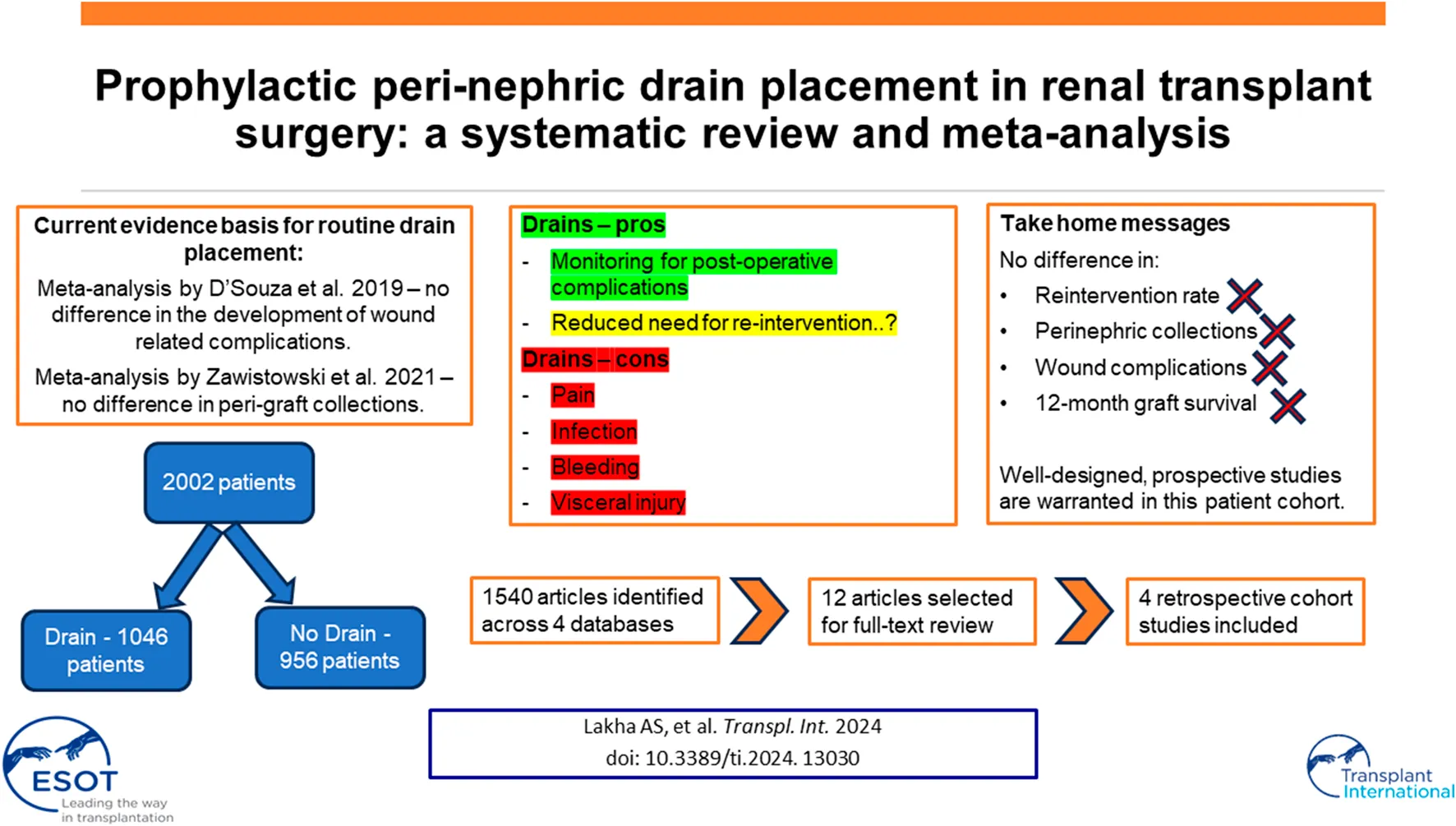
Abstract
Renal transplantation is common worldwide, with >25,000 procedures performed in 2022. Usage of prophylactic perinephric drains is variable in renal transplantation; drains are associated with risks, and there is a lack of consensus regarding benefit of routine drain placement in these patients. This meta-analysis assessed whether prophylactic drainage reduced need for reintervention postoperatively. This systematic review and meta-analysis was carried out using the Preferred Reporting Items in Systematic Reviews and Meta-Analysis, and prospectively registered on PROSPERO. Summary statistics for outcomes of interest underwent meta-analyses to a confidence interval (CI) of 95% and are presented as Forest Plots for Odds Ratio (OR). A systematic literature search in June 2023 revealed 1,540 unique articles across four databases. Of these, four retrospective cohort studies were selected. Meta-analysis of three studies showed no significant reduction in reintervention rate with pre-emptive drain placement, OR = 0.59 (95% CI: 0.16–2.23), p = 0.44. Meta-analysis did not show a significant reduction in perinephric collections with prophylactic drain insertion OR = 0.55 (95% CI: 0.13–2.37), p = 0.42. Finally, there is not good evidence that drain placement reduces superficial wound complications or improves 12-month graft survival. Further work is needed, including well-designed, prospective studies to assess the risks and benefits of drain placement in these patients.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023422685, Identifier PROSPERO CRD42021255795.
