Abstract
Background: Extracorporeal membrane oxygenation (ECMO) is frequently used during lung transplantation. Unfractionated heparin (UFH) is mainly used as part of ECMO support for anticoagulation. One of the most common perioperative complications is bleeding, which high-dose UFH can aggravate.
Methods: We retrospectively analyzed (n = 141) patients who underwent lung transplantation between 2020 and 2022. All subjects (n = 109) underwent central cannulated VA ECMO with successful intraoperative ECMO weaning. Patients on ECMO bridge, postoperative ECMO, heart-lung transplants and transplants without ECMO were excluded. The dose of UFH for the entire surgical procedure, blood loss and consumption of blood derivatives intraoperatively and 48 h after ICU admission were recorded. Surgical revision for postoperative bleeding were analyzed. Thrombotic complications, mortality and long-term survival were evaluated.
Results: Lower doses of UFH administered for intraoperative ECMO anticoagulation contribute to a reduction in intraoperative blood derivates consumption and blood loss with no thrombotic complications related to the patient or the ECMO circuit. Lower doses of UFH may lead to a decreased incidence of surgical revision for hemothorax.
Conclusion: Lower doses of UFH as part of intraoperative ECMO anticoagulation might reduce the incidence of complications and lead to better postoperative outcomes.

Introduction
Intraoperative extracorporeal membrane oxygenation (ECMO) is routinely used during lung transplant surgery to provide the patient with temporary respiratory and circulatory support [1–3]. Over the past two decades, many centres have switched from intraoperative support with cardiopulmonary bypass (CPB) to ECMO [1, 2, 4, 5]. The intraoperative ECMO approach allows surgeons to perform the procedure with greater precision and efficiency while minimizing the risk of lung injury [2, 4]. Most centres that use intraoperative ECMO, to a greater extent, support the idea that ECMO helps to control lung graft reperfusion [4, 6, 7]. Another advantage of intraoperative ECMO support is the possibility of transplanting more patients with comorbidities who would not be able to undergo surgery on selective unilateral ventilation without extracorporeal support. For example, patients with severe pulmonary hypertension have a high risk of left-sided heart failure, pulmonary fibrosis, and very low pulmonary compliance [8, 9]. ECMO has a much lower pro-inflammatory potential than CPB [1]. However, ECMO also carries risks of bleeding, infection, and thrombotic complications [10–13]. The entire team should decide to use intraoperative ECMO on a case-by-case basis, considering the patient’s medical history and condition [14, 15]. Our study aims to highlight that lower doses of anticoagulants administered during intraoperative ECMO support do not lead to increased risks and may be beneficial for the patient.
Intraoperative ECMO Anticoagulation During Lung Transplants
A certain amount of anticoagulation is crucial to prevent thrombotic complications during lung transplantation with intraoperative ECMO support [1, 2, 11]. Thrombotic events, such as clot formation, can cause significant harm to the patient and negatively impact the transplant’s success. However, the use of intraoperative anticoagulation is associated with a risk of bleeding, which can be problematic during surgery. Full heparinization with high activated clotting time (e.g., ACT >400 s) values are no longer necessary from CBP to ECMO support transition (also, thanks to heparin-coated ECMO cannulas and circuits). In contrast, the more knowledge we have about ECMO issues in the context of understanding coagulopathy, the more we strive for significantly lower doses of anticoagulation [4]. According to the current guidelines, the recommended procedure for ECMO cannulation is to administer a certain UFH bolus, usually 2000–5000 IU or 25–100 IU/kg, and then control the anticoagulation level using ACT [1, 11]. The ACT should be maintained in the range of 180–220 s [1, 11]. Other options (increasingly used over ACT) for anticoagulation monitoring are activated partial thrombin time (APTT) in the range of 60–90 s [16] and APTT ratio of 1.5–2.5 [16]. The anti-Xa assay is also a possible method for monitoring anticoagulation. The target values of the anti-Xa assay are 0.3–0.7 IU/mL [16]. According to the literature, it is also possible to perform anticoagulation monitoring using viscoelastic methods such as rotational thromboelastometry (ROTEM), explicitly using the CT INTEM/HEPTEM ratio [17].
