Abstract
Nighttime organ transplantation aims to decrease cold ischemia duration, yet conflicting data exists on its impact on graft function and perioperative complications. This multicenter TRANSPLANT’AFUF study including 2,854 patients, transplanted between 1 January 2011, and 31 December 2022, investigated nighttime kidney transplantation’s impact (8:00 p.m.–8:00 a.m.) versus daytime (8:00 a.m.–8:00 p.m.) on surgical complications and graft survival. Overall, 2043 patients (71.6%) underwent daytime graft, while 811 (28.4%) underwent nighttime graft. No impact was observed of timing of graft surgery on graft survival with a median survival of 98 months and 132 months for daytime and nightime grafting, respectively (p = 0.1749). Moreover, no impact was observed on early surgical complications (Clavien I-II = 20.95% for DG and 20.10% for NG; Clavien III-IV-V = 15.42% for DG and 12.94% for NG; p = 0.0889) and late complications (>30 days) (Clavien I-II = 6.80% for DG and 5.67% for NG; Clavien III-IV-V = 12.78% for DG and 12.82% for NG; p = 0.2444). Noteworthy, we found a significant increase in Maastricht 3 donors’ rates in nighttime transplantation (5.53% DG vs. 21.45% NG; p < 0.0001). In conclusion, nighttime kidney transplantation did not impact early/late surgical complications nor graft survival.
Introduction
Renal transplantation is an unplanned activity, heavily dependent on organ retrieval and the need to minimize cold ischemia time. Consequently, it is often performed outside of regular working hours. However, the safety of surgery during non-standard hours has raised concerns over the past several years, as highlighted in various studies [1], demonstrating increased morbidity and mortality among patients operated on at night [2–6], particularly attributed to practitioner fatigue [7].
Concerning renal transplantation, fewer than ten studies have been conducted in the last two decades to evaluate the impact of nighttime interventions on short- and long-term graft functionality, as well as early and late complications [8–14]. These studies were often outdated and do not account for recent transplantation data, including the use of perfusion machines, the rise of extended criteria kidneys, and Maastricht 3 donors.
The objective of this study was to evaluate the impact of nighttime renal transplantation on graft survival and early and late surgical complications.
Materials and Methods
Study Design
As part of the TRANSPLANT’AFUF group led by the AFUF (Association Française des Urologues en Formation), a multicenter French retrospective database involving 13 centers was established. Inclusions were carried out successively from 2022 until 2011 for some centers. In all these healthcare facilities, kidney transplant surgeries are exclusively performed by urologists. Nevertheless, there is a lack of consensus regarding the operational protocols for managing renal transplantation.
Patients were divided into two groups based on the timing of the graft incision: daytime grafts (8:00 a.m.–8:00 p.m.) (DG) and nighttime grafts (8:00 p.m.–8:00 a.m.) (NG). 8:00 a.m. was chosen as the cut-off time because it corresponds to the change of anesthetists and OR nurses. All time points (including the moment of skin incision, the duration of the anastomosis, and the overall procedure duration) were electronically recorded during surgery and available on operative schedule. To perform a secondary analysis, a deep night graft (DNG) (11:00 p.m.–4:00) subgroup was also established.
Parameters and Outcome Measures
Evaluated parameters included donor, graft, and recipient characteristics, graft incision and closure times, and vascular anastomosis times (time between the beginning of the first anastomosis to the end of the last anastomosis). Cold ischemia time was calculated between organ retrieval clamp and transplantation. According to the “Agence de Biomédecine” protocol, all extended criteria and Maastricht 3 kidneys must be put on hypothermic Perfusion Machine. All Maastricht 3 protocols in France are made using a normothermic extracorporeal circulation between the cardiac arrest and the retrievals of the organs [15].
The primary outcome measure was graft survival, the graft was considered non-functional in case of patient death, return to dialysis, or removal of the graft. Secondary outcome measures included graft function assessed by creatinine levels, delayed graft function (DGF, defined by the necessity of dialysis during the seven first day after transplantation), early complications (within the first postoperative month), and late complications assessed using the Clavien-Dindo classification. All these data were available on patient files.
We have distinguished between junior and senior surgeons for the analysis of complications. In our French centers, a senior surgeon has completed his or her post-internship formation.
Statistical Analyses
Statistical analyses were performed using SPSS software. Univariate comparison was made using Chi-squared test and Fisher’s exact test for categorical variables. Continuous variables were compared using Student’s t-test or Mann–Whitney U-test (when assumptions of Gaussian distribution were not met). Graft survival was estimated using Kaplan-Meier method. A significance level of p < 0.05 was considered for all statistical data.
Ethics
The study was a retrospective analysis and involved already available data on human participants and followed the 1964 Declaration of Helsinki and its later amendments. Data collection followed the French legislation concerning prospective non-interventional studies to evaluate routine care (Article Art.L1121-1-2 of French Public Health Code).
