Abstract
Simultaneous pancreas-kidney (SPK) transplantation improves quality of life and limits progression of diabetic complications. There is reluctance to accept pancreata from donors with abnormal blood tests, due to concern of inferior outcomes. We investigated whether donor amylase and liver blood tests (markers of visceral ischaemic injury) predict pancreas graft outcome using the UK Transplant Registry (2016-2021). 857 SPK recipients were included (619 following brainstem death, 238 following circulatory death). Peak donor amylase ranged from 8 to 3300 U/L (median = 70), and this had no impact on pancreas graft survival when adjusting for multiple confounders (aHR = 0.944, 95% CI = 0.754–1.81). Peak alanine transaminases also did not influence pancreas graft survival in multivariable models (aHR = 0.967, 95% CI = 0.848–1.102). Restricted cubic splines were used to assess associations between donor blood tests and pancreas graft survival without assuming linear relationships; these confirmed neither amylase, nor transaminases, significantly impact pancreas transplant outcome. This is the largest, most statistically robust study evaluating donor blood tests and transplant outcome. Provided other factors are acceptable, pancreata from donors with mild or moderately raised amylase and transaminases can be accepted with confidence. The use of pancreas grafts from such donors is therefore a safe, immediate, and simple approach to expand the donor pool to reach increasing demands.
Introduction
Diabetes Mellitus (DM) is a growing pandemic [1–3] associated with increased risks of developing life-limiting systemic complications. Diabetic patients may experience reduced quality of life and incur high healthcare-associated costs, particularly in patients with poorly controlled disease. Pancreas transplantation significantly improves the quality of life of patients, and can limit the progression of serious medical comorbidities [4–7].
The number of patients on the UK waiting list for pancreas transplantation is at an all-time high, and waiting time has worsened following the COVID-19 pandemic [8]. As of March 2023, 265 patients are actively waiting for a pancreas graft in the United Kingdom, representing a 27% increase from before the pandemic [9]. Taken together, there is a need to optimise decision-making surrounding organ utilisation and expand the donor pool to match the current demands for pancreas transplantation. It is essential to understand factors that predict transplant outcomes. It is equally important to identify factors that do not lead to poor outcomes, preventing the unwarranted rejection of donor organs based on these factors.
Initial screening of donors includes various blood tests, such as serum amylase and liver blood tests. Hyperamylaseamia (defined as serum amylase levels greater than 110 UI/L) can be seen in up to 40% of donors, and a markedly elevated serum amylase (more than three times the upper limit of normal) is generally considered to represent pancreatitis [10]. However, this blood test has low specificity, and can be raised due to a variety of aetiologies [11, 12].
Serum liver blood tests (LBTs) are markers of acute hepatocellular or cholangiocyte injury. The embryological development of the pancreas is closely related to the formation of foregut and midgut structures. The pancreas shares the same vascular supply with other foregut/midgut structures (including liver), receiving blood from both coeliac trunk and superior mesenteric artery. Therefore, markers of acute hypoxic injury to the liver could be a surrogate for hypoxic injury to the pancreas [13, 14].
This study aims to ascertain whether donor amylase and LBTs predict pancreas graft survival in patients undergoing SPK transplantation.
Materials and Methods
Data on adult simultaneous pancreas and kidney (SPK) transplants was retrieved from the UK Transplant registry, maintained by the National Health Service Blood and Transplant (NHSBT). Adult recipients (>16 years) from all 8 UK pancreas transplant centres, transplanted between January 2016 and December 2021, were included. These dates were chosen because, before January 2016, serial donor amylase and serial LBTs were not recorded. Recipients of grafts donated following circulatory or brain stem death [donation following brain stem death (DBD)/donation following circulatory death (DCD)] were included.
Data were provided in an anonymized form (patient identifiable information and transplant unit not provided) as per NHSBT approvals, and individual ethical or institutional review board approval was not required for this project. This project was approved by the pancreas advisory board.
Data were extracted from NHSBT in August 2023. Data were cleaned, and values that were deemed impossible were removed. Our primary aim was to compare the impact of donor serum amylase on 3-year pancreas graft survival. Secondary analyses compared the impact of donor alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALT) and bilirubin, as well as renal blood tests and lactate, on 3-year pancreas graft survival.
Graft loss was defined as retransplantation, pancreatectomy or return to insulin therapy due to graft failure and was analysed as time-to-event, death censored, and measured until July 2023 (the common closure date of the study).
Statistical Analysis
Missing data is summarised in Supplementary Table S1. Missing data were dealt with by multiple imputation using the fully conditional specification technique applied to generate 5 imputed datasets. Due to significant right skew, peak amylase, LBT, renal function test and serum lactate values were log transformed prior to performing multiple imputation. These imputed datasets were used for all multivariable models.
Our approach for constructing multivariable models matched that described previously [13]. When entering LBT values as predictors in the following models they were kept as continuous variables, rather than splitting into arbitrary categories; this approach improves power and is best practice. The blood tests were kept as continuous variables, which is superior to creating arbitrary categories [15–17]. To combat issues with skew, all blood test values were entered into models as log2 (blood test value).
Cox proportional hazards method was used to build multivariable graft survival models. Donor, graft, recipient and operative factors available from NHSBT registry were initially screened. Variables were selected based on clinical experience, if they had previously been reported to affect graft survival, or if they were significantly correlated with donor amylase and LBTs. Table 3 lists all considered variables. Automatic variable selection techniques (such as backwards stepwise selection) were avoided as these are recommended against in small datasets [18].
As there was significant correlation between each of the blood tests, there would be significant issues with multi-collinearity if they were entered into the same model. Therefore, separate multivariable models were built for donor amylase and each individual LBT, renal function test and serum lactate values. Results of these models are displayed as adjusted hazard ratios (aHR) with 95% confidence intervals. Interaction terms where introduced into these models to assess whether the impact of donor blood tests on pancreas graft survival differed in older donors or those with prolonged CIT.
Finally, we repeated our main cox regression models for graft survival, using a restricted cubic spline approach (3 knots located at 10/50/90th percentiles) to assess the impact of donor serum amylase and LBTs on outcome without assuming linear relationships [19].
For all tests performed p < 0.05 was deemed significant. Analyses were performed in SPSS™ version 26 (IBM Corp, Armonk, New York, United States) or R (R Foundation for Statistical Computing, Vienna, Austria). The latter was used to generate all figures.
Results
857 adult recipients of deceased donor pancreas (619 DBD and 238 DCDs) were included, with median follow up of 37.5 months. Median donor age was 34 (interquartile range 24–46). Cohort demographics are included in Table 1, with further details in Supplementary Table S1.
