- 1Department of Intensive Care Medicine, Maastricht University Medical Center+, Maastricht, Netherlands
- 2Heart and Vascular Center, Maastricht University Medical Center+, Maastricht, Netherlands
- 3Department of Surgery, Maastricht University Medical Center+, Maastricht, Netherlands
- 4School of Nutrition and Translational Research in Metabolism, Faculty of Health, Medicine and Life Sciences, Maastricht University, Maastricht, Netherlands
- 5Academy for Postgraduate Medical Training, Maastricht University Medical Center+, Maastricht, Netherlands
- 6School of Health Professions Education, Faculty of Health, Medicine and Life Sciences, Maastricht University, Maastricht, Netherlands
- 7Department of Anesthesiology, Pain and Palliative Medicine, Radboud University Medical Center, Nijmegen, Netherlands
- 8Care and Public Health Research institute (Caphri), Faculty of Health, Medicine and Life Sciences, Maastricht University, Maastricht, Netherlands
- 9Cardiovascular research institute Maastricht (Carim), Faculty of Health, Medicine and Life Sciences, Maastricht University, Maastricht, Netherlands
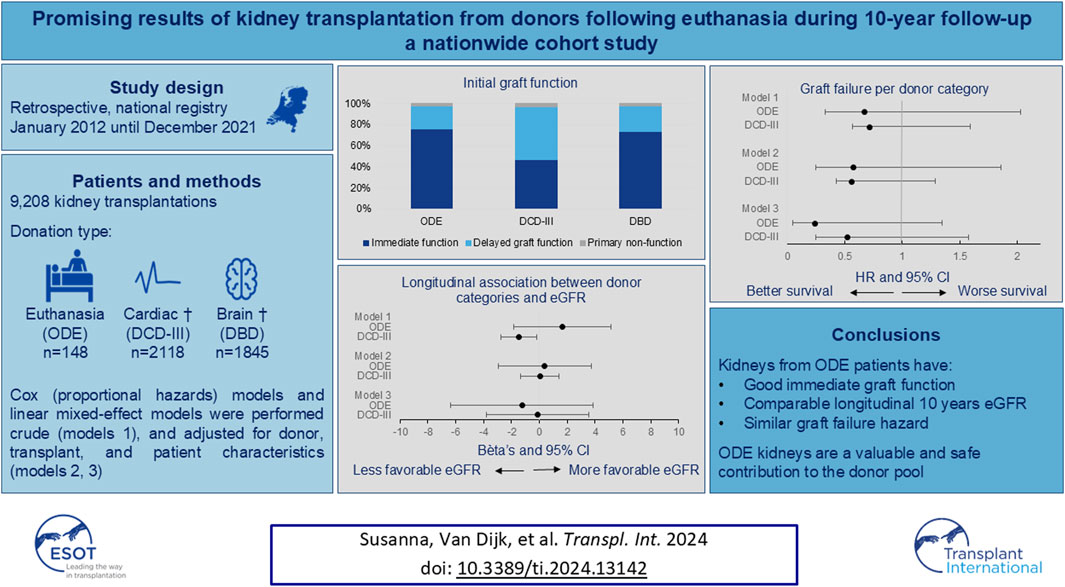
The outcome of kidneys transplanted following organ donation after euthanasia (ODE) remains unclear. This study analyzed all kidney transplantations in the Netherlands from January 2012 to December 2021, comparing the outcomes following ODE, donation after circulatory death (DCD-III), and donation after brain death (DBD). 9,208 kidney transplantations were performed: 148 ODE, 2118 DCD-III, and 1845 DBD. Initial graft function was compared between these categories. Immediate graft function, delayed graft function and primary non-function in ODE kidney recipients were 76%, 22%, and 2%, respectively, 47%, 50% and 3% in DCD-III kidney recipients and 73%, 25%, and 2% in DBD kidney recipients (overall p-value: p < 0.001). The number of kidneys transplanted over a median follow-up period of 4.0 years (IQR 2.0–6.6), was 1810, including 72 ODE, 958 DCD-III and 780 DBD kidneys. In this period, 213 grafts (11.8%) failed [7 grafts (9.7%) from ODE donors, 93 grafts (9.7%) from DCD-III donors, and 113 grafts (14.5%) from DBD donors]. Kidneys transplanted after euthanasia have a good immediate graft function, a comparable longitudinal 10 years eGFR, and similar graft failure hazard to kidneys from DCD-III and DBD. Kidney transplantation following ODE is a valuable and safe contribution to the donor pool.
Introduction
Post-mortem organ donation can be performed after brain death (Donation after Brain Death, DBD) or following circulatory death (Donation after Circulatory Death, DCD). DCD is categorized based on the Maastricht classification into four types, of which DCD-I, DCD-II, and DCD-IV are classified as uncontrolled donations; DCD-III is classified as donation following withdrawal of life-sustaining therapy and is a controlled donation [1]. Since July 2017, DCD-I and DCD-II procedures are no longer performed in the Netherlands.
