLTx, Double lung transplant for ILD. *Defined as concurrent/baseline FEV1 ≥ 90%.
Lung Function
Oscillometry measurements were performed using the tremoflo device (THORASYS® tremoflo® C-100 Airway Oscillometry System) according to European Respiratory taskforce recommendations [27]. Artefacts and tests that did not meet quality control (three measurements per patient with a R5 coefficient variation of <15%) were excluded [28]. Spirometry (Vmax Software, BreezeSuite) was performed as per American Thoracic Society/European Respiratory Society task force recommendations [29]. Standard oscillometry (R5, R5–19, X5, AX) and spirometry (FEV1, FVC, FEV1/FVC) parameters were reported. Z-scores for oscillometry and %-predicted values for spirometry measurements were calculated using published predictive equations [14, 30]. A normal Z-score was determined by ±one standard deviation from the mean (Z-score of ±1.64).
Chest Radiographs
Digital chest radiograph (CXR) measurements [lung height and width (cm)]) were obtained from the allograft and native lung in the SLTx recipients. CXR measurements were performed using in-software Cerner Enterprise Web Viewer 3.0 calipers. Lung height was measured from the mid-diaphragm to the lung apex and width was measured from the inside of the chest wall across the mid-height of the two diaphragms [31].
Modelling
Oscillometry measurements from patients with ILD and from the DLTx group were used in a standard model of respiratory impedance. ILD patients with an FVC measurement of <80% to match spirometry of the LTx groups were included. Patients with ILD (n = 25, male = 19) had a mean ± SD age of 72.2 ± 6.5 years and %-predicted FVC of 63.9% ± 10.6%.
In brief, the standard model obtained from oscillometry is typically expressed with separate resistive (R) and reactive (X) components (Figure 2). This model can be advanced to an inhomogeneous airway model with two parallel pathways (one for each lung) to examine resistance (Rrs) and reactance (Xrs) from each lung independently [32]. The model was used to determine the Rrs and Xrs contribution from a single lung in both the DLTx and ILD groups by using the median R5 and X5 from each group (Supplementary Equations S1, S2). Modelling of R5 and X5 for a SLTx recipient was derived by combining the results from a single lung from each of the DLTx and ILD groups (Figure 2). Further details are outlined in the Supplementary Material.
FIGURE 2
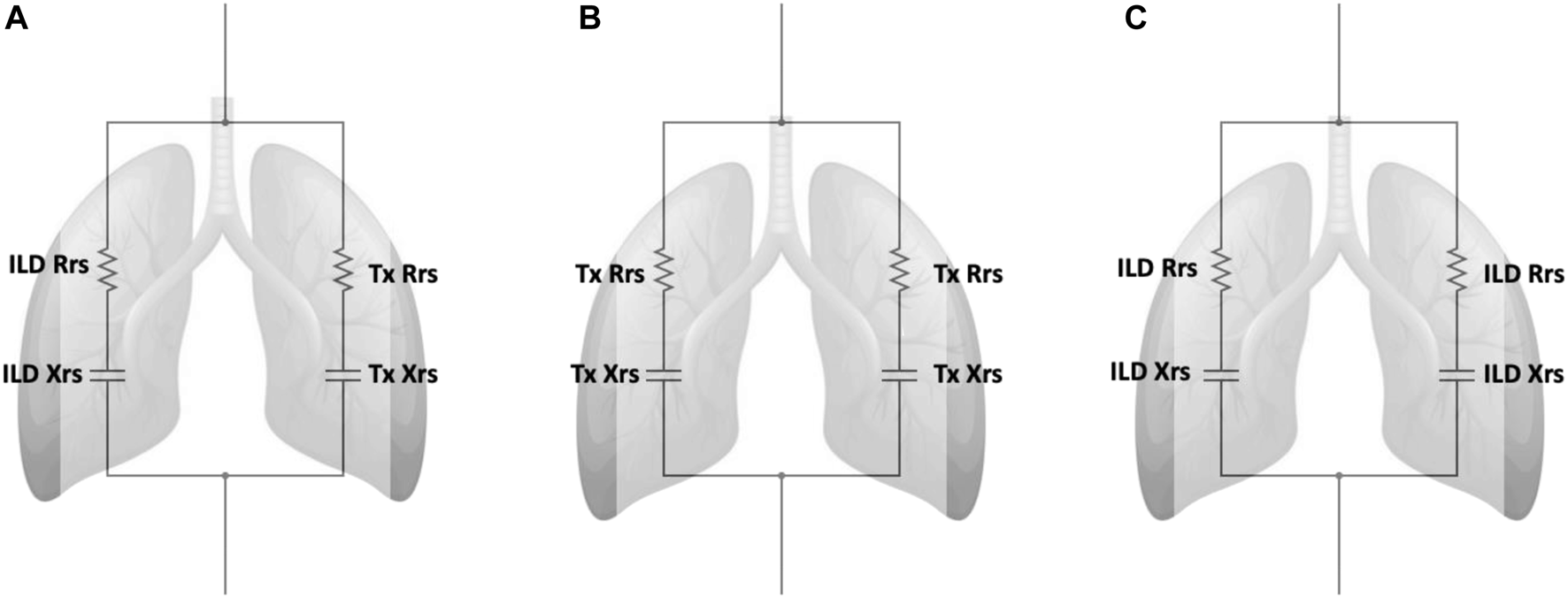
Inhomogeneous models with separate parallel pathways for each lung. Definition of abbreviations: ILD, Interstitial lung disease; Tx, Transplant; Rrs, Respiratory Resistance; Xrs, Respiratory Reactance. (A) Model of the single lung transplant group. This model contains separate parallel pathways with a single ILD lung and a single transplanted lung. (B) Model of the double lung transplant group. This model contains two parallel pathways of a single transplanted lungs. (C) Model of ILD group. This model contains two parallel pathways of a single ILD lungs. ILD Rrs and Tx Rrs are the mean resistance from a single lung from the ILD and Double lung transplant groups respectively. ILD Xrs and Tx Xrs are the mean reactance from a single lung from the ILD and Double lung transplant groups respectively.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism 8.4.2 and IBM SPSS Statistics 26. Descriptive statistics were summarized using mean with standard deviation or median with interquartile range for continuous variables for parametric and non-parametrically distributed data, respectively; and frequency (%) for categorical variables. Results were compared using the two-sample t-test for continuous variables and the chi-square test for categorical variables. Relationships between oscillometry and spirometry were assessed using Spearman’s correlation. Statistical significance was set at a 2-sided level of 0.05.