TABLE 1
| DBD (N = 619) | DCD (N = 238) | Overall (N = 857) | |
|---|---|---|---|
| Recipient Age | |||
| Median [Min, Max] | 42.0 [21.0, 64.0] | 42.0 [20.0, 61.0] | 42.0 [20.0, 64.0] |
| Recipient Sex | |||
| Female | 267 (43.1%) | 92 (38.7%) | 359 (41.9%) |
| Male | 352 (56.9%) | 146 (61.3%) | 498 (58.1%) |
| Recipient Ethnicity | |||
| White | 515 (83.2%) | 205 (86.1%) | 720 (84.0%) |
| Non-White | 97 (15.7%) | 32 (13.4%) | 129 (15.1%) |
| Recipient BMI | |||
| Median [Min, Max] | 24.6 [17.7, 36.5] | 25.1 [18.4, 36.9] | 24.8 [17.7, 36.9] |
| Type of Recipient Diabetes | |||
| Type 1 Diabetes Mellitus | 488 (78.8%) | 179 (75.2%) | 667 (77.8%) |
| Type 2 Diabetes Mellitus | 22 (3.6%) | 10 (4.2%) | 32 (3.7%) |
| Donor Sex | |||
| Female | 316 (51.1%) | 96 (40.3%) | 412 (48.1%) |
| Male | 303 (49.0%) | 142 (59.7%) | 445 (51.9%) |
| Donor Age | |||
| Median [Min, Max] | 35.0 [10.0, 63.0] | 29.0 [4.00, 54.0] | 34.0 [4.00, 63.0] |
| Donor BMI | |||
| Median [Min, Max] | 23.4 [14.5, 38.4] | 22.6 [11.3, 36.2] | 23.1 [11.3, 38.4] |
| Donor Ethnicity | |||
| White | 554 (89.5%) | 216 (90.8%) | 770 (89.8%) |
| Non-White | 53 (8.6%) | 21 (8.8%) | 74 (8.6%) |
| Donor Cause of Death | |||
| Hypoxic Brain Injury | 198 (32.0%) | 111 (46.6%) | 309 (36.1%) |
| Intrcranial Haemorrhage | 284 (45.9%) | 61 (25.6%) | 345 (40.3%) |
| Intrcranial Thrombosis | 27 (4.4%) | 9 (3.8%) | 36 (4.2%) |
| Trauma | 30 (4.8%) | 25 (10.5%) | 55 (6.4%) |
| Other | 55 (8.9%) | 17 (7.1%) | 72 (8.4%) |
| Cold Ischaemic Time (minutes) | |||
| Median [Min, Max] | 647 [223, 1,320] | 611 [339, 1,060] | 634 [223, 1,320] |
| 3-year Graft failure | |||
| No | 547 (88.4%) | 214 (89.9%) | 761 (88.8%) |
| Yes | 68 (11.0%) | 21 (8.8%) | 89 (10.4%) |
Summary of Cohort Demographics (N = 857).
DBD, donation following brainstem death; DCD, donation following circulatory death.
Summary of Donor Serum Amylase and Liver Blood Tests
Table 2 provides a summary of donor amylase and liver blood tests across the cohort (see Supplementary Material S2 for further details). Peak Amylase and ALT values are graphically displayed in Figure 1. A wide range of peak donor amylase were identified in our study. 465 donors had a peak amylase of <100 iu/L, 257 donors had a peak amylase of between 100 iu/L and 1000 iu/L, and five donors had peak amylase >1000 iu/L (130 were missing a value for peak amylase). Of all donors, a total of 197 had an amylase value of >130 iu/L (the P-PASS cut-off) [20].
TABLE 2
| DBD (N = 619) | DCD (N = 238) | Overall (N = 857) | |
|---|---|---|---|
| Amylase | |||
| Median [Min, Max] | 70 [8, 3,300] | 69 [10, 1,310] | 70.0 [8, 3,300] |
| ALT | |||
| Median [Min, Max] | 59 [8, 5,090] | 89 [9, 5,930] | 67.0 [8, 5,930] |
| AST | |||
| Median [Min, Max] | 65 [0, 2040] | 94.0 [10, 7,910] | 72.0 [0, 7,910] |
| ALP | |||
| Median [Min, Max] | 85 [31, 721] | 90.0 [35, 541] | 86.0 [31, 721] |
| Bilirubin | |||
| Median [Min, Max] | 12 [3, 124] | 11.5 [3, 65] | 12.0 [3, 124] |
Summary of Peak Donor Serum Amylase and Liver Blood Tests.
DBD, donation following brainstem death; DCD, donation following circulatory death; ALT, alanine transaminase; AST, aspartate transaminase; ALP, alkaline phosphatase.
FIGURE 1
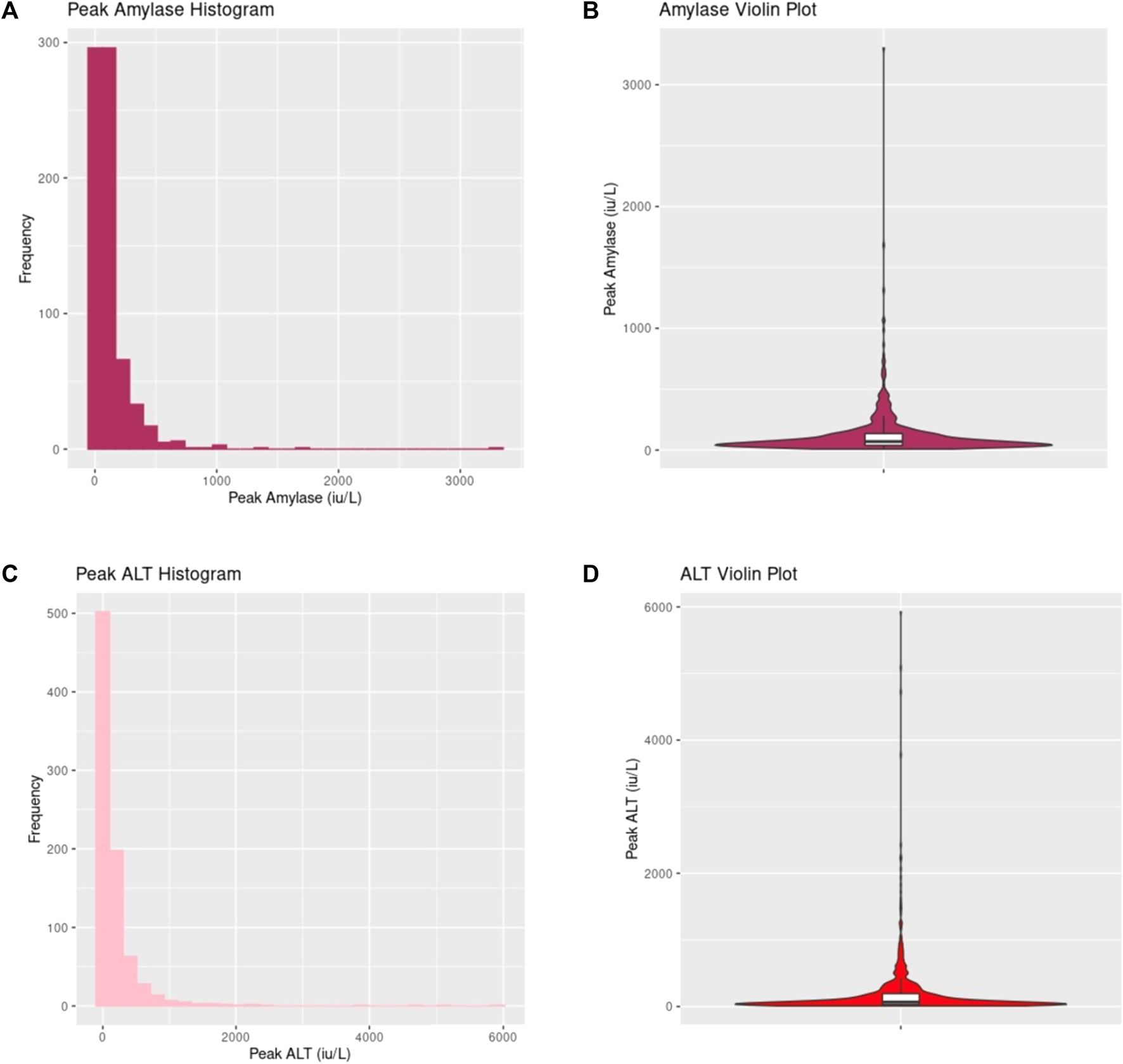
Donor peak amylase and peak alanine transaminase (ALT) distribution. (A,B) demonstrates values of peak amylase across the entire cohort displayed in histogram and violin plot respectively. (C,D) shows values of peak ALT across the entire cohort displayed in histogram and violin plot respectively.