In ODE, a patient dies in a controlled manner, following administration by a physician of euthanasia drugs. However, the dying process of these patients differs from that of patients who donate their organs after circulatory death (i.e., DCD) following withdrawal of life-sustaining treatments in the ICU (i.e., DCD-III) or following brain death (i.e., DBD), as these patients are critically ill [2]. Hence, the DCD classification recently proposed to include ODE patients in a separate category of highly controllable DCD: DCD-V [3, 4].
As the present study describes data from Netherlands, we use organ donation after euthanasia (ODE) as terminology rather than MAID (Medical Assistance in Dying), which is used in other countries [5, 6]. The ethical, legal, and logistical implications of ODE, in general, have been extensively discussed in both the scientific literature and public media [7, 8].
The possibility of ODE is expanding to more countries, and the number of procedures is increasing annually in most countries where ODE is already available [9, 10]. Organ donation after euthanasia may increase the number of donor organs and thus aid in narrowing the gap between the demand and availability of organs for transplantation. The next question to consider is whether the outcomes of the transplanted organs after ODE are sufficient to continue the procedure.
Data on the outcome of kidneys transplanted following ODE is scarce [11]. A conference abstract reported graft function of transplanted kidneys following ODE that was comparable to DCD-III and DBD over a 5-year follow-up period [12]. We hypothesized that transplant outcomes after ODE have favorable initial graft function, favorable estimated glomerular filtration rate, and less graft failure compared to transplant outcomes from DCD-III and DBD over a 10-year follow-up period. We investigated whether this was independent of a comprehensive set of donor, recipient, and transplant variables. This investigation provides the results of kidney transplants following ODE compared to kidney transplants from other forms of donation during a 10-year study period.
Patients and Methods
Data from the Dutch Transplant Foundation (Nederlandse Transplantatie Stichting, NTS) are recorded in the Netherlands Organ Transplantation Registry (NOTR), which includes all kidney transplantations performed in the Netherlands. The authors requested and obtained data on transplantations between 1st January 2012 (the year of the first ODE retrieval in the Netherlands), and 31st December 2021, from the NTS registry NOTR in accordance with their data registry governance.
These data were used to construct a retrospective cohort of patients who underwent a kidney transplantation, to compare the graft function between ODE, DCD-III, and DBD derived grafts.
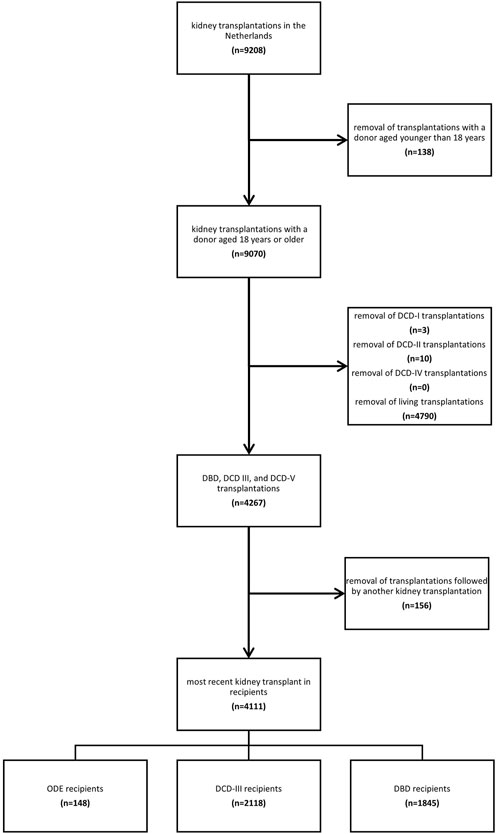
Next, we excluded organ transplantations from donors younger than 18 years, and DCD-I, DCD-II, and DCD-IV donations, as defined in the Maastricht Category [1], and living donation retrievals. No donors in the dataset were represented in multiple transplantation categories (e.g., a living transplantation followed by a post-mortem donation). This resulted in the following categories to be studied: organ donation after euthanasia (ODE, DCD-V); organ donation after circulatory death, Maastricht Category III (DCD-III); and organ donation after brain death (DBD) [1, 3].
Recipients may undergo multiple kidney transplantations during their disease course. For the primary investigation, we included and characterized the most recent transplant (i.e., the latest transplant) in a recipient. In this way any recipient with multiple kidney transplantations was included only once in the analyses.
We described the donors, the recipients, and transplantation and graft characteristics for ODE (DCD-V), DCD-III and DBD categories. Initial graft function, as well as estimated glomerular filtration (eGFR) rate over 10 years and graft failure, were described for ODE, DCD-III and DBD categories.