Introduction
Usage of prophylactic perinephric drains is variable in renal transplantation, and there is a lack of consensus as to the relative benefit of placing an abdominal drain intraoperatively in this patient cohort [1]. Drainage of post-operative fluid collections and prevention of the development of perinephric collections are the main indications for placing such drains in this cohort of immunosuppressed surgical patients [2]. However, there is debate over the necessity of these drains, and whether they may introduce more risks. For example, placement of a drain can result in several complications, including but not limited to post-operative pain, visceral injury, surgical site infection, bleeding or malposition [3, 4]. Prospective studies in general and colorectal surgery have shown a higher surgical site infection risk when drains are inserted intraoperatively [4]. Furthermore, meta-analysis of randomised trials as well as prospective interventional studies suggest drain insertion results in more pain for patients who received intraoperative drain placement [5, 6].
The pathological basis for the development of collections is multifactorial, however immunosuppression, increasing age, obesity, smoking, difficulty of the operation such as bleeding or damage to surrounding structures such as lymphatic tissue in the recipient’s iliac lymph trunk are all thought to contribute to fluid collections post-operatively [7, 8]. Placing a drain during the index transplantation operation therefore is thought to serve as prophylaxis against these relatively common surgical complications. However, these complications are often sub-clinical, may occur after a surgical drain is removed, and not all post-operative collections require drainage. In addition, intraoperative haemostatic techniques may also be utilised to minimise fluid effusion post renal transplantation [9].
This systematic review aims to investigate the impact of prophylactic perinephric drains placed during renal transplantation surgery on immediate and short-term post-operative surgical complication rates. In addition, the broader impact on graft function will be assessed, as well as relevant important outcomes such as deep wound complications, and surgical site infection.
Methods
This study was carried out following the Preferred Reporting Items in Systematic Reviews and Meta-Analysis (PRISMA) [10]. The protocol was prospectively registered on the PROSPERO system from the University of York (CRD42021255795) on 10th May 2023 [11].
Literature Search
A literature search was carried out on 1st June 2023, using a combination of Medical Subject Heading (MeSH) terms, free text and keywords to limit the search to renal transplantation operations and drain placement. Complete search strategy is available in Appendix 1. Cochrane protocols, trials and reviews, Transplant Library, Embase, and Medline were all searched on the same date. Each article was assessed using the inclusion criteria outlined below, and any disagreement regarding the eligibility of an article was discussed. Agreement was reached by consensus with a third, and independent, reviewer.
Inclusion and Exclusion Criteria
There were no language or time-period restrictions. Abstract-only and conference presentation publications were excluded, as were studies assessing paediatric populations and combined transplantation procedures such as simultaneous pancreas-kidney. We included papers which compared outcomes of patients who had a perinephric drain placed intraoperatively during renal transplantation. Patients with drains placed superficial to the musculofascial layer (superficial drain), or patients with drains inserted percutaneously, were excluded.
Quality Assessment
Methodological quality of included studies was assessed using the Newcastle-Ottawa Score (NOS) tool, a validated scale for assessing the quality of cohort studies [12]. Two independent reviewers performed quality assessment with discrepancies discussed.
Data Extraction
Data were extracted using a standardised and predesigned data collection form. Data were extracted, where available, on study design characteristics (type of study design, follow-up length), donor kidney type (live or deceased), and outcomes of interest. Post-operative reintervention rate of any kind (either percutaneous image guided drainage, or return to theatre) was the primary outcome for comparison between drain and drain-free patient groups. Additional outcomes such as superficial and deep wound complications, graft survival at 12 months (where available) and delayed graft function were also collected.
Data Synthesis
Data analyses were performed and figures were extracted from Microsoft Excel and the statistical package RevMan Version 5.8.0, The Cochrane Collaboration, 2020. Heterogeneity was calculated for the meta-analyses using the I2 statistic, with the Mantel-Haenszel method and random-effects model utilised due to heterogeneity between the studies.
Summary statistics for outcomes of interest underwent meta-analyses to a confidence interval (CI) of 95% and are presented as Forest Plots for Odds Ratio (OR).
Results
Across all four databases, 1,627 papers were identified, of which 87 were identified as duplicates and discarded. Our search therefore revealed 1,540 unique titles and abstracts across all four databases. Of these, four retrospective cohort studies were selected according to the methodology outlined above, and these are presented in Table 1. Figure 1 outlines a PRISMA flow diagram in selecting articles for inclusion. Across the four studies selected, a total of 2,002 patients’ outcomes data were extracted for analysis. 1,046 had an intraoperative drain placed, 956 did not. Drains were removed when the output recorded less than <50 mL/24 h consistently across three of the studies, and was not reported in the remaining study. Only Farag et al. reported the type of drain used (a Jackson-Pratt suction drain). Furthermore, three out of the four studies reported complete data on type of donor (live vs. deceased), Table 1.