Supplementary Figure S1 provides a graphical display of peak donor AST, ALP and bilirubin values. There were no significant differences in the blood tests between DBD and DCD donors (Table 2).
Impact of Amylase and Liver Blood Tests on Pancreas Graft Survival
Table 3 displays the multivariable cox regression model for 3-year pancreas graft survival. Peak donor amylase, peak transaminases (ALT and AST), peak ALP, and peak bilirubin did not predict pancreas graft survival, even when adjusting for a range of factors (Table 3).
TABLE 3
| Adjusted HR (95% CI) | p-value | |
|---|---|---|
| Blood Tests | ||
| Amylase (Peak) | 0.944 (0.754–1.181) | 0.602 |
| ALT (Peak) | 0.967 (0.848–1.102) | 0.616 |
| AST (Peak) | 0.908 (0.771–1.070) | 0.247 |
| ALP (Peak) | 0.865 (0.594–1.261) | 0.451 |
| Bilirubin (Peak) | 1.229 (0.930–1.624) | 0.148 |
| Cold Ischaemic Time (hours) | 1.338 (0.611–2.930) | 0.467 |
| Donor Age (years) | 1.009 (0.992–1.026) | 0.322 |
| Donor Type | 0.731 (0.430–1.243) | 0.247 |
| Donor BMI | 1.078 (1.015–1.144) | 0.014 |
| Transplant Year | 0.948 (0.820–1.096) | 0.472 |
| Recipient Age (years) | 0.960 (0.935–0.986) | 0.003 |
| Recipient BMI | 0.992 (0.918–1.073) | 0.842 |
3-Year Graft Survival Cox regression using pooled data on peak donor amylase and liver blood tests from imputed datasets.
For blood tests, logs were taken before inclusion in this model, due to all blood tests results being right-skewed. The effect estimates relate to a unit increase in log2 (blood tests value). Results from the various LBTs (ALT, AST, ALP, and bilirubin) could not be included in a single model because of multicollinearity; therefore, multivariable results for each LBT are from a separate multivariable model. Multivariable results for variables other than LBTs are from the model including peak Amylase.
ALT, alanine transaminase; AST, aspartate transaminase; ALP, alkaline phosphatase; CI, confidence interval; LBT, liver blood test; HR, hazard ratio; DBD, donation following brainstem death; DCD, donation following circulatory death.
The impact of blood tests on outcome was then assessed separately in DBD and DCD cohorts. Repeating the model in Table 3 in the DBD cohort, confirmed that donor amylase did not predict pancreas graft survival in this group (aHR = 0.965, 0.760–1.227, p = 0.768). For DCD graft recipients, a further multivariable model was created, with the addition of normothermic regional perfusion (NRP) as a confounder; again, this confirmed no impact of donor amylase on pancreas graft survival (aHR = 0.984, 0.609–1.590, p = 0.948). Similar analyses found no impact of peak donor liver blood tests in either the DBD or DCD subgroup.
We have also performed a multivariable analysis on those with amylase values greater than 130 (the cut-off used in the P-PASS score) [20], adjusting for all of the factors in Table 3. Pancreases from donors with peak amylase >130 were not at higher risk of graft loss compared with those with amylase ≤130 (aHR = 0.730, 95% CI 0.460–1.733, p = 0.730). This is a sensitivity analysis only, as using arbitrary cut-offs for continuous variables significantly reduces the power of analyses.
Donor amylase and transaminases may have a greater impact in older donors and pancreases with prolonged cold ischaemic time. This hypothesis was tested by the addition of interaction terms to the model shown in Table 3. There was no evidence that the impact of donor peak amylase or peak ALT on pancreas graft survival differed based on donor age (interaction p = 0.340 & p = 0.890 respectively), or prolonged cold ischaemic time (interaction p = 0.699 & p = 0.924 respectively).
The relationship between peak amylase/LBT values and graft survival was also modelled using restricted cubic splines (Figures 2A, B). This avoids assumptions about the nature of the relationship between peak blood test values and outcome, whilst also adjusting for all the confounders listed in Table 3. As shown in Figures 2A, B, this confirms no impact of peak amylase or peak ALT on outcome. By way of counter example, a restricted cubic spline analysis was also performed for recipient age which is a known prognostic factor; this showed that younger recipients have worse outcome (Figure 2C).
