Abstract
Solid organ transplant (SOT) recipients are particularly susceptible to infections caused by multidrug-resistant organisms (MDRO) and are often the first to be affected by an emerging resistant pathogen. Unfortunately, their prevalence and impact on morbidity and mortality according to the type of graft is not systematically reported from high-as well as from low and middle-income countries (HIC and LMIC). Thus, epidemiology on MDRO in SOT recipients could be subjected to reporting bias. In addition, screening practices and diagnostic resources may vary between countries, as well as the availability of new drugs. In this review, we aimed to depict the burden of main Gram-negative MDRO in SOT patients across HIC and LMIC and to provide an overview of current diagnostic and therapeutic resources.
Introduction
Solid organ transplant (SOT) recipients are at high risk for acquiring colonization and/or infection with multi-drug resistant organisms (MDRO) with associated high morbidity and mortality rates [1–3].
In the last 10 years, Enterobacterales, P. aeruginosa, and Acinetobacter baumannii have emerged as critical threats due to a progressive widespread pattern of resistance, impacting patient survival, mainly among vulnerable populations [4]. The present review will focus on these pathogens.
The objective is to provide an overview of the epidemiology of these MDROs in SOT recipients in different regions of the world. Diagnostic and treatment strategies will be also reviewed considering differences in the access to new diagnostic tools and new antibiotics across high- and low-medium-income countries.
Methods
We conducted a narrative review by a computer-based PubMed search using as keywords “Solid Organ Transplantation,” “multidrug resistance,” “extended-spectrum β-lactamase producing” or “extended-spectrum cephalosporin resistance,” “carbapenem resistance” or “carbapenemase-producing,” “difficult to treat resistant P. aeruginosa,” “carbapenem-resistant A. baumannii” to identify published all-language literature between June 2013 and June 2023. A pre-established chart was used to extract epidemiological data. MDRO was defined according to Magiorakos criteria and new DTR concept [5,6]. HIC and LMIC were defined according to world bank classification [7]. To estimate MDRO prevalence in SOT recipients across countries, we included studies reporting the number of infections by each specific MDRO, as well as the number of transplanted patients during the same period. Studies that only reported colonization or laboratory-based descriptions without clinical data were excluded.
Results
Epidemiology of MDRO Infections After SOT
Compared to high-income countries (HICs), data on MDRO infections among SOT patients is relatively scarce in low and middle-income countries (LMICs). In these regions, the number of transplants per million people is lower when compared to Western Europe and the US. However, in absolute terms, 39% of all transplants are performed in these countries (see Figure 1) [8]. Significant discrepancies in donor referral and transplantation exist between HICs and LMICs. In the latter, the proportion of living-donor transplants is higher, especially in Asia [9]. Moreover, the rates of MDRO infections among SOT recipients are highly influenced by the local epidemiology. For instance, Brazil, Turkey, India, China, and Argentina are described as countries with the highest prevalence of CRAB infection [10] Moreover, India and China have a high prevalence of ESCR-E and CRE, mainly NDM-producing [10,11]. Thus, it is expected that LMIC bear a high burden of these diseases, which are likely underreported due to deficiencies in diagnosis, lack of microbiology laboratory infrastructure, and limited resources to make post-transplant infection rates public. Finally, there is a lack of representativity from countries in the Middle East and Africa. Taking into account these considerations, an overview of the worldwide prevalence of infection by most common MDROs per 1,000 transplant-recipients is shown in Figure 2.
FIGURE 1
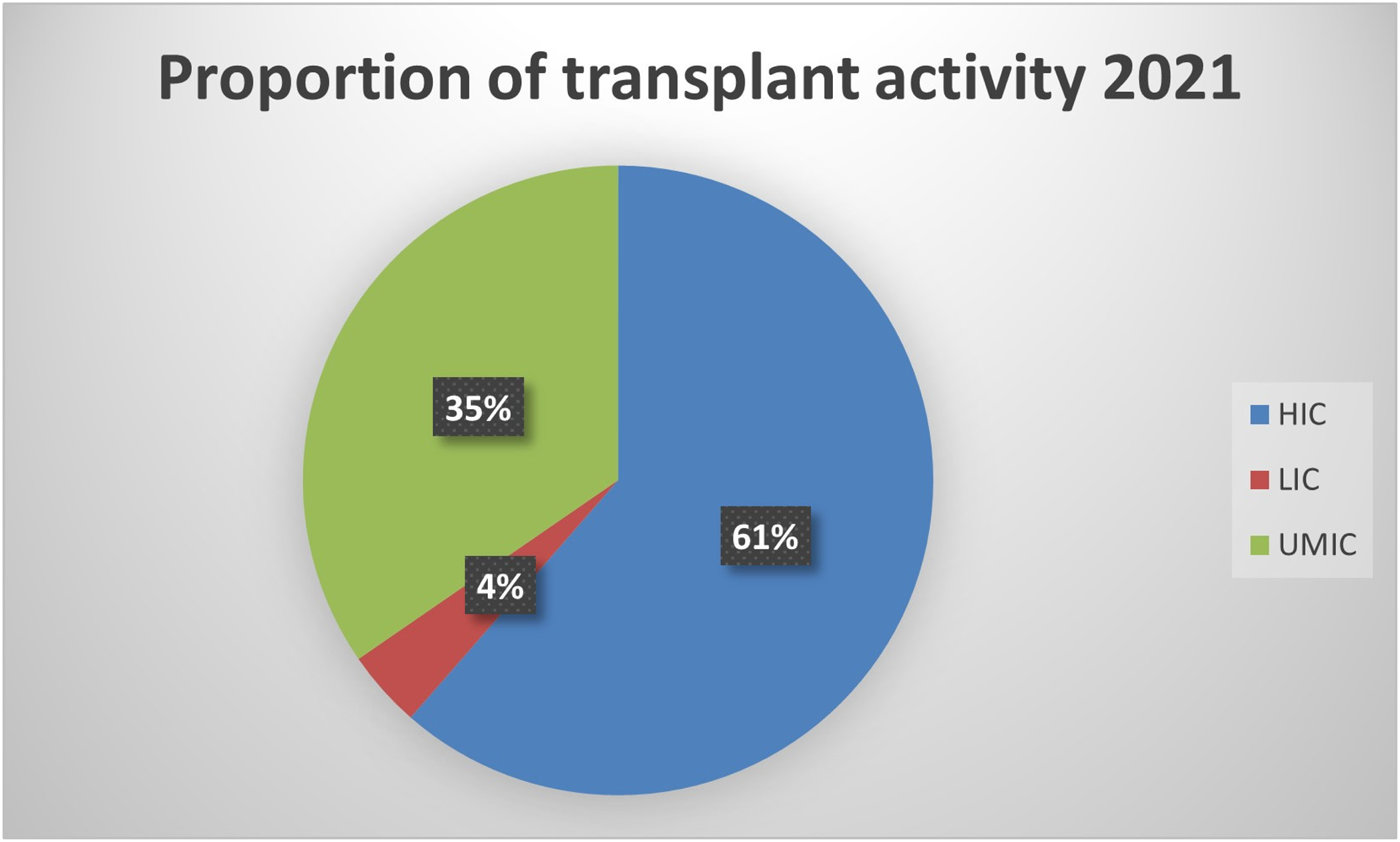
Proportion of transplant activity in high-, lower- and upper middle-income countries.
FIGURE 2
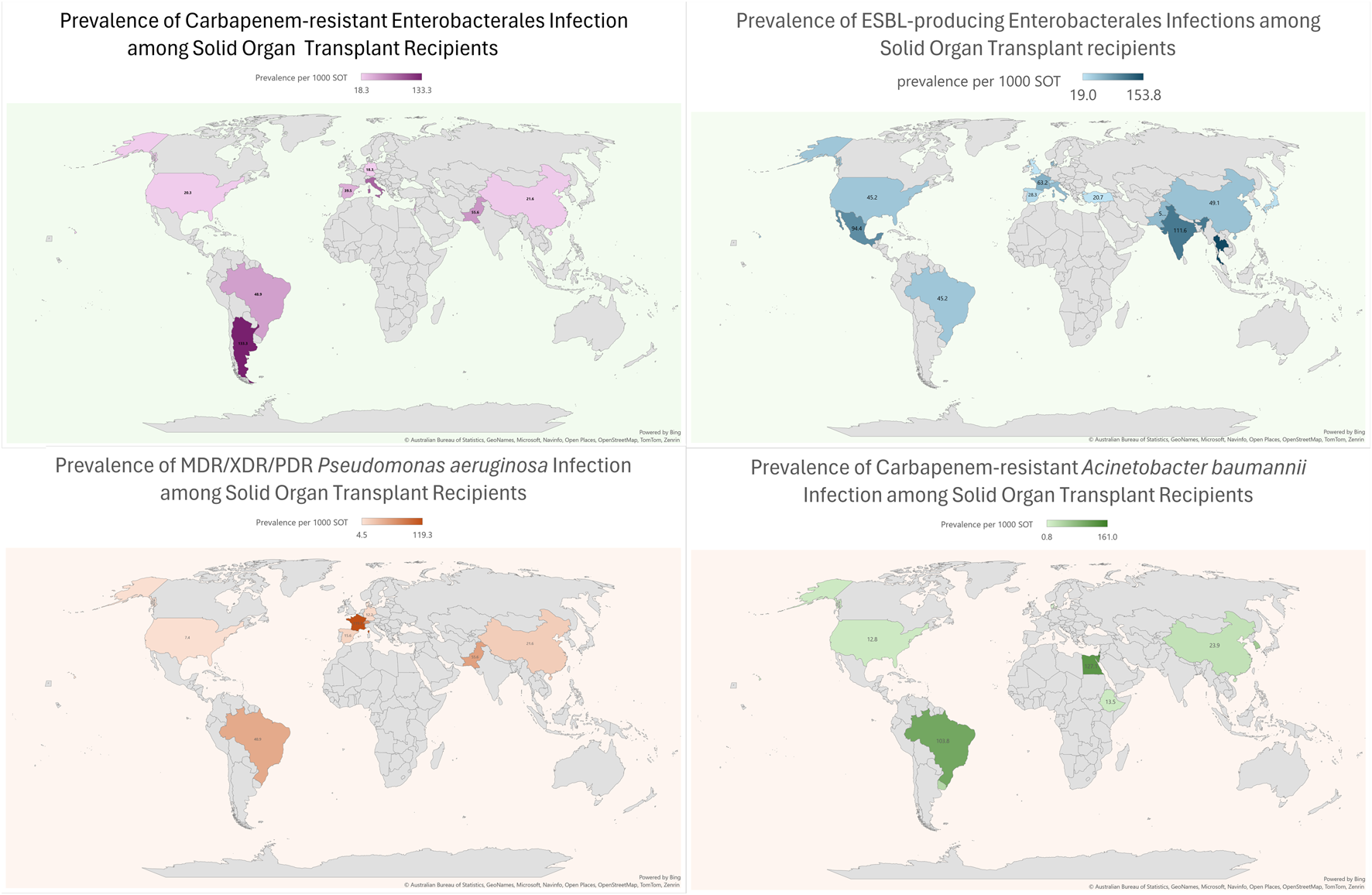
Prevalence of Multidrug resistant Gram-negative bacterial infections in SOT recipients across the world.
ESBL-Producing Enterobacterales
ESBL-E infection is the most commonly reported MDR Gram-negative infection, with a prevalence ranging from 3% to 11% and an aggregate rate of 7% among all bacterial infections in all types of SOT; however, in KT recipients the prevalence of ESBL-E, mainly in urinary tract infections (UTIs) may be >30% in high endemic centers [12–33] (Table 1). Data from the Swiss Transplant Cohort showed that, ESBL production was observed in 11.4% Enterobacterales isolated from 1072 SOT recipients [70]. Enterobacterales infections occurred at a median of 69 days after transplant, interestingly patients were predominantly outpatients. Higher prevalence of ESBL-E has been reported in studies analyzing SOT recipients with BSI and UTI [36,37,71]. In a study assessing the epidemiology of UTI in a cohort of 4388 SOT recipients in Spain, the prevalence of ESBL in E. coli was 26% [38].
TABLE 1
| Low and medium-income countries | High-income countries | |||||||
|---|---|---|---|---|---|---|---|---|
| Type of resistance | All infections | BSI | UTIa | LRTI | All infections | BSI | UTI | LRTI |
| ESBL | 7.0% (4.4%–11.2%) | 3.4% (0.9%–11.7%) | 14.4% (5.6%–21.6%) | NA | 5.5% (2.2%–13.6%) | 12.8% (7%–40%) | 5% (1%–6%) | NA |
| CRE | 4.0% (0.9%–15.7%) | 2.0% (0.9%–7.8%) | 2.8% (0.8%–7.7%) | 1.3% (1%–2.1%) | 6% (1.9%–10.3%) | 8% | NA | NA |
| DTR-Pa | 1.4% (0.8%–3.9%) | 3.1% (1.5%–8.0%) | 1.1% (0.8%–1.5%) | 3.2% | 7% | 10% | NA | 9% (3%–15%) |
| CR-Ab | 4.1% (0.8%–28.6%) | 1.4% (1.1%–28.6%) | NA | 5.8% (3.8%–9.7%) | 1%–6% | NA | NA | NA |
Prevalence of MDRO infections in SOT recipients reported in studies from low- and medium-income and high-income countries.