Donor Characteristics
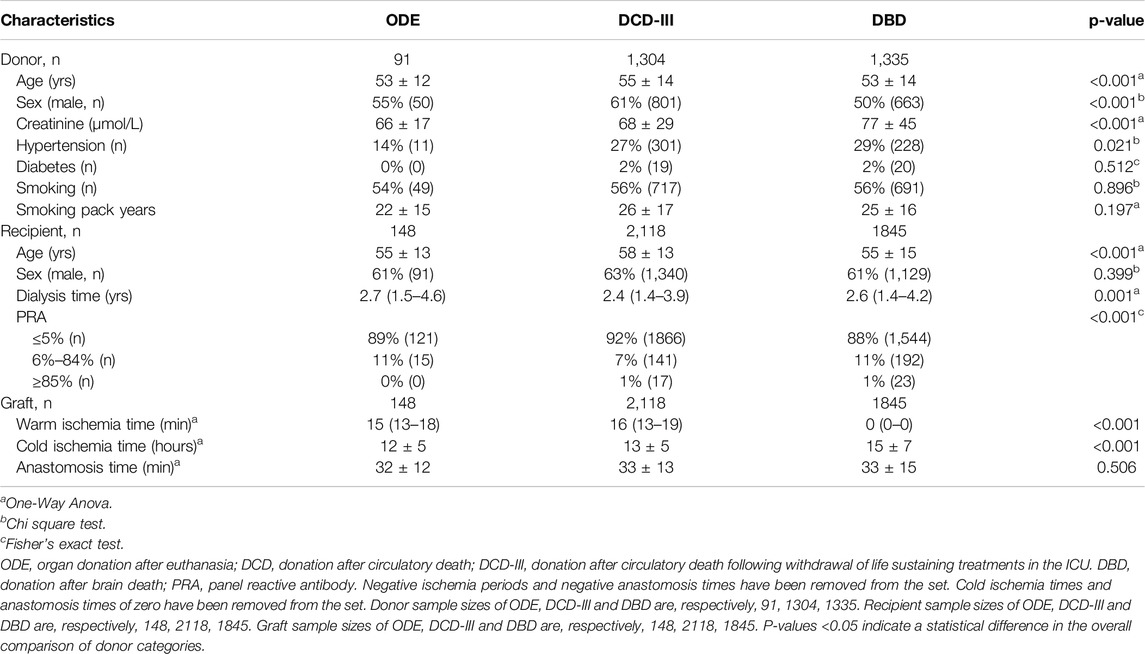
For donor characteristics, we described age in years, sex, serum creatinine concentrations in µmol/L, medical history of hypertension, diabetes mellitus, and smoking status (dichotomous outcome measures and in pack years), as reported in the NOTR.
Recipient Characteristics
For recipient characteristics, age was defined as the recipient’s age at transplantation in years. Furthermore, we described sex, dialysis time and panel reactive antibody (PRA). The PRA test was used to estimate the degree of sensitization in recipients’ blood to donor-specific antibodies. Traditionally, the recipient’s serum is exposed to a panel of random donor lymphocytes. The PRA test indicates the risk of transplant failure to the host response to transplantation [13, 14]. PRA is classified as low (≤5%), intermediate (6%–84%), and high (≥85%). Dialysis time was measured as the days of dialysis of the recipient before transplantation and presented in years by dividing by 365.25.
Transplantation Characteristics
The warm ischemia time (WIT) is defined as the time between the circulatory arrest (e.g., loss of cardiac output in a DCD-III and ODE (DCD-V), and arterial clamping in DBD until the start of cold aortic flush (in situ preservation) or the start of normothermic aortic flush in case of normothermic machine perfusion of the donor [15]. The cold ischemia time (CIT) is defined as the start of cold aortic flush (in situ preservation) until cessation of hypothermic machine perfusion respectively taken off ice. The anastomosis time (AT) is defined as the time between the end of the hypothermic state and reperfusion of the kidney in the recipient.
Graft Characteristics
Initial Graft Function
Graft function within the first week post-transplantation was categorized into primary non-function, delayed graft function, and immediate graft function. Kidney transplantations that failed (e.g., non-viable kidneys, or graft loss) in the first week post-transplantation were categorized as primary non-function. Kidney transplantations that required dialysis the first week post-transplantation were categorized as delayed graft function. The remaining kidney transplantations were categorized as immediate graft function.
Graft Failure
Graft failure and its causes were pre-scored in the NOTR and included hyperacute rejection, infection (not graft-related), infection of graft, non-viable kidney, patient dying with a functioning transplant, permanent non-function, recurrent primary renal disease, rejection after stopping all immunosuppressive drugs, rejection while taking immunosuppressive drugs (acute/chronic), removal of functioning graft, technical problems, thrombosis/infarction, vascular or ureteric problems, vascular problems: none-operative or rejection related, other (renal) and unknown (Supplementary Table S1).