TABLE 1
| Drain insertion donor type | No drain insertion donor type | |||||||
|---|---|---|---|---|---|---|---|---|
| Study | Methodology | Drain insertion, n | No drain insertion, n | Live donor (%) | Deceased donor (%) | Live donor (%) | Deceased donor (%) | Overall recommendation |
| Derweesh et al. | Single centre, retrospective cohort study | 81 | 84 | 56 | 44 | 64 | 36 | Use drain in patients receiving sirolimus |
| Cimen et al. | Single centre, retrospective cohort study | 374 | 283 | 38 | 62 | 39 | 61 | No benefit with drain insertion |
| Farag et al. | Single centre, retrospective cohort study | 112 | 388 | 13 | 87 | 42 | 58 | No benefit with drain insertion |
| Sidebottom et al. | Single centre, retrospective cohort study | 479 | 201 | — | — | — | — | No benefit with drain insertion |
Summary of studies included, and overall recommendations regarding prophylactic drainage.
FIGURE 1
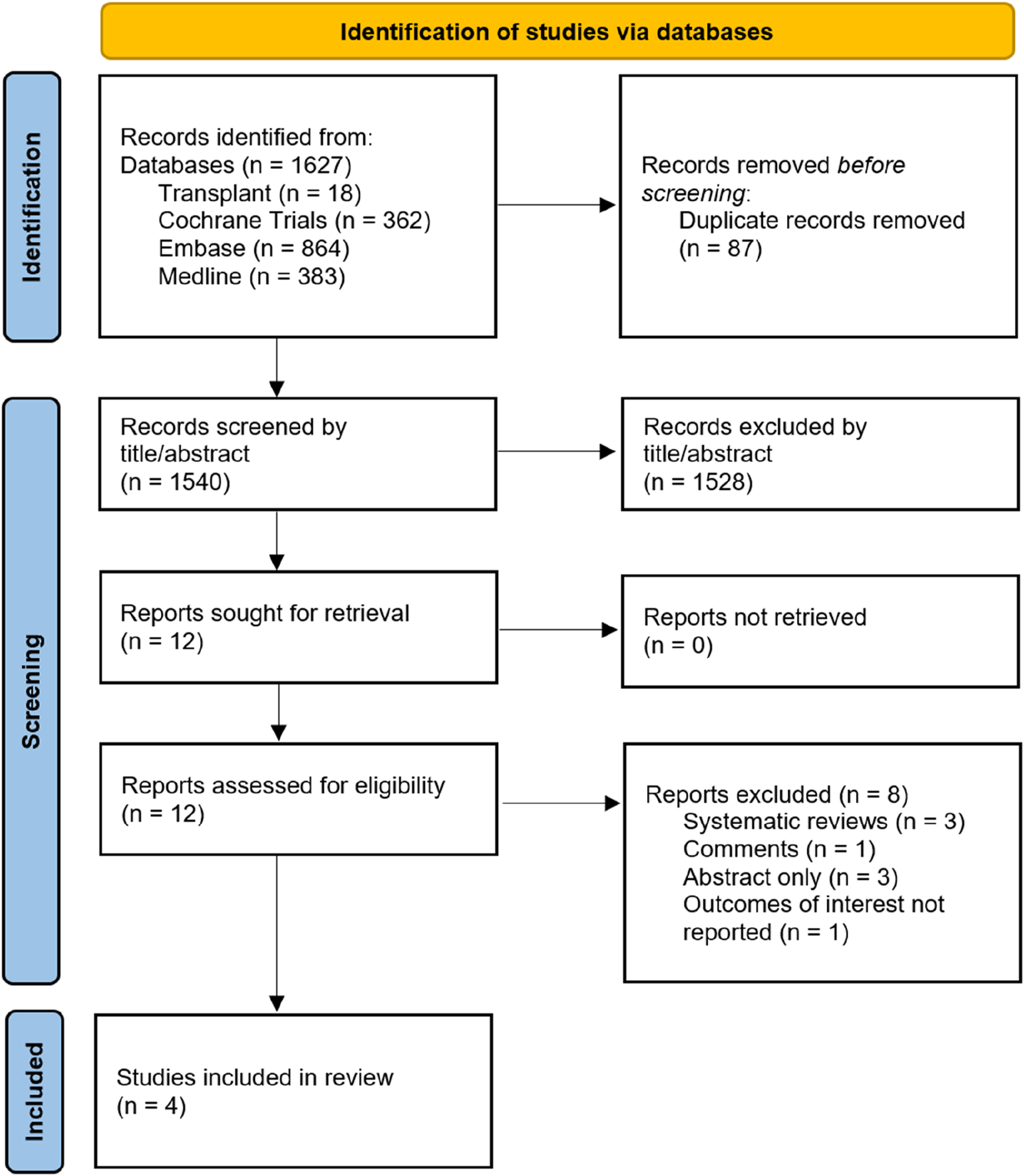
PRISMA flow diagram.
Quality Assessment
Methodological quality of included studies was assessed using the Newcastle-Ottawa Score (NOS), and Table 2 shows all included studies and their respective quality assessments. All studies were rated as “good quality” when NOS scores were converted to Agency for Healthcare Research and Quality (AHRQ) descriptors according to the following threshold: three or four stars in the selection domain, and one or two stars in the comparability domain, and two or three stars in the outcome/exposure domain.