Results
Population Description
Out of the 2,972 patients in the TRANSPLANT’AFUF database, 118 patients were excluded due to missing graft timing data, resulting in the inclusion of 2,854 patients between 01 January 2011 and 31 December 2022. Of these, 2,043 were daytime grafts (DG—8:00 a.m.–8:00 p.m.) and 811 were nighttime grafts (NG—8:00 p.m.–8:00 a.m.). 218 grafts were realized in deep night (DNG—11:00 p.m.–4:00 a.m.) (Figure 1).
FIGURE 1
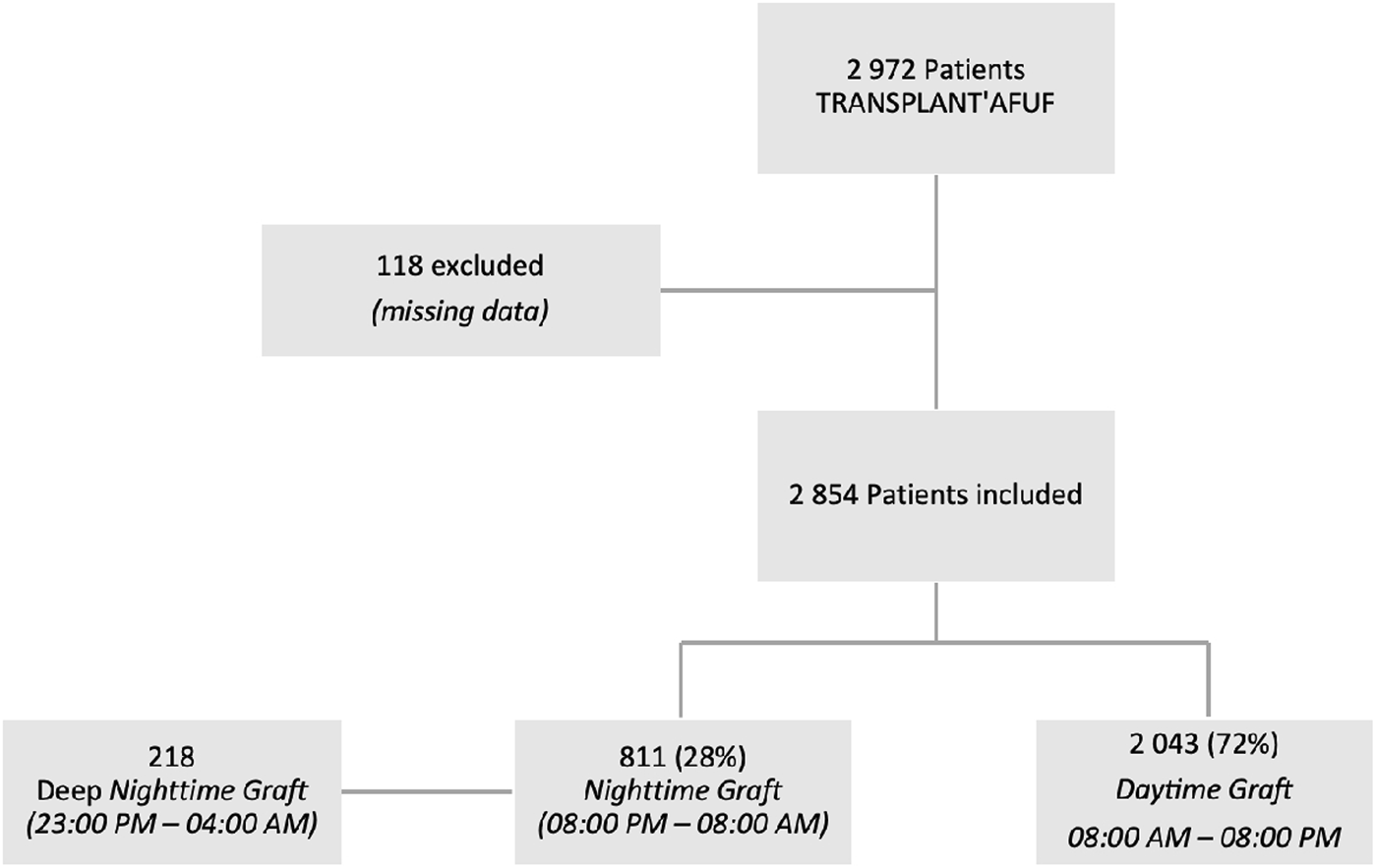
Flow chart.
Table 1 displays the characteristics of donors, showing significant differences in donor type with Maastricht 3 kidneys being more frequently transplanted at night (21.45% NG vs. 5.53% DG, p < 0.0001). Additionally, 20.80% of DG were from living donors. No differences were observed in preoperative creatinine, expanded criteria kidneys, or donor age. Regarding transplanted kidney characteristics (Table 2), NG kidneys were more often placed on machines (54.38% NG vs. 37.98% DG, p < 0.0001) and exhibited more arterial calcifications (27.37% NG vs. 22.47% DG, p < 0.017). However, no differences were found in the number of arteries or veins.