Estimated Glomerular Filtration Rate
Graft function was studied using 10-year follow-up on serum creatinine. The eGFR was calculated using the re-expressed MDRD-4-formula [16]. The concentration of serum creatinine (in µmol/L) was converted to serum creatinine in mg/dL by using the molecular weight of creatinine (113.12 g/mol). Increased age of recipients for creatinine measurements post-transplant was considered (e.g., for eGFR estimation 2 years post-transplant, the following age was used: age at date of transplantation plus 2 × 365.25). The eGFR was presented as mean and standard error (SE) to indicate that time moments may have more or fewer observations in the ODE, DCD-III, and DBD groups. Invalid (negative) creatinine values were removed from the dataset (n = 1,021). The number of invalid creatinine values at 3 months, 1 year, 2 years, 3 years, 4 years, 5 years, 6 years, 7 years, 8 years, 9 years, and 10 years were, respectively, 42, 36, 110, 178, 167, 140, 111, 102, 67, 43, and 25.
Statistical Analysis
This observational study is reported in accordance with the STROBE guideline [17].
Continuous data was visually inspected for normality and presented as mean ± standard deviation or as median (interquartile range). Categorical variables were presented as percentages. One-way ANOVA, Chi-square test, and Fisher’s exact test were used to test overall differences between ODE, DCD-III and DBD categories. Pairwise comparisons were conducted as post-hoc analysis to identify differences between 2 of the 3 categories if an overall test indicated statistical significance.
First, we used linear mixed-effects models to analyze whether longitudinal kidney function over 10 years, based on eGFR, differed between donor categories, with DBD as the reference category. We investigated a model containing donor category and time as independent variables (model 1). Model 1 was subsequently adjusted for donor age, donor sex, donor smoking, recipient age, recipient sex, and transplant ischemic times (CIT and AT) (model 2). Next, this model was further adjusted for donor hypertension, donor diabetes mellitus, WIT and transplant PRA (model 3). Recipient ID was added as a random effect to the models. The longitudinal mixed-effects models were repeated with time as random slopes. Fixed effects were presented as coefficients (β) and 95% CI, with a negative coefficient indicating a lower eGFR per donor category, as compared to the reference category.
Then, we used Cox proportional hazard models for the main analyses to investigate the association between donor categories and graft failure, with DBD as reference category. Grafts in which primary non-function occurred within the first week post-transplant, and therefore failing grafts, were excluded from the primary Cox analyses, because it is considered a short-term outcome with another presumed mechanism than those involved over the longer periods of time. Death of the recipient was considered a censored event in the main analyses when the recipient died with a functioning graft. Crude (model 1) models were adjusted (models 2 and 3) for the same set of variables in accordance with adjustments for the linear-mixed effects models above. For the Cox models, we report hazard ratios (HR) with their 95% confidence intervals (CI), with an HR higher than 1 indicating an increased hazard per donor category as compared to the reference category. The proportional hazards assumption was checked using the scaled Schoenfeld residuals.
We performed four sensitivity analyses and re-analyzed the above Cox models by first replacing the recipients for organs donated (i.e., including all transplantations of each recipient; sensitivity analysis 1) to determine whether the outcomes remain consistent with the primary models. Next, we also re-analyzed model 1 and model 2, in which recipient death was not censored, but included as an event (sensitivity analysis 2). A third sensitivity analysis was performed to re-analyze model 1 and model 2, in which primary non-function, which was assumed to have a separate etiology from graft failure occurring after a prolonged period, was included (sensitivity analysis 3). Finally, a fourth sensitivity analysis was performed re-analyzing model 1 and model 2, in which only the first transplantation within a recipient was used in the analyses, instead of the last transplantation within a recipient in the primary analyses. Although we assumed that matching a kidney between the donor and recipient is independent, HLA mismatch and antibody production could change due to re-transplantations. The fourth sensitivity analysis excluded such mechanisms by showing similar results (Supplementary Table S2) [18]. In addition to regression coefficients, hazard ratios and their 95% confidence intervals, we report p-values, which were considered statistically significant at p < 0.05. We analyzed the data using IBM SPSS Statistics 27 and R x64 i4.1.3 and R studio 2023.
Results
Of the 9,208 kidney transplantations performed in the Netherlands between 1st January 2012 and 31st December 2021, 9,070 were from donors aged 18 years and older. After excluding 4,790 transplantations from living donors and 13 transplantations from DCD-I, DCD-II, and DCD-IV donors, and after excluding previous transplantations within the recipients, 4,111 kidney transplant recipients remained, with kidney transplants originating from 2,730 unique donors (Figure 1). In total, 148 recipients received a kidney from a donor after ODE, 2,118 from a donor after DCD-III, and 1,845 from a donor after DBD (Figure 1).
Donor recipient and transplantation baseline characteristics are presented in Table 1. ODE donors had lower serum creatinine concentrations (p = 0.046) compared to DBD donors. ODE recipients were younger (p = 0.034) than DCD-III recipients. A minor, not clinically relevant difference, was found in WIT between ODE transplantations and DCD-III transplantations (p = 0.022). As expected, WIT was longer in ODE as compared to DBD transplantations (p < 0.001). CIT was shorter in ODE transplantations as compared to DBD (p < 0.001), no difference was found for CIT in ODE and DCD-III (Supplementary Table S3).