Rates of UTI, are mainly obtained from studies including kidney transplant recipients.
Two large studies have investigated molecular characteristics of MDR-E isolated in SOT recipients, from Spain and US each. In the Spanish study, 541 MDR-E isolates were collected. The main microorganisms were E. coli (46.2%), K. pneumoniae (35.3%), E. cloacae (6.5%) and C. freundii (6.3%). Overall, 78.0% of strains harbored ESBL genes, CTX-M-group-1 being the most prevalent (53.3%) followed by CTX-M-group-9 in 15.4%. Among ESBL-producers, 2.1% of E. coli, 47.3% of K. pneumoniae and 11.1% of E. cloacae harbored a carbapenemase gene. Hyperproduction of chromosomal-AmpC was detected in E. cloacae (57.7%), C. freundii (82.6%) and other MDR-E species (39.1%) [72]. In the US study on 88 transplant recipients, 20% of patients were colonized with MDR-E (ESCR-E only n = 23; CRE only n = 12; both n = 5), 52% of ESCR-E carried blaCTX-M. Post-transplant MDR-E infection rate was 10%, the attack rate was higher following CRE than ESCR-E colonization (53% vs. 21%, p = .05) [39].
Main risk factors for ESBL-E infections after SOT are reported in Table 2 [73]. In this regard, the role of targeted perioperative antibiotic prophylaxis (T-PAP) is still an open issue [74]. Two studies, from France and Thailand each, showed a reduction of ESBL-E infections in patients receiving T-PAP after OLT and KT, respectively [13,40]. However, both of them showed several limitations including observational design, heterogeneity in drugs used in the OLT study, and consideration of asymptomatic bacteriuria as an endpoint in the KT cohort. Furthermore, it should be remarked that carbapenem exposure is the main driver for carbapenem resistant infections.
TABLE 2
| Type of resistance | Risk factors for infection | Risk factors for mortality |
|---|---|---|
| ESBL-E [13,34,40,70,71,73] |
General Characteristics
- Female gender - Kidney Transplant - MELD score >25 Colonization status - ESBL-Enterobacterales carriage in the prior 1 year Pre-SOT antibiotic exposure/prophylaxis - Pre-operative prophylaxis for spontaneous bacterial peritonitis - Carbapenems prophylaxis - Exposure to third-generation cephalosporin, TMP/SMX or echinocandins in the prior 6 months Post-SOT condition - Acute rejection in prior 3 months - Reoperation - Corticosteroid containing immunosuppressive regimen |
Severity of patient and/or condition
- Pitt bacteremia score - Mechanical ventilation at the time of infection diagnosis |
| CRE [42,43,48,74–83] |
General Characteristics
- Male gender - Older age - Time of hospitalization - Lung transplant - Liver transplant - Multiple infected organisms or sites - Previous infections - Dialysis - MELD score >32 - Median lymphocytes count under 700 cell/mm3 Colonization status - Pre/post-transplant CRE carriage - Multisite colonization - Colonization by more than one species of CRE Pre-SOT antibiotic exposure - Carbapenem use (OR 2.53, OR 2.80) Post-SOT condition - Combined transplant - Prolonged mechanical ventilation - Possible donor-derived infection - Delayed kidney function/Ureteral stent - CMV infection - Re-transplantation - Rejection - Mycophenolate use |
General Characteristics
- older age - CMV disease - Lymphocytes ≤600 U/mm3 - Pitt bacteremia score - Graft failure Severity of patient and/or condition - Septic shock - High SOFA score - Multiple infected organisms or sites - Genitourinary source - No source control - INCREMENT-CPE mortality score ≥8 Antibiotic exposure - Appropriate empiric therapy (protective) - Polymyxin exposure in the prior 6 months |
| DTR-Pa [54,59–61,84,85] |
General Characteristics
- Hospital stay > 10 days - Lower median lymphocyte counts - Central venous catheter - Urinary catheter - Prior transplantation - ICU admission in previous year - Septic shock Pre-SOT antibiotic exposure - Prior carbapenem use - Prior ciprofloxacin use Post-SOT condition - re-transplantation - urological surgical procedure after Kidney transplant |
Severity of patient and/or condition
- Bacteremia - creatinine >1,5 - onset of BSI while in ICU |
| CR-Ab [20,44,62,86–89] |
General Characteristics
- Liver Transplant performed because of fulminant hepatitis - high preoperative serum levels of BUN - pre-operative hypoalbuminemia Post-SOT condition - Fungal culture positivity after SOT - long duration of surgery - tracheal intubation twice - longer cold ischemia time - post-Liver transplant need for dialysis |
Severity of patient and/or condition
- Platelet count < 50,000/mm3 - Mechanical ventilation at the onset of CRAb - ICU-acquired infection Antibiotic exposure - Inappropriate empiric therapy - Colistin-carbapenem regimens |
Risk factors for MDRO infection and for mortality.
ESBL-E infections are associated with increased length of stay, mainly in case of initial inappropriate therapy [34,35]. In addition, high recurrence rates have been reported ranging from 25% for BSI to 79% for UTI, mainly in KT recipients [34,90]. Factors associated with relapse were inappropriate empirical therapy, advanced age, and persistent bacteriuria [41,70].
Carbapenem-Resistant Enterobacterales
The prevalence of CRE infection after SOT varies according to the type of organ, being higher among liver (2%–10%) and lung (5%–7%) transplant recipients [91]. These rates seem to be a little higher in HIC than in LMIC (see Table 1) [42–45,49–52]. The rate of CRE infection is on average 30% among CRE carriers [53]. Usually, CRE infection occurs in the first 4–8 weeks after transplant, earlier infections (within 2 weeks) are observed in pre-transplant carriers and/or in donor derived infections (DDI) [48,53,92]. Notably, incidence of DDI due to CRE is high in China, one study focused on KT patients reported that possible DDI increased the risk of CRE infection by more than six times [75]. Authors reported varying prevalence rates of CRE among donor or preservation fluid cultures, ranging from 1.6% to 19.2% [93–95].
CRE infection after SOT often presents as severe infection with BSI and/or lung involvement [76,91].
Carbapenemases show significant geographical variation—K. pneumoniae carbapenemases (KPC) remain the commonest in United States, the metallo-beta-lactamases (MBLs) are most common in the countries of South and Southeast Asia and OXA-48-type carbapenemases in the Middle East, Mediterranean and northern African countries [96–98]. In the two studies assessing molecular characteristics of MDR-E isolated from SOT recipients, the main mechanisms of carbapenem resistance were OXA-48 in Spain accounting for 78% of the isolates, and KPC in US detected in 72% of CRE [39,72]. These mechanisms were mostly detected in K. pneumoniae isolates. Few studies in LMICs investigated this issue. The proportion of strains with carbapenemase-producing is reported to be 46%–84% among OLT recipients and 83% among KT recipients. KPC-producing CRE appears to be the most frequent. The second most common carbapenemase is NDM, which corresponded to 28% in an OLT cohort in China and 2% in a KT cohort in Brazil. Despite, CRE post-transplant infection rates are high in India, details about the proportion of NDM and KPC are not available [99]. Other carbapenemases, such as IMP, are less frequent and often associated with outbreaks [77,78,100,101].
Risk factors for CRE infection have been usually investigated in specific organ transplant settings and most commonly in OLT recipients (see Table 2) [48,75,79,80]. Carriage, either acquired before or after transplant, and peri-surgical complications have been associated with highest risk of developing CRE infection [48,77]. For pre-transplant carriage, shorter the time of detection before SOT, higher is the risk of infection after SOT [81]. For post-transplant carriage, it is worth mentioning that this occurs 2-3 times as more frequently than pre-transplant carriage, thus in high endemic areas it could be considered to repeat the screening for rectal carriage, which is usually done before or at transplant time, also during the post-transplant period during ICU or hospital stay. Conversely, the role of T-PAP for CRE is under debate [74,82].
Rates of mortality and graft failure in patients developing CRE infection after SOT are as high as on average 40% and 20%, respectively. After adjusting for confounding variables CRE infection was found as a significant predictor of poor outcome [83].
Difficult-To-Treat Resistant Pseudomonas aeruginosa
Assessing the burden of difficult-to-treat resistant (DTR) P. aeruginosa (DTR-Pa) in SOT recipients is difficult for several reasons including: i) different drug resistance definitions used across centers and study periods; ii) analysis of respiratory isolates generally available only for LuT recipients while for other types of transplant most data come from studies on BSI; iii) cumulative data on drug resistance provided including also other pathogens; and iv) lack of large multicenter studies.
With this premise, DTR-Pa appears to be the MDRO with the lowest prevalence among SOT in LMIC, described from 0.8% to 3.9% in KT and OLT recipients (Table 1) [54–58]. In HIC, Pa generally ranked first among pathogens isolated from LuT recipients, with rates of MDR ranging from 7% to 50% [59,60,84]. In a single-center Spanish study, including 318 consecutive episodes of BSI in a cohort of non-lung SOT recipients, 44 (15%) BSI were caused by Pa with 31 (63%) strains classified as XDR [61]. The most frequent source was UTI, and the median time from transplantation to BSI was shorter for XDR episodes (66 vs. 278 days). Independent risk factors for XDR Pa BSI were prior transplantation, nosocomial acquisition and septic shock [85]. Only colistin and amikacin maintained activity against XDR strains. Compared to patients with susceptible-Pa BSI, those with XDR-Pa BSI received more frequently inappropriate empirical treatment (58% vs. 22%), and had higher 7-day (20.7% vs. 8.5%) and 30-day (38% vs. 16%) mortality rates.
Few data are available about the mechanisms underlying DTR and CR phenotypes in Pa. In a recent study including CR-Pa from 972 individuals (USA n = 527, China n = 171, south and central America n = 127, Middle East n = 91, Australia and Singapore n = 56), almost a quarter of strains were shown to produce a carbapenemase, mostly consisting of KPC-2 (49%) or VIM-2 (36%), with a prevalence varying across south and central America 69%, Australia and Singapore 57%, China 32%, Middle East 30%, US 2% [4]. In a study on 163 clinical P. aeruginosa isolates in adult cystic fibrosis and LuT in Australia, 32 (19.6%) were XDR, 82% of strains were susceptible to ceftolozane/tazobactam [102].
Mortality risk associated with DTR/XDR or CR Pa infection after SOT seems to be higher in patients with septic shock and/or multiorgan failure and ICU stay [61].
Carbapenem-Resistant Acinetobacter baumannii
The overall rate of CRAb infection among SOT recipients varies from 1% to 6% in HIC [66–69]. In a study conducted in US on 248 patients with A. baumannii infection, CRAb rates were higher among SOT compared to non-SOT patients (43% vs. 14%) [103].
CRAb prevalence after SOT in China and Brazil can reach 29% [10,16,62,63,86]. A systematic review focused on uro-pathogens among KT recipients highlighted A. baumannii as the third most frequently encountered Gram-negative bacteria, displaying a prevalence rate of 8% in the Middle-East [104] Additionally, 4%–10% of OLT recipients have pneumonia attributed to CRAb in China, Brazil, Egypt and Uruguay [64,65,87,88] A Chinese study involving 107 LuT recipients found that CRAb was the predominant MDRO infection agent, accounting for 35% of Gram-negative MDRO [63]. Thus local epidemiology is pivotal in planning screening for CRAb before and after SOT.
A. baumannii is intrinsically resistant to a wide range of antibiotic classes, caused by simultaneous mechanisms of resistance [105]. Among these, decreased outer-membrane porins, constitutional expression of efflux pumps, intrinsic harboring of β-lactamases and plasmidial carbapenemase, has been widely described. Among carbapenemases, OXA-23-like are the most common. However, CRAb isolates harboring MBL, such as blaNDM-1 genes, has been associated with increased mortality rates in a study conducted from Pakistan [106–108]. Resistance to polymyxin is infrequent and appears to be linked to outbreaks [46,62,86,109].
Data about risk factors for CRAb infections were exclusively reported for OLT recipients from LMICs [87,88]. (Table 2) CRAb infection mortality rates are the highest among SOT MDRO infections and often exceed 40% (ranging from 20% to 47%) [62,86,87,89,110].
Diagnosis of MDRO Infections After SOT
Timely diagnosis of MDRO infections in SOT recipients is critically important to patient and allograft survival. Advanced diagnostic methodologies may aid in shortening the time to narrowest appropriate antibiotic administration; however, data on their optimal use and interpretation in this specific population are limited [111]. In addition, the availability of rapid diagnostics may vary by location.