TABLE 2
| Study | Selcection of cohorts | Comparability | Outcome | |||||
|---|---|---|---|---|---|---|---|---|
| Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts on the basis of the design or analysis | Assessment of outcome | Was follow up long enough for outcomes to occur | Adequacy of follow up of cohorts | |
| Derweesh et al. 2008 [2] | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ |
| Sidebottom et al. 2014 [13] | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | — | ☆ |
| Cimen et al. 2016 [14] | ☆ | ☆ | ☆ | ☆ | ☆ ☆ | ☆ | — | ☆ |
| Farag et al. 2021 [15] | ☆ | — | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ |
Quality assessments using the NOS.
Reintervention Rate
We performed a meta-analysis to ascertain whether intraoperative perinephric drain placement was associated with a reduced need for either image-guided percutaneous drainage or return to theatre post renal transplantation. Meta-analysis of three studies showed no evidence of a significant reduction in reintervention rate with drain placement, OR = 0.59 (95% CI: 0.16–2.23), p = 0.44, Figure 2. The study from Sidebottom et al. did not report reintervention rate post renal transplant, therefore was not included in the meta-analysis.
FIGURE 2
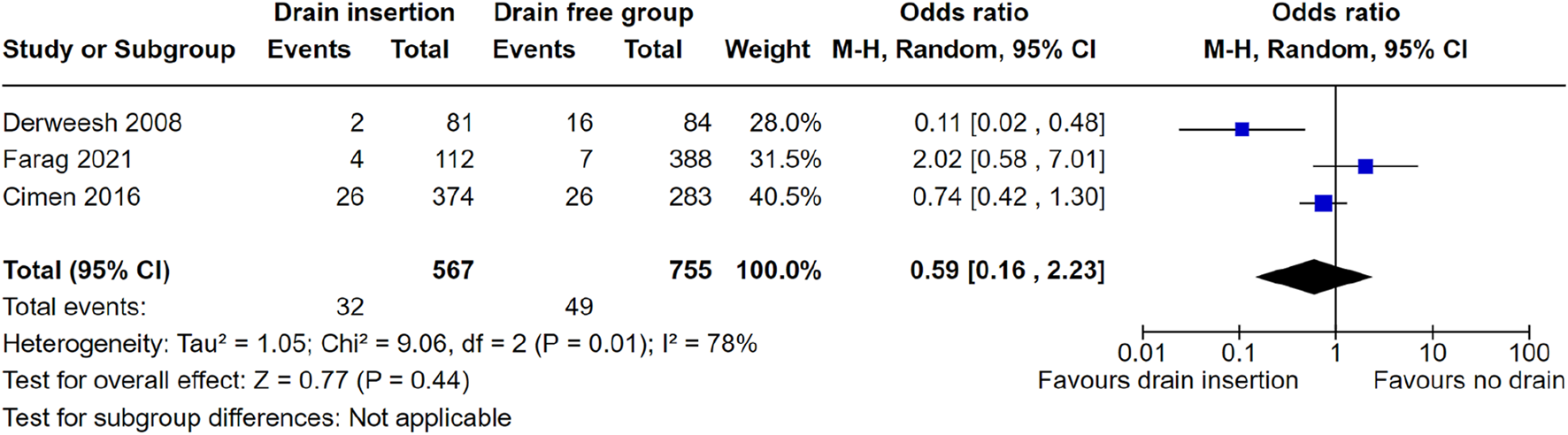
Meta-analysis of reintervention rate.
Deep Wound Complications
Three studies reported figures for deep wound complications and were therefore included for meta-analysis. Meta-analysis did not show a significant reduction in perinephric collections with prophylactic drain insertion OR = 0.55 (95% CI: 0.13–2.37) p = 0.42, Figure 3. One study could not be included in meta-analysis as they only reported an odds ratio (rather than raw patient-level data) for reduced risk, favouring drain insertion due to lower rates of peri-graft collections OR = 0.62 (95% CI: 0.43–0.88), p = 0.01.
FIGURE 3
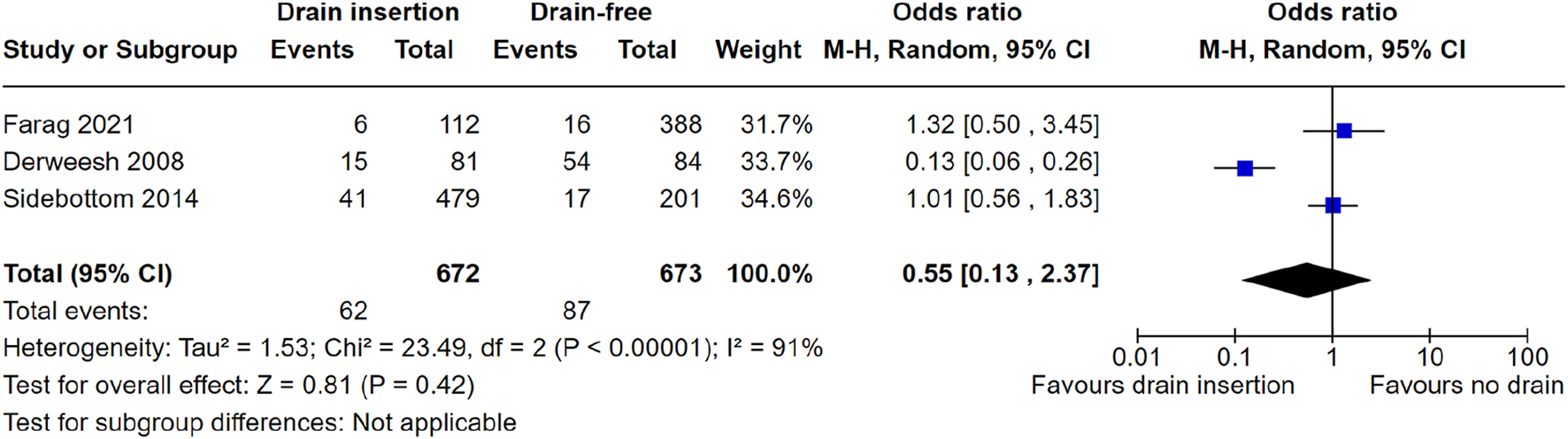
Meta-analysis of deep wound complications.