Guidelines
The study was approved by the St Vincent’s Hospital Human Research Ethics Committee (2019/ETH12765) and the Alfred Health Human Research Ethics Committee (HREC 50035).
Results
A total of 45 stable recipients after LTx for ILD were recruited (23 SLTx and 22 DLTx recipients). Baseline demographics (Table 1) between the SLTx and DLTx groups were similar with regards to recipient gender (87.0% versus 77.3% males) and recipient and donor age [median (IQR) age for recipients: 63.0 (57.0–67.0) versus 63.0 (58.0–66.3) years and mean ± SD age for donors: 44.6 ± 12.9 versus 49.9 ± 18.1 years, for SLTx and DLTx, respectively]. Recipient height, weight and BMI, donor-recipient height difference and donor smoking history were similar between SLTx and DLTx groups. Concurrent FEV1/baseline FEV1% were also similar between the two groups [median (IQR) 96.0 (92.5–101.0)% versus 98.3 (94.5–100.0)% for SLTx and DLTx, respectively], indicating lung function stability and no evidence of chronic allograft dysfunction. The duration post-LTx was significantly shorter in the SLTx compared to the DLTx group [median (IQR) 1.0 (0.7–1.9) versus 1.6 (1.0–2.7) years (p < 0.05), for SLTx and DLTx, respectively]. Donor height was significantly taller in the SLTx compared to the DLTx group (mean ± SD 176.0 ± 6.7 versus 167.0 ± 11.0 cm) (p < 0.01). CXR measurements in the SLTx group demonstrated smaller height (169.2 ± 26.9 cm) and width (89.3 ± 13.0 cm) in the native lung compared to the allograft (207.0 ± 31.4 cm and 127.0 ± 22.0 cm, for height and width, respectively) (p < 0.01). Most CXRs (18/23 patients) were performed on the same day or within a month of lung function measurements. Three patients in the SLTx group had bronchial complications—two with left bronchial stenoses requiring stent insertion at four and 6 months prior to lung function measurements. One patient had a left anastomotic stricture.
TABLE 1
| Patient characteristics | SLTx (n = 23) | DLTx (n = 22) | p-value |
|---|---|---|---|
| Recipient age (years) | 63.0 (57.0–67.0)* | 63.0 (58.0–66.3)* | 0.78 |
| Recipient height (cm) | 172.0 (10.6) | 171.0 (8.2) | 0.60 |
| Recipient weight (kg) | 80.3 (12.7) | 77.0 (15.7) | 0.44 |
| Recipient BMI (kg/m2) | 26.9 (3.9) | 26.5 (5.1) | 0.61 |
| Gender (n, % total) | |||
| Males | 20 (87.0%) | 17 (77.3%) | 0.46 |
| Females | 3 (13.0%) | 5 (22.7%) | |
| Duration Post-transplant (years) | 1.0 (0.7–1.9)* | 1.6 (1.0–2.7)* | <0.05 |
| Allograft side | |||
| Left | 8 | 22 | - |
| Right | 14 | 22 | - |
| Types of ILD | |||
| Idiopathic pulmonary fibrosis | 14 | 18 | - |
| Hypersensitivity pneumonitis | 5 | 1 | - |
| Connective tissue disease-ILD | 1 | 0 | - |
| Combined pulmonary fibrosis emphysema | 1 | 1 | - |
| Nonspecific interstitial pneumonia | 1 | 1 | - |
| Lymphoid interstitial pneumonia | 1 | 0 | - |
| Niemann-pick type B | 0 | 1 | - |
| Donor age (years) | 44.6 (12.9) | 49.9 (18.1) | 0.28 |
| Donor height (cm) | 176.0 (6.7) | 167.0 (11.0) | <0.01 |
| Donor-recipient height difference (cm) | 5.9 (4.0) | 8.0 (5.4) | 0.17 |
| Donor smoking history (n, % total) | |||
| No | 9 (39.1%) | 14 (63.6%) | 0.20 |
| Yes | 11 (47.8%) | 6 (27.3%) | |
| Not reported | 3 (13.0%) | 2 (9.1%) | |
| Spirometry post-LTx | |||
| Concurrent FEV1/baseline FEV1 (%) | 96.0 (92.5–101.0)* | 98.3 (94.5–100.0)* | 0.45 |
| FEV1-% predicted | 70.0 (14.5) | 93.5 (26.0) | <0.01 |
| FVC-% predicted | 70.5 (16.8) | 90.7 (12.8) | <0.01 |
| Concurrent FEV1/FVC | 0.80 (0.098) | 0.80 (0.080) | 0.81 |
Baseline recipient and donor demographics of single and double lung transplant groups.