Initial graft function was available for ODE 127 out of 148 (86%), for DCD-III 1940 out of 2,118 (92%), and for DBD 1636 out of 1845 kidneys (89%) (Table 2). First-week post-transplantation outcomes showed that immediate graft function was higher in ODE, which was similar to DBD, when compared to DCD-III (overall p-value: p < 0.001) (Table 2). DCD-III showed more delayed graft function as compared to ODE and DBD, whereas primary non-function was similar (overall p-value: p < 0.001) (Table 2; Supplementary Table S1).
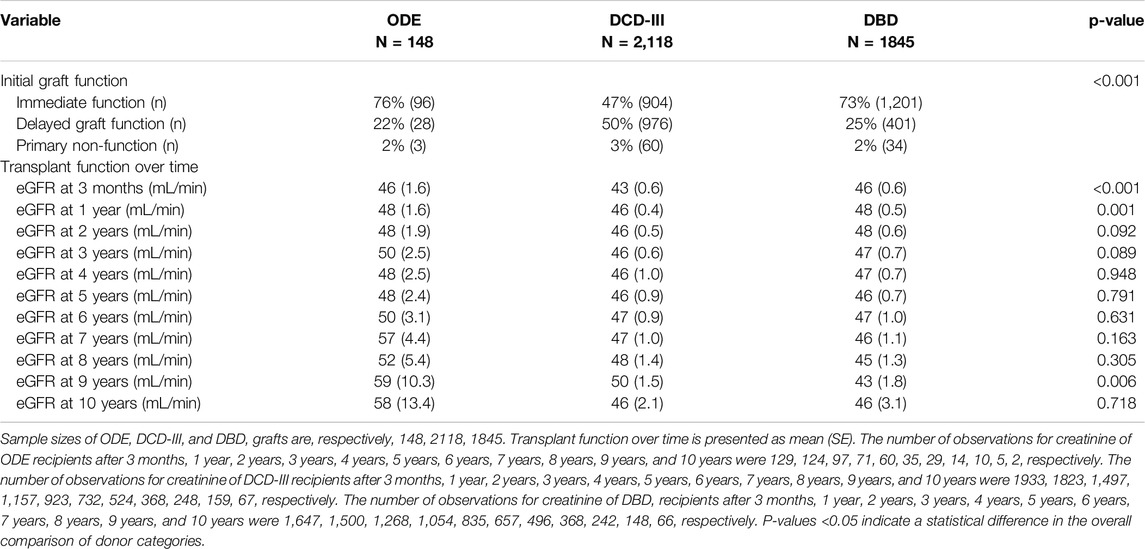
Table 2. Initial graft function and transplant function using estimated glomerular filtration rate (eGFR) up until 10 years after transplantation.
Mean graft function over time by eGFR is shown in Table 2. Longitudinal mixed-effects regression analyses adjusted for donor category and time (model 1, Table 3) showed that, compared to DBD, longitudinal eGFR for ODE was (β: 95% CI) 1.64 mL/min/1.73 m2 (−1.84; 5.13) and for DCD-III was −1.47 mL/min/1.73m2 (−2.74;-0.20) over the 10-year period. After additional adjustments for donor sex, donor age, donor smoking, recipient age, recipient sex, cold ischemic period, anastomosis time, and initial graft function (model 2, Table 3), and further adjustment for donor hypertension, donor diabetes mellitus, WIT and transplant PRA (model 3, Table 3) this association disappeared. Mixed-effects analyses with random slopes for time showed similar results (model 1–3, Supplementary Table S4).
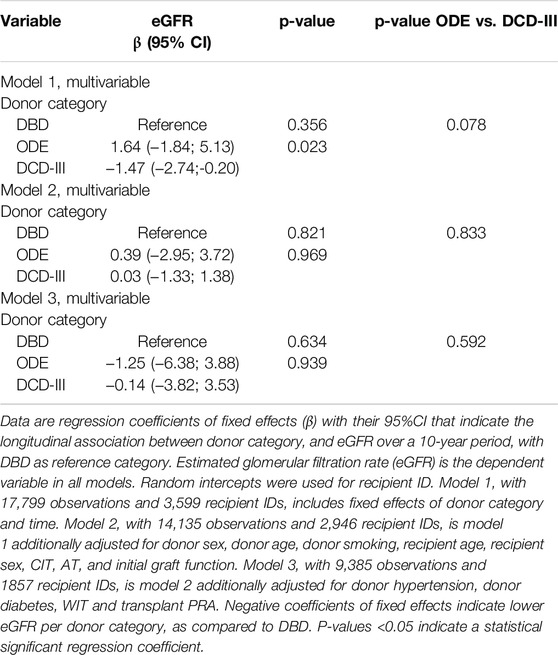
Table 3. Longitudinal association between donor categories and estimated glomerular filtration rate over 10 years.