Superficial Wound Complications
Only two studies reported the rates of superficial wound complications with a standardised definition, with superficial complications inclusive of wound evisceration, infection and dehiscence. Derweesh et al. reported no significant difference between the percentage of wound complications in the drain (13.6%) and no drain group (22.6%), p = 0.13. Farag et al. reported superficial wound complications (inclusive of subcutaneous seroma or wound dehiscence), with no statistically significant difference in the incidence of wound complications between the drain and drain-free groups (p = 0.35).
Graft Survival at 12 months
Finally, we intended to assess graft survival at 12 months and whether or not there was any difference between drain and drain-free cohorts. Sidebottom et al. reported a 30 days follow up, and Cimen et al. reported 1 month longest follow up data. Farag et al. reported 98.5% and 96.4% graft survival rates in drain-free and drainage groups, respectively (p = 0.20). Similarly, Derweesh et al. reported graft survival rates of 83% and 88% in drain-free and drainage groups, respectively (p = 0.43).
Discussion
This review found no overall benefit when placing perinephric drains prophylactically during renal transplantation, including when assessing need for re-intervention post-operatively. Similarly, this review found no overall benefit of prophylactic drainage on reducing superficial or deep wound complications. Finally, there is not good evidence that perinephric drain placement is associated with improved graft survival outcomes at 12 months post renal transplantation.
Current literature demonstrates the range of complications associated with prophylactic drain insertion. One prospective study suggests that surgical site infection risk is increased when drains are inserted during general surgery procedures (OR 2.41, 95% CI 1.32–4.30, p = 0.004), however less of an effect is seen in vascular and orthopaedic surgery [4]. Furthermore, a systematic review and meta-analysis of twelve randomised controlled trials involving 1763 patients showed patients who underwent drainage had significantly higher pain scores as measured by the visual analogue scale (MD 10.08, 95% CI 5.24 to 14.92; p < 0.00001) [5]. There are limited studies reporting the incidence of bleeding and iatrogenic visceral injury secondary to perinephric drain placement. In one case series of deep pelvic collection drainage, a 2% haemorrhage rate was reported [16]. Fluid collections within the liver parenchyma may be amenable to percutaneous drainage, however this carries a reported 4% risk of major complications such as hepatocolic fistula creation, biliary peritonitis, and arterioportal fistula formation [17–19]. For retroperitoneal perinephric drains, a treatment failure rate exceeding 30% has been reported, often due to drain malposition [17].
Early post-operative collections such as seromas and haematomas occur post-transplant but the majority are discovered incidentally and are usually managed conservatively. The incidence of post operative surgical site haemorrhage detected by imaging and associated with a concurrent serum haemoglobin drop of more than 20 g/L over a 24 h period is relatively low (4.9%), with 90% of cases occurring within 1 day of implantation [20]. Collections more likely to require intervention such as urinomas, abscesses and lymphoceles, typically present later in the post-operative course, and the association with drain insertion is unclear. Lymphoceles in particular are common post renal transplant, with an incidence of 0.6%–51% reported in the literature, and 6.4% according to one recent retrospective study [21]. Urine leak has a reported incidence of 0.6%–6% and generally appears in the early post-transplant period [22, 23].
There have been two similar reviews in this area published previously. In 2019, D’Souza et al. showed that drain placement is associated with a higher incidence of peri-transplant fluid collections (RR 0.62; 95% confidence interval, 0.42–0.90), however no significant difference in the development of wound related complications [24]. A later review by Zawistowski et al. provided an update with the inclusion of a 2021 retrospective single-centre cohort study by Farag et al. The primary end-point in the Zawistowski meta-analysis was also perigraft collections [1]. No significant difference was seen between drain-free and drainage groups (pooled unadjusted OR = 0.77, 95% CI: 0.28–2.17). Similarly, there was no statistically significant difference in the secondary end points of surgical site infection, lymphocele, haematoma, and wound dehiscence between patients who did or did not receive prophylactic drainage. This review provides the most recent and extensive review of the current literature assessing the role of prophylactic perinephric drainage on short and long term clinically significant complications post kidney transplant. While previous reviews focused on the incidence of common post-operative complications, these are not necessarily clinically significant, as not all collections require drainage. By focusing our primary outcome on reintervention rate for post-operative collections, we aimed to better demonstrate the clinical significance of prophylactic drainage on renal transplant patients. More generally, the search criteria were robust and consistent across a range of generic and transplant-specific databases, with no language or time-period restrictions applied during article selection. All studies were rated as “good quality” when rated for quality via the Newcastle-Ottawa Score.