All data are reported as mean (SD) or median (IQR)*. Definition of abbreviations: SLTx, Single Lung Transplant; DLTx, Double Lung Transplant; BMI, body mass index; FEV1, forced expiratory volume in one second; FVC, forced vital capacity.
Lung Function
FEV1 and FVC were significantly lower in the SLTx group compared to the DLTx group (Table 1). Mean ± SD FEV1-% predicted was 70.0 ± 14.5 versus 93.5% ± 26.0% (p < 0.01) and FVC-% predicted was 70.5 ± 16.8 versus 90.7% ± 12.8% (p < 0.01), in SLTx and DLTx groups, respectively. Oscillometry demonstrated that R5 in both SLTx and DLTx groups were within normal limits (median Z-score <1.64). However, X5 and Ax were abnormal in the SLTx group (median Z-scores of −2.26 and 2.22 for X5 and Ax, respectively) and within normal limits in the DLTx group (Table 2).
TABLE 2
| SLTx n = 23 | DLTx n = 22 | p-value | ILD n = 25 | Model data (single lung) | |
|---|---|---|---|---|---|
| R5 (cmH2O.s/L) | 3.06 (2.67–3.83) | 3.06 (2.48–3.84) | 0.94 | 3.41 (2.85–3.69) | 3.23 |
| Z-score | 0.61 (−0.18 to 1.29) | 0.11 (−0.79 to 1.27) | 0.54 | 0.002 (−0.60 to 1.29) | — |
| Z-score >1.64, n | 3 | 4 | — | 4 | — |
| R5-19 (cmH2O.s/L) | 0.66 (0.45–1.08) | 0.36 (0.08–0.78) | 0.11 | 0.81 (0.63–1.20) | — |
| X5 (cmH2O.s/L) | −1.88 (−2.89 to −1.39) | −1.22 (−1.87 to −0.86) | <0.01 | −2.24 (−2.74 to −1.97) | −1.73 |
| Z-score | −2.26 (−3.76 to −0.83) | −0.36 (−1.44 to 0.37) | <0.01 | −2.52 (−3.53 to −1.42) | — |
| Z-score <−1.64, n | 14 | 4 | — | 16 | — |
| Ax (cmH2O/L) | 13.00 (9.73–18.50) | 7.58 (3.55–13.50) | 0.01 | 17.0 (13.65–22.22) | — |
| Z-score | 2.22 (1.52–2.68) | 1.17 (0.44–2.25) | 0.01 | 2.21 (1.62–2.68) | — |
| Z-score >1.64, n | 17 | 9 | — | 19 | — |
Oscillometry data in the single and double lung transplant groups and ILD group.
All data are reported as median (IQR)* Definition of abbreviations: SLTx, Single Lung Transplant; DLTx, Double Lung Transplant; ILD, interstitial lung disease; R5, resistance at 5 Hz; R5–19, Resistance between 5 and 19 Hz; X5, Reactance at 5 Hz; Ax, Reactance Area.
Oscillometry showed similar measurements in resistance (R5 and R5–19) between both groups. Median (IQR) R5 was 3.06 (2.67–3.83) versus 3.06 (2.48–3.84) cmH2O.s/L (p = 0.94) and R5-19 was 0.66 (0.45–1.08) versus 0.36 (0.08–0.74) cmH2O.s/L (p = 0.11) in the SLTx and DLTx groups, respectively. Reactance (X5) was significantly lower and Ax significantly higher (i.e., more abnormal) in the SLTx group compared to the DLTx group. Median (IQR) X5 was −1.88 (−2.89 to −1.39) versus −1.22 (−1.87 to −0.86) cmH2O.s/L (p < 0.01) and Ax was 13.00 (9.73–18.50) versus 7.58 (3.55–13.50) cmH2O/L (p = 0.01) in the SLTx and DLTx groups, respectively (Table 2; Figure 3). Z-score comparisons of oscillometry measurements between SLTx and DLTx groups were similar to that observed with raw values (Table 2).