TABLE 1
| Daytime graft | Nighttime graft | p-value | |
|---|---|---|---|
| Sex (Male/Female) | 1,065/978 | 503/308 | <0.0001 a |
| Age (years)b | 55 [45–56–67] | 54 [47–56–65] | 0.4862 |
| Donor Type | <0.0001 a | ||
| Deceased after Brain Death (DBD) | 1,492 (73.03%) | 619 (76.33%) | |
| Maastricht Category 3 (M3) | 113 (5.53%) | 174 (21.45%) | |
| Maastricht Category 2 (M2) | 9 (0.44%) | 10 (1.23%) | |
| Living-Related Donor (LRD) | 425 (20.80%) | 0 (0%) | |
| Expanded Criteria Donors | 809 (39.60%) | 330 (40.69%) | 0.8548 |
| Preoperative Creatinine (µmol/L) | 75 (54–86) | 74 (50–86) | 0.4823 |
Donor characteristics.
Statistically significant.
Mean [first interquartile—median—third interquartile].
Italic values means number of patients.
TABLE 2
| Daytime graft | Nighttime graft | p-value | |
|---|---|---|---|
| Perfusion Machine | 776 (37.98%) | 441 (54.38%) | <0.0001 a |
| Multiple Arteries (>1) | 481 (23.54%) | 211 (26.88%) | 0.1164 |
| Arterial Calcification | 459 (22.47%) | 211 (27.37%) | 0.0217 a |
| Multiple Veins (>1) | 1915 (93.73%) | 773 (95.31%) | 0.4993 |
Renal graft characteristics.
Statistically significant.
Regarding recipients, only preemptive transplantation was statistically significant (14.05% for DG vs. 6.29% for NG, p < 0.0001). There were no differences in terms of gender, age, BMI, cause of renal insufficiency, type of dialysis, residual diuresis, or the number of transplants. There was no difference between the mean follow up between DG and NG (30 months for DG; 28 months for NG; p = 0.2064) (Table 3).
TABLE 3
| Daytime graft | Nighttime graft | p-value | |
|---|---|---|---|
| Male | 1,289 (63.09%) | 525 (64.73%) | 0.4112 |
| Age (years)b | 53 [42–54–65] | 54 [44–55–65] | 0.3618 |
| BMI | 25.48 (22.08–28.41) | 25.74 (22.65–28.69) | 0.1879 |
| Cause of ESRD | 0.3296 | ||
| Glomerular | 737 (36.07%) | 309 (38.10%) | |
| Vascular | 227 (11.11%) | 96 (11.84%) | |
| CTIN | 159 (7.78%) | 56 (6.91%) | |
| Polycystic | 365 (17.87%) | 120 (14.80%) | |
| Uropathy | 166 (8.13%) | 61 (7.52%) | |
| Undetermined | 214 (10.47%) | 90 (11.10%) | |
| Other | 139 (6.80%) | 67 (8.26%) | |
| Preemptive Graft | 287 (14.05%) | 51 (6.29%) | <0.0001 a |
| Dialysis Type | (N = 1,643) | (N = 731) | 0.1608 |
| Peritoneal | 199 (12.11%) | 74 (10.12%) | |
| Hemodialysis | 1,444 (87.89%) | 657 (89.88%) | |
| Residual Diuresis >50 mL/day | 1,217 (59.57%) | 488 (60.17%) | 0.7314 |
| ≥3rd Transplant | 31 (1.52%) | 11 (1.36%) | 0.6116 |
| Mean follow up (months)b | 30 [13–25–40] | 28 [13–25–39] | 0.2064 |
Recipient characteristics.
Statistically significant. BMI, body mass index; ESRD, end stage renal disease; CTIN, chronic tubulo-interstitial nephropathy.
Mean [first interquartile–median–third interquartile].
Italic values means number of patients.
Regarding surgery, there were more Lich-Gregoir (LG) ureteric anastomoses performed at night and more pyelo-ureteral (PU) anastomoses during the day (LG 78.56% for DG and 92.11% for NG; PU 14.93% for DG and 3.70% for NG; p < 0.0001). The duration of vascular anastomosis was significantly shorter during the day (50.5 min for DG vs. 55 min for NG, p < 0.005). The duration of cold ischemia was not statistically different between the two groups [777 min for DG (493–1,052) vs. 810 min for NG (570–1,005); p = 0.0541] (Table 4).
TABLE 4
| Daytime graft | Nighttime graft | p-value | |
|---|---|---|---|
| Implantation Site Calcification | 317 (15.52%) | 112 (13.81%) | 0.2783 |
| Operation Duration (min) | 180 (145–210) | 178 (146–204) | 0.1911 |
| Bleeding (mL) | 205 (50–300) | 221 (50–300) | 0.1391 |
| Intraoperative Transfusion | 98 (4.80%) | 31 (3.82%) | 0.3163 |
| Senior Surgeon | 829 (40.58%) | 361 (44.51%) | 0.0503 |
| Urinary Anastomosis | <0.0001 a | ||
| Lich Gregoir | 1,605 (78.56%) | 747 (92.11%) | |
| Pyeloureteral | 305 (14.93%) | 30 (3.70%) | |
| Leadbetter | 12 (0.59%) | 30 (0.37%) | |
| Other | 1 (0.05%) | 0 (0%) | |
| Vascular Anastomosis Duration (min) | 50.5 (35–61) | 55 (39–67) | 0.0097 a |
| Cold Ischemia Duration (min) | 777 (493–1,052) | 810 (570–1,005) | 0.0541 |
Perioperative data.