In a survey among American Society Transplant (AST) members, 19 respondents indicated frequently ordering multiplexed molecular assays (82%) and antimicrobial susceptibility to new antibiotics (76%), and >80% of respondents reported to change treatment according to the results of such tests [112]. However, data from other countries are missing.
Preliminary data on the use of a multiplex PCR panel in 29 transplant recipients with 45 bloodstream infections remarked the possibility of off-target pathogens [113]. Indeed, a consensus conference to define the utility of these new diagnostics in SOT concluded that prospective multicenter studies are needed to investigate their performance and reproducibility compared to reference standards, the optimal timing of testing to predict and/or diagnose disease, the impact on clinical outcomes, and the cost-effectiveness also for point-of care applications [112].
ESBL-Producing Enterobacterales
Molecular detection of ESBL genes may aid in decreasing the time to diagnosis and initiation of targeted antimicrobials in SOT recipients. Several systems capable of detecting ESBL-producing Enterobacterales in lower respiratory tract specimens and blood are commercially available; however, not all genes responsible for ESBL production, including blaTEM and blaSHV are included on all panels. Moreover, assays used for rapid genotypic resistance detection display reduced accuracy in polymicrobial infections [111,112]. Rapid phenotypic antimicrobial susceptibility testing has also been demonstrated to reduce the time to optimal therapy among bacteremic non-transplant patients [111,114].Current recommendations underscore the need for conventional antimicrobial susceptibility testing to verify results of rapid genotypic and phenotypic testing when there is concern for a highly resistant phenotype and for polymicrobial infection [1,111].
Carbapenem-Resistant Enterobacterales
Several rapid diagnostic tests for carbapenem resistance are commercially available and include real‐time polymerase chain reaction and nucleic acid tests such as the Xpert® Carba‐R (Cepheid), Verigene® BC‐GN (Luminex), and BioFire® FilmArray® Blood Culture Identification 2 Panel, which test for blaKPC, blaNDM, blaOXA, blaVIM, and blaIMP gene sequences [115,116].However, this assays display reduced accuracy in polymicrobial infections [111,112]. Other methods for rapid diagnosis of CRE include chromogenic assays as RAPIDEC® CARBA NP (bioMérieux) and Rapid CARB Blue (Rosco Diagnostics) and matrix‐assisted laser desorption ionization‐time-of-flight mass spectroscopy (MALDI‐TOF MS) [117–119]. While rapid diagnostic assays for the detection of carbapenem resistance may reduce the time to effective antimicrobial therapy, current guidelines and expert consensus recommendations recommend conventional antimicrobial susceptibility testing to confirm the diagnosis of a CRE infection [1,111].
According to local availability, antimicrobial susceptibility to old and new agents is advisable not only on the clinical isolate but also on the colonizing strain in order to start promptly an appropriate treatment upon the onset of infection symptoms/signs.
Difficult-To-Treat Resistant Pseudomonas aeruginosa
Difficult-to-treat resistance has been defined as P. aeruginosa which exhibits non-susceptibility to aztreonam, piperacillin-tazobactam, ciprofloxacin, levofloxacin, ceftazidime, cefepime, meropenem, imipenem-cilastatin [120].
Rapid diagnostic tests for the identification of P. aeruginosa are commercially available and include nucleic acid tests, MALDI-TOF MS, and peptide nucleic acid fluorescent in situ hybridization (PNA FISH; AdvanDx) [121]. However, given that DTR-Pa evolves due to multiple resistance mechanisms, current guidelines recommend against rapid diagnostic testing to guide empiric treatment [1].
Carbapenem-Resistant Acinetobacter baumannii
In low-prevalence areas, use of rapid diagnostic and phenotypic tests for the detection of CRAB has posed clinical challenges. A recent study comparing the NG-Test CARBA 5 (NG-Biotech) version2 with the Xpert-Carba-R assay, modified carbapenem inactivation method (mCIM), and the CIMTris assay with whole-genome sequencing as the reference standard demonstrated that the NG-Test CARBA 5 and Xpert Carba-R had an overall percentage agreement of 6.2%, noting OXA-type carbapenemases are not included, and the CIMTris had an overall percentage agreement of 99%. In addition, approximately 96% of isolates incorrectly tested positive for IMP on NG-Test CARBA 5 [122]. Supplementary studies are being undertaken to identify opportunities for rapid diagnostics for CR-Ab infections [123,124].
Management of MDROs in SOT patients
The management of MDRO infections in SOT patients is not different from that recommended in other patients in view of choice agent/regimen and treatment duration. The outsized burden of AMR in the LMICs is further complicated by non-availability of recently approved antibacterial agents. For example, in the South Asian region, where carbapenem-resistant infections are very common, cefiderocol, sulbactam-durlobactam, meropenem-vaborbactam, imipenem-relebactam, eravacycline and plazomicin are not yet available. The treatment for severe infections, with bacteraemia as a prototype, is discussed here. Overall, spectrum of activity of various antimicrobial agents is shown in Figure 3. Selection of agents should be based on in vitro activity and local availability. An algorithm for treatment approaches is proposed in Figure 4.
FIGURE 3
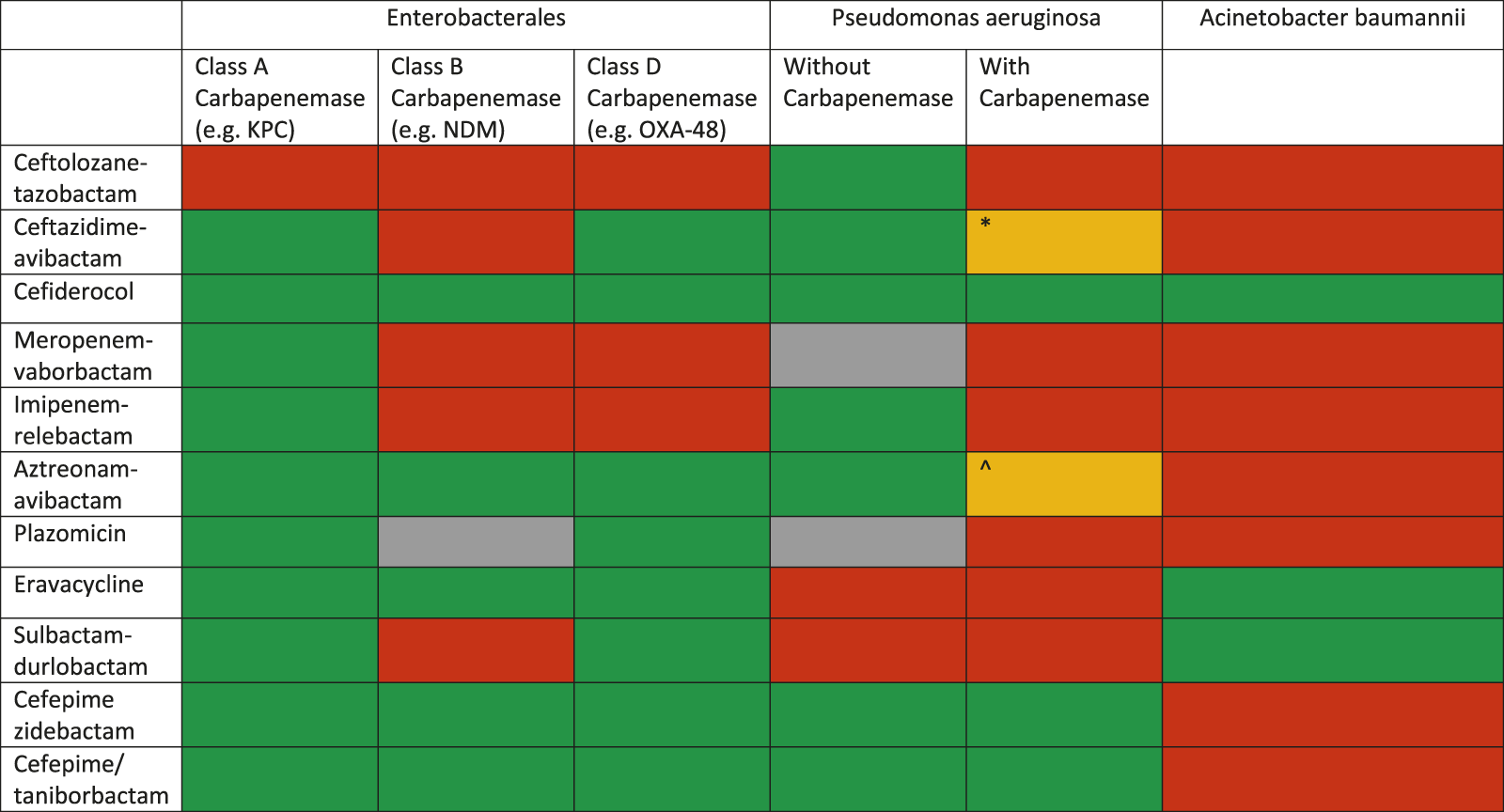
Spectrum of various novel agents active against carbapenem-resistant Gram-negative organisms (modified from: European Respiratory Review 2022 31: 220119) *This drug may retain activity against serine-type carbapenemases (e.g., GES) but are inactivated by metallobetalactamases ^This combination has been shown to be active in vitro against some MBL producing P. aeruginosa strains.
FIGURE 4
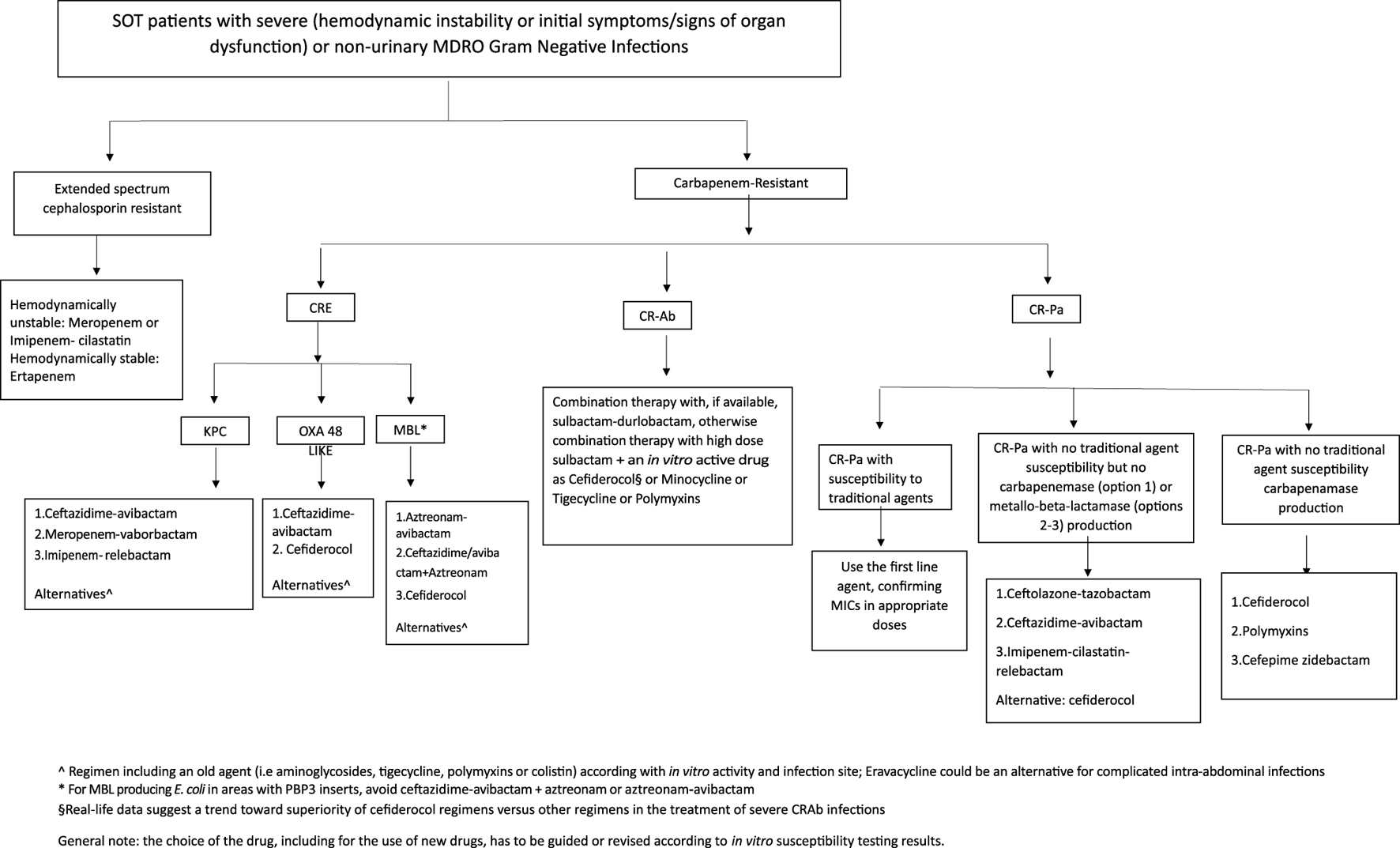
Treatment flowchart.