Materials and Methods
This study was approved by the Local Ethics Committee (reference number EK-786/23) and was registered in the clinical trial database at ClinicalTrials.gov (identifier number NCT06054997). This study was designed as a single-centre, retrospective, observational study that included all lung transplants performed between January 2020 and December 2022 within the Prague Lung Transplant Program Motol University Hospital. A total of 141 patients underwent transplantation during the study period. The exclusion criteria were lung transplantation without intraoperative ECMO support, block heart-lung transplantation, ECMO bridge-to-lung transplant, and planned postoperative ECMO. The inclusion criteria were lung transplantation performed with intraoperative ECMO support, central ECMO cannulation, and successful ECMO support termination at the end of surgery. According to the study inclusion criteria, only lung transplants performed under intraoperative central ECMO cannulation with successful ECMO weaning were included. In total, 109 patients fulfilled the inclusion criteria. Thirty-two patients were excluded based on the following criteria: heart-lung transplant (HLTx), n = 4; ECMO bridge, n = 4; transplantation (Tx) without ECMO, n = 8; and planned prolonged ECMO, n = 16. UFH was used for ECMO anticoagulation in all the patients. The subjects were divided into two groups for further analysis according to the UFH dose administered during the entire surgical procedure. In the first group, the UFH dose was ≤60 IU/kg/surgery. In the second group, the UFH dose was greater than 61 IU/kg/surgery. A cutoff value of 60 IU/kg was determined based on the available literature review. Values ≤60 IU/kg/surgery were considered relatively lower doses, and values >61 IU/kg/surgery were considered higher doses of UFH [2, 4, 10–13, 16–19]. The UFH effect was monitored using activated clotting time (ACT) values. The intraoperative haemoglobin level target for red blood cell (RBC) substitution was 100 g/L. Coagulopathy was managed according to the clinical experience of the anesthesiologist and viscoelastic Point of care methods (ROTEM, PFA). The parameters followed up intraoperatively in both groups were total blood loss in milliliters and related to the patient’s weight during the surgery (assessed by the amount of blood in a calibrated suction device); the total amount of UFH administered to the patient during surgery in the international unit (IU) and related to patient weight; and the consumption of blood derivatives during the surgical procedure, such as RBC, fresh frozen plasma (FFP), and platelets (PLT). In both groups, ACT values were monitored after the administration of UFH before ECMO cannulation and then every 60 min. In both groups, protamine was administered at the end of the surgical procedure until physiological ACT values below 120 s were achieved (if needed). No type of biological glue to seal the anastomosis was used. In both groups, intraoperative VA ECMO was implanted electively, and no patient underwent urgent ECMO cannulation due to cardiac or pulmonary reasons. The Maquet Rotaflow RF-32 centrifugal pump provided Intraoperative VA ECMO support. Heparin-coated cannulas and a heparin-coated tubing system were used for cannulation. According to internal guidelines, the ECMO flow was maintained at 1/2 to 2/3 of the calculated cardiac output. In the postoperative period, we followed up on the development of hemothorax requiring surgical revision. We considered surgical revision for hemothorax to be a significant bleeding complication. We also evaluated blood product consumption in the period 48 h after ICU admission, PGD grade three in 72 h after LUTx, 30-day and 90-day mortality, long-term survival and ECMO circuit-related and patient-related thrombotic complications. The basic, standard points of care in the Intensive care unit (ICU) are displayed in Table 1.
TABLE 1
| Mechanical ventilation |
|---|
| • Maximum effort to achieve early extubation |
| • Upon admission to the Intensive Care Unit |
| • Pressure control mode mechanical ventilation (initially PEEP 8–10 cmH2O) |
| • Pressure support mode mechanical ventilation (PEEP 5 cmH2O) |
| • extubation |
| Vasopressoric support |
| • First choice—Norepinephrine |
| • Additional—Vasopressin |
| Immunosuppressives medication |
| • Tacrolimus |
| • Mycophenolate-mofetil |
| • Methylprednisolone |
| • Basiliximab |
| Antibiotics and antivirotics |
| • Piperaciline-Tazobactam i.v. |
| • Amphotericin inh. |
| • Ganciclovir i.v. |
| Analgesia |
| combination, according to a visual analogue scale and patient needs |
| • Bilateral ESPB |
| • Sufentanil |
| • Paracetamol |
| • NSAID |
| • Dexmedetomidine |
| • LMWH (Enoxaparine) s.c. based on antiXa assay |
| • Ketamin |
| Thrombosis prevention |
| • LMWH (Enoxaparine) s.c. based on antiXa assay |
| Specific examination and procedures |
| • Chest X-ray every 24 h |
| • Physiotherapy 4 times/day or based on the needs of the patient |
| • Microbiology findings every 24 h |
| • Pulmonary artery pressure continual monitoring |
| • Continual hemodynamic monitoring |
| • Early enteral feeding |
Standard, essential post-transplant ICU care.
Abbreviations: PEEP, Positive end-expiratory pressure; cmH2O centimeter of water; i.v. intravenous; inh. inhalation; NSAID, non-steroidal anti-inflammatory drugs; LMWH, low molecular weight heparin, s.c. subcutaneous.
Recipient and Donor Characteristics
Both groups of recipients were relatively homogenous, even though the number of subjects was not the same in both groups (lower dose UFH/kg group, n = 44; higher dose UFH/kg group, n = 65). The p-values for sex, age, height and mean pulmonary artery pressure (mPAP) were above the significance level of 0.05. The lower dose UFH/kg group recipients had a slightly higher weight (p = 0.048) and a higher BMI (p = 0.0093). The distribution of diagnoses for which the recipients were transplanted was also homogeneous (p > 0.05). These numbers are listed in Table 2. Donor characteristics were completely homogeneous in both groups. The p-values of sex, age, weight, height, BMI, and cause of death were above the significance level of 0.05 (Table 2).