However, this review and analysis has several limitations. Given the retrospective nature of the studies identified in the literature, it is not possible to confidently demonstrate causality between our exposure (drain placement) and outcome (reintervention rate) of interest. The control groups included in the studies (no drain placement) would likely also be affected by selection bias. For instance, the Derweesh et al. study shows significant differences between the groups with respect to patient body mass index (BMI) and immunosuppression use (specifically sirolimus). These are both factors which are known to affect wound healing, surgical site infection, and wound complications specifically in renal transplantation, therefore the results cannot reliably be interpreted due to the selection bias present in the cohorts [25, 26]. Owing to the small number of studies included in this analysis (less than 10), publication bias could not be accurately assessed using Egger’s regression test for funnel plot asymmetry [27]. We found significant heterogeneity in the reporting of outcomes, and so meta-analyses were performed where specific outcomes were published. We also intended to record outcomes such as post-operative pain around the wound or drain site, opiate usage, length of hospital stay, and overall mortality, however these data were not available in the published literature in relation to drain use. Analysis of these outcomes would allow us to more effectively examine the complications associated with drain insertion, however due to the lack of availability we were not able to do so. Patient-reported outcomes and measures following drain insertion in particular would be an important aspect of drain insertion to assess and report upon, and one which we advocate should be investigated in future prospective studies. Regarding Figure 3, we intended to include Cimen et al. results in our meta-analysis, however were unable to contact the authors to obtain raw data to include in the meta-analysis. This represents a drawback to our review because Cimen et al. found lower odds of peri-graft collection, thus favouring drain insertion (p = 0.01). Finally, there was heterogeneity in the definitions of parameters such as “wound complications,” whereby authors divided into either clinically significant vs. not significant, or superficial vs. deep, or specifically looking at individual complications such as surgical site infection, wound dehiscence, or superficial wound collection. We therefore only included data from studies where we were confident that the data reflected the specific outcomes of interest described above.
One of the key rationales for intraoperative drain placement is pre-emptive control of post-operative collections such as lymphocele, seroma, haematoma, urinoma or infected tissue fluid. Ongoing monitoring for bleeding and infective collection around the graft site are the main indication for routine placement of a perinephric drain, however placement of the drain itself is associated with risks. In a meta-analysis of 28 randomised trials involving 3,659 patients, Gurusamy et al. showed that a drain-free approach to open cholecystectomy was associated with significantly lower wound infection rates, and no difference in the incidence of post-operative abdominal collection [28]. Partly as a result of this, drains are now no longer placed for uncomplicated open cholecystectomy operations. Furthermore, a single-centre experience of combined liver-kidney transplants showed no difference in the incidence of superficial/deep wound complications, collection size, intervention rate, graft failure, and overall patient survival between drainage and non-drain patient cohorts [29].
Better access to cross-sectional imaging provides a non-invasive tool for surgeons to utilise in the investigation for post-operative collections. Ultrasound provides accurate assessment of vascular flow to the graft, and can assess the presence of perinephric fluid collection and associated graft parenchymal compression [30]. Imaging is not always performed routinely, however in association with symptoms such as fever or pain, signs of graft failure such as high serum creatinine, ipsilateral leg swelling or hydronephrosis, drainage of these collections is indicated [31].
Current practice also shows a variety approaches to prophylactic drainage. In 2020, a survey of 43 renal transplant surgeons across Australia and New Zealand revealed 61% of surgeons practising routine drain insertion, while 21% rarely inserted drains [32]. A more recent (2023) survey of UK-based transplant surgeon practices suggests over two-thirds of respondents routinely insert one drain, while 8.3% indicated insertion of two or more drains on a routine, prophylactic basis. Only one-fifth of surgeons insert drains selectively as reported in this study [33]. This suggests the need for a paradigm shift in how prophylactic drainage in renal transplantation is viewed, especially in the absence of overwhelming evidence supporting its impact on favourable post-operative outcomes.
Given the lack of clear benefit of placing perinephric drains intraoperatively during renal transplantation, negative impact on patient experience, and the potential risks, we advocate for a an approach whereby drains are only placed for specific indications on a case by case basis. Prospective data is needed to support this position, and trial-level evidence is warranted to support or discourage routine perinephric drainage.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
AL: first author, study design, data collection, data analysis, manuscript write up. SA: study design, data collection, data analysis, manuscript write up. JH: study design, data analysis, manuscript write up. JO’C: lead investigator, study design, data collections, data analysis, overall responsibility for the study. All authors contributed to the article and approved the submitted version.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We acknowledge the essential help of Carolyn Smith, Bodleian Health Care Libraries, University of Oxford, United Kingdom with the search strategy, choice of databases, and use of reference management software and systematic review software.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
Zawistowski M Nowaczyk J Domagała P . Prophylactic Intra-Abdominal Drainage Following Kidney Transplantation: A Systematic Review and Meta-Analysis. Pol Przegl Chir (2021) 93(4):1–10. 10.5604/01.3001.0014.9166
2.