Third-Generation Cephalosporin-Resistant Enterobacterales
Carbapenems are considered the drug of choice for the management of severe ESBL-E infections in SOT patients [120,125]. The MERINO trial compared piperacillin-tazobactam versus meropenem for the management of ceftriaxone-resistant E. coli and Klebsiella spp. bacteremia. Thirty-day mortality, was higher in the piperacillin-tazobactam group (12% vs. 4%; absolute risk difference 9%), failing to meet the non-inferiority margin [126]. The carbapenem superiority appears to be related to elevated piperacillin-tazobactam MICs with co-occurrence of narrow spectrum oxacillinases [127]. Ertapenem is generally deferred as an upfront therapy in critically ill patients [128]. Carbapenems including ertapenem, fluoroquinolones, TMP-SMX and aminoglycosides are options for stable patients with pyelonephritis and other UTI. Switch to oral regimens can be considered once clinical stability is achieved and susceptible oral agents with good intestinal absorption are available [125].
Klebsiella aerogenes, Enterobacter cloacae complex, and Citrobacter freundii are commonly associated with higher risk of AmpC-β-lactamase production [129]. Despite its limited ability to induce AmpC-β-lactamases, piperacillin-tazobactam is considered inferior for treatment due to the risk of hydrolysis [130]. MERINO 2, a small RCT evaluating piperacillin-tazobactam versus meropenem in bacteremia with presumed AmpC-producing organisms showed no difference between the two agents in clinical failure and mortality [131]. However, some observational data point to poorer outcomes with piperacillin-tazobactam [132–134]. Cefepime minimally induces AmpC β-lactamases and is relatively stable to AmpC hydrolysis. Some observational studies show higher mortality with cefepime MICs 4–8 μg/mL (susceptible dose-dependent range), probably correlating with co-production of ESBLs [135]. Carbapenems are stable against AmpC β-lactamases and are the drugs of choice for severe infections and/or upon isolates with MICs ≥4 μg/mL for cefepime [120].
Studies addressing the role of intestinal decolonization for SOT recipients colonized with ESCR-E are limited. One case-control study described the successful use of a 5-day course of norfloxacin in reducing the burden of ESBL-E in stool samples obtained from OLT recipients during an outbreak in a transplant unit [136]. However, other studies have described the development of colistin- and tobramycin-resistant K. pneumoniae after attempted decolonization with orally administered colistin [137,138]. Given the risk of selecting resistant organisms, this approach is not recommended [139].
Carbapenem-Resistant Enterobacterales
Once the CRE is confirmed, carbapenamase testing and antimicrobial susceptibility for all available agents are recommended. For KPC-producing CRE isolates, ceftazidime-avibactam, meropenem-vaborbactam, and imipenem-relebactam are the first line options of therapy [125]. Cefiderocol can be used provided susceptibility testing is available. For OXA-48 type carbapenemase-producing CRE, ceftazidime-avibactam is the preferred agent of choice. Cefiderocol is an alternative [140,141]. NDM-producing CRE is best treated with a combination of ceftazidime-avibactam and aztreonam. Aztreonam retains activity against MBL but is inactivated by coexistent ESBLs, AmpCs or OXA-48 like enzymes. Avibactam protects the aztreonam from these mechanisms. Cefiderocol is a potential option for treatment of NDM- and other MBL-producers if the isolate is susceptible to this agent. In MBL-producing E coli, presence of four-amino acid (YRIN or YRIK) inserts in Penicillin binding protein 3 (PBP3) are common in countries like India and China, reducing the interaction of aztreonam at that site, leading to higher MICs [142,143]. The efficacy of ceftazidime-avibactam plus aztreonam may not be retained in MBL producing E coli isolates with PBP3 inserts.
Few studies have assessed the efficacy of the new drugs specifically in SOT recipients. Most data are available for ceftazidime-avibactam as it was first introduced in Europe and US. A multicentre observational study of 210 SOT recipients with BSI due carbapenemase producing K. pneumoniae, 149 received active primary therapy with CAZ-AVI (66/149) or best available treatment (BAT) (83/149). Patients treated with CAZ-AVI had higher 14-day (80.7% vs. 60.6%, p = 0.011) and 30-day (83.1% vs. 60.6%, p = 0.004) clinical success and lower 30-day mortality (13.25% vs. 27.3%, p = .053) than those receiving BAT. In the adjusted analysis, CAZ-AVI increased the probability of clinical success; in contrast, it was not independently associated with 30-day mortality. In the CAZ-AVI group, combination therapy was not associated with better outcomes [144].
There is a paucity of data regarding intestinal decolonization of SOT recipients colonized with CRE. A clinical trial on SOT colonized with MDRO failed to show a benefit from decolonization with oral colistin plus neomycin, conversely decolonization was associated with adverse events [145]. Thus, this approach is currently not recommended. The role of fecal microbiota transplantation in restoring intestinal microbial diversity in SOT recipients colonized with MDROs seems promising; however, more data on clinical effectiveness and safety are needed [146].
Difficult to Treat Resistant P. aeruginosa
Treatment of Pa with carbapenem resistance can be approached in three ways. If a traditional agent like piperacillin-tazobactam, cefepime, ceftazidime or fluoroquinolones remains susceptible with carbapenem resistance, they can be used in optimal doses [147]. This is primarily due to lack of functional OprD which is required for carbapenem entry.
If there is resistance to traditional agents and to carbapenems (e.g., a XDR or DTR strain), it is important to check for carbapenemases [148,149]. If carbapenemase testing is negative, ceftolozane-tazobactam is considered the drug of choice when in vitro activity is confirmed. For CR-Pa where resistance is mediated by a non-MBL carbapenemase (e.g., KPC, GES) ceftazidime-avibactam or imipenem-relebactam could be used; cefiderocol is an alternative option.
For CR-Pa isolates with documented MBL production, the therapeutic options are limited. Cefiderocol or polymyxins are generally the only drugs maintaining in vitro activity. However, data on clinical efficacy are controversial for cefiderocol, and generally poor for polymyxin/colistin mainly due to toxicity. The combination of ceftazidime-avibactam and aztreonam could be an option although clinical experience is limited [150,151]. Cefepime-zidebactam has been reported as a salvage option in these patients [152,153].
Carbapenem Resistant Acineotacter baumannii
The therapy of CRAb infections is particularly complex in view of difficulty in differentiating between colonization and invasive infection, especially in the lung, with extremely limited therapeutic options. There is no single antibiotic available as a preferred agent in the management of CRAb infections. One of the recent promising agents is sulbactam-durlobactam. In a phase 3 RCT, 28-day all-cause mortality was 19% in the sulbactam–durlobactam group and 32% in the colistin group, an absolute difference of −13.2%, meeting the non-inferiority criteria. In both groups, combination with imipenem-cilastatin was used. Most guidelines currently recommend sulbactam based therapy and wherever possible in combination with other in-vitro active agents [125]. Sulbactam is a competitive betalactamase-inhibitor with independent anti-Acinetobacter activity via saturation of PBP1 and PBP3 in high doses [154]. But the susceptible MIC range for sulbactam is not established. Also, changes in the above PBPs can decrease its affinity and result in resistance. Few studies have supported the benefit of ampicillin-sulbactam especially against polymyxins [105]. The options for combination therapy with sulbactam include minocycline, tigecycline, polymyxins and cefiderocol. Colistin is frequently active in vitro; however, the unfavourable PK/PD profile of this drug results in low efficacy and high toxicity rates. Two large randomized controlled studies have shown the addition of high-dose meropenem to colistin does not result in clinical benefit [155,156]. Nebulised polymyxins are not currently recommended in view of preferential distribution to the unaffected areas of the lung, absence of benefit in randomized trials and potential for bronchospasm [157–159]. The role of cefiderocol is debated [160,161]. This drug shows high rates of in vitro activity and, despite it was associated with higher mortality compared with standard treatment (mostly consisting of colistin-based regimens) in patients with CR-Ab infections in the phase III CREDIBLE-CR trial [161], it has been shown to be more or equally effective than older regimens, with a significantly lower toxicity, in several real-word observational studies [162].
Conclusion
To conclude, to draw the global burden of MDROs in SOT recipients is difficult due to the lack of standardization in screening and reporting colonization and/or infections with such pathogens; and the access to diagnostic and therapeutic resources could be variable across countries. To improve outcomes associated with MDRO colonization and/or infections in SOT recipients, new rapid advanced diagnostics could be supportive, as well as the prompt availability of phenotypic susceptibility to old and new drugs. Use of these tests should be guided by local epidemiology and patient risk factors, their impact on outcome should be investigated.
Statements
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
The author(s) declare(s) that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
AM received scientific grants from the Wellcome Trust DBT alliance and the Indian Council of Medical Research. MG received a research grant from Pfizer, payment for lecture from Shionogi, MSD, Pfizer and BioMerieux.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviation
SOT, solid organ transplantation; MDRO, multi drug resistant organisms; ESBL-E, extended spectrum beta-lactamase producing enterobacterales; ESCR-E, extended-spectrum cephalosporin resistant-enterobacterales; CRE, carbapenem resistant enterobacterales; CPE, carbapenemase producing enterobacterales; KPC, Klebsiella pneumoniae carbapenemase; MBL, metallo-beta-lactamase; NDM, New Delhi metallobetalactamase; VIM, verona-integron-metallo beta lactamase; IMP, imipenemase; MDR, multi-drug-resistant; XDR; extensively drug resistant; PDR, pan-drug resistant; DTR, difficult-to treat resistance; CR-Pa, carbapenem resistant Pseudomonas aeruginosa; CR-Ab, carbapenem resistant Acinetobacter baumannii; HIC, high income countries; LMIC, low and medium income countries; OLT, orthotopic liver transplantation; KT, kidney transplantation; LuT, lung transplantation; HT, heart transplantation; UTI, urinary tract infection; DDI, donor derived infection.
References
1.
Pouch SM Patel G , AST Infectious Diseases Community of Practice. Multidrug-Resistant Gram-Negative Bacterial Infections in Solid Organ Transplant Recipients—Guidelines From the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transpl (2019) 33(9):e13594. 10.1111/ctr.13594
2.
Johnson LE D’Agata EMC Paterson DL Clarke L Qureshi ZA Potoski BA et al Pseudomonas Aeruginosa Bacteremia Over a 10-Year Period: Multidrug Resistance and Outcomes in Transplant Recipients. Transpl Infect Dis (2009) 11(3):227–34. 10.1111/j.1399-3062.2009.00380.x
3.
Falcone M Tiseo G Galfo V Giordano C Leonildi A Marciano E et al Bloodstream Infections in Patients With Rectal Colonization by Klebsiella Pneumoniae Producing Different Type of Carbapenemases: A Prospective, Cohort Study (CHIMERA Study). Clin Microbiol Infect (2021) 28:298.e1–298.e7. 10.1016/j.cmi.2021.06.031
4.
Reyes J Komarow L Chen L Ge L Hanson BM Cober E et al Global Epidemiology and Clinical Outcomes of Carbapenem-Resistant Pseudomonas Aeruginosa and Associated Carbapenemases (POP): A Prospective Cohort Study. The Lancet Microbe (2023) 4(3):e159–e170. 10.1016/S2666-5247(22)00329-9
5.
Magiorakos A-PP Srinivasan A Carey RBB Carmeli Y Falagas ME Giske CG et al Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin Microbiol Infect (2012) 18(3):268–81. 10.1111/j.1469-0691.2011.03570.x
6.
Kadri SS Adjemian J Lai YL Spaulding AB Ricotta E Prevots DR et al Difficult-to-Treat Resistance in Gram-Negative Bacteremia at 173 US Hospitals: Retrospective Cohort Analysis of Prevalence, Predictors, and Outcome of Resistance to All First-Line Agents. Clin Infect Dis (2018) 67:1803–14. 10.1093/cid/ciy378
7.
Hamadeh N Van Rompaey C Metreau E . World Bank Country Classification by Income Levels (2023). Available from: https://blogs.worldbank.org/opendata/new-world-bank-country-classifications-income-level-2022-2023 (Accessed January 2, 2024).
8.
Organización Nacional de Trasplantes, World Health Organization. European Directorate for the Quality of Medicines and HealthCare, Iberoamerican Network/Counci, Transplantation WN for B& M. Global Observatory on Donation and Transplantation (2023). Available from: https://www.transplant-observatory.org/ (Accessed October 23, 2023).
9.
Gurakar A Muhammad H Gurakar M Aslan A Dao D . Liver Transplantation in Developing Countries. Hepatol Forum (2022) 3:103–7. 10.14744/hf.2022.2022.0014
10.
Oldenkamp R Schultsz C Mancini E Cappuccio A . Filling the Gaps in the Global Prevalence Map of Clinical Antimicrobial Resistance. Proc Natl Acad Sci (2021) 118(1):e2013515118. 10.1073/pnas.2013515118
11.
Friedman ND Carmeli Y Walton AL Schwaber MJ . Carbapenem-Resistant Enterobacteriaceae: A Strategic Roadmap for Infection Control. Infect Control Hosp Epidemiol (2017) 38(5):580–94. 10.1017/ice.2017.42
12.