TABLE 2
| Recipient-characteristic variable | ≤60 IU/kg UFH group (n = 44) | >61 IU/kg UFH group (n = 65) | p-value |
|---|---|---|---|
| Male sex, n (%) | 26 (59.09%) | 44 (67.69%) | 0.36 |
| Age (years; mean ± SD) | 54.14 ± 11.53 | 52.09 ± 12.47 | 0.59 |
| Weight (kg; mean ± SD) | 79.91 ± 15.47 | 73.93 ± 15.26 | 0.048 |
| Height (cm; mean ± SD) | 172.1 ± 7.39 | 173.4 ± 7.72 | 0.39 |
| Body mass index (mean ± SD) | 26.9 ± 4.43 | 24.6 ± 4.57 | 0.0093 |
| mPAP (torr; mean ± SD) | 26.3 ± 9.6 | 27.8 ± 13.4 | 0.528 |
| thoracotomy prior LUTx (sum) | 1 | 1 | 0.78 |
| Transplant indication | |||
| COPD, n (%) | 15 (34%) | 26 (40%) | 0.59 |
| Pulmonary fibrosis, n (%) | 18 (40.9%) | 23 (35.38%) | 0.50 |
| Cystic fibrosis, n (%) | 3 (6.8%) | 5 (7.69%) | 0.89 |
| Others, n (%) | 8 (18.18%) | 11 (16.92%) | 0.82 |
| Donor-characteristic variable | ≤60 IU/kg UFH group (n = 44) | >61 IU/kg UFH group (n = 65) | p-value |
|---|---|---|---|
| Male sex, n (%) | 21 (41.72%) | 37 (56.92%) | 0.34 |
| Age (years; mean ± SD) | 47.41 ± 16.05 | 44.78 ± 16.32 | 0.41 |
| Weight (kg; mean ± SD) | 70.9 ± 13.62 | 76.7 ± 17.12 | 0.059 |
| Height (cm; mean ± SD) | 170.3 ± 10.12 | 172.6 ± 10.95 | 0.26 |
| Body mass index (mean ± SD) | 24.1 ± 3.64 | 25.6 ± 4.7 | 0.074 |
| Cause of death | |||
| Subarachnoid haemorrhage, n (%) | 10 (22.7%) | 14 (21.5%) | 0.88 |
| Intracerebral bleeding, n (%) | 8 (18.2%) | 14 (21.5%) | 0.67 |
| Traumatic brain injury, n (%) | 15 (34.1%) | 16 (24.6%) | 0.28 |
| Anoxic brain injury, n (%) | 8 (18.2%) | 13 (20.0%) | 0.81 |
| Others, n (%) | 3 (6.8%) | 8 (12.3%) | 0.35 |
Recipient and donor characteristics.
Abbreviations: IU, international unit; UFH, unfractionated heparin; SD, standard deviation; n, Number of Subjects; kg, Kilogram(s); COPD, chronic obstructive pulmonary disease; mPAP, mean pulmonary arterial pressure.
Statistical Analysis
Statistical analyses were performed with version 8.0.1 (244) of GraphPad Prism statistical software. Statistical significance was set at p < 0.05. The unpaired t-test was used to statistically evaluate blood loss, UFH dose, consumption of blood derivatives, and ACT values. For the statistical evaluation of surgical revision for hemothorax and PGD, we chose chi-square test. Kaplan-Mayer curve and log-rank test have been performed for 30-day and 90-day” and long-term survival assessment (long-term survival time endpoint October/2023).
Results
Patients were recruited between January 2020 and December 2022, and based on the exclusion criteria, a total of 32/141 patients were excluded from the study. A flow diagram based on the Consolidated Standards of Reporting Trials (CONSORT) is shown in Figure 1. The lower dose of UFH (≤60 IU/kg/surgical procedure) and higher dose of UFH (>61 IU/kg/surgical procedure) groups ultimately consisted of 44 and 65 patients, respectively. For most parameters, we obtained surprising results, clearly in favour of the administration of lower doses of UFH (≤60 IU/kg). Total blood loss during surgery was significantly lower in the group treated with lower doses of UFH (≤60 IU/kg). The mean total intraoperative blood loss was 753 and 1,470 mL, respectively (p < 0.0001) (Table 3). Blood loss related to body weight was also significantly lower in the UFH group ≤60 IU/kg). The mean intraoperative blood loss/patient body weight was 9.628 mL/kg and 20.97 mL/kg, respectively (p < 0.0001) (Table 3). The total UFH dose was significantly lower in the UFH group (≤60 IU/kg). The mean total intraoperative UFH doses were 3491 IU and 8694 IU, respectively (p < 0.0001) (Table 3). The total UFH dose, based on body weight, was significantly lower in the UFH group (≤60 IU/kg). The mean dose of UFH/patient bodyweight intraoperatively was 43.81 IU/kg and 116.6 IU/kg, respectively (p < 0.0001) (Table 3). We also noticed a significant difference in favour of reducing the consumption of blood derivatives in the group with lower doses of UFH ≤60 IU/kg. The consumption of RBCs during the surgical procedure was 0.5581 and 1.908 units, respectively (p = 0.0009) (Table 3). The FFP consumption during surgery was 0.4186 and 1.862 units, respectively (p = 0.0009) (Table 3). The platelet consumption during surgery was 0.1628 and 0.4154 units, respectively (p = 0.1461) (Table 3). The mean ACT values before ECMO cannulation and 3 min after the administration of UFH were lower in the UFH group ≤60 IU/kg (156.3 and 209.1) (p < 0.0001) (Table 3). There was a significant reduction in bleeding complications in terms of surgical revision for hemothorax in the lower UFH dose group ≤60 IU/kg, with only one revision (2.27%) and nine revisions (13.85%), respectively (p = 0.040) (Table 3). In the postoperative period 48 h after admission to the ICU, we did not observe a significant decrease in the consumption of blood derivatives (Table 4). However, there was a significantly lower incidence of third-degree PGD 72 h after LUTx in the group where a lower dose of UFH was administered (p = 0.038) (Table 4; Figure 2). The 30-day, 90-day, and long-term survivals were not different (Figure 3). The log-rank test was p = 0,6879 (Figure 3). Mortality rates were not different in either group. We did not record any thrombotic complications arising from the ECMO circuit or patient-related complications in any group.