Derweesh IH Ismail HR Goldfarb DA Araki M Zhou L Modlin C et al Intraoperative Placing of Drains Decreases the Incidence of Lymphocele and Deep Vein Thrombosis After Renal Transplantation. BJU Int (2008) 101(11):1415–9. 10.1111/j.1464-410X.2007.07427.x
3.
Findik UY Topcu SY Vatansever O . Effects of Draıns on Pain, Comfort and Anxiety in Patıents Undergone Surgery. Int J Caring Sci (2013) 6(3).
4.
Mujagic E Zeindler J Coslovsky M Hoffmann H Soysal SD Mechera R et al The Association of Surgical Drains With Surgical Site Infections - A Prospective Observational Study. Am J Surg (2019) 217(1):17–23. 10.1016/j.amjsurg.2018.06.015
5.
Wong CS Cousins G Duddy JC Walsh SR . Intra-Abdominal Drainage for Laparoscopic Cholecystectomy: A Systematic Review and Meta-Analysis. Int J Surg (2015) 23:87–96. 10.1016/j.ijsu.2015.09.033
6.
Agarwal S Duara BK Ahmed R Bhaskar Das BD . Ultrasound-Guided Percutaneous Drainage of Intra-Abdominal Collection and Its Clinical Outcome: A Prospective Interventional Study. JCDR (2022) 16(12):TC05–TC09. 10.7860/jcdr/2022/59959.17328
7.
Mehrabi A Fonouni H Wente M Sadeghi M Eisenbach C Encke J et al Wound Complications Following Kidney and Liver Transplantation. Clin Transpl (2006) 20(Suppl. 17):97–110. 10.1111/j.1399-0012.2006.00608.x
8.
Inoue T Saito M Narita S Numakura K Tsuruta H Maeno A et al Evaluation of Persistent Lymphatic Fluid Leakage Using a Strategy of Placing a Drain After Kidney Transplantation: A Statistical Analysis to Assess Its Origin. Transplant Proc (2017) 49(8):1786–90. 10.1016/j.transproceed.2017.06.021
9.
Tammaro V Vernillo A Dumani X Florio I Pelosio L Jamshidi A et al Prevention of Fluid Effusion in Kidney Transplantation With the Use of Hemostatic Biomaterials. Transplant Proc (2014) 46(7):2203–6. 10.1016/j.transproceed.2014.07.048
10.
Page MJ McKenzie JE Bossuyt PM Boutron I Hoffmann TC Mulrow CD et al The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ (2021) 372:n71. 10.1136/bmj.n71
11.
Lakha AS Ahmed S Hunter J O’Callaghan J . Do Prophylactic Perinephric Drains Placed at the Time of Renal Transplantation Reduce Surgical Complications?PROSPERO 2023 CRD42023422685 (2023). Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023422685 (Accessed December 26, 2023).
12.
Wells G Shea B O’Connell D Robertson J Peterson J Losos M et al The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analysis (2000).
13.
Sidebottom RC Parsikia A Chang PN Berhane Z Campos S Khanmoradi K et al No Benefit When Placing Drains After Kidney Transplant: A Complex Statistical Analysis. Exp Clin Transplant (2014) 12(2):106–12.
14.
Cimen S Guler S Tennankore K Imamoglu A Alwayn I . Surgical Drains Do Not Decrease Complication Rates But Are Associated With a Reduced Need for Imaging After Kidney Transplant Surgery. Ann Transpl (2016) 21:216–21. 10.12659/aot.898260
15.
Farag A Gaynor JJ Serena G Ciancio G . Evidence to Support a Drain-Free Strategy in Kidney Transplantation Using a Retrospective Comparison of 500 Consecutively Transplanted Cases at a Single center. BMC Surg (2021) 21(1):74. 10.1186/s12893-021-01081-x
16.
Harisinghani MG Gervais DA Maher MM Cho CH Hahn PF Varghese J et al Transgluteal Approach for Percutaneous Drainage of Deep Pelvic Abscesses: 154 Cases. Radiology (2003) 228(3):701–5. 10.1148/radiol.2283020924
17.
Lorenz J Thomas JL . Complications of Percutaneous Fluid Drainage. Semin Intervent Radiol (2006) 23(2):194–204. 10.1055/s-2006-941450
18.
Satoh H Matsuyama S Mashima H Lmoto A Hidaka K Hisatsugu T . A Case of Hepatocolic Fistula After Percutaneous Drainage for a Gas-Containing Pyogenic Liver Abscess. J Gastroenterol (1994) 26(6):782–5. 10.1007/BF02349288
19.
Tazawa J Sakai Y Maekawa S Ishida Y Maeda M Marumo F et al Solitary and Multiple Pyogenic Liver Abscesses: Characteristics of the Patients and Efficacy of Percutaneous Drainage. Am J Gastroenterol (1997) 92(2):271–4.
20.
Hachem LD Ghanekar A Selzner M Famure O Li Y Kim SJ . Postoperative Surgical-Site Hemorrhage After Kidney Transplantation: Incidence, Risk Factors, and Outcomes. Transpl Int (2017) 30(5):474–83. 10.1111/tri.12926
21.