Khah AN Hakemi-Vala M Samavat S Nasiri MJ . Prevalence, Serotyping and Drug Susceptibility Patterns of Escherichia Coli Isolates From Kidney Transplanted Patients With Urinary Tract Infections. World J Biol Chem (2020) 11(3):112–8. 10.4331/wjbc.v11.i3.112
13.
Aramwittayanukul S Malathum K Kantachuvesiri S Arpornsujaritkun N Chootip P Bruminhent J . Impact of Carbapenem Peri-Transplant Prophylaxis and Risk of Extended-Spectrum Cephalosporin-Resistant Enterobacterales Early Urinary Tract Infection in Kidney Transplant Recipients: A Propensity Score-Matched Analysis. Front Med (2022) 9:841293. 10.3389/fmed.2022.841293
14.
Hamid R Javaid S Khan M Lal N Luxmi S Sarfaraz S . Multiple Drug Resistant Urinary Tract Infection in Kidney Transplant Recipients: A Retrospective Cohort Study. Saudi J Kidney Dis Transpl (2020) 31(5):905–16. 10.4103/1319-2442.301197
15.
Kiros T Asrat D Ayenew Z Tsige E . Bacterial Urinary Tract Infection Among Adult Renal Transplant Recipients at St. Paul’s Hospital Millennium Medical College, Addis Ababa, Ethiopia. BMC Nephrol (2019) 20(1):289. 10.1186/s12882-019-1485-9
16.
Jin M Zeng L Zhang WW Deng X Li J Zhang WW . Clinical Features of Multidrug-Resistant Organism Infections in Early Postoperative Solid Organ Transplantation in a Single Center. Ann Palliat Med (2021) 10(4):4555–62. 10.21037/apm-21-777
17.
Gong L Zhang L Liu X Odilov B Li S Hu Z et al Distribution and Antibiotic Susceptibility Pattern of Multidrug-Resistant Bacteria and Risk Factors Among Kidney Transplantation Recipients With Infections Over 13 Years: A Retrospective Study. Infect Drug Resist (2021) 14:5661–9. 10.2147/IDR.S318941
18.
Kose A Altunisik Toplu S Akbulut S Yasar S Sarici KB Duman Y et al Evaluation of Clinical Characteristics and Outcomes of Postoperative Infections in Living Liver Donors. Int J Clin Pract (2021) 75(8):e14324. 10.1111/ijcp.14324
19.
Siritip N Nongnuch A Dajsakdipon T Thongprayoon C Cheungprasitporn W Bruminhent J . Epidemiology, Risk Factors, and Outcome of Bloodstream Infection Within the First Year After Kidney Transplantation. Am J Med Sci (2021) 361(3):352–7. 10.1016/j.amjms.2020.10.011
20.
Qiao B Wu J Wan Q Zhang S Ye Q . Factors Influencing Mortality in Abdominal Solid Organ Transplant Recipients With Multidrug-Resistant Gram-Negative Bacteremia. BMC Infect Dis (2017) 17(1):171. 10.1186/s12879-017-2276-1
21.
Rosado-Canto R Parra-Avila I Tejeda-Maldonado J Kauffman-Ortega C Rodriguez-Covarrubias FT Trujeque-Matos M et al Perioperative Fosfomycin Disodium Prophylaxis Against Urinary Tract Infection in Renal Transplant Recipients: A Randomized Clinical Trial. Nephrol Dial Transpl (2020) 35(11):1996–2003. 10.1093/ndt/gfz261
22.
Møller DL Sørensen SS Perch M Gustafsson F Rezahosseini O Knudsen AD et al Bacterial and Fungal Bloodstream Infections in Solid Organ Transplant Recipients: Results From a Danish Cohort With Nationwide Follow-Up. Clin Microbiol Infect (2022) 28(3):391–7. 10.1016/j.cmi.2021.07.021
23.
Bert F Larroque B Paugam-Burtz C Dondero F Durand F Marcon E et al Pretransplant Fecal Carriage of Extended-Spectrum β-Lactamase–producing Enterobacteriaceae and Infection After Liver Transplant, France. Emerg Infect Dis (2012) 18(6):908–16. 10.3201/eid1806.110139
24.
Boscolo A Sella N Pettenuzzo T De Cassai A Crociani S Schiavolin C et al Multidrug-Resistant and Extended-Spectrum β-Lactamase Gram-Negative Bacteria in Bilateral Lung Transplant Recipients: Incidence, Risk Factors, and In-Hospital Mortality. Chest (2022) 162(6):1255–64. 10.1016/j.chest.2022.06.046
25.
Gagliotti C Morsillo F Moro ML Masiero L Procaccio F Vespasiano F et al Infections in Liver and Lung Transplant Recipients: A National Prospective Cohort. Eur J Clin Microbiol Infect Dis (2018) 37(3):399–407. 10.1007/s10096-018-3183-0
26.
Yamamoto M Takakura S Iinuma Y Hotta G Matsumura Y Matsushima A et al Changes in Surgical Site Infections After Living Donor Liver Transplantation. PLoS One (2015) 10(8):e0136559–14. 10.1371/journal.pone.0136559
27.
Lim JH Cho JH Lee JH Park YJ Jin S Park GY et al Risk Factors for Recurrent Urinary Tract Infection in Kidney Transplant Recipients. Transpl Proc (2013) 45(4):1584–9. 10.1016/j.transproceed.2012.12.011
28.
Kim S-H Mun SJ Ko J-H Huh K Cho SY Kang CI et al Poor Outcomes of Early Recurrent Post-Transplant Bloodstream Infection in Living-Donor Liver Transplant Recipients. Eur J Clin Microbiol Infect Dis (2021) 40(4):771–8. 10.1007/s10096-020-04074-5
29.
Sanclemente G Bodro M Cervera C Linares L Cofán F Marco F et al Perioperative Prophylaxis With Ertapenem Reduced Infections Caused by Extended-Spectrum Betalactamase-Producting Enterobacteriaceae After Kidney Transplantation. BMC Nephrol (2019) 20(1):274. 10.1186/s12882-019-1461-4
30.
Chapman FA Dickerson JE Daly C Clancy M Geddes C . Impact of Increased Duration of Trimethoprim-Sulfamethoxazole Prophylaxis for Pneumocystis Pneumonia After Renal Transplant. Ann Transpl (2019) 24:625–30. 10.12659/AOT.918195
31.
Shendi AM Wallis G Painter H Harber M Collier S . Epidemiology and Impact of Bloodstream Infections Among Kidney Transplant Recipients: A Retrospective Single-Center Experience. Transpl Infect Dis (2018) 20(1). 10.1111/tid.12815
32.
Ramadas P Rajendran PP Krishnan P Alex A Siskind E Kadiyala A et al Extended-Spectrum-Beta-Lactamase Producing Bacteria Related Urinary Tract Infection in Renal Transplant Recipients and Effect on Allograft Function. PLoS One (2014) 9(3):e91289. 10.1371/journal.pone.0091289
33.
Bui KT Mehta S Khuu TH Ross D Carlson M Leibowitz MR et al Extended Spectrum β-Lactamase-Producing Enterobacteriaceae Infection in Heart and Lung Transplant Recipients and in Mechanical Circulatory Support Recipients. Transplantation (2014) 97(5):590–4. 10.1097/01.TP.0000436928.15650.59
34.
Aguiar EB Maciel LC Halpern M de Lemos AS Ferreira ALP Basto ST et al Outcome of Bacteremia Caused by Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae After Solid Organ Transplantation. Transpl Proc (2014) 46(6):1753–6. 10.1016/j.transproceed.2014.05.003
35.
Gupta A Baveja U Tandon N Patel S Saigal S Soin A et al Epidemiological Analysis of Extended-Spectrum Beta-Lactamase-Producing Bacterial Infections in Adult Live Donor Liver Transplant Patients. Indian J Crit Care Med (2018) 22(4):290–6. 10.4103/ijccm.IJCCM_206_17
36.
Oriol I Sabé N Simonetti AF Lladó L Manonelles A González J et al Changing Trends in the Aetiology, Treatment and Outcomes of Bloodstream Infection Occurring in the First Year After Solid Organ Transplantation: A Single-Centre Prospective Cohort Study. Transpl Int (2017) 30(9):903–13. 10.1111/tri.12984
37.
Neofytos D Stampf S Hoessly LD D'Asaro M Tang GN Boggian K et al Bacteremia During the First Year After Solid Organ Transplantation: An Epidemiological Update. Open Forum Infect Dis (2023) 10(6):ofad247. 10.1093/ofid/ofad247
38.
Vidal E Torre-Cisneros J Blanes M Montejo M Cervera C Aguado JM et al Bacterial Urinary Tract Infection After Solid Organ Transplantation in the RESITRA Cohort. Transpl Infect Dis (2012) 14(6):595–603. 10.1111/j.1399-3062.2012.00744.x
39.
Hong Nguyen M Shields RK Chen L William Pasculle A Hao B Cheng S et al Molecular Epidemiology, Natural History and Long-Term Outcomes of Multi-Drug Resistant Enterobacterales Colonization and Infections Among Solid Organ Transplant Recipients. Clin Infect Dis (2021) 15261:1–12. 10.1093/cid/ciab427
40.
Logre E Bert F Khoy-Ear L Janny S Giabicani M Grigoresco B et al Risk Factors and Impact of Perioperative Prophylaxis on the Risk of Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae-Related Infection Among Carriers Following Liver Transplantation. Transplantation (2020) 105(2):338–45. 10.1097/TP.0000000000003231
41.
Pilmis B Scemla A Join-Lambert O Mamzer MF Lortholary O Legendre C et al ESBL-Producing Enterobacteriaceae-Related Urinary Tract Infections in Kidney Transplant Recipients: Incidence and Risk Factors for Recurrence. Infect Dis (Auckl) (2015) 47(10):714–8. 10.3109/23744235.2015.1051107
42.
Wu D Chen C Liu T Wan Q . Risk Factors for Acquisition of Carbapenem-Resistant Klebsiella Pneumoniae and Mortality Among Abdominal Solid Organ Transplant Recipients With K. Pneumoniae Infections. Med Sci Monit (2020) 26:e922996. 10.12659/MSM.922996
43.
Freire MP Carvalho LB Reusing JO Spadão F Lopes MIBF Nahas WC et al Carbapenem-Resistant Enterobacteriaceae Among Kidney Transplant Recipients – Insights on the Risk of Acquisition and CRE Infection. Infect Dis (Auckl) (2021) 53(6):430–9. 10.1080/23744235.2021.1887511
44.
Chen F Pang X-Y Shen C Han LZ Deng YX Chen XS et al High Mortality Associated With Gram-Negative Bacterial Bloodstream Infection in Liver Transplant Recipients Undergoing Immunosuppression Reduction. World J Gastroenterol (2020) 26(45):7191–203. 10.3748/wjg.v26.i45.7191
45.
Rodrigues dos Santos BG Amaral ES Fernandes PF Oliveira CM Rodrigues JL Perdigão Neto LV et al Urinary Tract Infections and Surgical Site Infections Due to Carbapenem-Resistant Enterobacteriaceae in Renal Transplant. Transpl Proc (2016) 48(6):2050–5. 10.1016/j.transproceed.2016.05.005
46.
Yuan X Liu T Wu D Wan Q . Epidemiology, Susceptibility, and Risk Factors for Acquisition of MDR/XDR Gram-Negative Bacteria Among Kidney Transplant Recipients With Urinary Tract Infections. Infect Drug Resist (2018) 11:707–15. 10.2147/IDR.S163979
47.
Cicora F Mos F Paz M Allende NGG Roberti J . Infections With BlaKPC-2-Producing Klebsiella Pneumoniae in Renal Transplant Patients: A Retrospective Study. Transpl Proc (2013) 45(9):3389–93. 10.1016/j.transproceed.2013.07.064
48.
Giannella M Bartoletti M Campoli C Rinaldi M Coladonato S Pascale R et al The Impact of Carbapenemase-Producing Enterobacteriaceae Colonization on Infection Risk After Liver Transplantation: A Prospective Observational Cohort Study. Clin Microbiol Infect (2019) 25:1525–31. 10.1016/j.cmi.2019.04.014
49.
Adelman MW Connor AA Hsu E Saharia A Mobley CM Victor DW 3rd et al Bloodstream Infections After Solid Organ Transplantation: Clinical Epidemiology and Antimicrobial Resistance (2016–21). Jac-antimicrobial Resist (2023) 6(1):dlad158. 10.1093/jacamr/dlad158
50.
Pouch SMM Kubin CJJ Satlin MJJ Tsapepas DS Lee JR Dube G et al Epidemiology and Outcomes of Carbapenem-Resistant Klebsiella Pneumoniae Bacteriuria in Kidney Transplant Recipients. Transpl Infect Dis (2015) 17(6):800–9. 10.1111/tid.12450
51.