FIGURE 2
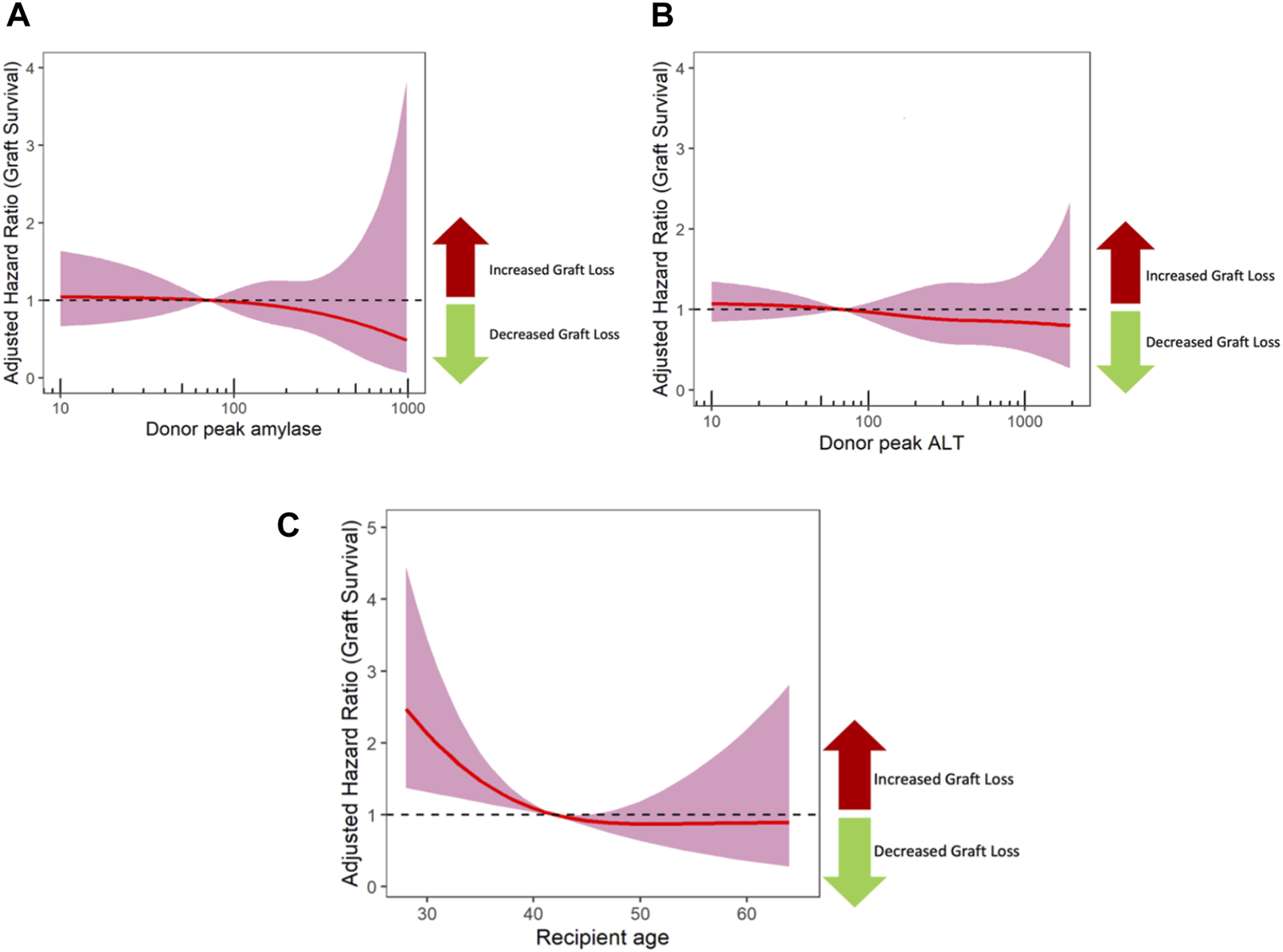
The impact of peak donor Amylase (A) and ALT (B) on graft survival using cox regression models with restricted cubic splines. The shaded area represents the 95% confidence interval, and a dashed line at 1 represents no impact on outcome. For comparison, a separate model was performed for recipient age (C), which showed that younger recipients have worse outcome. ALT, alanine transaminase.
It may be argued that the terminal value (the value closest to donation) is more predictive of outcome. As serum amylase and LBT levels closest to donation (rather than peak values) may represent the cumulative effect of ischaemic injury during donation, we built further models using terminal values in an identical fashion to Table 3. This is shown in Table 4, where terminal values of amylase, LBTs, renal function tests and serum lactate were not significant in outcomes.
TABLE 4
| Adjusted HR (95% CI) | p-value | |
|---|---|---|
| Blood Tests | ||
| Amylase (Terminal) | 0.979 (0.776–1.236) | 0.857 |
| ALT (Terminal) | 0.965 (0.828–1.124) | 0.646 |
| AST (Terminal) | 0.895 (0.669–1.198) | 0.430 |
| ALP (Terminal) | 1.011 (0.713–1.434) | 0.950 |
| Bilirubin (Terminal) | 1.067 (0.798–1.428) | 0.661 |
| Cold Ischaemic Time (hours) | 1.348 (0.614–2.957) | 0.457 |
| Donor Age (years) | 1.009 (0.992–1.026) | 0.297 |
| Donor Type | 0.736 (0.433–1.251) | 0.258 |
| Donor BMI | 1.077 (1.015–1.144) | 0.015 |
| Transplant Year | 0.949 (0.821–1.098) | 0.484 |
| Recipient Age (years) | 0.960 (0.935–0.986) | 0.003 |
| Recipient BMI | 0.993 (0.919–1.073) | 0.857 |
Sensitivity Analyses with terminal values of amylase and liver blood tests.
For blood tests, logs were taken before inclusion in this model, due to all blood tests results being right-skewed. The effect estimates relate to a unit increase in log2 (blood tests value). Results from the various LBTs (ALT, AST, ALP, and bilirubin) could not be included in a single model because of multicollinearity; therefore, multivariable results for each LBT are from a separate multivariable. Multivariable results for variables other than LBTs are from the model including peak Amylase.
ALT, alanine transaminase; AST, aspartate transaminase; ALP, alkaline phosphatase; CI, confidence interval; LBT, liver blood test; HR, hazard ratio; DBD, donation following brainstem death; DCD, donation following circulatory death.
There may be specific concern where donor amylase values are extremely elevated (>1000 iu/L, 10 times the upper limit of normal). Follow-up data was available for 4 pancreas transplants which used grafts from donors with peak amylase over 1,000; all of these were functioning at last follow-up (Figure 3).