Statistically significant.
Italic values means number of patients.
In a sub-group analysis, we found that, compared with DG, seniors realized significantly more DNG (40.72% DG vs. 49.08% DNG; p = 0.0172).
Primary Outcome Measure
Graft survival was observed, considering the graft non-functional in case of patient death, return to dialysis, or removal of the graft. There was no significant difference in graft survival between DG and NG. The time of 75% survival was 67.5 months for DG and 63.5 for NG [p = 0.1749; HR (day/night): 0.8695; 95% CI (0.7049; 1.073)] (Figure 2).
FIGURE 2
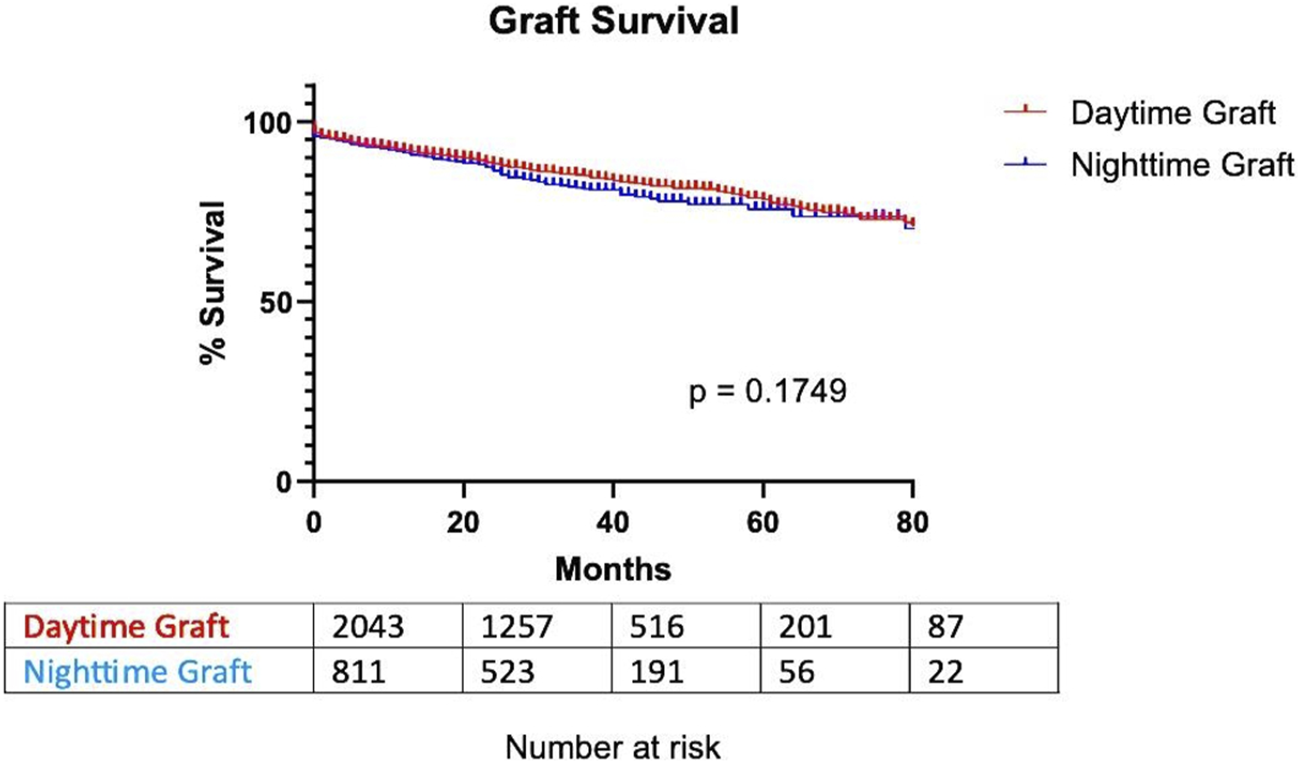
Graft survival based on transplantation timing.
Secondary Outcome Measures
Creatinine levels at 1-year post-transplant did not significantly differ between the two groups [DG: 137 μmol/L (101–157); NG: 140 μmol/L (103–158); p = 0.3019].
Regarding DGF, there was no statistically significant difference observed between the daytime and nighttime groups [410 DGF for DG (20.21%); 173 DGF for NG (21.54%); p = 0.4276] (Table 5). The occurrence of DGF was found to be correlated with prolonged anastomosis timing, irrespective of whether it occurred during the daytime (no DGF 52 min vs. DGF 64 min; p < 0.0001) or nighttime (no DGF 55 min vs. DGF 63 min; p = 0.0024).