FIGURE 1
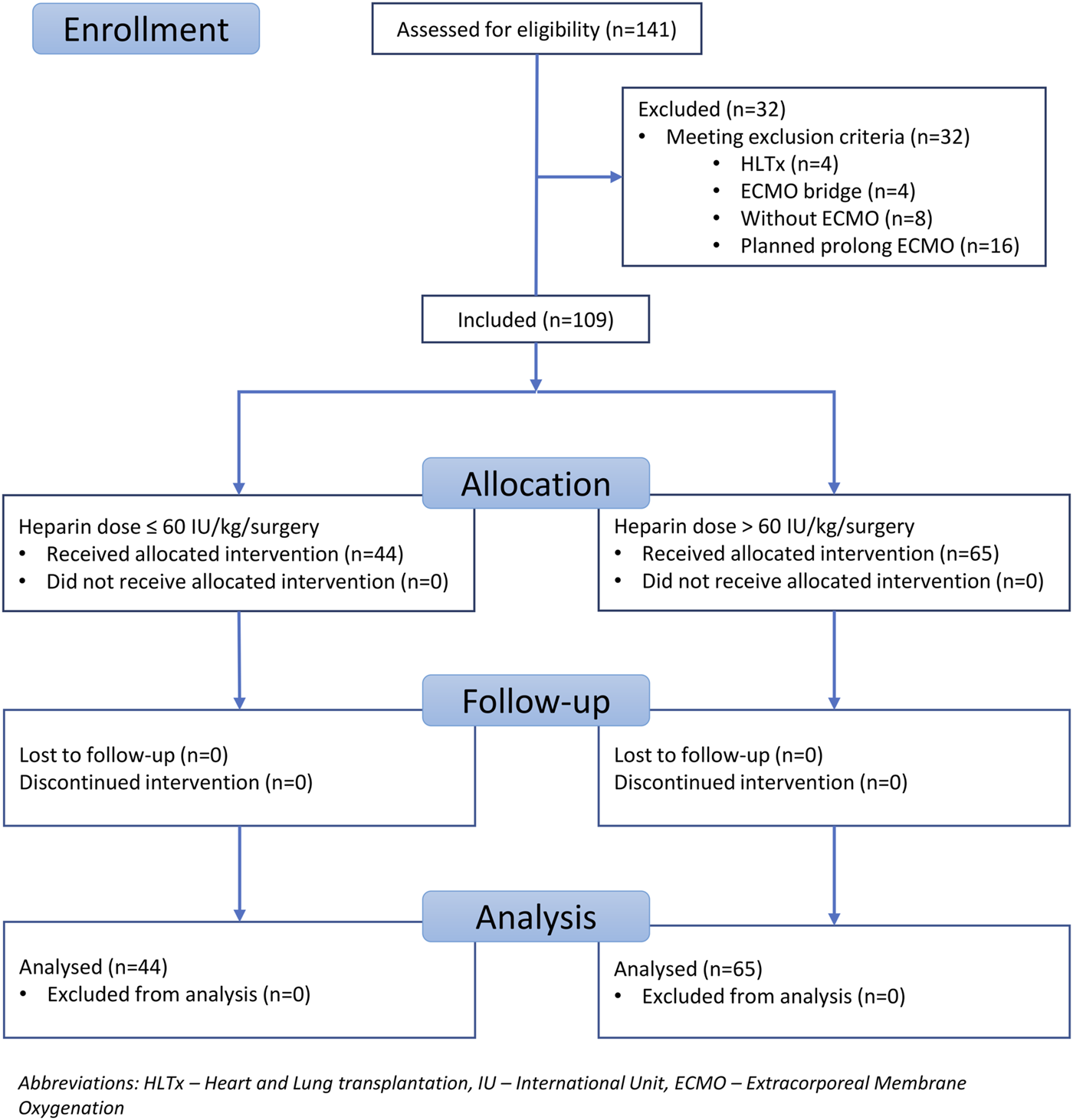
Flow chart of the study population.