FIGURE 3
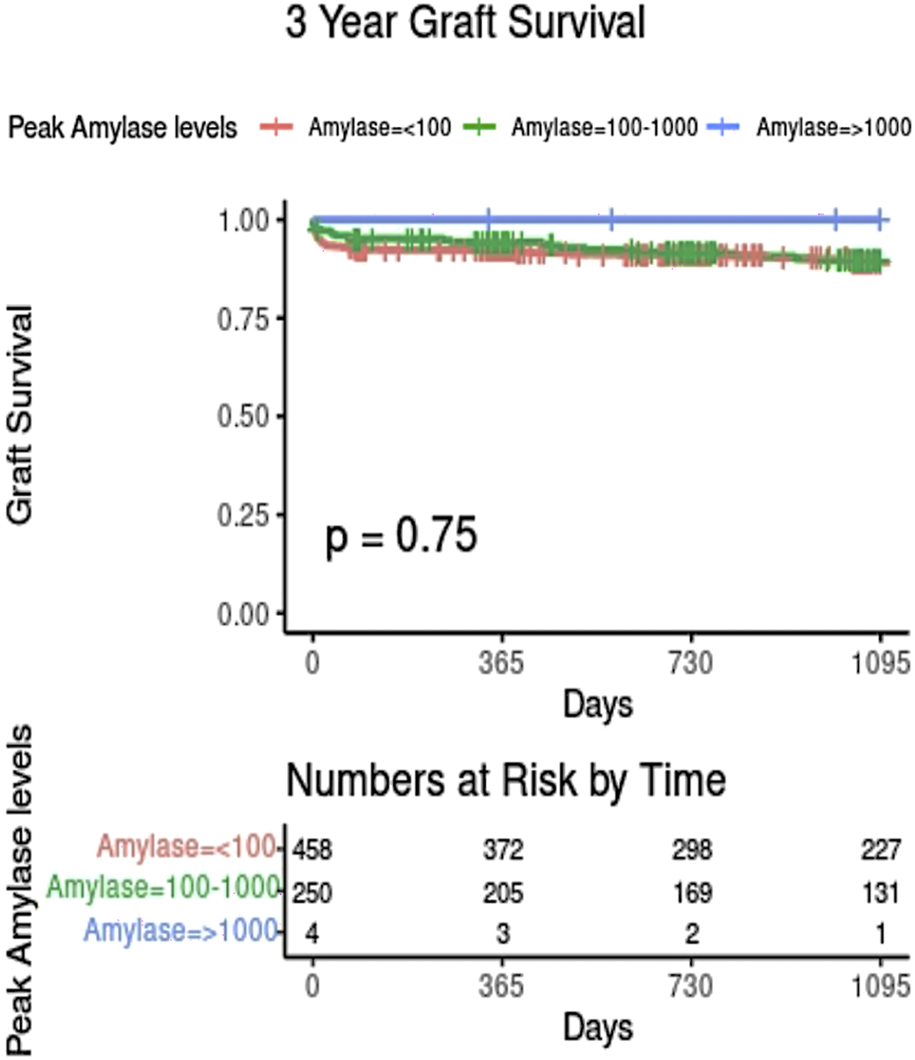
Kaplan-Meier plot showing graft survival based on donor peak amylase level. Only those with complete amylase and graft survival data are shown.
Sensitivity analyses were performed where raw amylase and LBT values (rather than log-transformed values) were entered into the cox regression model. Again, peak donor amylase and LBTs did not show significant impact in recipient outcomes.
We also assessed the impact of donor renal function tests and lactate, as the function of the transplanted kidney can impact pancreas graft function. Donor HbA1c was not recorded for more than 90% of the donors and therefore could not be assessed in this study. Donor peak creatinine, peak urea, peak estimated glomerular filtration rate (eGFR), and serum lactate did not predict pancreas graft survival (Supplementary Table S3). None of the examined blood tests predicted kidney graft survival in multivariable models. However, kidney graft survival may be better assessed in a study dedicated to kidney grafts, with much larger cohorts of kidney transplants alone.
Discussion
This large, statistically robust cohort study (619 DBD and 238 DCDs) has found no association between donor amylase and pancreas graft survival in SPK transplantation, on adjusted analyses. Although there was no evidence of an impact on outcome at any donor amylase level, relatively few pancreases were transplanted from donors with extreme increases in amylase (>1,000). Therefore, the impact of extreme elevations in amylase remain uncertain and such donor should be assessed on a case-by-case basis. It is reassuring that all four pancreases transplanted from donors with amylase >1,000 were functioning well at last follow up.
Additionally, our study has also found no association between donor LBTs and pancreas graft outcome. Hence, donor amylase and LBTs alone should not be a determining factor in organ utilisation in the modern era of pancreas transplantation.
With the rising demand for pancreas transplantation due to the increasing global disease burden of diabetes mellitus [1–3] and longer waiting lists there is a need to widen access to pancreas transplantation through improved utilisation of grafts. Further knowledge and evidence-based organ assessment is crucial in quantifying extended-criteria and marginal donor organs [21]. At the time of organ selection, some serological markers such as amylase levels and liver blood tests can be useful taken together with other markers of increasing risk when deciding the suitability and quality of a pancreas allograft but it is important to note they are non-specific and that there are other donor variables that may affect these blood tests [10–12, 22]. Nonetheless, surgeons remain reluctant to accept pancreas grafts from donor with raised serum amylase due to concerns of inferior outcomes. This becomes more important during an era of DCD transplantation as these are more prone to ischaemic damage but represent an underused resource [23–25].
Vinkers et al established the Pre-procurement Pancreas Allocation Suitability Score (P-PASS) in 2008, where a total of nine clinical parameters were used to predict the odds of a donor allograft being accepted for transplantation. The P-PASS score includes donor body mass index, age, duration of intensive care stay, serum amylase, lipase, sodium, duration of donor cardiac arrest, and whether or not the donor was on vasopressor support. Liver function tests, cold ischaemia time and type of donor, i.e., DCDs vs. DBDs are excluded in P-PASS. A low P-PASS score of 17 and below were three times more likely to be accepted as pancreas donors than donor grafts that scored above 17 [20]. The P-PASS score has been utilised by Eurotransplant since 2009 [26]. Amylase levels were among the nine parameters in this scoring system, where raised Amylase of ≥130 iu/L contributes to a higher P-PASS score, which is associated with high odds of organ discard [20]. It is important to note that the P-PASS score was developed based on chance of organ decline, and not based on outcome in transplanted pancreases. It therefore reflects what clinicians perceive as high risk, rather than factors which actually predict pancreas quality.
Interestingly, two retrospective analyses by Schenker et al and Blok JJ et al [27] revealed that there is no significant difference in long-term patient and graft survivals between donors with low (≤17) and high (≥17) P-PASS scores [28]. This supports our findings and further reiterates that donor pancreas allografts should not be rejected based solely on high P-PASS scores and the parameters that deem a subgroup of donors as marginal donors.
In the US the Pancreas Donor Risk index was developed from data taken from the Scientific Registry of Transplant Recipients database and is linked to graft survival. It has also been validated in the UK cohort [29]. It may offer better predictions for more marginal pancreases and some studies have confirmed it is a better predictor of pancreas graft survival after SPK rather than after solitary pancreas transplantation [30]. It is also a better predictor than the P-PASS for pancreas graft survival [27]. Age, and cold ischaemia are included but amylase and lipase are excluded from the PDRI, as they were not associated with outcome. A recent systematic review conducted by Ling et al have shown that both P-PASS and PDRI are inadequate risk indices for use in solid pancreas transplantation due inadequate reporting of model performance metrics outside of current externally validated cohorts. P-PASS was derived for pancreas graft acceptance and not for prediction of graft survival. PDRI was validated for the outcomes of 1-year pancreas survival, and limited to graft survival for SPK transplants only [31]. These studies also did not focus on donor blood tests, and their impact on outcome, our study fills these gaps.
Liver function tests and amylase are both included in the North American Islet donor score which was developed to guide decision making as to whether to accept a particular pancreas to improve isolation outcomes [32, 33]. However, both amylase and transaminases were shown in the same Wang 2016 paper to have no impact on success of islet isolation from 1,056 donors. This mirrors our results in whole pancreas transplantation.