FIGURE 3
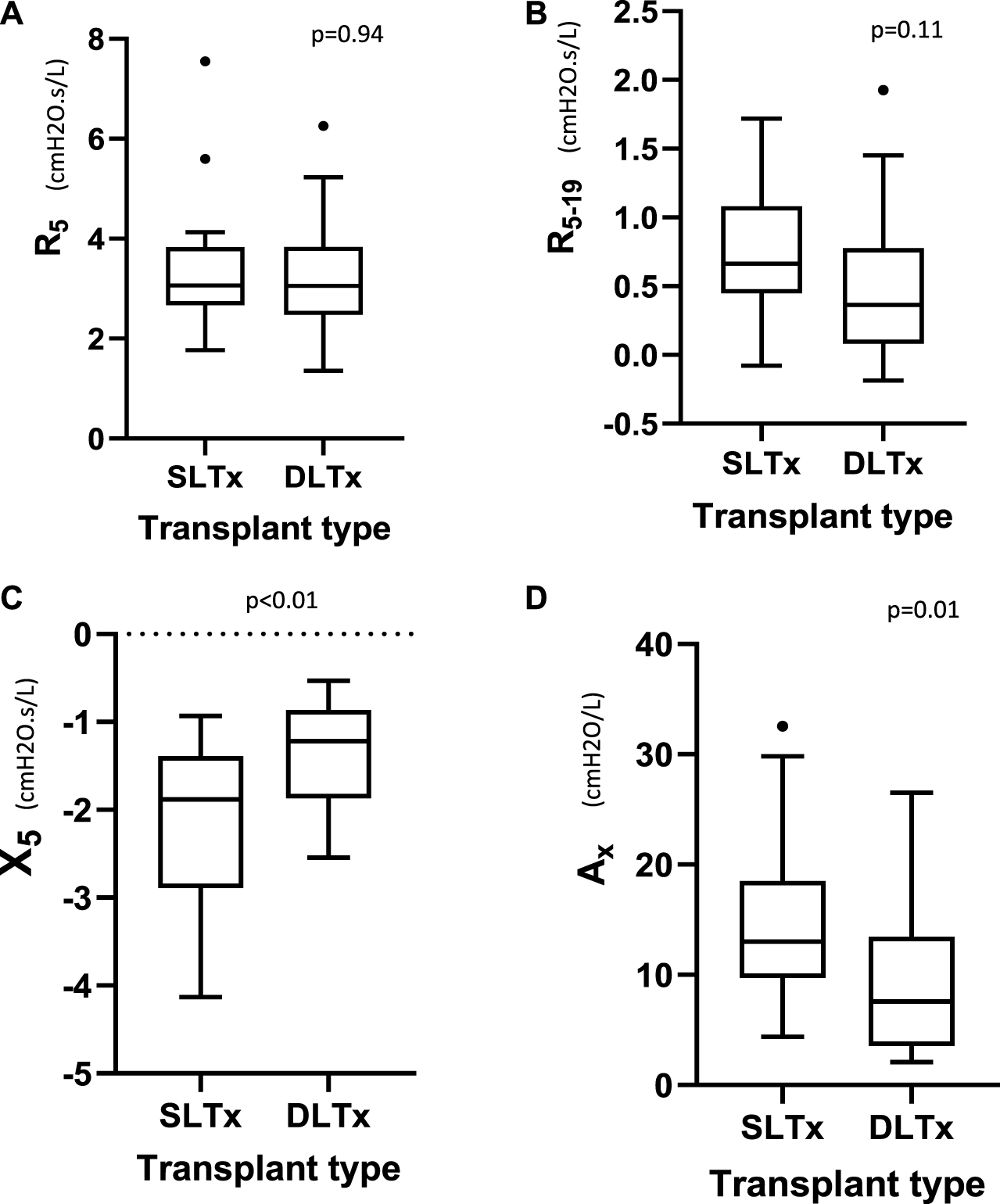
Tukey boxplot comparing the oscillometry indices of (A) R5, (B) R5-19, (C) X5, (D) Ax in 23 SLTx and 22 DLTx patients. Outliers are marked with dots outside the boxplots. Definition of abbreviations: SLTx, Single Lung Transplant; DLTx, Double Lung Transplant; R5, resistance at 5Hz; R5-19, Resistance between 5Hz and 19Hz; X5, Reactance at 5Hz; Ax, Reactance Area.
There were significant associations between oscillometry parameters (R5, R5–19, X5 and Ax) and FVC in the SLTx group [R5 (rs = −0.47, p = 0.02), R5-19 (rs = −0.45, p = 0.03), X5 (rs = 0.72, p < 0.01) and Ax (rs = −0.70, p < 0.01)]. In the DLTx group, significant correlations with FVC were only demonstrated between X5 (rs = 0.65, p < 0.01) and Ax (rs = −0.52, p = 0.01). Similar correlations were observed when comparing FEV1 with oscillometry indices for both SLTx and DLTx groups.
Modelling
The derived single lung values of R5 and X5 for DLTx and ILD groups are displayed in Table 2. There was close agreement between the inhomogeneous oscillometry model predicted R5 (3.23 cmH2O.s/L) and X5 (−1.73 cmH2O.s/L) with the measured R5 (3.06 cmH2O.s/L) and X5 (−1.88 cmH2O.s/L) in the SLTx group.
Discussion
Our multicenter cross-sectional study is the first study, to our knowledge, to report oscillometry measurements in stable single (SLTx) and double (DLTx) lung transplantation recipients, exclusively in patients with ILD as their native lung disease. Our novel findings demonstrate that resistance (R5 and R5–19) measured by oscillometry was similar between SLTx and DTLx recipients despite FEV1 and FVC being significantly lower in the SLTx group. Furthermore, reactance at 5 Hz (X5) and Ax were significantly worse in the SLTx recipients compared to the DLTx recipients. These findings were replicated using a simple mathematical model based on real-life data obtained from DLTx recipients and patients with ILD. Our data, suggests that the differences in respiratory mechanics after SLTx and DLTx may be predominantly attributed to changes in the elastic properties rather than airway caliber.