After exclusion of primary non-functioning grafts, over a median follow-up period of 4.0 years (IQR 2.0–6.6), 1810 grafts were transplanted, which included 72 ODE, 958 DCD-III, and 780 DBD grafts. Over the median follow-up period, 213 grafts (11.8%) failed. This included 7 grafts (9.7%) for ODE, 93 grafts (9.7%) for DCD-III, and 113 grafts (14.5%) for DBD. Median follow-up periods of each donor category were 3.7 years (IQR 2.0–5.8) for ODE, 4.0 years (IQR 2.0–6.3) for DCD-III and 4.1 years (IQR 2.1–7.0) for DBD grafts.
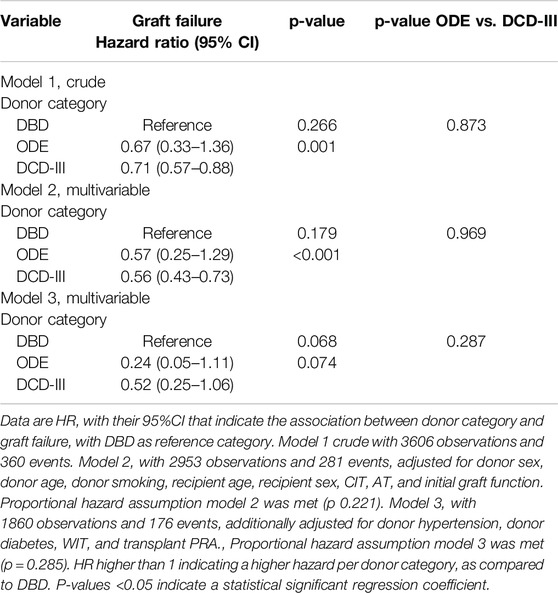
When studying the association between graft failure and donor category, compared to DBD, the hazard ratio for ODE was (HR: 95% CI) 0.67 (0.33–1.36) and for DCD-III was 0.71 (0.57–0.88) using crude Cox regression analysis (model 1, Table 4). After adjustment for donor sex, donor age, donor smoking, recipient age, recipient sex, cold ischemic period, anastomosis time and initial graft function, the hazard ratio was, compared to DBD, 0.57 (0.25–1.29) for ODE and 0.56 (0.43–0.73) for DCD-III. After additional adjustments for donor hypertension, donor diabetes mellitus, WIT, and transplant PRA, the statistically significant difference between DBD and DCD-III grafts disappeared. The proportional hazards assumption was met (Supplementary Figures S1–S3).
The four sensitivity analyses, re-analyzing the above models by replacing the recipients for organs donated (i.e., including all kidneys transplanted in each recipient; sensitivity analysis 1, Supplementary Table S2); in which recipient death was not censored, but included as an event; (sensitivity analysis 2, Supplementary Table S2); in which primary non-function was included (sensitivity analysis 3, Supplementary Table S2); and in which only the first transplantation of each recipient was included (sensitivity analysis 4, Supplementary Table S2), all showed similar results as the primary analyses.
Discussion
This study addresses the transplant outcomes of kidneys donated after euthanasia over a 10-year study period, compared to DCD-III and DBD, and has three main findings. First, immediate graft function was higher in ODE, when compared to DCD-III, and similar to DBD. Second, longitudinally, eGFR for ODE did not differ from eGFR for DBD and eGFR for DCD-III over 10 years, after adjustment for donor sex, donor age, donor smoking, recipient age, recipient sex, CIT, AT, initial graft function and donor hypertension, donor diabetes mellitus, WIT and transplant PRA. Third, graft failure for ODE did not differ from graft failure for DBD and graft failure for DCD-III, after adjustment for donor sex, donor age, donor smoking, recipient age, recipient sex, CIT, AT, and initial graft function, donor hypertension, donor diabetes mellitus, WIT and transplant PRA.
Human kidney transplantation remains the treatment of choice for the majority of patients with end-stage renal failure [19–21]. Despite increased numbers of donor organs due to expanded donor criteria, organs from living donors, and donation after circulatory death, the gap between the demand and availability of kidneys for transplantation remains substantial [22–25]. Although the results for ODE kidneys regarding longitudinal eGFR and graft failure were not statistically significantly different compared to those for DBD or DCD-III kidneys, the overall results for ODE support the concept that ODE kidneys are a promising extension of the donor pool. Notably, extension of the donor pool is not the primary goal of the procedure, because ODE is the patient’s final altruistic wish.
Previous research on the outcomes of kidney transplantations following ODE was done in smaller cohorts and case series [11, 12], while no study has assessed longitudinal eGFR and graft function over 10 years. In contrast to others who included data that did not measure an extensive set of potential confounders [18] or only studied ODE compared to DCD-III [14], we focused on DBD, DCD-III, and ODE, using comprehensive data from a nationwide registry.