Additionally, it is worth noting that a previous smaller study by Hesse and Sutherland have demonstrated that an isolated elevation of amylase is not usually related to the functional status of the pancreas allograft, unless there was overt pancreatic trauma or pancreatitis. Graft function post-transplantation was found to be comparable in the recipients, regardless of whether the donor had normal or elevated amylase levels [34]. Krieger and others further echoes this, as they have shown that SPK graft survival rates in recipients of grafts from donors who had raised serum amylase compared favourably to outcomes in recipients of “ideal” donor grafts [35].
There are some limitations to the studies discussed above; both were performed in the early phases of pancreas transplantation, and only confined to the United States. Furthermore, the sample sizes in both studies were smaller than the present study. Both studies also reviewed graft outcomes based on arbitrary categories of normal and abnormal serum amylase, which reduces the power of the study [15–18]. Despite the limitations, these studies support our findings that hyperamylaseamia in donors is not a contraindication for pancreas organ donation. To our knowledge, our work is the largest cohort study to date, looking at the relationship between serum amylase and liver function tests upon pancreas graft survival in the modern era of pancreas transplantation. We have incorporated prospectively collected data from a large cohort, with robust statistical analysis as detailed above.
With the increased use of DCD grafts, there is an increased vulnerability towards inevitable ischaemic-reperfusion injury during procurement [36, 37]. Due to the close anatomical relationship between the pancreas and its partially shared vascular supply with the foregut, raised donor LBTs may represent ischaemic injury to abdominal viscera [14, 38–42]. Raised liver blood tests (LBTs) in liver donors were frequently used to define extended-criteria donors, in the context of liver transplantation [43, 44]. Due to the partially shared vascular supply [14, 38–42] between liver and pancreas we hypothesised that elevations in LBTs, especially transaminases, reflect hypoxic injury to the liver and are therefore a surrogate for hypoxic injury to the pancreatic allograft. This is supported by work showing that donors dying from hypoxic brain injury have far higher transaminase levels [11, 12].
Parajuli and others have found that delayed kidney graft function represented a significant risk factor for early pancreas graft loss (<90 days post-transplant) in SPK transplant recipients [45]. In view of this, we have therefore separately assessed the impact of peak donor renal function tests in our study, as the function of the transplanted kidney can impact pancreas graft function [45, 46]. We have found that donor renal blood tests did not predict pancreas graft survival (Supplementary Table S3). However, transplanted kidney graft survival may be better assessed in a study dedicated to kidney grafts, with much larger cohorts of kidney transplants alone.
More recently, our group explored the significance of deranged LBTs in liver transplantation and found that raised donor transaminases do not predict post-liver transplant outcomes [13]. Our study mirrors these findings in pancreas transplant, as there were no associations between abnormal LBTs and pancreas graft survival. Since routine liver function tests are carried out as part of the work up for a potential transplant donor, our findings reinforces that rises in LBTs should not be considered as a limiting factor in pancreas allograft allocation.
Furthermore, whilst in intensive care units, some donors may be given insulin in response to donor hyperglycaemia of varying aetiologies [47, 48]. A recent, large cohort study by Shapey et al suggests that donor insulin use is associated with a higher risk of graft loss due to islet failure and a lower risk of graft loss due to thrombosis in pancreas transplant recipients [49]. This suggests that actual markers of organ function and pancreas physiology may be more predictive of pancreas transplant outcomes, rather than non-specific enzyme release, such as amylase.
This study is limited by the retrospective design. Specifically, we lack granularity of data regarding imaging and clinical features of acute pancreatitis, or details regarding pancreatic trauma. As we only included donated pancreas grafts which were accepted and used for transplantation, the vast majority will be from donors without clinical or radiological features of pancreatitis or pancreatic trauma. Therefore, we cannot comment on the suitability of pancreases from donors where these features are present. We also lack information on serum lipase. Though we acknowledge it is a more specific marker of pancreatic injury, it is not routinely performed in the UK setting. Further study into the effects of lipase and pancreas graft transplantation outcomes, in a healthcare system that routinely measures donor serum lipase, may be a point in future research.
There is also a degree of selection bias, as various clinicians have different thresholds for donor amylase when it comes to discarding grafts at the time of organ procurement. As described in our results section, there is a wide range of donor amylase values in the pancreas grafts that were transplanted in our study. Hence multivariable analysis was performed to adjust for key confounders.
Finally, the right skewed distribution of serum blood tests translates to smaller number of donors in the extremely elevated results. This is reflected in the marked increase in the confidence intervals of the cox regression model adjusted hazard ratios with restricted cubic splines (Figure 2). The low number of donor with high amylase may affect the power of our study, and the most powerful way of assessing this was by using restricted cubic splines (Figure 2). The confidence intervals around these splines reveal uncertainty as amylase level increases. These are confidence fairly narrow up to a peak amylase value of 500, and then sharply increase due to the lower numbers of pancreases transplanted from donors with amylase values greater than 500. Although pancreases from donors with severely increased peak amylase (>1,000) all performed well in this study, this is a small group. Therefore it remains uncertain whether large increases in amylase (>1,000) impact on graft survival, and such donors should be assessed, on a case-by-case basis.
In conclusion, our study has demonstrated that the use of pancreas grafts from donors with hyperamylasaemia and raised liver blood tests is not associated with inferior outcomes. Mild or moderately raised donor amylase and liver blood tests should therefore not be considered a barrier to transplantation and organ utilisation when other donor factors are considered acceptable. This knowledge should prevent unnecessary organ discard, and provides a simple method to expand the donor pool to meet current demands.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
Study concept and design: ST, CW, and SW. Acquisition of data: NH, ST, and SW from the National Health Service Blood and Transplant Registry. Data analysis: ST and NH. Data interpretation: All authors. Drafting of the manuscript: NH and ST. Critical revision of manuscript: All authors. Study Supervision: SW and CW. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study is supported by the National Institute for Health and Care Research (NIHR) Blood and Transplant Research Unit in Organ Donation and Transplantation (NIHR203332), a partnership between NHS Blood and Transplant, University of Cambridge and Newcastle University. The views expressed are those of the author(s) and not necessarily those of the NIHR, NHS Blood and Transplant or the Department of Health and Social Care.
Conflict of interest
Author ST worked on this project during an MRC Clinical Research Training Fellowship (MR/Y000676/1) at Newcastle University.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/ti.2024.12864/full#supplementary-material
Abbreviations
ALT, alanine transaminase; AST, aspartate transaminase; ALP, alkaline phosphatase; CIT, Cold Ischaemic Time; DCD, Donor after Circulatory Death; DBD, Donor after Brainstem Death; DM, Diabetes Mellitus; LBT, Liver Blood Tests; NHS, National Health Service (United Kingdom); NHSBT, National Health Service Blood and Transplant; SPK, Simultaneous Pancreas-Kidney transplantation; WIT, Warm Ischaemic Time.