Macesic N Gomez-Simmonds A Sullivan SB Giddins MJ Ferguson SA Korakavi G et al Genomic Surveillance Reveals Diversity of Multidrug-Resistant Organism Colonization and Infection: A Prospective Cohort Study in Liver Transplant Recipients. Clin Infect Dis (2018) 67(6):905–12. 10.1093/cid/ciy199
52.
Ferst PG Filmann N Heilgenthal EM Schnitzbauer AA Bechstein WO Kempf VAJ et al Colonization With Multidrug-Resistant Organisms Is Associated With in Increased Mortality in Liver Transplant Candidates. PLoS One (2021) 16:1–13. 10.1371/journal.pone.0245091
53.
Giannella M Freire M Rinaldi M Abdala E Rubin A Mularoni A et al Development of a Risk Prediction Model for Carbapenem-Resistant Enterobacteriaceae Infection After Liver Transplantation: A Multinational Cohort Study. Clin Infect Dis (2021) 73(4):e955–e966. 10.1093/cid/ciab109
54.
Wan Q Luo A Zhong Z Ye Q . The Distribution and Resistance of Pathogens Among Solid Organ Transplant Recipients With Pseudomonas Aeruginosa Infections. Med Sci Monit (2016) 22:1124–30. 10.12659/MSM.896026
55.
Freire MP Martinho L Mendes CV Spadão F De Paula FJ Nahas WC et al Institutional Protocol Adherence in the Incidence of Recurrent Urinary Tract Infection After Kidney Transplantation. J Glob Antimicrob Resist (2020) 23:352–8. 10.1016/j.jgar.2020.10.013
56.
Freire MP Song ATW Oshiro ICV Andraus W D’Albuquerque LAC Abdala E . Surgical Site Infection After Liver Transplantation in the Era of Multidrug-Resistant Bacteria: What New Risks Should Be Considered?Diagn Microbiol Infect Dis (2021) 99(1):115220. 10.1016/j.diagmicrobio.2020.115220
57.
Chueiri Neto F Emídio LA Perales SR Stucchi RSB Dragosavac D Falcao ALE et al Bloodstream Infections in Early Postsurgery Liver Transplant: An Analysis of 401 Patients Over 10 Years. Transpl Proc (2019) 51(6):1972–7. 10.1016/j.transproceed.2019.03.040
58.
Su H Ye Q Wan Q Zhou J . Predictors of Mortality in Abdominal Organ Transplant Recipients With Pseudomonas Aeruginosa Infections. Ann Transpl (2016) 21:86–93. 10.12659/AOT.896269
59.
Tebano G Geneve C Tanaka S Grall N Atchade E Augustin P et al Epidemiology and Risk Factors of Multidrug-Resistant Bacteria in Respiratory Samples After Lung Transplantation. Transpl Infect Dis (2016) 18(1):22–30. 10.1111/tid.12471
60.
Stjärne AA Hammarström H Inghammar M Larsson H Hansson L Riise GC et al Microbiological Findings in Bronchoalveolar Lavage Fluid From Lung Transplant Patients in Sweden. Transpl Infect Dis (2018) 20(6):e12973. 10.1111/tid.12973
61.
Bodro M Sabé N Tubau F Lladó L Baliellas C González-Costello J et al Extensively Drug-Resistant Pseudomonas Aeruginosa Bacteremia in Solid Organ Transplant Recipients. Transplantation (2015) 99(3):616–22. 10.1097/TP.0000000000000366
62.
Wu D Chen C Liu T Jia Y Wan Q Peng J . Epidemiology, Susceptibility, and Risk Factors Associated With Mortality in Carbapenem-Resistant Gram-Negative Bacterial Infections Among Abdominal Solid Organ Transplant Recipients: A Retrospective Cohort Study. Infect Dis Ther (2021) 10(1):559–73. 10.1007/s40121-021-00411-z
63.
Meng D Chang R Zhu R . Analysis of Nosocomial Infection and Risk Factors in Lung Transplant Patients: A Case-Control Study. Ann Transl Med (2022) 10(14):804. 10.21037/atm-22-3023
64.
Prieto AJ Lopez M Rando K Castelli J Medina Presentado J . Early Bacterial Pneumonia After Hepatic Transplantation: Epidemiologic Profile. Transpl Proc (2018) 50(2):503–8. 10.1016/j.transproceed.2017.11.047
65.
Mukhtar A Abdelaal A Hussein M Dabous H Fawzy I Obayah G et al Infection Complications and Pattern of Bacterial Resistance in Living-Donor Liver Transplantation: A Multicenter Epidemiologic Study in Egypt. Transpl Proc (2014) 46(5):1444–7. 10.1016/j.transproceed.2014.02.022
66.
Shields RK Clancy CJ Gillis LM Kwak EJ Silveira FP Massih RCA et al Epidemiology, Clinical Characteristics and Outcomes of Extensively Drug-Resistant Acinetobacter Baumannii Infections Among Solid Organ Transplant Recipients. PLoS One (2012) 7(12):e52349. 10.1371/journal.pone.0052349
67.
Reddy P Zembower TR Ison MG Baker TA Stosor V . Carbapenem-Resistant Acinetobacter Baumannii Infections After Organ Transplantation. Transpl Infect Dis (2010) 12(1):87–93. 10.1111/j.1399-3062.2009.00445.x
68.
Biderman P Bugaevsky Y Ben-Zvi H Bishara J Goldberg E . Multidrug-Resistant Acinetobacter Baumannii Infections in Lung Transplant Patients in the Cardiothoracic Intensive Care Unit. Clin Transpl (2015) 29(9):756–62. 10.1111/ctr.12575
69.
Kim YJ Kim SI Lee Yd. Choi HJ Choi JY Yoon SK et al Carbapenem-Resistant Acinetobacter Baumannii Bacteremia in Liver Transplant Recipients. Transpl Proc (2018) 50(4):1132–5. 10.1016/j.transproceed.2018.01.043
70.
Kohler P Wolfensberger A Stampf S Brönnimann A Boggian K van Delden C et al Temporal Trends, Risk Factors and Outcomes of Infections Due to Extended-Spectrum β-Lactamase Producing Enterobacterales in Swiss Solid Organ Transplant Recipients Between 2012 and 2018. Antimicrob Resist Infect Control (2021) 10(1):50. 10.1186/s13756-021-00918-7
71.
Anesi JA Lautenbach E Tamma PD Thom KA Blumberg EA Alby K et al Risk Factors for Extended-Spectrum β-Lactamase-Producing Enterobacterales Bloodstream Infection Among Solid-Organ Transplant Recipients. Clin Infect Dis (2021) 72(6):953–60. 10.1093/cid/ciaa190
72.
Fernández-Martínez M González-Rico C Gozalo-Margüello M Marco F Gracia-Ahufinger I Aranzamendi M et al Molecular Characterization of Multidrug Resistant Enterobacterales Strains Isolated From Liver and Kidney Transplant Recipients in Spain. Sci Rep (2021) 11(1):11875–9. 10.1038/s41598-021-90382-5
73.
Alevizakos M Nasioudis D Mylonakis E . Urinary Tract Infections Caused by ESBL-Producing Enterobacteriaceae in Renal Transplant Recipients: A Systematic Review and Meta-Analysis. Transpl Infect Dis (2017) 19(6):e12759. 10.1111/tid.12759
74.
Righi E Mutters NT Guirao X Del Toro MD Eckmann C Friedrich AW et al ESCMID/EUCIC Clinical Practice Guidelines on Perioperative Antibiotic Prophylaxis in Patients Colonized by Multidrug-Resistant Gram-Negative Bacteria Before Surgery. Clin Microbiol Infect (2023) 29(4):463–79. 10.1016/j.cmi.2022.12.012
75.
Zheng M-M Guo M-X Shang L-M Zhang J Lin J Tian Y et al Effect of Carbapenem-Resistant Klebsiella Pneumoniae Infection on the Clinical Outcomes of Kidney Transplant Recipients. Infect Drug Resist (2022) 15:6471–83. 10.2147/IDR.S381265
76.
Lu J Zhang A Han L Guo Z Cui W Jiang Y et al Clinical Outcomes and Risk Factors for Death Following Carbapenem-Resistant Klebsiella Pneumoniae Infection in Solid Organ Transplant Recipients. Microbiol Spectr (2023) 11(1):e0475522. 10.1128/spectrum.04755-22
77.
Freire MP Oshiro IC Pierrotti LC Bonazzi PR de Oliveira LM Song AT et al Carbapenem-Resistant Enterobacteriaceae Acquired Before Liver Transplantation: Impact on Recipient Outcomes. Transplantation (2017) 101(4):811–20. 10.1097/TP.0000000000001620
78.
Freire MP de Oliveira Garcia D Cury AP Spadão F Di Gioia TS Francisco GR et al Outbreak of IMP-Producing Carbapenem-Resistant Enterobacter Gergoviae Among Kidney Transplant Recipients. J Antimicrob Chemother (2016) 71(9):2577–85. 10.1093/jac/dkw165
79.
Taminato M Fram D Pereira RRF Sesso R Belasco AGS Pignatari AC et al Infection Related to Klebsiella Pneumoniae Producing Carbapenemase in Renal Transplant Patients. Rev Bras Enferm (2019) 72(3):760–6. 10.1590/0034-7167-2019-0009
80.
Zhang F Zhong J Ding H Pan J Yang J et al Analysis of Risk Factors for Carbapenem-Resistant Klebsiella Pneumoniae Infection and its Effect on the Outcome of Early Infection After Kidney Transplantation. Front Cel Infect Microbiol (2021) 11:726282. 10.3389/fcimb.2021.726282
81.
Taimur S Pouch SM Zubizarreta N Mazumdar M Rana M Patel G et al Impact of Pre-Transplant Carbapenem-Resistant Enterobacterales Colonization And/or Infection on Solid Organ Transplant Outcomes. Clin Transpl (2021) 35(4):e14239. 10.1111/ctr.14239
82.
Bonazzetti C Rinaldi M Cosentino F Gatti M Freire MP Mularoni A et al Survey on the Approach to Antibiotic Prophylaxis in Liver and Kidney Transplant Recipients Colonized With “Difficult to Treat” Gram-Negative Bacteria. Transpl Infect Dis (2024) 26:e14238. 10.1111/tid.14238
83.
Anesi JA Lautenbach E Thom KA Tamma PD Blumberg EA Alby K et al Clinical Outcomes and Risk Factors for Carbapenem-Resistant Enterobacterales Bloodstream Infection in Solid Organ Transplant Recipients. Transplantation (2023) 107(1):254–63. 10.1097/TP.0000000000004265
84.
Palacio F Reyes LF Levine DJ Sanchez JF Angel LF Fernandez JF et al Understanding the Concept of Health Care-Associated Pneumonia in Lung Transplant Recipients. Chest (2015) 148(2):516–22. 10.1378/chest.14-1948
85.
Freire MP Camargo CH Yamada AY Nagamori FO Reusing Junior JO Spadão F et al Critical Points and Potential Pitfalls of Outbreak of IMP-1-Producing Carbapenem-Resistant Pseudomonas Aeruginosa Among Kidney Transplant Recipients: A Case–Control Study. J Hosp Infect (2021) 115:83–92. 10.1016/j.jhin.2021.05.006
86.
Nie XM Huang PH Ye QF Wan QQ . The Distribution, Drug Resistance, and Clinical Characteristics of Acinetobacter Baumannii Infections in Solid Organ Transplant Recipients. Transpl Proc (2015) 47(10):2860–4. 10.1016/j.transproceed.2015.09.037
87.
Guo W Sheng J Gu Y Xing T-H Peng Z-H Zhong L . Analysis and Forecast for Multidrug-Resistant Acinetobacter Baumannii Infections Among Liver Transplant Recipients. Transpl Proc (2014) 46(5):1448–52. 10.1016/j.transproceed.2014.02.027
88.
Freire MP Pierrotti LC Oshiro IC Bonazzi PR Oliveira LM Machado AS et al Carbapenem-Resistant Acinetobacter Baumannii Acquired Before Liver Transplantation: Impact on Recipient Outcomes. Liver Transpl (2016) 22(5):615–26. 10.1002/lt.24389
89.
Serifoglu I Dedekarginoglu BE Bozbas SS Akcay S Haberal M . Clinical Characteristics of Acinetobacter Baumannii Infection in Solid-Organ Transplant Recipients. Exp Clin Transpl (2018) 16:171–5. 10.6002/ect.TOND-TDTD2017.P51
90.
Bartoletti M Vandi G Furii F Bertuzzo V Ambretti S Tedeschi S et al Management of Immunosuppressive Therapy in Liver Transplant Recipients Who Develop Bloodstream Infection. Transpl Infect Dis (2018) 20(5):e12930–9. 10.1111/tid.12930
91.
Giannella M Bartoletti M Conti M Righi E . Carbapenemase-Producing Enterobacteriaceae in Transplant Patients. J Antimicrob Chemother (2021) 76(Suppl. ment_1):i27–i39. 10.1093/jac/dkaa495
92.