TABLE 5
| Daytime graft | Nighttime graft | p-value | |
|---|---|---|---|
| Delayed Graft Function | 410 (20.21%) | 173 (21.54%) | 0.4276 |
| Complication < 30 Days | 0.0889 | ||
| Clavien I-II | 428 (20.95%) | 163 (20.10%) | |
| Immediate postoperative transfusion | 344 (16.84%) | 134 (16.52%) | 0.8388 |
| Clavien III-IV-V | 315 (15.42%) | 105 (12.94%) | |
| Transplantectomy | 75 (3.67%) | 26 (3.21%) | |
| Thrombosis | 80 (3.92%) | 35 (4.32%) | |
| Surgical reintervention | 241 (11.80%) | 87 (10.73%) | |
| Urinoma | 68 (3.33%) | 23 (2.84%) | |
| Complication > 30 Days | 0.2444 | ||
| Clavien I-II | 139 (6.80%) | 46 (5.67%) | |
| Clavien III-IV-V | 261 (12.78%) | 104 (12.82%) | |
| Ureteric stenosis | 69 (3.38%) | 35 (4.32%) | 0.2276 |
Postoperative data.
No significant difference was observed in surgical complications based on graft timing, either for early complications <30 days (Clavien I-II DG 20.95% vs. NG 20.10%; Clavien III-IV-V DG 15.42% vs. NG 12.94%; p = 0.0889) or late >30 days (Clavien I-II DG 6.80% vs. NG 5.67%; Clavien III-IV-V DG 12.78% vs. NG 12.82%; p = 0.2444). Looking in detail at early and late Clavien III complications (IIIa vs. IIIb), we also found no difference between DG and NG (<30 days: DG Clavien IIIa 1.57%—Clavien IIIb 9.83% vs. NG Clavien IIIa 0.97%—Clavien IIIb 8.75%; p = 0.4072. >30 day: DG Clavien IIIa 1.81%—Clavien IIIb 9.15% vs. NG Clavien IIIa 0.97%—Clavien IIIb 9.73%; p = 0.1217) (Table 5).
There was also no difference in surgical complications: immediate postoperative transfusion, graft removal, thrombosis, surgical reintervention, urinoma or ureteric stenosis (Table 5). In subgroup analysis, no significant difference was observed in surgical complications Clavien III-V for early complication <30 days (16.06% DNG vs. 12.78% DG; p = 0.1724).
Figures 3, 4 show the association between surgical complications with cold ischemia time and transplantation timing, which revealed no difference between the two groups.
FIGURE 3
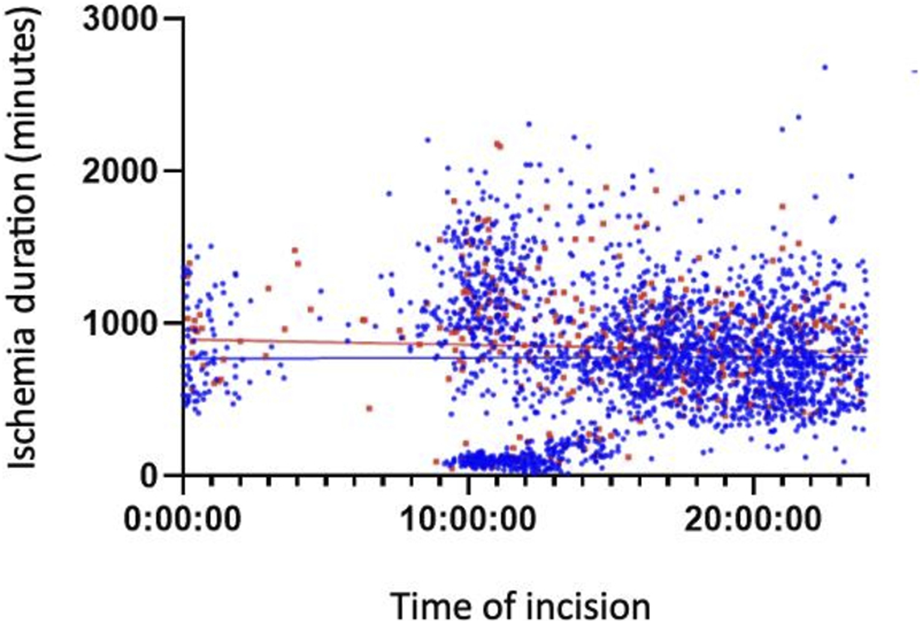
Early Complications based on cold ischemia time and transplantation timing.