Resistance (R5 and R5–19) was not increased (i.e., not more abnormal) in the SLTx compared to DLTx recipients. This may be due to patients in our study having stable disease as indicated by the preserved spirometric ratio and concurrent/baseline FEV1 being greater than 90% [25] and thus suggesting the absence of spirometric obstruction and acute or chronic lung allograft dysfunction. Chronic allograft dysfunction is commonly due to bronchiolitis obliterans (BO) [33] with the underlying pathology being fibroproliferative airway plugging [34]. Airway plugging may lead to a reduction in airway caliber and an increase in airway resistance. As resistance was similar between SLTx and DLTx recipients, allograft dysfunction due to BO seems unlikely. This is supported by our cohort being spirometrically-stable. The underlying pathology in the native single ILD lung typically affects the lung parenchyma rather than the airways. However, airway epithelial cell proliferation and expansion in a number of bronchioles can also occur in the distal airways of those with ILD [35]. We speculate that changes in the distal airways may increase airway caliber in the native single ILD lung and thus explain the similarities in resistance between the SLTx and DLTx recipients. Our results are consistent with recent oscillometry studies demonstrating normal resistance in ILD [19, 20]. However, data is conflicting as other studies report resistance to be increased or impaired in patients with ILD in those with more severe lung restriction and lung function impairment [17]. Comparatively, in our study, spirometry demonstrated that lung function impairment was worse in our SLTx recipients compared to DLTx recipients, yet resistance derived from oscillometry was not. Comparisons with other studies are limited because previous oscillometry studies examined ILD patients that did not include LTx recipients.
In contrast to resistance, reactance (X5) was significantly lower, and Ax was significantly higher (i.e., X5 and Ax were more impaired) in the SLTx compared to the DLTx recipients. These findings are consistent with previous studies showing more abnormal reactance in patients with ILD compared to healthy controls [17, 20] and in those with ILD and more severe lung restriction [15]. Reduced lung volume due to the diseased native ILD lung could account for X5 and Ax being more abnormal as these parameters are dependent on lung volume [36]. In the SLTx recipients the native ILD lung was significantly smaller compared to the allograft, which we confirmed using chest radiograph measurements. The allograft side may have contributed to lung volume differences in the SLTx group because left-sided allografts are typically smaller because of the position of the heart. However, a majority of our SLTx recipients underwent a right-sided LTx thus unlikely to contribute to our results (Table 1). Differences in lung volumes between the native lung and allograft in SLTx recipients may lead to asynchrony and altered lung mechanics during respiration. This phenomenon has not been demonstrated in SLTx recipients with ILD, but asynchrony can occur in SLTx recipients with emphysema. The native emphysematous lung and allograft can inflate and empty at different rates and subsequently lead to chest wall asymmetry and mediastinal shift during respiration [12]. The reduced lung volumes may therefore explain a more abnormal reactance. The forced maneuver during spirometry versus tidal breathing during oscillometry measurement needs to be taken into consideration, however the impact on the resulting physiological measurements remains elusive. Additionally, asynchrony in muscle forces, which may result from diaphragm dysfunction, can develop between the two sides of the chest after SLTx [37] and may exacerbate chest wall asymmetry and alter chest wall and lung mechanics. Studies assessing reactance measured via oscillometry in patients with SLTx, respiratory muscle dysfunction and/or chest wall deformities are lacking therefore we can only speculate these mechanisms.
Our study included a simple model that incorporated measurements from real-life ILD and LTx patients to support our in vivo findings in SLTx recipients. The inhomogeneous model shows that in the SLTx group, the single transplanted lung has low reactance while the non-transplanted lung has high reactance (i.e., an increased X5) which corroborates our novel findings. Agreement between the predicted X5 from the model and the measured X5 in the actual SLTx group further ascertains that the increased X5 measured in the SLTx group is indeed attributed to the increased reactance in the native ILD lung. While there is close agreement between the predicted and measured median X5, the measured X5 was slightly more abnormal (−1.73 versus −1.88 cmH2O.s/L, respectively). The more negative X5 may be a reflection of more advanced disease in the SLTx group before transplantation. Using the ILD group’s single lung reactance in the SLTx model, we may have underestimated the reactance in the single native ILD lung. The results derived from this model replicates and provides further evidence to support our in vivo findings in a small number of ILD patients after single and double lung transplantation.
Spirometry was significantly lower or more impaired in our SLTx recipients compared to DLTx recipients as demonstrated in other studies [9, 10]. Anthropometrics in the SLTx and DLTx recipients were similar and thus unlikely to contribute to differences in spirometry. Donor height was significantly taller in the SLTx recipients however is unlikely to be relevant because there was no difference in donor-recipient height matching between the two groups, suggesting appropriate lung size matching. The maximal spirometry measurement achieved is typically lower in SLTx compared to DLTx recipients [10, 38] and thought to be related to the remaining single diseased native lung. The disease pathology in the native lung is also reflected in the normal FEV1/FVC ratio in SLTx, consistent with that of restrictive lung disease.