References
1.
(WHO) WHO Diabetes. Geneva, Switzerland: World Health Organization (2023). Available from: https://www.who.int/health-topics/diabetes (Accessed October 7, 2023).
2.
Cho NH Shaw JE Karuranga S Huang Y da Rocha Fernandes JD Ohlrogge AW et al IDF Diabetes Atlas: Global Estimates of Diabetes Prevalence for 2017 and Projections for 2045. Diabetes Res Clin Pract (2018) 138:271–81. 10.1016/j.diabres.2018.02.023
3.
Ong KL Stafford LK McLaughlin SA Boyko EJ Vollset SE Smith AE et al Global, Regional, and National Burden of Diabetes From 1990 to 2021, With Projections of Prevalence to 2050: A Systematic Analysis for the Global Burden of Disease Study 2021. The Lancet (2023) 402:203–34. 10.1016/s0140-6736(23)01301-6
4.
Larsen JL . Pancreas Transplantation: Indications and Consequences. Endocr Rev (2004) 25(6):919–46. 10.1210/er.2002-0036
5.
Fridell JA Stratta RJ Gruessner AC . Pancreas Transplantation: Current Challenges, Considerations, and Controversies. J Clin Endocrinol Metab (2023) 108(3):614–23. 10.1210/clinem/dgac644
6.
White SA Shaw JA Sutherland DE . Pancreas Transplantation. The Lancet (2009) 373(9677):1808–17. 10.1016/S0140-6736(09)60609-7
7.
Gruessner AC Gruessner RW . The 2022 International Pancreas Transplant Registry Report—A Review. Transpl Proc. (2022) 54(7):1918–43. 10.1016/j.transproceed.2022.03.059
8.
Annual report on pancreas and islet transplantation. Annual Report on Pancreas and Islet Transplantation 2022-2023. United Kingdom: NHS Blood and Transplant; 2023Available from: https://www.odt.nhs.uk/statistics-and-reports/organ-specific-reports/. Accessed 18 January 2024.
9.
Parsons J Counter C . Annual Report on Pancreas and Islet Transplantation. United Kingdom: NHS Blood and Transplant (2023). Available from: https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/30883/nhsbt-pancreas-and-islet-transplantation-report-2223.pdf (Accessed October 7, 2023).
10.
Pieper-Bigelow C Strocchi A Levitt MD . Where Does Serum Amylase Come From and Where Does It Go?Gastroenterol Clin North America (1990) 19(4):793–810. 10.1016/s0889-8553(21)00514-8
11.
Salt WB Schenker S . Amylase--its Clinical Significance: A Review of the Literature. Medicine (1976) 55:269–89. 10.1097/00005792-197607000-00001
12.
Lam R Muniraj T . Hyperamylasemia. In: StatPearls. St. Petersburg, Florida, United States: StatPearls Publishing (2022).
13.
Tingle SJ Bramley R Goodfellow M Thompson ER McPherson S White SA et al Donor Liver Blood Tests and Liver Transplant Outcomes: UK Registry Cohort Study. Transplantation (2023) 10–97. 10.1097/TP.0000000000004610
14.
Ehrhardt JD Gomez F . Embryology, Pancreas. In: StatPearls. Treasure Island (FL): StatPearls Publishing (2023). Available from: https://www.ncbi.nlm.nih.gov/books/NBK545243/ (Accessed October 7, 2023).
15.
Bennette C Vickers A . Against Quantiles: Categorization of Continu-Ous Variables in Epidemiologic Research, and its Discontents. BMC Med Res Methodol (2012) 12:21. 10.1186/1471-2288-12-21
16.
Royston P Altman DG Sauerbrei W . Dichotomizing Continuous Predic- Tors in Multiple Regression: A Bad Idea. Stat Med (2006) 25:127–41. 10.1002/sim.2331
17.
Collins GS Ogundimu EO Cook JA Manach YL Altman DG . Quantifying the Impact of Different Approaches for Handling Continuous Predictors on the Perfor-Mance of a Prognostic Model. Stat Med (2016) 35:4124–35. 10.1002/sim.6986
18.
Heinze G Dunkler D . Five Myths About Variable Selection. Transpl Int (2017) 30(1):6–10. 10.1111/tri.12895
19.
Harrell FE. Regression Modeling Strategies With Applications to Linear Models, Logistic Regression, and Survival Analysis. 2nd ed. New York, NY: Springer (2015).
20.
Vinkers MT Rahmel AO Slot MC Smits JM Schareck WD . How to Recognize a Suitable Pancreas Donor: A Eurotransplant Study of Preprocurement Factors. Transplant Proc (2008) 40(5):1275–8. 10.1016/j.transproceed.2008.03.142
21.
Neuberger J Callaghan C . Organ Utilization–The Next Hurdle in Transplantation?Transpl Int (2020) 33(12):1597–609. 10.1111/tri.13744
22.
Fridell JA Stratta RJ . Expanding the Pancreas Donor Pool. Curr Transplant Rep (2014) 1:100–12. 10.1007/s40472-014-0015-8
23.
Salvalaggio PR Davies DB Fernandez LA Kaufman DB . Outcomes of Pancreas Transplantation in the United States Using Cardiac-Death Donors. Am J Transplant (2006) 6(5):1059–65. 10.1111/j.1600-6143.2006.01310.x
24.
Muthusamy AS Vaidya A . Expanding the Donor Pool in Pancreas Transplantation. Curr Opin Organ Transplant (2011) 16(1):123–7. 10.1097/MOT.0b013e328341b123
25.
Singh RP Rogers J Farney AC Moore PS Hartmann EL Reeves-Daniel A et al Outcomes of Extended Donors in Pancreatic Transplantation With Portal-Enteric Drainage. Transplant Proc (2008) 40(2):502–5. 10.1016/j.transproceed.2008.02.014
26.
Arbogast H . Pancreas Allocation in the Eurotransplant Area. In: Transplantation of the Pancreas. Cham: Springer International Publishing (2023). p. 129–39.
27.
Blok JJ Kopp WH Verhagen MJ Schaapherder AF de Fijter JW Putter H et al The Value of PDRI and P-PASS as Predictors of Outcome After Pancreas Transplantation in a Large European Pancreas Transplantation Center. Pancreas (2016) 45(3):331–6. 10.1097/MPA.0000000000000485
28.
Schenker P Vonend O Ertas N Wunsch A Viebahn R . Preprocurement Pancreas Allocation Suitability Score Does Not Correlate With Long-Term Pancreas Graft Survival. Transplant Proc (2010) 42(1):178–80. 10.1016/j.transproceed.2009.12.036
29.
Mittal S Smilevska R Franklin R Hammer C Knight S Vrakas G et al An Analysis of the Association Between Older Recipient Age and Outcomes After Whole‐Organ Pancreas Transplantation–A Single‐Centre, Retrospective Study. Transpl Int (2020) 33(5):529–35. 10.1111/tri.13575
30.