FIGURE 4
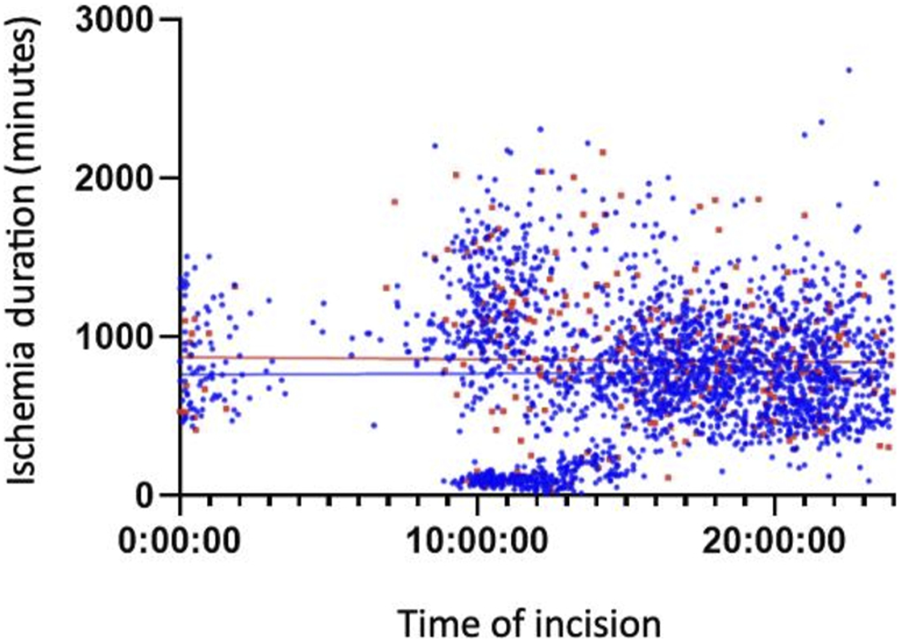
Late Complications based on Cold ischemia time and transplantation timing.
Discussion
This French multicenter study is one of the most comprehensive analyses to date regarding the impact of transplantation timing on renal graft survival and early and late surgical complications. Organ transplantation outcomes are inconsistent, with liver grafts showing negative effects of nocturnal interventions [16], while cardiac and lung grafts remain unaffected [17]. Cold ischemia time significantly influences long-term renal graft survival [18–21]. However, surgical complications necessitating subsequent interventions have been linked to reduced graft survival [8, 22, 23], particularly vascular complications [24] and increased operative time [25]. Despite multiple retrospective analyses, these studies are often limited by small cohorts, leading to non-significant results [11–14]. Only three retrospective studies have yielded significant outcomes concerning complication rates and graft survival for nighttime kidney transplants.
Brunschot et al. [9] analyzed 4,519 kidney transplants performed between 2000 and 2013 in the Netherlands, of which 1,480 occurred at night. The results showed significantly lower technical failure rates for nighttime grafts (1%) compared to daytime grafts (2.6%). Another single-center study by Shaw TM et al. [10], involving 633 kidney transplants from 2000 to 2008, revealed increased urinary complications for grafts between 3:00 a.m. and 6:00 a.m., without a significant difference in 1-year graft survival. Fechner et al. [8] conducted a third study in 2008, comparing 260 daytime and nighttime kidney transplants between 1994 and 2004, demonstrating an elevated risk of delayed graft function recovery and vascular complications for nighttime grafts, without differences in cold ischemia time. In our study, the observed rate of surgical complications aligns with literature findings [26], showing no significant increase in surgical complications based on graft timing, despite a significant extension in vascular anastomosis time during the night.
Kidney transplants from Maastricht 3 donors were more frequently performed at night. This trend is largely attributed to the need to minimize cold ischemia time for expanded criteria grafts. However, no national protocol exists for the optimal timing of Limitation of Active Therapies (LAT) in France. The reduction of nighttime transplants could be considered by scheduling LAT for these donors earlier in the morning.
Additionally, the significant use of perfusion machines for nighttime grafts might be explained by the proportion of living-related donors (LRDs) during the day, who do not require perfusion machines, as well as the increased proportion of Maastricht 3 donors. The utilization of these machines could contribute to improved graft function [27, 28]. Similarly, the higher proportion of Maastricht 3 donors at night probably explains the gender difference, since these donors are mainly men [29].
Finally, Figures 2, 3 illustrate a cluster of morning grafts characterized by a cold ischemia window of 16–30 h, which could correspond to grafts rescheduled for the following morning to avoid procedures during the deep nighttime hours (12:00 p.m. to 08:00 a.m.). This observed behavior may be attributed to concerns regarding potential surgical complications and hesitancy within surgical teams. Our study indicates that, despite a somewhat slower pace, as evidenced by prolonged anastomosis time, potentially linked to fatigue, the initial concerns may not be substantiated. It is established that, beyond a 6-h threshold, each additional hour of cold ischemia time does impact graft survival [18–21]. Unfortunately, the limitations of our retrospective study design and the extended follow-up duration hinder our ability to conclusively demonstrate the enduring effects of cold ischemia. Facilitating easier and more direct access to the operating room holds promise for enhancing long-term graft survival, all the while maintaining organizational efficiency and ensuring the quality of work performed by surgical teams.
In conclusion, this study demonstrates that nighttime transplantations do not result in delayed graft function recovery, despite an extended vascular anastomosis time. Furthermore, these nocturnal interventions do not lead to an increased risk of early or late surgical complications. However, it’s important to note that most grafts are already scheduled for the early morning to avoid procedures during the deep nighttime period (12:00 p.m. to 08:00 a.m.), and this choice does not negatively impact graft functional recovery. However, our study could not conclude on the impact of this choice on very long-term graft survival. To reduce the number of early-night grafts, earlier LAT for Maastricht 3 donors could be considered. Additionally, the significant use of perfusion machines for nighttime grafts could contribute to the favorable graft functional recovery. We also believe that by prioritizing access to the operating room for kidney transplants, we could reduce the number of transplants delayed until the morning, and thus their cold ischemia.