Limitations that must be acknowledged include the small sample size in our study. The patient cohort was small, as we only included patients with ILD as their native disease. Only one other study has measured oscillometry in SLTx and DLTx recipients however these authors assessed LTx recipients with various forms of native lung diseases, with COPD comprising the majority of their patient cohort [39]. Limiting our study participants to one native disease, ILD, avoids confounding factors from including various diseases. Furthermore, our study groups were matched for age and gender and there were no significant differences between recipient baseline characteristics to confound our results. Differences in lung volume likely contribute to our findings and additionally we did not report lung volume measurements. As a surrogate we showed that there was a significant difference in lung size between the native and allograft lung in the SLTx group using a standardized technique of chest radiograph measurements [31]. The effect of significant differences between donor and recipient height must also be acknowledged however, optimum size matching was performed in accordance with local guidelines. There was no significant difference in smoking history between the two groups and the effect of donor smoking is not known but donor smoking history must also be acknowledged.
The time post-LTx was statistically significantly shorter in the SLTx compared to the DLTx group, however it is clinically insignificant since both groups should have achieved and maintained their maximal spirometry at the time of measurement during the study [9]. The specific effect of relevant clinical parameters such as bronchial stenosis and/or other bronchial or pleural complications were not examined in this cross-sectional study and require further evaluation. Furthermore, the trajectory of oscillometry measurements is not established and will likely alter over time. Spirometry declines more rapidly in SLTx than in DLTx recipients [9, 40] and whether this also occurs in oscillometry is yet to be determined.
Conclusion
In summary, in SLTx recipients, oscillometry measurement of resistance is similar to that observed in DLTx recipients. However, similarly to spirometry, reactance is more impaired in SLTx compared to DLTx recipients. This is likely attributed to changes in the elastance due to reduced alveolar volume in the native ILD lung in SLTx recipients and may lead to asynchrony in respiratory mechanics. Whether the breathing maneuver performing during lung function testing impacts respiratory mechanics is yet to be elucidated but “quiet” tidal breathing may be a more attractive measurement compared to the forced maneuver used in spirometry.
These cross-sectional findings highlight the physiological complexities of LTx that are not completely understood. The significance of normal resistance, yet abnormal spirometry and abnormal reactance as a predictor of clinical outcomes, requires reliable reference values and further longitudinal investigation. Further study in LTx recipients with obstructive lung disease would also improve our understanding. A better understanding of the physiological changes after SLTx and DLTx is vital for developing novel diagnostic and therapeutic approaches to improve LTx outcomes.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the St Vincent’s Hospital Human Research Ethics Committee (2019/ETH12765) and the Alfred Health Human Research Ethics Committee (HREC 50035). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
KN, DD, GS, MP, and KT contributed to the conception and design of the work; JS, KN, DD, BB, BL, JV, SE, and KT contributed to data acquisition and analysis. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Lungitude Foundation’s (patient philanthropic group) donation and the ANZSRS Jeff Pretto Memorial Research Grant 2019.
Acknowledgments
We thank all investigators, study teams, staff and patients for participating in these studies. We acknowledge the pulmonary function laboratories at St Vincent’s Sydney and the Alfred Hospital Melbourne.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/ti.2023.11758/full#supplementary-material
Abbreviations
Ax, Reactance Area; BO, Bronchiolitis obliterans; DLTx, Double Lung Transplantation; FEV1, Forced Expiratory Volume in one second; FVC, Forced Vital Capacity; ILD, Interstitial Lung Disease; R5, Resistance at 5 Hz; R5–19, Resistance between 5 Hz and 19 Hz; SLTx, Single Lung Transplantation; TLC, Total Lung Capacity; X5, Reactance at 5 Hz.
References
1.
De Oliveira NC Osaki S Maloney J Cornwell RD Meyer KC . Lung Transplant for Interstitial Lung Disease: Outcomes for Single Versus Bilateral Lung Transplantation. Interact Cardiovasc Thorac Surg (2012) 14(3):263–7. 10.1093/icvts/ivr085
2.
Kapnadak SG Raghu G . Lung Transplantation for Interstitial Lung Disease. Eur Respir Rev (2021) 30(161):210017. 10.1183/16000617.0017-2021
3.
Li D Liu Y Wang B . Single Versus Bilateral Lung Transplantation in Idiopathic Pulmonary Fibrosis: A Systematic Review and Meta-Analysis. PLOS ONE (2020) 15(5):e0233732. 10.1371/journal.pone.0233732
4.
Chambers DC Cherikh WS Harhay MO Hayes D Jr. Hsich E Khush KK et al The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-Sixth Adult Lung and Heart-Lung Transplantation Report-2019; Focus Theme: Donor and Recipient Size Match. J Heart Lung Transpl (2019) 38(10):1042–55. 10.1016/j.healun.2019.08.001
5.
Schaffer JM Singh SK Reitz BA Zamanian RT Mallidi HR . Single-vs. Double-Lung Transplantation in Patients With Chronic Obstructive Pulmonary Disease and Idiopathic Pulmonary Fibrosis Since the Implementation of Lung Allocation Based on Medical Need. JAMA (2015) 313(9):936–48. 10.1001/jama.2015.1175
6.
Black MC Trivedi J Schumer EM Bousamra M van Berkel V . Double Lung Transplants Have Significantly Improved Survival Compared With Single Lung Transplants in High Lung Allocation Score Patients. Ann Thorac Surg (2014) 98(5):1737–41. 10.1016/j.athoracsur.2014.05.072
7.