Sevmis M Aktas S Alkara U Kilercik H Uyar M Sevmis S . Risk Factors, Diagnosis, and Treatment of Lymphocele After Renal Transplantation: A Retrospective Study. Transplant Proc (2021) 53(3):1040–7. 10.1016/j.transproceed.2021.01.028
22.
Buttigieg J Agius-Anastasi A Sharma A Halawa A . Early Urological Complications After Kidney Transplantation: An Overview. World J Transpl (2018) 8(5):142–9. 10.5500/wjt.v8.i5.142
23.
Whang M Benson M Salama G Geffner S Sun H Aitchison S et al Urologic Complications in 4000 Kidney Transplants Performed at the Saint Barnabas Health Care System. Transpl Proc. (2020) 52(1):186–90. 10.1016/j.transproceed.2019.10.008
24.
D’Souza K Crowley SP Hameed A Lam S Pleass HC Pulitano C et al Prophylactic Wound Drainage in Renal Transplantation: A Systematic Review. Transplant Direct (2019) 5(7):e468. 10.1097/TXD.0000000000000908
25.
Lu H Zheng P Chen RY Chen M . Analysis of Risk Factors for Impaired Wound Healing After Kidney Transplantation. Int Wound J (2023) 20(1):140–4. 10.1111/iwj.13848
26.
Dean PG Lund WJ Larson TS Prieto M Nyberg SL Ishitani MB et al Wound-Healing Complications After Kidney Transplantation: A Prospective, Randomized Comparison of Sirolimus and Tacrolimus. Transplantation (2004) 77(10):1555–61. 10.1097/01.tp.0000123082.31092.53
27.
Egger M Davey Smith G Schneider M Minder C . Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ (1997) 315:629–34. 10.1136/bmj.315.7109.629
28.
Gurusamy KS Samraj K . Routine Abdominal Drainage for Uncomplicated Open Cholecystectomy. Cochrane Database Syst Rev (2007) 2007(2):CD006003. 10.1002/14651858.CD006003.pub2
29.
Vincenzi P Gaynor JJ Chen LJ Figueiro J Morsi M Selvaggi G et al No Benefit of Prophylactic Surgical Drainage in Combined Liver and Kidney Transplantation: Our Experience and Review of the Literature. Front Surg (2021) 8:690436. 10.3389/fsurg.2021.690436
30.
Irshad A Ackerman S Sosnouski D Anis M Chavin K Baliga P . A Review of Sonographic Evaluation of Renal Transplant Complications. Curr Probl Diagn Radiol (2008) 37(2):67–79. 10.1067/j.cpradiol.2007.06.001
31.
Ghonge NP Goyal N Vohra S Chowdhury V . Renal Transplant Evaluation: Multimodality Imaging of Post-Transplant Complications. Br J Radiol (2021) 94(1124):20201253. 10.1259/bjr.20201253
32.
Mugino M Lee T Lam S Hameed A Sandroussi C Chadban S et al Prophylactic Wound Drainage in Renal Transplant: A Survey of Practice Patterns in Australia and New Zealand. Exp Clin Transplant (2020) 18(7):771–7. 10.6002/ect.2020.0071
33.
Amer A Scuffell C Dowen F Wilson CH Manas DM . A National Survey on Enhanced Recovery for Renal Transplant Recipients: Current Practices and Trends in the UK. Ann R Coll Surgeons Engl (2023) 105(2):166–72. 10.1308/rcsann.2021.0365
APPENDIX 1
Transplant Library Search URL: https://ovidsp.ovid.com/ovidweb.cgi?T=JS&NEWS=N&PAGE=main&SHAREDSEARCHID=3TvHv11Dt4cw1NlxqWFB6MvMmZDjDjslUOaG2iETazua2DxHJlBL1wKyGfIVYFHQn.
Medline Search URL: https://ovidsp.ovid.com/ovidweb.cgi?T=JS&NEWS=N&PAGE=main&SHAREDSEARCHID=31LygHgPjlVo7WTjuxhYBSXyljJHgkIVLLuds7cIY2hvyaKpJoHIjjvfZJJeXOJf5.
Embase search URL: https://ovidsp.ovid.com/ovidweb.cgi?T=JS&NEWS=N&PAGE=main&SHAREDSEARCHID=6sWADoa0ntU0FTQmkdaeO5QBXlCWubLBzIi7HG9w9P3AEztaoVShf2nSuZ4TDUKCJ.
Summary
Keywords
drain, renal transplant, prophylactic drainage, collection, perinephric drain
Citation
Lakha AS, Ahmed S, Hunter J and O’Callaghan J (2024) Prophylactic Peri-Nephric Drain Placement in Renal Transplant Surgery: A Systematic Review and Meta-Analysis. Transpl Int 37:13030. doi: 10.3389/ti.2024.13030
Received
23 March 2024
Accepted
09 July 2024
Published
02 August 2024
Volume
37 - 2024
Updates
Copyright
© 2024 Lakha, Ahmed, Hunter and O’Callaghan.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adil S. Lakha, asl48@cantab.ac.uk
‡ Present address: Adil S. Lakha, Nuffield Department of Surgical Sciences, University of Oxford, Oxford, United Kingdom
ORCID: Adil S. Lakha, orcid.org/0000-0002-9525-127X
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.