Statements
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The study was a retrospective analysis and involved already available data on human participants and followed the 1964 Declaration of Helsinki and its later amendments. Data collection followed the French legislation concerning prospective non-interventional studies to evaluate routine care (Article Art.L1121-1-2 of French Public Health Code).
Author contributions
MU: redactor. PD: senior of MU for the article, head of department. TW: contributor of the database, statistics, corrections. ES: project leader of TRANSPLANT’AFUF. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
BMI, Body Mass Index; CTIN, chronic tubulo-interstitial nephropathy; DCD, Deceased after Cardiac Death; DG, Daytime Graft; DGF, Delayed Graft Function; ESRD, end stage renal disease; LAT, Limitation of Active Therapies; LG, Lich-Gregoir; LRD, Living-Related Donor; M2, Maastricht Category 2; M3, Maastricht Category 3; NG, Nighttime Graft; PU, pyelo-ureteral.
References
1.
McKee M Priest P Ginzler M Black N . Which General Surgical Operations Must Be Done at Night?Ann R Coll Surg Engl (1991) 73(5):295–302. discussion 301-302.
2.
Gray A . United Kingdom National Confidential Enquiry Into Perioperative Deaths. Minerva Anestesiol (2000) 66(5):288–92.
3.
Chacko AT Ramirez MA Ramappa AJ Richardson LC Appleton PT Rodriguez EK . Does Late Night Hip Surgery Affect Outcome?J Trauma (2011) 71(2):447–53. discussion 453. 10.1097/TA.0b013e3182231ad7
4.
Olson EJ Drage LA Auger RR . Sleep Deprivation, Physician Performance, and Patient Safety. Chest (2009) 136(5):1389–96. 10.1378/chest.08-1952
5.
Rothschild JM Keohane CA Rogers S Gardner R Lipsitz SR Salzberg CA et al Risks of Complications by Attending Physicians After Performing Nighttime Procedures. JAMA (2009) 302(14):1565–72. 10.1001/jama.2009.1423
6.
Kelz RR Freeman KM Hosokawa PW Asch DA Spitz FR Moskowitz M et al Time of Day Is Associated With Postoperative Morbidity: An Analysis of the National Surgical Quality Improvement Program Data. Ann Surg (2008) 247(3):544–52. 10.1097/SLA.0b013e31815d7434
7.
Lockley SW Barger LK Ayas NT Rothschild JM Czeisler CA Landrigan CP et al Effects of Health Care Provider Work Hours and Sleep Deprivation on Safety and Performance. Jt Comm J Qual Patient Saf (2007) 33(11):7–18. 10.1016/s1553-7250(07)33109-7
8.
Fechner G Pezold C Hauser S Gerhardt T Müller SC . Kidney’s Nightshift, Kidney’s Nightmare? Comparison of Daylight and Nighttime Kidney Transplantation: Impact on Complications and Graft Survival. Transpl Proc (2008) 40(5):1341–4. 10.1016/j.transproceed.2008.02.072
9.
Brunschot DMDÖ Hoitsma AJ van der Jagt MFP d’Ancona FC Donders RART van Laarhoven CJHM et al Nighttime Kidney Transplantation Is Associated With Less Pure Technical Graft Failure. World J Urol (2016) 34(7):955–61. 10.1007/s00345-015-1679-0
10.
Shaw TM Lonze BE Feyssa EL Segev DL May N Parsikia A et al Operative Start Times and Complications After Kidney Transplantation. Clin Transpl (2012) 26(3):E177–83. 10.1111/j.1399-0012.2012.1622.x
11.
Sugünes N Bichmann A Biernath N Peters R Budde K Liefeldt L et al Analysis of the Effects of Day-Time vs. Night-Time Surgery on Renal Transplant Patient Outcomes. J Clin Med (2019) 8(7):E1051. 10.3390/jcm8071051
12.
Kienzl-Wagner K Schneiderbauer S Bösmüller C Schneeberger S Pratschke J Ollinger R . Nighttime Procedures Are Not Associated With Adverse Outcomes in Kidney Transplantation. Transpl Int (2013) 26(9):879–85. 10.1111/tri.12125
13.
Seow YY Alkari B Dyer P Riad H . Cold Ischemia Time, Surgeon, Time of Day, and Surgical Complications. Transplantation (2004) 77(9):1386–9. 10.1097/01.tp.0000122230.46091.e2
14.
Fockens MM Alberts VP Bemelman FJ Idu MM . Renal Transplantation at Night. Ned Tijdschr Geneeskd (2014) 158:A7779.
15.