Van Muylem A Mélot C Antoine M Knoop C Estenne M . Role of Pulmonary Function in the Detection of Allograft Dysfunction After Heart-Lung Transplantation. Thorax (1997) 52(7):643–7. 10.1136/thx.52.7.643
8.
Cooper JD Billingham M Egan T Hertz MI Higenbottam T Lynch J et al A Working Formulation for the Standardization of Nomenclature and for Clinical Staging of Chronic Dysfunction in Lung Allografts. International Society for Heart and Lung Transplantation. J Heart Lung Transpl (1993) 12(5):713–6.
9.
Mason DP Rajeswaran J Murthy SC McNeill AM Budev MM Mehta AC et al Spirometry After Transplantation: How Much Better Are Two Lungs Than One? Ann Thorac Surg (2008) 85(4):1193–201. 10.1016/j.athoracsur.2007.12.023
10.
Meyers BF Lynch JP Trulock EP Guthrie T Cooper JD Patterson GA . Single Versus Bilateral Lung Transplantation for Idiopathic Pulmonary Fibrosis: A Ten-Year Institutional Experience. J Thorac Cardiovasc Surg (2000) 120(1):99–107. 10.1067/mtc.2000.106322
11.
Pêgo-Fernandes PM Abrão FC Fernandes FLA Caramori ML Samano MN Jatene FB . Spirometric Assessment of Lung Transplant Patients: One Year Follow-Up. Clinics (Sao Paulo). (2009) 64(6):519–25. 10.1590/s1807-59322009000600006
12.
De Groote A Van Muylem A Scillia P Cheron G Verleden G Paiva M et al Ventilation Asymmetry After Transplantation for Emphysema: Role of Chest Wall and Mediastinum. Am J Respir Crit Care Med (2004) 170(11):1233–8. 10.1164/rccm.200403-323OC
13.
Dubois AB Brody AW Lewis DH Burgess BF Jr . Oscillation Mechanics of Lungs and Chest in Man. J Appl Physiol (1956) 8(6):587–94. 10.1152/jappl.1956.8.6.587
14.
Quanjer PH Stanojevic S Cole TJ Baur X Hall GL Culver BH et al Multi-Ethnic Reference Values for Spirometry for the 3-95-yr Age Range: The Global Lung Function 2012 Equations. Eur Respir J (2012) 40(6):1324–43. 10.1183/09031936.00080312
15.
van Noord JA Clement J Cauberghs M Mertens I Van de Woestijine KP Demedts M . Total Respiratory Resistance and Reactance in Patients With Diffuse Interstitial Lung Disease. Eur Resp J (1989) 2:846–52. 10.1183/09031936.93.02090846
16.
Yamamoto Y Miki K Tsujino K Kuge T Okabe F Kawasaki T et al Evaluation of Disease Severity in Bronchiectasis Using Impulse Oscillometry. ERJ Open Res (2020) 6(4):00053-2020. 10.1183/23120541.00053-2020
17.
Sugiyama A Hattori N Haruta Y Nakamura I Nakagawa M Miyamoto S et al Characteristics of Inspiratory and Expiratory Reactance in Interstitial Lung Disease. Respir Med (2013) 107(6):875–82. 10.1016/j.rmed.2013.03.005
18.
Mori K Shirai T Mikamo M Shishido Y Akita T Morita S et al Respiratory Mechanics Measured by Forced Oscillation Technique in Combined Pulmonary Fibrosis and Emphysema. Respir Physiol Neurobiol (2013) 185(2):235–40. 10.1016/j.resp.2012.10.009
19.
Mori Y Nishikiori H Chiba H Yamada G Kuronuma K Takahashi H . Respiratory Reactance in Forced Oscillation Technique Reflects Disease Stage and Predicts Lung Physiology Deterioration in Idiopathic Pulmonary Fibrosis. Respir Physiol Neurobiol (2020) 275:103386. 10.1016/j.resp.2020.103386
20.
Wu JKY Ma J Nguyen L Dehaas EL Vasileva A Chang E et al Correlation of Respiratory Oscillometry With CT Image Analysis in a Prospective Cohort of Idiopathic Pulmonary Fibrosis. BMJ Open Respir Res (2022) 9(1):e001163. 10.1136/bmjresp-2021-001163
21.
Cho E Wu JKY Birriel DC Matelski J Nadj R DeHaas E et al Airway Oscillometry Detects Spirometric-Silent Episodes of Acute Cellular Rejection. Am J Respir Crit Care Med (2020) 201(12):1536–44. 10.1164/rccm.201908-1539OC
22.
Bates JHT Suki B . Assessment of Peripheral Lung Mechanics. Respir Physiol Neurobiol (2008) 163(1-3):54–63. 10.1016/j.resp.2008.03.012
23.
Zhu MZL Levvey BJ McGiffin DC Snell GI . An Intention-To-Treat View of Lung Transplantation for Interstitial Lung Disease: Successful Strategies to Minimize Waiting List and Posttransplant Mortality. Transplantation (2022) 106(1):188–99. 10.1097/TP.0000000000003664
24.
Levvey BJ Harkess M Hopkins P Chambers D Merry C Glanville AR et al Excellent Clinical Outcomes From a National Donation-After-Determination-Of-Cardiac-Death Lung Transplant Collaborative. Am J Transpl (2012) 12(9):2406–13. 10.1111/j.1600-6143.2012.04193.x
25.