With regard to other organs donated following ODE, preliminary studies on graft function of transplanted lungs after ODE [26–28] yielded outcome results comparable to DCD-III grafts, and similar results were reported for transplanted livers [29–31]. Recently, the first successful heart transplantation after donation after euthanasia was published [32]. The current study thus found comparable transplant outcomes between ODE and DCD-III [29] and between ODE, DCD-III, and DBD [26] regarding graft failure for kidneys transplanted.
In the future, more patients will request to donate their organs after euthanasia, and it is expected that an increasing number of countries will allow this procedure. Observational data showed that approximately 10% of all patients undergoing euthanasia might be medically eligible to donate at least one organ [33].
However, the vast majority of patients who undergo euthanasia are suffering from end-stage malignancy, which makes them unsuitable as a donor. “Euthanasia donors” often suffer from neurodegenerative or psychiatric disorders, which are not primarily associated with deterioration of organ function of potentially transplantable organs, such as the heart, lungs, liver, kidneys and pancreas [4, 34]. DCD-III donors, who die after withdrawal from life-sustaining therapy, inevitably suffer from hypoxia, hypotension, and inadequate organ perfusion during the progression to circulatory arrest (agonal phase) and the mandatory 5-min period of warm, pulseless ischemia [35, 36]. Donors after brain death suffer from a systemic inflammatory response with a potentially negative impact on graft outcomes [37].
This study has strengths and limitations. A major strength is the large nationwide registry including all donations and transplantations in the Netherlands with a comprehensive set of donor, recipient and transplantation variables that created the opportunity to investigate potential confounding in the associations under investigation. Indeed, different studies have shown associations between either prolonged CIT or prolonged AT or both, and both kidney function and post-transplant graft failure [38, 39]. AT has also been associated with delayed graft function [40]. Hence the adjustments for these variables in the models of the present study. Furthermore, the Cox models were adjusted for transplant PRA as it indicates the risk of transplant failure to the host-response to transplantation [13, 14]. In addition, hypertension and diabetes mellitus, and tobacco exposure, have each been associated to worse eGFR [41]. Therefore, models were adjusted for recipient hypertension and diabetes mellitus and donor smoking behavior. As no information on smoking behavior in recipients was available, residual confounding might have caused us to underestimate the present associations, although most likely recipient smoking behavior is not dependent on ODE, DBD or DCD-III donors [42]. The criteria for HLA-mismatch are different for the first transplantation and for later transplantations and we chose to study the population that comprises the most recent transplantation of recipients in the analyses. Therefore, the associations could not be adjusted for HLA mismatch and this could have led to an underestimation of the present results.
Another strength is a total of four sensitivity analyses that were conducted. In sensitivity analysis 1, all kidney transplantations within recipients were included to investigate whether including multiple transplants for the same recipients changed the results. In the second sensitivity analysis, recipient death with functioning transplant, was included as an event, as graft survival and recipient deaths may be related. In sensitivity analysis 3, primary non-function was included as an event, despite it being considered a short-term transplant outcome. The fourth sensitivity analysis, considering only the first kidney transplantation per recipient, was performed as HLA matching is not independent of the number of transplantations within a recipient. The presence of donor-specific HLA antibodies before transplantation is considered a risk factor for graft rejection. Furthermore, waiting time until transplantation increases the risk of higher sensitization levels. Organ transplantation induces HLA alloimmunization, affecting the matching of a re-transplant and waiting time until transplantation [18].
Another strength lies in the approach of investigating the 10-year post-transplantation outcome in two different ways: using Cox proportional hazards analyses for the association between donor categories and graft failure and longitudinal analyses for the association between donor categories and eGFR, which together increase the validity of the results. The study has several limitations as well. In the Netherlands, post-mortem donation allocation is based on blood and tissue match between the donor and recipient of the organ, the medical urgency of the recipient, and other circumstances related to the condition of the organ or the background of the recipient. Neither the donor nor their relatives are allowed to choose a recipient [43]. One donor could have donated two kidneys to two different recipients. However, we did not take into account the potential dependency between recipients who received a kidney from the same donor, which is a limitation of this study. This dependency between recipients could potentially have affected transplant outcomes, although the direction of its effect is difficult to assess. With regard to sensitivity analysis 1, to investigate whether including multiple transplants for the same recipients changed the results, it needs to be recognized that multiple kidney transplantations within a recipient during the disease course are dependent and this was not accounted for in the Cox models.
Furthermore, the current study’s ODE sample size decreased considerably after 6 years of follow-up, potentially compromising the reliability of the longer-term findings. This limitation requires a cautious interpretation of results during the extended follow-up period of ODE grafts, suggesting that conclusions towards 10 years should be less strongly conveyed. Future research should thus focus on larger cohorts to enhance the robustness of long-term conclusions. Given the contemporary annual increase in the number of ODE procedures, it is, however, estimated that an analysis of the first 300 kidney transplantations will take at least 5 additional years.