TABLE 3
| Variables | ≤60 IU/kg UFH group (n = 44) | > 61 IU/kg UFH group (n = 65) | p-value |
|---|---|---|---|
| Total blood loss (ml; mean ± SD) | 753.5 ± 522.5 | 1,470 ± 1,014 | <0.0001 |
| Blood loss per kg (ml/kg; mean ± SD) | 9.63 ± 6.59 | 20.97 ± 15.64 | <0.0001 |
| Total dose of heparin (IU; mean ± SD) | 3,491 ± 1,088 | 8,694 ± 3,790 | <0.0001 |
| Dose of heparin per kg (IU/kg; mean ± SD) | 43.81 ± 12.08 | 116.6 ± 44.98 | <0.0001 |
| RBC consumption (unit) | 0.5581 ± 1.119 | 1.908 ± 2.435 | 0.0009 |
| FFP consumption (unit) | 0.4186 ± 1.349 | 1.862 ± 2.543 | 0.0009 |
| PLT consumption (unit) | 0.1628 ± 0.5314 | 0.4154 ± 1.044 | 0.1461 |
| Surgical revision due to haemothorax (% of revision) | 2.27 | 13.85 | 0.040 |
Variables blood loss, intraoperative blood product consumption, UFH dose, and surgical revision for haemothorax.
Abbreviations: IU, international unit; UFH, unfractionated heparin, mL, millilitre; kg, kilogram; SD, standard deviation; RBC, red blood cells; FFP, fresh frozen plasma; PLT, platelets.
Bold values are statistically significant.
TABLE 4
| Variables | ≤60 IU/kg UFH group (n = 44) | > 61 IU/kg UFH group (n = 65) | p-value |
|---|---|---|---|
| PGD grade 3 (total, percentage) | 0 (0%) | 6 (9.23%) | 0.038 |
| RBCs consumption mean (unit) | 1.43 ± 2.0 | 1.55 ± 2.4 | 0.7996 |
| FFP consumption mean (unit) | 0.41 ± 1.4 | 1.12 ± 3.0 | 0.1567 |
| PLT consumption mean (unit) | 0.23 ± 1.1 | 0.35 ± 1.1 | 0.5615 |
Variables post LUTx (PGD 3 in 72hours post LUTx), (FFP, RBC, PLT 48 h post LUTx).
Abbreviations: IU, international unit; UFH, unfractionated heparin; LUTx, lung transplantation; PGD, primary graft dysfunction; FFP, fresh frozen plasma; RBCs, red blood cells; PLT, platelets; IU, international unit.
Bold values are statistically significant.
FIGURE 2
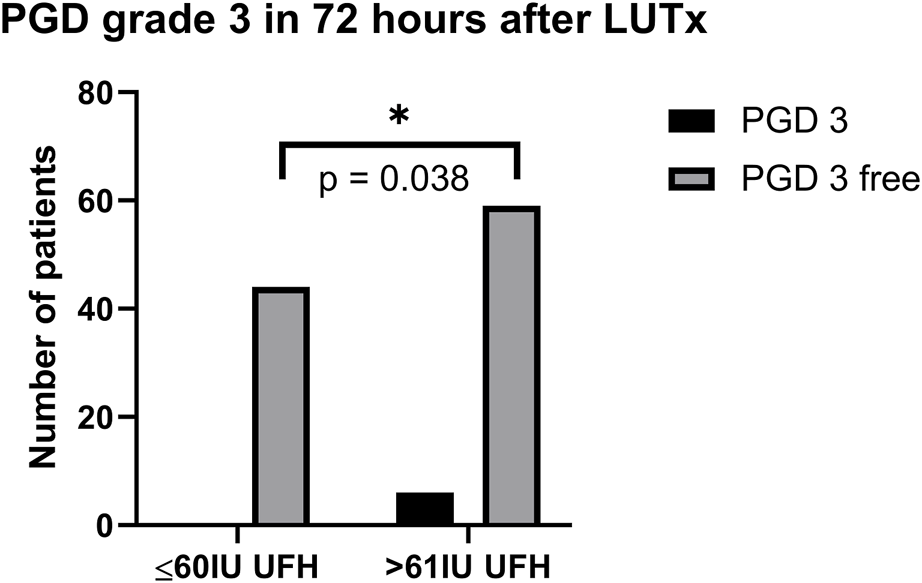
PGD grade 3 in 72 h after LUTx. Abbreviations: PGD, primary graft dysfunction; UFH, unfractionated heparin; LUTx, lung transplantation.
FIGURE 3
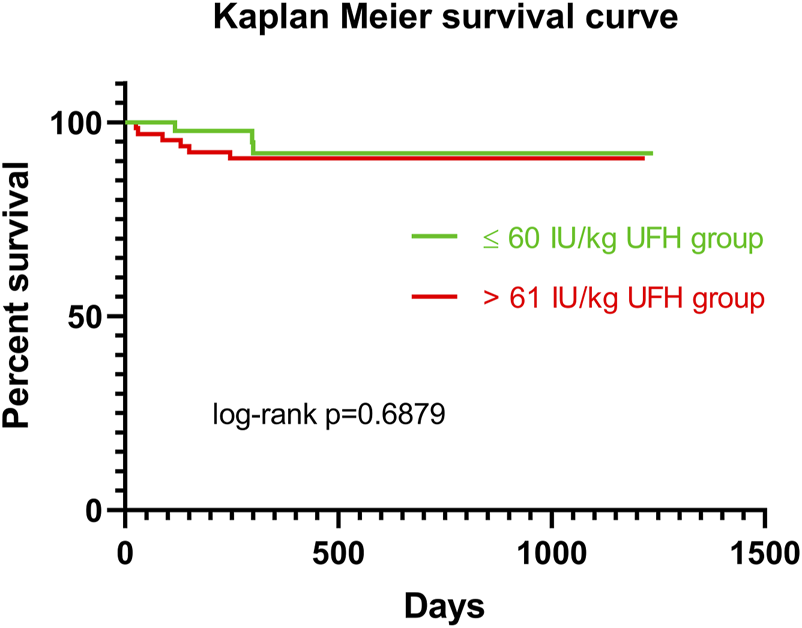
Kaplan Meier survival curve for patients in ≤60 IU/kg UFH group (green line) vs >61 IU/kg UFH group (red line). Abbreviations: IU, international unit; UFH, unfractionated heparin.