Corinne A Denis S Gérard A Lionel B Benoit B Laurent B et al Conditions à Respecter Pour Réaliser des Prélèvements D’organes sur des Donneurs Décédés Près Arrêt Cardio-Circulatoire de la Catégorie III de Maastricht Dans un Etablissement de Santé (2021). Available From: https://www.agence-biomedecine.fr/IMG/pdf/v9_guide_ddac_miii_-_juillet_2021.pdf (Accessed, 2021).
16.
Lonze BE Parsikia A Feyssa EL Khanmoradi K Araya VR Zaki RF et al Operative Start Times and Complications After Liver Transplantation. Am J Transpl (2010) 10(8):1842–9. 10.1111/j.1600-6143.2010.03177.x
17.
George TJ Arnaoutakis GJ Merlo CA Kemp CD Baumgartner WA Conte JV et al Association of Operative Time of Day With Outcomes After Thoracic Organ Transplant. JAMA (2011) 305(21):2193–9. 10.1001/jama.2011.726
18.
Debout A Foucher Y Trébern-Launay K Legendre C Kreis H Mourad G et al Each Additional Hour of Cold Ischemia Time Significantly Increases the Risk of Graft Failure and Mortality Following Renal Transplantation. Kidney Int (2015) 87(2):343–9. 10.1038/ki.2014.304
19.
van der Vliet JA Warlé MC . The Need to Reduce Cold Ischemia Time in Kidney Transplantation. Curr Opin Organ Transpl (2013) 18(2):174–8. 10.1097/MOT.0b013e32835e2a08
20.
van der Vliet JA Warlé MC Cheung CLS Teerenstra S Hoitsma AJ . Influence of Prolonged Cold Ischemia in Renal Transplantation. Clin Transpl (2011) 25(6):E612–6. 10.1111/j.1399-0012.2011.01510.x
21.
Salahudeen AK Haider N May W . Cold Ischemia and the Reduced Long-Term Survival of Cadaveric Renal Allografts. Kidney Int (2004) 65(2):713–8. 10.1111/j.1523-1755.2004.00416.x
22.
Agüera Fernandez LG Robles JE Rosell D Rodríguez-Rubio FI Abad JI Zudaire JJ et al Multivariate Analysis of the Impact of Surgical Complications in Renal Transplant. Arch Esp Urol (1994) 47(10):999–1006.
23.
Król R Cierpka L Ziaja J Pawlicki J Budziński G . Surgically Treated Early Complications After Kidney Transplantation. Transpl Proc (2003) 35(6):2241–2. 10.1016/s0041-1345(03)00769-3
24.
Osman Y Shokeir A Ali-el-Dein B Tantawy M Wafa EW el-Dein ABS et al Vascular Complications After Live Donor Renal Transplantation: Study of Risk Factors and Effects on Graft and Patient Survival. J Urol (2003) 169(3):859–62. 10.1097/01.ju.0000050225.74647.5a
25.
Sandid MS Assi MA Hall S . Intraoperative Hypotension and Prolonged Operative Time as Risk Factors for Slow Graft Function in Kidney Transplant Recipients. Clin Transpl (2006) 20(6):762–8. 10.1111/j.1399-0012.2006.00567.x
26.
Timsit MO Kleinclauss F Richard V Thuret R . Surgical Complications of Renal Transplantation. Prog Urol (2016) 26(15):1066–82. 10.1016/j.purol.2016.09.052
27.
Opelz G Döhler B . Multicenter Analysis of Kidney Preservation. Transplantation (2007) 83(3):247–53. 10.1097/01.tp.0000251781.36117.27
28.
Jochmans I Moers C Smits JM Leuvenink HGD Treckmann J Paul A et al Machine Perfusion Versus Cold Storage for the Preservation of Kidneys Donated After Cardiac Death: A Multicenter, Randomized, Controlled Trial. Ann Surg (2010) 252(5):756–64. 10.1097/SLA.0b013e3181ffc256
29.
Agence de la biomédecine. Organs - Kidney Transplant (2022). Available From: https://rams.agence-biomedecine.fr/greffe-renale-0 (Accessed January 6, 2024).
Summary
Keywords
renal transplantation, graft survival, nighttime, surgery, surgical complication
Citation
Uhl M, Waeckel T, Seizilles De Mazancourt E, Taha F, Kaulanjan K, Goujon A, Beretta A, Papet J, Dupuis H, Panis A, Peyrottes A, Lemaire A, Larose C, Bettler L, Pues M, Joncour C, Stempfer G, Ghestem T and De Sousa P (2024) Impact of Transplantation Timing on Renal Graft Survival Outcomes and Perioperative Complications. Transpl Int 37:12202. doi: 10.3389/ti.2024.12202
Received
07 October 2023
Accepted
29 January 2024
Published
14 February 2024
Volume
37 - 2024
Updates
Copyright
© 2024 Uhl, Waeckel, Seizilles De Mazancourt, Taha, Kaulanjan, Goujon, Beretta, Papet, Dupuis, Panis, Peyrottes, Lemaire, Larose, Bettler, Pues, Joncour, Stempfer, Ghestem and De Sousa.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: M. Uhl, marine.uhl@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.