Verleden GM Raghu G Meyer KC Glanville AR Corris P . A New Classification System for Chronic Lung Allograft Dysfunction. J Heart Lung Transpl (2014) 33(2):127–33. 10.1016/j.healun.2013.10.022
26.
Darley DR Nilsen K Vazirani J Borg BM Levvey B Snell G et al Airway Oscillometry Parameters in Baseline Lung Allograft Dysfunction: Associations From a Multicenter Study. J Heart Lung Transpl (2023) 42:767–77. 10.1016/j.healun.2022.12.026
27.
King GG Bates J Berger KI Calverley P de Melo PL Dellaca RL et al Technical Standards for Respiratory Oscillometry. Eur Respir J (2020) 55(2):1900753. 10.1183/13993003.00753-2019
28.
Wu JK DeHaas E Nadj R Cheung AB Dandurand RJ Hantos Z et al Development of Quality Assurance and Quality Control Guidelines for Respiratory Oscillometry in Clinic Studies. Respir Care (2020) 65(11):1687–93. 10.4187/respcare.07412
29.
Miller MR Hankinson J Brusasco V Burgos F Casaburi R Coates A et al Standardisation of Spirometry. Eur Respir J (2005) 26(2):319–38. 10.1183/09031936.05.00034805
30.
Oostveen E MacLeod D Lorino H Farre R Hantos Z Desager K et al The Forced Oscillation Technique in Clinical Practice: Methodology, Recommendations and Future Developments. Eur Respir J (2003) 22(6):1026–41. 10.1183/09031936.03.00089403
31.
Li D Weinkauf J Hirji A Nagendran J Kapasi A Lien D et al Chest X-Ray Sizing for Lung Transplants Reflects Pulmonary Diagnosis and Body Composition and Is Associated With Primary Graft Dysfunction Risk. Transplantation (2021) 105(2):382–9. 10.1097/TP.0000000000003238
32.
Otis AB McKerrow CB Bartlett RA Mead J McIlroy MB Selver-Stone NJ et al Mechanical Factors in Distribution of Pulmonary Ventilation. J Appl Physiol (1956) 8(4):427–43. 10.1152/jappl.1956.8.4.427
33.
Estenne M Maurer JR Boehler A Egan JJ Frost A Hertz M et al Bronchiolitis Obliterans Syndrome 2001: An Update of the Diagnostic Criteria. J Heart Lung Transplant (2002) 21(3):297–310. 10.1016/s1053-2498(02)00398-4
34.
de Jong PA Dodd JD Coxson HO Storness-Bliss C Pare PD Mayo JR et al Bronchiolitis Obliterans Following Lung Transplantation: Early Detection Using Computed Tomographic Scanning. Thorax (2006) 61(9):799–804. 10.1136/thx.2005.053249
35.
Plantier L Cazes A Dinh-Xuan A-T Bancal C Marchand-Adam S Crestani B . Physiology of the Lung in Idiopathic Pulmonary Fibrosis. Eur Respir Rev (2018) 27(147):170062. 10.1183/16000617.0062-2017
36.
Kaminsky DA Simpson SJ Berger KI Calverley P de Melo PL Dandurand R et al Clinical Significance and Applications of Oscillometry. Eur Respir Rev (2022) 31(163):210208. 10.1183/16000617.0208-2021
37.
Ratnovsky A Kramer MR Elad D . Breathing Power of Respiratory Muscles in Single-Lung Transplanted Emphysematic Patients. Respir Physiol Neurobiol (2005) 148(3):263–73. 10.1016/j.resp.2005.08.002
38.
Mason DP Rajeswaran J Li L Murthy SC Su JW Pettersson GB et al Effect of Changes in Postoperative Spirometry on Survival After Lung Transplantation. J Thorac Cardiovasc Surg (2012) 144(1):197–203. 10.1016/j.jtcvs.2012.03.028
39.
Ochman M Wojarski J Wiórek A Slezak W Maruszewski M Karolak W et al Usefulness of the Impulse Oscillometry System in Graft Function Monitoring in Lung Transplant Recipients. Transplant Proc (2018) 50(7):2070–4. 10.1016/j.transproceed.2017.12.060
40.
Rubin AS Nascimento DZ Sanchez L Watte G Holand AR Fassbind DA et al Functional Improvement in Patients With Idiopathic Pulmonary Fibrosis Undergoing Single Lung Transplantation. J Bras Pneumol (2015) 41(4):299–304. 10.1590/S1806-37132015000000057
Summary
Keywords
interstitial lung disease, resistance, oscillometry, single and double lung transplantation, reactance
Citation
Sim JPY, Nilsen K, Borg BM, Levvey B, Vazirani J, Ennis S, Plit M, Snell GI, Darley DR and Tonga KO (2023) Oscillometry in Stable Single and Double Lung Allograft Recipients Transplanted for Interstitial Lung Disease: Results of a Multi-Center Australian Study. Transpl Int 36:11758. doi: 10.3389/ti.2023.11758
Received
30 June 2023
Accepted
14 November 2023
Published
05 December 2023
Volume
36 - 2023
Updates
Copyright
© 2023 Sim, Nilsen, Borg, Levvey, Vazirani, Ennis, Plit, Snell, Darley and Tonga.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katrina O. Tonga, katrina.tonga@sydney.edu.au
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.