Discussion
Intraoperative ECMO support during lung transplantation is a routine method frequently used to facilitate surgical procedures. This enables the procedure to be performed in significantly more polymorbid patients who cannot handle selective ventilation. Another indisputable advantage of ECMO is the possibility of controlled reperfusion of lung grafts. During ECMO support, the blood is in contact with allogeneic materials such as cannulas, circuits, and oxygenators. All of these factors can cause potential complications, including bleeding and thrombosis. Therefore, balancing the edge between anticoagulation and procoagulation is very important to minimize complications during intraoperative ECMO support. UFH remains the most widely used drug for ECMO anticoagulation. From the available recommendations of thoracosurgery societies, we know the recommendation for UFH dosing, most often between 25–100 IU/kg and effect control with ACT in the range of 180–220 s. However, the literature and the guidelines more frequently report the possibility of reducing the dose of UFH and, very importantly, without an increase in thrombotic complications. According to the recommendations of the thoracosurgery societies, in the case of bleeding complications or anticipated intraoperative bleeding (for example, significant intrapleural adhesions), it is recommended to minimize the dose of UFH or completely eliminate UFH and perform heparin-free ECMO. For example, Bernhardt et al. mentioned in The International Society for Heart and Lung Transplantation/Heart Failure Society of America Guidelines on Acute Mechanical Circulatory Support that “bleeding complications in acute mechanical circulatory support (MCS) are common and frequently necessitate withdrawal of anticoagulation” and stated that “in the settings of life-threatening bleeding, full discontinuation of all anticoagulation may be necessary” [20]. Hartwig et al. stated in The American Association for Thoracic Surgery guidelines that “low or no heparin regimes are recommended for patients with significant adhesions and impaired coagulation status” [1]. Additionally, Lorusso et al., in the EACTS/ELSO/STS/AATS expert consensus, mentioned that “anticoagulation is required during prolonged ECLS to prevent circuit thrombus formation with embolization and/or circuit failure. However, bleeding remains the most frequent complication associated with ECLS. UFH infusion is typically delayed until haemostasis is achieved, often within 24–48 h. Reports that suggest the safety of prolonged withdrawal of anticoagulation for as long as 3 days when faced with bleeding are important” [21]. This trend was also confirmed by our study, which suggests that a lower dose of UFH as part of intraoperative ECMO support may not pose a risk but, on the contrary, can have substantial benefits for the patient. Using lower doses of UFH below 60 IU/kg (43.81 IU/kg based on our study) can benefit the patient and the entire perioperative period. Nonetheless, these results must be interpreted with caution, and numerous limitations should be considered. The major limitation is that the dosage of the UFH used in the study (high or low dose) has been based on subjective criteria, i.e., the clinical experience of the anesthesiologist guides its dosage. Another possible limitation of this study may be the division of subjects according to UFH into groups below and above 60 IU/kg since the available literature does not define the exact dose of UFH but only the range. Therefore, it was necessary to set a limit for dividing the patients into groups. However, the medians of the two groups were very far apart 43.81 IU/kg vs. 116.6 IU/kg; we presume this is more of a minor bias. Furthermore, having a more significant number of investigated subjects would be advisable, which would add even more weight to the entire study. Similarly, a particular bias may have been introduced into this study because the primary hemostasis disorder was not investigated; primary hemostasis can be disturbed as part of ECMO support during lung transplantation, thereby potentiating intraoperative bleeding [22]. The authors hypothesize that the significantly higher incidence of PGD grade 3 in 72 h postop in the group with higher doses of UFH may be caused by the need to administer a larger number of blood derivatives, which is one of the possible reason for the development of PGD. The anesthesiologist plays a crucial role in the decision-making process and is mostly responsible for anticoagulation management of intraoperative ECMO support [15, 23, 24]. Such management depends not only on their experience but also on their knowledge of the latest findings and recommended practices. The decision to withhold anticoagulation therapy or decrease its dosage involves balancing the competing risks between bleeding and clotting.
Conclusion
The results of this study generates a hypothesis that lower doses of UFH (mean dose of UFH: 43.81 IU/kg) administered for intraoperative ECMO anticoagulation may contribute to a reduction in intraoperative blood loss and decrease the incidence of surgical revisions for haemothorax. Furthermore, lower doses of UFH may reduce the intraoperative consumption of blood derivatives such as RBCs and FFP. Notably, the concept of lower doses of UFH did not have a negative effect on 30-day, 90-day and long-term survival. No thrombotic complications of the ECMO circuit or thrombotic complications related to the patient were observed. Further investigation in this area is needed to provide deeper insights into the potential use of lower doses of UFH during ECMO.