Axelrod DA Sung RS Meyer KH Wolfe RA Kaufman DB . Systematic Evaluation of Pancreas Allograft Quality, Outcomes and Geographic Variation in Utilization. Am J Transplant (2010) 10(4):837–45. 10.1111/j.1600-6143.2009.02996.x
31.
Ling JE Coughlan T Polkinghorne KR Kanellis J . Risk Indices Predicting Graft Use, Graft and Patient Survival in Solid Pancreas Transplantation: A Systematic Review. BMC Gastroenterol (2021) 21(1):80–20. 10.1186/s12876-021-01655-2
32.
Wang LJ Kin T O'gorman D Shapiro AJ Naziruddin B Takita M et al A Multicenter Study: North American Islet Donor Score in Donor Pancreas Selection for Human Islet Isolation for Transplantation. Cel Transplant (2016) 25(8):1515–23. 10.3727/096368916X691141
33.
O'Gorman D Kin T Murdoch T Richer B McGhee-Wilson D Ryan E et al The Standardization of Pancreatic Donors for Islet Isolation. InTransplantation Proc (2005) 37(2):1309–10. 10.1016/j.transproceed.2004.12.087
34.
Hesse UJ Gores PF Sutherland DE . Serum Amylase and Plasma Glucose Levels in Pancreas Cadaver Donors: Correlation With Func- Tional Status of the Pancreatic Graft. Transpl Proc (1989) 21(1 Pt 3):2765–6.
35.
Krieger NR Odorico JS Heisey DM D’Alessandro AM Knechtle SJ Pirsch JD et al Underutilization of Pancreas Donors. Transplantation (2003) 75(8):1271–6. 10.1097/01.TP.0000061603.95572.BF
36.
Taylor R Allen E Richards JA Goh MA Neuberger J Collett D et al Survival Advantage for Patients Accepting the Offer of a Circulatory Death Liver Transplant. J Hepatol (2019) 70:855–65. 10.1016/j.jhep.2018.12.033
37.
Dar WA Sullivan E Bynon JS Eltzschig H Ju C . Ischaemia Reperfusion Injury in Liver Transplantation: Cellular and Molecular Mechanisms. Liver Int (2019) 39(5):788–801. 10.1111/liv.14091
38.
Bertelli E Di Gregorio F Bertelli L Mosca S . The Arterial Blood Supply of the Pancreas: A Review. I. The Superior Pancreaticoduodenal and the Anterior Superior Pancreaticoduodenal Arteries. An Anatomical and Radiological Study. Surg Radiol Anat (1995) 17:97–106. 10.1007/BF01627566
39.
Bertelli E Di Gregorio F Bertelli L Civeli L Mosca S . The Arterial Blood Supply of the Pancreas: A Review. II. The Posterior Superior Pancreaticoduodenal Artery. An Anatomical and Radiological Study. Surg Radiologic Anat SRA (1996) 18(1):1–9. 10.1007/BF03207753
40.
Bertelli E Di Gregorio F Bertelli L Civeli L Mosca S . The Arterial Blood Supply of the Pancreas: A Review. III. The Inferior Pancreaticoduodenal Artery. An Anatomical Review and a Radiological Study. Surg Radiologic Anat SRA (1996) 18(2):67–74. 10.1007/BF01795221
41.
Bertelli E Di Gregorio F Bertelli L Orazioli D Bastianini A . The Arterial Blood Supply of the Pancreas: A Review: IV. The Anterior Inferior and Posterior Pancreaticoduodenal aa., and Minor Sources of Blood Supply for the Head of the Pancreas. An Anatomical Review and Radiologic Study. Surg Radiologic Anat (1997) 19:203–12. 10.1007/s00276-997-0203-7
42.
Bertelli E Di Gregorio F Mosca S Bastianini A . The Arterial Blood Supply of the Pancreas: A Review. V. The Dorsal Pancreatic Artery. An Anatomic Review and a Radiologic Study. Surg Radiologic Anat (1999) 20:445–52. 10.1007/BF01653138
43.
European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Liver Transplantation. J Hepatol (2016) 64:433–85. 10.1016/j.jhep.2015.10.006
44.
Vodkin I Kuo A . Extended Criteria Donors in Liver Transplantation. Clin Liver Dis (2017) 21:289–301. 10.1016/j.cld.2016.12.004
45.
Parajuli S Muth BL Astor BC Redfield RR Mandelbrot DA Odorico JS et al Delayed Kidney Graft Function in Simultaneous Pancreas-Kidney Transplant Recipients Is Associated With Early Pancreas Allograft Failure. Am J Transplant (2020) 20(10):2822–31. 10.1111/ajt.15923
46.
Hamed MO Chen Y Pasea L Watson CJ Torpey N Bradley JA et al Early Graft Loss After Kidney Transplantation: Risk Factors and Consequences. Am J Transplant (2015) 15(6):1632–43. 10.1111/ajt.13162
47.
Ellger B Langouche L Richir M Debaveye Y Vanhorebeek I Teerlink T et al Modulation of Regional Nitric Oxide Metabolism: Blood Glucose Control or Insulin? Intensive Care Med (2008) 34(8):1525–33. 10.1007/s00134-008-1118-4
48.
Aljada A Ghanim H Mohanty P Kapur N Dandona P . Insulin Inhibits the Pro-Inflammatory Transcription Factor Early Growth Response Gene-1 (Egr)-1 Expression in Mononuclear Cells (MNC) and Reduces Plasma Tissue Factor (TF) and Plasminogen Activator Inhibitor-1 (PAI-1) Concentrations. J Clin Endocrinol Metab (2002) 87(3):1419–22. 10.1210/jcem.87.3.8462
49.
Shapey IM Summers A Khambalia H Yiannoullou P Fullwood C Hanley NA et al Donor Insulin Therapy in Intensive Care Predicts Early Outcomes After Pancreas Transplantation. Diabetologia (2021) 64(6):1375–84. 10.1007/s00125-021-05411-9
Summary
Keywords
pancreas transplantation, SPK transplantation, registry study, donor blood tests, graft surival, organ utilisation
Citation
Ho NX, Tingle SJ, Malik AK, Thompson ER, Kourounis G, Amer A, Pandanaboyana S, Wilson C and White S (2024) Donor Blood Tests do Not Predict Pancreas Graft Survival After Simultaneous Pancreas Kidney Transplantation; a National Cohort Study. Transpl Int 37:12864. doi: 10.3389/ti.2024.12864
Received
18 February 2024
Accepted
07 May 2024
Published
20 May 2024
Volume
37 - 2024
Updates
Copyright
© 2024 Ho, Tingle, Malik, Thompson, Kourounis, Amer, Pandanaboyana, Wilson and White.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ning Xuan Ho, ning.ho1@nhs.net
†These authors share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.