Mularoni A Bertani A Vizzini G Gona F Campanella M Spada M et al Outcome of Transplantation Using Organs From Donors Infected or Colonized With Carbapenem-Resistant Gram-Negative Bacteria. Am J Transpl (2015) 15(10):2674–82. 10.1111/ajt.13317
93.
Zhang F Zhong J Ding H Liao G . Effects of Preservative Fluid Associated Possible Donor-Derived Carbapenem-Resistant Klebsiella Pneumoniae Infection on Kidney Transplantation Recipients. BMC Nephrol (2022) 23(1):101. 10.1186/s12882-022-02733-7
94.
He L Fu Z Wang M Wang X Wang L Li G et al Prevention and Treatment of Carbapenem-Resistant Organism Bacilli From Liver Transplantation Donors – Single Center Experience. Infect Drug Resist (2022) 15:47–52. 10.2147/IDR.S346494
95.
Wang Z Guo Z Feng H Fu C Zhao GY Ma K et al Treatment of Donor-Derived Carbapenem-Resistant Klebsiella Pneumoniae Infection After Renal Transplantation With Tigecycline and Extended-Infusion Meropenem. Curr Med Sci (2021) 41(4):770–6. 10.1007/s11596-021-2397-z
96.
Aguado JM Silva JT Fernández-Ruiz M Cordero E Fortún J Gudiol C et al Management of Multidrug Resistant Gram-Negative Bacilli Infections in Solid Organ Transplant Recipients: SET/GESITRA-SEIMC/REIPI Recommendations. Transpl Rev (2018) 32(1):36–57. 10.1016/j.trre.2017.07.001
97.
Boyd SE Livermore DM Hooper DC Hope WW . Metallo-β-Lactamases: Structure, Function, Epidemiology, Treatment Options, and the Development Pipeline. Antimicrob Agents Chemother (2020) 64(10):e00397–20. 10.1128/AAC.00397-20
98.
van Duin D Doi Y . The Global Epidemiology of Carbapenemase-Producing Enterobacteriaceae. Virulence (2016) 0(0):460–9. 10.1080/21505594.2016.1222343
99.
Khillan V Kale P Pamecha V Rathor N Sarin SK . Infections in Live Donor Liver Transplant Recipients: A Study of Timeline, Aetiology and Antimicrobial Resistance of Bacterial and Fungal Infections From the Developing World. Indian J Med Microbiol (2017) 35(4):604–6. 10.4103/ijmm.IJMM_17_295
100.
Freire MP de Oliveira Garcia D Lima SG Pea CRD Reusing Junior JO Spadão F et al Performance of Two Methods of Carbapenem-Resistant Enterobacterales Surveillance on a Kidney Transplant Ward: Selective Culture of and Real-Time PCR Directly From Rectal Swabs. Infection (2022) 50(6):1525–33. 10.1007/s15010-022-01839-2
101.
Tian F Li Y Wang Y Yu B Song J Ning Q et al Risk Factors and Molecular Epidemiology of Fecal Carriage of Carbapenem Resistant Enterobacteriaceae in Patients With Liver Disease. Ann Clin Microbiol Antimicrob (2023) 22(1):10. 10.1186/s12941-023-00560-8
102.
Kostoulias X Chang CC Wisniewski J Abbott IJ Zisis H Dennison A et al Antimicrobial Susceptibility of Ceftolozane-Tazobactam Against Multidrug-Resistant Pseudomonas Aeruginosa Isolates From Melbourne, Australia. Pathology (2023) 55(5):663–8. 10.1016/j.pathol.2023.03.009
103.
Garnacho-Montero J Ortiz-Leyba C Jimenez-Jimenez FJ Barrero-Almodóvar AE García-Garmendia JL Bernabeu-WittelI M et al Treatment of Multidrug-Resistant Acinetobacter Baumannii Ventilator-Associated Pneumonia (VAP) With Intravenous Colistin: A Comparison With Imipenem-Susceptible VAP. Clin Infect Dis (2003) 36(9):1111–8. 10.1086/374337
104.
Behzad D Hakimeh A Hossein R Khaledi A . A Middle East Systematic Review and Meta-Analysis of Bacterial Urinary Tract Infection Among Renal Transplant Recipients; Causative Microorganisms. Microb Pathog (2020) 148:104458. 10.1016/j.micpath.2020.104458
105.
Piperaki ET Tzouvelekis LS Miriagou V Daikos GL . Carbapenem-Resistant Acinetobacter Baumannii: In Pursuit of an Effective Treatment. Clin Microbiol Infect (2019) 25(8):951–7. 10.1016/j.cmi.2019.03.014
106.
Liu B Liu L . Molecular Epidemiology and Mechanisms of Carbapenem-Resistant Acinetobacter Baumannii Isolates From ICU and Respiratory Department Patients of a Chinese University Hospital. Infect Drug Resist (2021) 14:743–55. 10.2147/IDR.S299540
107.
Wong D Nielsen TB Bonomo RA Pantapalangkoor P Luna B Spellberg B . Clinical and Pathophysiological Overview of Acinetobacter Infections: A Century of Challenges. Clin Microbiol Rev (2017) 30(1):409–47. 10.1128/CMR.00058-16
108.
Ejaz H Qamar MU Junaid K Younas S Taj Z Bukhari SNA et al The Molecular Detection of Class B and Class D Carbapenemases in Clinical Strains of Acinetobacter Calcoaceticus-Baumannii Complex: The High Burden of Antibiotic Resistance and the Co-Existence of Carbapenemase Genes. Antibiotics (2022) 11(9):1168. 10.3390/antibiotics11091168
109.
Freire MP Van Der Heijden IM do Prado GVB Cavalcante LS Boszczowski I Bonazzi PR et al Polymyxin Use as a Risk Factor for Colonization or Infection With Polymyxin-Resistant Acinetobacter Baumannii After Liver Transplantation. Transpl Infect Dis (2014) 16(3):369–78. 10.1111/tid.12210
110.
Oh DH Kim YC Kim EJ Jung IY Jeong SJ Kim SY et al Multidrug-Resistant Acinetobacter Baumannii Infection in Lung Transplant Recipients: Risk Factors and Prognosis. Infect Dis (Auckl) (2019) 51(7):493–501. 10.1080/23744235.2018.1556400
111.
Turbett SE Banach DB Bard JD Gandhi RG Letourneau AR Azar MM . Rapid Antimicrobial Resistance Detection Methods for Bloodstream Infection in Solid Organ Transplantation: Proposed Clinical Guidance, Unmet Needs, and Future Directions. Transpl Infect Dis (2023) 25:e14113. 10.1111/tid.14113
112.
Azar MM Turbett S Gaston D Gitman M Razonable R Koo S et al A Consensus Conference to Define the Utility of Advanced Infectious Disease Diagnostics in Solid Organ Transplant Recipients. Am J Transpl (2022) 22(12):3150–69. 10.1111/ajt.17147
113.
Palacios CF Peaper D Malinis MF Malinis MF Perreault S Cohen EA et al 535. Evaluation of the BioFire Blood Culture Identification (BCID2) Panel for Transplant Recipients With a Bloodstream Infection. Open Forum Infect Dis (2022) 9(Suppl. ment_2). 10.1093/ofid/ofac492.588
114.
Bhalodi AA MacVane SH Ford B Ince D Kinn PM Percival KM et al Real-World Impact of the Accelerate PhenoTest BC Kit on Patients With Bloodstream Infections in the Improving Outcomes and Antimicrobial Stewardship Study: A Quasiexperimental Multicenter Study. Clin Infect Dis (2022) 75(2):269–77. 10.1093/cid/ciab921
115.
Rood IGH Li Q . Review: Molecular Detection of Extended Spectrum-β-Lactamase- and Carbapenemase-Producing Enterobacteriaceae in a Clinical Setting. Diagn Microbiol Infect Dis (2017) 89(3):245–50. 10.1016/j.diagmicrobio.2017.07.013
116.
Rhoads DD Pournaras S Leber A Balada-Llasat JM Harrington A Sambri V et al Multicenter Evaluation of the BIOFIRE Blood Culture Identification 2 Panel for Detection of Bacteria, Yeasts, and Antimicrobial Resistance Genes in Positive Blood Culture Samples. J Clin Microbiol (2023) 61(6):e0189122. 10.1128/jcm.01891-22
117.
Ghebremedhin B Halstenbach A Smiljanic M Kaase M Ahmad-Nejad P . MALDI-TOF MS Based Carbapenemase Detection From Culture Isolates and From Positive Blood Culture Vials. Ann Clin Microbiol Antimicrob (2016) 15(1):5. 10.1186/s12941-016-0120-x
118.
AbdelGhani S Thomson GK Snyder JW Thomson KS . Comparison of the Carba NP, Modified Carba NP, and Updated Rosco Neo-Rapid Carb Kit Tests for Carbapenemase Detection. J Clin Microbiol (2015) 53(11):3539–42. 10.1128/JCM.01631-15
119.
Poirel L Nordmann P . Rapidec Carba NP Test for Rapid Detection of Carbapenemase Producers. J Clin Microbiol (2015) 53(9):3003–8. 10.1128/JCM.00977-15
120.
Tamma PD Aitken SL Bonomo RA Mathers AJ van Duin D Clancy CJ . Infectious Diseases Society of America Antimicrobial Resistant Treatment Guidance: Gram-Negative Bacterial Infections. Clin Infect Dis (2023) 72:e169–e183. 10.1093/cid/ciaa1478
121.
Bauer KA Perez KK Forrest GN Goff DA . Review of Rapid Diagnostic Tests Used by Antimicrobial Stewardship Programs. Clin Infect Dis (2014) 59(Suppl. l_3):S134–45. 10.1093/cid/ciu547
122.
Khoo BY Hon PY Leong J Sai Rama Sridatta P Thevasagayam NM Loy SQD et al Evaluation of NG-Test CARBA 5 Version 2, Cepheid Xpert Carba-R, and Carbapenem Inactivation Methods in Comparison to Whole-Genome Sequencing for the Identification of Carbapenemases in Non-Fermenting Gram-Negative Bacilli. J Clin Microbiol (2023) 61(9):e0031623. 10.1128/jcm.00316-23
123.
Li P Niu W Fang Y Zou D Liu H Qin Y et al Development and Evaluation of a Loop-Mediated Isothermal Amplification Assay for Rapid and Specific Identification of Carbapenem-Resistant Acinetobacter Baumannii Strains Harboring Bla OXA-23, and the Epidemiological Survey of Clinical Isolates. Microb Drug Resist (2020) 26(12):1458–65. 10.1089/mdr.2019.0441
124.
Mertins S Higgins PG Thunissen C Magein H Gilleman Q Mertens P et al Development of an Immunochromatographic Lateral Flow Assay to Rapidly Detect OXA-23-OXA-40-OXA-58- and NDM-Mediated Carbapenem Resistance Determinants in Acinetobacter Baumannii. J Med Microbiol (2023) 72(4). 10.1099/jmm.0.001681
125.
Paul M Carrara E Retamar P Tängdén T Bitterman R Bonomo RA et al European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Guidelines for the Treatment of Infections Caused by Multidrug-Resistant Gram-Negative Bacilli (Endorsed by European Society of Intensive Care Medicine). Clin Microbiol Infect (2022) 28(4):521–47. 10.1016/j.cmi.2021.11.025
126.
Harris PNA Tambyah PA Lye DC Lee TH Yilmaz M Alenazi TH et al Effect of Piperacillin-Tazobactam vs Meropenem on 30-Day Mortality for Patients With E Coli or Klebsiella Pneumoniae Bloodstream Infection and Ceftriaxone Resistance: A Randomized Clinical Trial. JAMA (2018) 320(10):984–94. 10.1001/jama.2018.12163
127.
Henderson A Paterson DL Chatfield MD Tambyah PA Lye DC De PP et al Association Between Minimum Inhibitory Concentration, Beta-Lactamase Genes and Mortality for Patients Treated With Piperacillin/Tazobactam or Meropenem From the MERINO Study. Clin Infect Dis (2021) 73(11):e3842–e3850. 10.1093/cid/ciaa1479
128.
Zusman O Farbman L Tredler Z Daitch V Lador A Leibovici L et al Association Between Hypoalbuminemia and Mortality Among Subjects Treated With Ertapenem Versus Other Carbapenems: Prospective Cohort Study. Clin Microbiol Infect (2015) 21(1):54–8. 10.1016/j.cmi.2014.08.003
129.
Kohlmann R Bähr T Gatermann SG . Species-Specific Mutation Rates for AmpC Derepression in Enterobacterales With Chromosomally Encoded Inducible AmpC β-Lactamase. J Antimicrob Chemother (2018) 73(6):1530–6. 10.1093/jac/dky084
130.
Akata K Muratani T Yatera K Naito K Noguchi S Yamasaki K et al Induction of Plasmid-Mediated AmpC β-Lactamase DHA-1 by Piperacillin/Tazobactam and Other β-Lactams in Enterobacteriaceae. PLoS One (2019) 14(7):e0218589. 10.1371/journal.pone.0218589
131.