Statements
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Local Ethics Committee (reference number EK-786/23). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
JaV designed the study; JaV and GH collected the study data; JaV analyzed the data and wrote the major part of the manuscript; GH, JiV, MS, RN, TV, and RL participated in the research performance and/or substantially contributed to the writing of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Institutional Project Cooperation Program, Research Area INCA. These funding agencies played no role in the data analysis or the presentation of this article.
Acknowledgments
The authors thank all the anesthesiologists and intensivists involved in this study, especially Filip Spisak, Tomas Kopanko, Eva Pokorna, Kristyna Petrovicova, Karel Dana, Lenka Laitnerova, Vladimir Bicek, Vojtech Simecek, Michal Garaj, Martina Krecmerova, Martin Malosek, Jan Berousek, Miroslav Durila, Zuzana Prikrylova, Jan Sipek, Jan Bures, Vlasta Vlasakova, Jaroslav Hylmar, and Zdenek Havelka. The authors also thank all surgeons involved in this study, especially Jan Simonek, Jiri Pozniak, Jan Kolarik, and Jan Burkert. The authors also thank all perfusionists involved in this study, especially Michal Hlavacek, Pavel Hedvicak, Vojtech Veverka, Tomas Smetak, Pavol Kovac, and Adam Necesany.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
ACT, activated clotting time; APTT, activated partial thrombin time; BMI, body mass index; CONSORT, Consolidated Standards for Reporting Trials; CPB, cardiopulmonary bypass; ECMO, extracorporeal membrane oxygenation; FFP, fresh frozen plasma; HLTx, heart-lung transplant; ICU, intensive care unit; LUTx, lung transplant; MCS, mechanical circulatory support; NSAID, non-steroidal anti-inflammatory drugs; PEEP, positive end-expiratory pressure; PFA, platelet function assay; RBCs, red blood cells; ROTEM, rotational thromboelastometry; Tx, transplantation; UFH, unfractionated heparin.
References
1.
Hartwig M van Berkel V Bharat A Cypel M Date H Erasmus M et al The American Association for Thoracic Surgery (AATS) 2022 Expert Consensus Document: The Use of Mechanical Circulatory Support in Lung Transplantation. J Thorac Cardiovasc Surg (2023) 165(1):301–26. 10.1016/j.jtcvs.2022.06.024
2.
Moreno Garijo J Cypel M McRae K Machuca T Cunningham V Slinger P . The Evolving Role of Extracorporeal Membrane Oxygenation in Lung Transplantation: Implications for Anesthetic Management. J Cardiothorac Vasc Anesth (2019) 33(7):1995–2006. 10.1053/j.jvca.2018.10.007
3.
Thomas M Martin AK Allen WL Makey IA Renew JR Rodrigues ES et al Lung Transplantation Using a Hybrid Extracorporeal Membrane Oxygenation Circuit. ASAIO J Am Soc Artif Intern Organs (2020) 66(10):e123–5. 10.1097/MAT.0000000000001157
4.
Hoetzenecker K Benazzo A Stork T Sinn K Schwarz S Schweiger T et al Bilateral Lung Transplantation on Intraoperative Extracorporeal Membrane Oxygenator: An Observational Study. J Thorac Cardiovasc Surg (2020) 160(1):320–7.e1. 10.1016/j.jtcvs.2019.10.155
5.
Martin AK Yalamuri SM Wilkey BJ Kolarczyk L Fritz AV Jayaraman A et al The Impact of Anesthetic Management on Perioperative Outcomes in Lung Transplantation. J Cardiothorac Vasc Anesth (2020) 34(6):1669–80. 10.1053/j.jvca.2019.08.037
6.
Loor G Huddleston S Hartwig M Bottiger B Daoud D Wei Q et al Effect of Mode of Intraoperative Support on Primary Graft Dysfunction After Lung Transplant. J Thorac Cardiovasc Surg (2022) 164(5):1351–61.e4. 10.1016/j.jtcvs.2021.10.076
7.
Porteous MK Diamond JM Christie JD . Primary Graft Dysfunction: Lessons Learned About the First 72 h After Lung Transplantation. Curr Opin Organ Transpl (2015) 20(5):506–14. 10.1097/MOT.0000000000000232
8.
George PM Patterson CM Reed AK Thillai M . Lung Transplantation for Idiopathic Pulmonary Fibrosis. Lancet Respir Med (2019) 7(3):271–82. 10.1016/S2213-2600(18)30502-2
9.
Chicotka S Pedroso FE Agerstrand CL Rosenzweig EB Abrams D Benson T et al Increasing Opportunity for Lung Transplant in Interstitial Lung Disease With Pulmonary Hypertension. Ann Thorac Surg (2018) 106(6):1812–9. 10.1016/j.athoracsur.2018.04.068
10.
Aubron C DePuydt J Belon F Bailey M Schmidt M Sheldrake J et al Predictive Factors of Bleeding Events in Adults Undergoing Extracorporeal Membrane Oxygenation. Ann Intensive Care (2016) 6(1):97. 10.1186/s13613-016-0196-7
11.
Bartlett R Arachichilage DJ Chitlur M Hui SKR Neunert C Doyle A et al The History of Extracorporeal Membrane Oxygenation and the Development of Extracorporeal Membrane Oxygenation Anticoagulation. Semin Thromb Hemost (2023) 50:81–90. 10.1055/s-0043-1761488
12.
Olson SR Murphree CR Zonies D Meyer AD Mccarty OJT Deloughery TG et al Thrombosis and Bleeding in Extracorporeal Membrane Oxygenation (ECMO) Without Anticoagulation: A Systematic Review. ASAIO J Am Soc Artif Intern Organs (2021) 67(3):290–6. 10.1097/MAT.0000000000001230
13.
Rajsic S Breitkopf R Oezpeker UC Bukumirić Z Dobesberger M Treml B . The Role of Excessive Anticoagulation and Missing Hyperinflammation in ECMO-Associated Bleeding. J Clin Med (2022) 11(9):2314. 10.3390/jcm11092314
14.
Fessler J Finet M Fischler M Le Guen M . New Aspects of Lung Transplantation: A Narrative Overview Covering Important Aspects of Perioperative Management. Life Basel Switz (2022) 13(1):92. 10.3390/life13010092
15.
Martin AK Reed AK Hoetzenecker K Fessler J . How We Would Treat Our Own Lung Transplantation: A Multidisciplinary and International Perspective. J Cardiothorac Vasc Anesth (2023) 37:2207–14. 10.1053/j.jvca.2023.07.042
16.
McMichael ABV Ryerson LM Ratano D Fan E Faraoni D Annich GM . 2021 ELSO Adult and Pediatric Anticoagulation Guidelines. ASAIO J Am Soc Artif Intern Organs (2022) 68(3):303–10. 10.1097/MAT.0000000000001652
17.
Vajter J Garaj M Lischke R Durila M . (710) Bolus Dosage of Heparin According to ACT Versus Continuous Dosage of Heparin According to ROTEM for ECMO Anticoagulation in Lung Transplantation - Effect on Perioperative Blood Loss. J Heart Lung Transpl (2023) 42(4):S314–5. 10.1016/j.healun.2023.02.724
18.
Kumar G Maskey A . Anticoagulation in ECMO Patients: An Overview. Indian J Thorac Cardiovasc Surg (2021) 37(2):241–7. 10.1007/s12055-021-01176-3
19.
Rajsic S Breitkopf R Jadzic D Popovic Krneta M Tauber H Treml B . Anticoagulation Strategies During Extracorporeal Membrane Oxygenation: A Narrative Review. J Clin Med (2022) 11(17):5147. 10.3390/jcm11175147
20.
Bernhardt AM Copeland H Deswal A Gluck J Givertz MM , Task Force 1. The International Society for Heart and Lung Transplantation/Heart Failure Society of America Guideline on Acute Mechanical Circulatory Support. J Card Fail (2023) 29(3):304–74. 10.1016/j.cardfail.2022.11.003
21.
Lorusso R Whitman G Milojevic M Raffa G McMullan DM Boeken U et al 2020 EACTS/ELSO/STS/AATS Expert Consensus on Post-Cardiotomy Extracorporeal Life Support in Adult Patients. Eur J Cardio-thorac Surg Off J Eur Assoc Cardio-thorac Surg (2021) 59(1):12–53. 10.1093/ejcts/ezaa283
22.
Garaj M Durila M Vajter J Solcova M Marecek F Hrachovinová I . Extracorporeal Membrane Oxygenation Seems to Induce Impairment of Primary Hemostasis Pathology as Measured by a Multiplate Analyzer: An Observational Retrospective Study. Artif Organs (2022) 46(5):899–907. 10.1111/aor.14142
23.
Durila M Vajter J Garaj M Pollert L Berousek J Vachtenheim J Jr et al Rotational Thromboelastometry Reduces Blood Loss and Blood Product Usage After Lung Transplantation. J Heart Lung Transpl Off Publ Int Soc Heart Transpl (2021) 40(7):631–41. 10.1016/j.healun.2021.03.020
24.
Vajter J Vachtenheim J Prikrylova Z Berousek J Vymazal T Lischke R et al Effect of Targeted Coagulopathy Management and 5% Albumin as Volume Replacement Therapy During Lung Transplantation on Allograft Function: A Secondary Analysis of a Randomized Clinical Trial. BMC Pulm Med (2023) 23(1):80. 10.1186/s12890-023-02372-0
Summary
Keywords
ECMO, UFH, anticoagulation, lung transplantation, anesthesiology
Citation
Vajter J, Holubova G, Novysedlak R, Svorcova M, Vachtenheim J, Vymazal T and Lischke R (2024) Anaesthesiologic Considerations for Intraoperative ECMO Anticoagulation During Lung Transplantation: A Single-Centre, Retrospective, Observational Study. Transpl Int 37:12752. doi: 10.3389/ti.2024.12752
Received
27 January 2024
Accepted
06 March 2024
Published
20 March 2024
Volume
37 - 2024
Updates
Copyright
© 2024 Vajter, Holubova, Novysedlak, Svorcova, Vachtenheim, Vymazal and Lischke.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jaromir Vajter, jaromir.vajter@fnmotol.cz
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.