Stewart AG Paterson DL Young B Lye DC Davis JS Schneider K et al Meropenem Versus Piperacillin-Tazobactam for Definitive Treatment of Bloodstream Infections Caused by AmpC β-Lactamase-Producing Enterobacter Spp, Citrobacter Freundii, Morganella Morganii, Providencia Spp, or Serratia Marcescens: A Pilot Multicenter Randomized Controlled Trial (MERINO-2). Open Forum Infect Dis (2021) 8(8):ofab387. 10.1093/ofid/ofab387
132.
Chaubey VP Pitout JDD Dalton B Gregson DB Ross T Laupland KB . Clinical and Microbiological Characteristics of Bloodstream Infections Due to AmpC β-Lactamase Producing Enterobacteriaceae: An Active Surveillance Cohort in a Large Centralized Canadian Region. BMC Infect Dis (2014) 14:647. 10.1186/s12879-014-0647-4
133.
Cheng L Nelson BC Mehta M Seval N Park S Giddins MJ et al Piperacillin-Tazobactam Versus Other Antibacterial Agents for Treatment of Bloodstream Infections Due to AmpC β-Lactamase-Producing Enterobacteriaceae. Antimicrob Agents Chemother (2017) 61(6):e00276–17. 10.1128/AAC.00276-17
134.
da Cunha Ferreira T Martins IS . Risk Factors of Death in Bloodstream Infections Caused by AmpC β-Lactamase-Producing Enterobacterales in Patients With Neoplasia. Infect Drug Resist (2021) 14:3083–97. 10.2147/IDR.S312920
135.
Lee N-Y Lee C-C Li C-W Li MC Chen PL Chang CM et al Cefepime Therapy for Monomicrobial Enterobacter Cloacae Bacteremia: Unfavorable Outcomes in Patients Infected by Cefepime-Susceptible Dose-Dependent Isolates. Antimicrob Agents Chemother (2015) 59(12):7558–63. 10.1128/AAC.01477-15
136.
Paterson DLL Singh N Rihs JDD Squier C Rihs BLL Muder RRR . Control of an Outbreak of Infection Due to Extended-Spectrum Beta-Lactamase-Producing Escherichia Coli in a Liver Transplantation Unit. Clin Infect Dis (2001) 33(1):126–8. 10.1086/320882
137.
Halaby T al Naiemi N Kluytmans J van der Palen J Vandenbroucke-Grauls CMJE . Emergence of Colistin Resistance in Enterobacteriaceae After the Introduction of Selective Digestive Tract Decontamination in an Intensive Care Unit. Antimicrob Agents Chemother (2013) 57(7):3224–9. 10.1128/AAC.02634-12
138.
Strenger V Gschliesser T Grisold A Zarfel G Feierl G Masoud L et al Orally Administered Colistin Leads to Colistin-Resistant Intestinal Flora and Fails to Prevent Faecal Colonisation With Extended-Spectrum β-Lactamase-Producing Enterobacteria in Hospitalised Newborns. Int J Antimicrob Agents (2011) 37(1):67–9. 10.1016/j.ijantimicag.2010.09.010
139.
Tacconelli E Mazzaferri F de Smet AM Bragantini D Eggimann P Huttner BD et al ESCMID-EUCIC Clinical Guidelines on Decolonization of Multidrug-Resistant Gram-Negative Bacteria Carriers. Clin Microbiol Infect (2019) 25(7):807–17. 10.1016/j.cmi.2019.01.005
140.
Castanheira M Doyle TB Collingsworth TD Sader HS Mendes RE . Increasing Frequency of OXA-48-Producing Enterobacterales Worldwide and Activity of Ceftazidime/Avibactam, Meropenem/Vaborbactam and Comparators Against These Isolates. J Antimicrob Chemother (2021) 76(12):3125–34. 10.1093/jac/dkab306
141.
Ackley R Roshdy D Meredith J Minor S Anderson WE Capraro GA et al Meropenem-Vaborbactam Versus Ceftazidime-Avibactam for Treatment of Carbapenem-Resistant Enterobacteriaceae Infections. Antimicrob Agents Chemother (2020) 64(5):e02313–19. 10.1128/AAC.02313-19
142.
Livermore DM Mushtaq S Vickers A Woodford N . Activity of Aztreonam/Avibactam Against Metallo-β-Lactamase-Producing Enterobacterales From the UK: Impact of Penicillin-Binding Protein-3 Inserts and CMY-42 β-Lactamase in Escherichia Coli. Int J Antimicrob Agents (2023) 61(5):106776. 10.1016/j.ijantimicag.2023.106776
143.
Bhagwat SS Hariharan P Joshi PR Palwe SR Shrivastava R Patel MV et al Activity of Cefepime/Zidebactam Against MDR Escherichia Coli Isolates Harbouring a Novel Mechanism of Resistance Based on Four-Amino-Acid Inserts in PBP3. J Antimicrob Chemother (2020) 75(12):3563–7. 10.1093/jac/dkaa353
144.
Pérez-Nadales E Fernández-Ruiz M Natera AM Gutiérrez-Gutiérrez B Mularoni A Russelli G et al Efficacy of Ceftazidime-Avibactam in Solid Organ Transplant Recipients With Bloodstream Infections Caused by Carbapenemase-Producing Klebsiella Pneumoniae. Am J Transpl (2023) 23(7):1022–34. 10.1016/j.ajt.2023.03.011
145.
Fariñas MC González-Rico C Fernández-Martínez M Fortún J Escudero-Sanchez R Moreno A et al Oral Decontamination With Colistin Plus Neomycin in Solid Organ Transplant Recipients Colonized by Multidrug-Resistant Enterobacterales: A Multicentre, Randomized, Controlled, Open-Label, Parallel-Group Clinical Trial. Clin Microbiol Infect (2021) 27(6):856–63. 10.1016/j.cmi.2020.12.016
146.
Gopalsamy SN Woodworth MH Wang T Carpentieri CT Mehta N Friedman-Moraco RJ et al The Use of Microbiome Restoration Therapeutics to Eliminate Intestinal Colonization With Multidrug-Resistant Organisms. Am J Med Sci (2018) 356(5):433–40. 10.1016/j.amjms.2018.08.015
147.
Zeng Z-R Wang W-P Huang M Shi L-N Wang Y Shao H-F . Mechanisms of Carbapenem Resistance in Cephalosporin-Susceptible Pseudomonas Aeruginosa in China. Diagn Microbiol Infect Dis (2014) 78(3):268–70. 10.1016/j.diagmicrobio.2013.11.014
148.
Pragasam AK Veeraraghavan B Anandan S Narasiman V Sistla S Kapil A et al Dominance of International High-Risk Clones in Carbapenemase-Producing Pseudomonas Aeruginosa: Multicentric Molecular Epidemiology Report From India. Indian J Med Microbiol (2018) 36(3):344–51. 10.4103/ijmm.IJMM_18_294
149.
Jovcic B Lepsanovic Z Suljagic V Rackov G Begovic J Topisirovic L et al Emergence of NDM-1 Metallo-β-Lactamase in Pseudomonas Aeruginosa Clinical Isolates From Serbia. Antimicrob Agents Chemother (2011) 55(8):3929–31. 10.1128/AAC.00226-11
150.
Sempere A Viñado B Los-Arcos I Campany D Larrosa N Fernández-Hidalgo N et al Ceftazidime-Avibactam Plus Aztreonam for the Treatment of Infections by VIM-Type-Producing Gram-Negative Bacteria. Antimicrob Agents Chemother (2022) 66(10):e0075122. 10.1128/aac.00751-22
151.
Mularoni A Mezzatesta ML Pilato M Medaglia AA Cervo A Bongiorno D et al Combination of Aztreonam, Ceftazidime–Avibactam and Amikacin in the Treatment of VIM-1 Pseudomonas Aeruginosa ST235 Osteomyelitis. Int J Infect Dis (2021) 108:510–2. 10.1016/j.ijid.2021.05.085
152.
Tirlangi PK Wanve BS Dubbudu RR Yadav BS Kumar LS Gupta A et al Successful Use of Cefepime-Zidebactam (WCK 5222) as a Salvage Therapy for the Treatment of Disseminated Extensively Drug-Resistant New Delhi Metallo-β-Lactamase-Producing Pseudomonas Aeruginosa Infection in an Adult Patient With Acute T-Cell Leukemia. Antimicrob Agents Chemother (2023) 67(8):e0050023. 10.1128/aac.00500-23
153.
Rigatto MH Oliveira MS Perdigão-Neto LV Levin AS Carrilho CM Tanita MT et al Multicenter Prospective Cohort Study of Renal Failure in Patients Treated With Colistin Versus Polymyxin B. Antimicrob Agents Chemother (2016) 60(4):2443–9. 10.1128/AAC.02634-15
154.
Penwell WF Shapiro AB Giacobbe RA Gu RF Gao N Thresher J et al Molecular Mechanisms of Sulbactam Antibacterial Activity and Resistance Determinants in Acinetobacter Baumannii. Antimicrob Agents Chemother (2015) 59(3):1680–9. 10.1128/AAC.04808-14
155.
Paul M Daikos GL Durante-Mangoni E Yahav D Carmeli Y Benattar YD et al Colistin Alone Versus Colistin Plus Meropenem for Treatment of Severe Infections Caused by Carbapenem-Resistant Gram-Negative Bacteria: An Open-Label, Randomised Controlled Trial. Lancet Infect Dis (2018) 18(4):391–400. 10.1016/S1473-3099(18)30099-9
156.
Kaye KS Marchaim D Thamlikitkul V Carmeli Y Chiu CH Daikos G et al Colistin Monotherapy Versus Combination Therapy for Carbapenem-Resistant Organisms. NEJM Evid (2023) 2(1). 10.1056/evidoa2200131
157.
Niederman MS Alder J Bassetti M Boateng F Cao B Corkery K et al Inhaled Amikacin Adjunctive to Intravenous Standard-of-Care Antibiotics in Mechanically Ventilated Patients With Gram-Negative Pneumonia (INHALE): A Double-Blind, Randomised, Placebo-Controlled, Phase 3, Superiority Trial. Lancet Infect Dis (2020) 20(3):330–40. 10.1016/S1473-3099(19)30574-2
158.
Kollef MH Ricard J-D Roux D Francois B Ischaki E Rozgonyi Z et al A Randomized Trial of the Amikacin Fosfomycin Inhalation System for the Adjunctive Therapy of Gram-Negative Ventilator-Associated Pneumonia: IASIS Trial. Chest (2017) 151(6):1239–46. 10.1016/j.chest.2016.11.026
159.
Rattanaumpawan P Lorsutthitham J Ungprasert P Angkasekwinai N Thamlikitkul V . Randomized Controlled Trial of Nebulized Colistimethate Sodium as Adjunctive Therapy of Ventilator-Associated Pneumonia Caused by Gram-Negative Bacteria. J Antimicrob Chemother (2010) 65(12):2645–9. 10.1093/jac/dkq360
160.
Wunderink RG Matsunaga Y Ariyasu M Clevenbergh P Echols R Kaye KS et al Cefiderocol Versus High-Dose, Extended-Infusion Meropenem for the Treatment of Gram-Negative Nosocomial Pneumonia (APEKS-NP): A Randomised, Double-Blind, Phase 3, Non-Inferiority Trial. Lancet Infect Dis (2021) 21(2):213–25. 10.1016/S1473-3099(20)30731-3
161.
Bassetti M Echols R Matsunaga Y Ariyasu M Doi Y Ferrer R et al Efficacy and Safety of Cefiderocol or Best Available Therapy for the Treatment of Serious Infections Caused by Carbapenem-Resistant Gram-Negative Bacteria (CREDIBLE-CR): A Randomised, Open-Label, Multicentre, Pathogen-Focused, Descriptive, Phase 3 Trial. Lancet Infect Dis (2021) 21(2):226–40. 10.1016/S1473-3099(20)30796-9
162.
Gatti M Cosentino F Giannella M Viale P Pea F . Clinical Efficacy of Cefiderocol-Based Regimens in Patients With Carbapenem-Resistant Acinetobacter Baumannii Infections: A Systematic Review With Meta-Analysis. Int J Antimicrob Agents (2024) 63(2):107047. 10.1016/j.ijantimicag.2023.107047
Summary
Keywords
solid organ transplant, multidrug-resistant Gram-negative bacteria, epidemiology, clinical impact, diagnosis and treatment, new anti-infective agents
Citation
Freire MP, Pouch S, Manesh A and Giannella M (2024) Burden and Management of Multi-Drug Resistant Organism Infections in Solid Organ Transplant Recipients Across the World: A Narrative Review. Transpl Int 37:12469. doi: 10.3389/ti.2024.12469
Received
24 November 2023
Accepted
07 May 2024
Published
17 June 2024
Volume
37 - 2024
Updates
Copyright
© 2024 Freire, Pouch, Manesh and Giannella.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maddalena Giannella, maddalena.giannella@unibo.it
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.