Another limitation of the study is that recipient ethnicity could not be used in the MDRD4 equation, due to lack of information. This has potentially led to a small underestimation of follow-up eGFR. However, since the same error has been made within each recipient, this will not make major difference in the trend over time.
In conclusion, kidneys transplanted after euthanasia have a good immediate graft function and a comparable longitudinal eGFR over 10 years and comparable hazard for graft failure when compared to kidneys transplanted after brain death or circulatory arrest. Overall, these results support the concept that ODE kidneys are a promising contribution to the donor pool, and ODE should be continued.
Data Availability Statement
The data analyzed in this study is subject to the following licenses/restrictions: The dataset is available upon specific request to the NTS (Netherlands Transplantation Society). Requests to access these datasets should be directed to Cynthia Konijn-Janssen, ZGF0YXZlcnpvZWtAdHJhbnNwbGFudGF0aWVzdGljaHRpbmcubmw=.
Ethics Statement
Ethical approval was not required for the studies involving humans because the data were generated from a national dataset and anonymized beforehand. The data represent routine care. Therefore, ethical approval is not required. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from a by-product of routine care or industry. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author Contributions
WJ, JB, and WM established the study setup and design and collected the data. CS and BB analyzed the data and wrote the initial draft of the manuscript, after which subsequent drafts were collaboratively revised with ND, WM, HV, and JB. BB provided statistical support. WM coordinated the team efforts. ND submitted the manuscript. CS and ND contributed equally to this manuscript. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. Maarten H.L. Christiaans, nephrologist, Maastricht UMC+, for his valuable input during the first revision of the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/ti.2024.13142/full#supplementary-material
References
1. Kootstra, G, Daemen, JH, and Oomen, AP. Categories of Non-Heart-Beating Donors. Transpl Proc (1995) 27(5):2893–4.
2. Kootstra, G, and van Heurn, E. Non-Heartbeating Donation of Kidneys for Transplantation. Nat Clin Pract Nephrol (2007) 3(3):154–63. doi:10.1038/ncpneph0426
3. Detry, O, Le Dinh, H, Noterdaeme, T, De Roover, A, Honore, P, Squifflet, JP, et al. Categories of Donation After Cardiocirculatory Death. Transpl Proc (2012) 44(5):1189–95. doi:10.1016/j.transproceed.2012.05.001
4. Bollen, J, de Jongh, W, Hagenaars, J, van Dijk, G, ten Hoopen, R, Ysebaert, D, et al. Organ Donation After Euthanasia: A Dutch Practical Manual. Am J Transplant (2016) 16(7):1967–72. doi:10.1111/ajt.13746
5. Silva, ESV, Silva, AR, Rochon, A, Lotherington, K, Hornby, L, Wind, T, et al. Organ Donation Following Medical Assistance in Dying, Part II: A Scoping Review of Existing Processes and Procedures. JBI Evid Synth (2024) 22(2):195–233. doi:10.11124/jbies-22-00140
6. Weiss, MJ, Dupras-Langlais, M, Lavigne, MJ, Lavigne, S, Martel, AC, and Chaudhury, P. Organ Donation After Medical Assistance in Dying: A Descriptive Study From 2018 to 2022 in Quebec. CMAJ (2024) 196(3):E79–E84. doi:10.1503/cmaj.230883
7. Allard, J, and Fortin, MC. Organ Donation After Medical Assistance in Dying or Cessation of Life-Sustaining Treatment Requested by Conscious Patients: The Canadian Context. J Med Ethics (2017) 43(9):601–5. doi:10.1136/medethics-2016-103460
8. Bollen, J, Ten Hoopen, R, Ysebaert, D, van Mook, W, and van Heurn, E. Legal and Ethical Aspects of Organ Donation After Euthanasia in Belgium and the Netherlands. J Med Ethics (2016) 42(8):486–9. doi:10.1136/medethics-2015-102898
9. van Dijk, N, Stärcke, P, de Jongh, W, Jansen, N, Shaw, D, Bollen, J, et al. Organ Donation After Euthanasia in Patients Suffering From Psychiatric Disorders: 10-Years of Preliminary Experiences in the Netherlands. Transpl Int (2023) 9(36):10934–2277. doi:10.3389/ti.2023.10934
10. Silva, ESV, Silva, A, Rochon, A, Lotherington, K, Hornby, L, Wind, T, et al. Outcomes from Organ Donation Following Medical Assistance in Dying: A Scoping Review. Transpl Rev (Orlando) (2023) 37(1):100748. doi:10.1016/j.trre.2023.100748
11. Luke, PP, Skaro, A, Sener, A, Tang, E, Levine, M, Sami, S, et al. Kidney Transplant Outcomes after Medical Assistance in Dying. Can Urol Assoc J. (2022) 16(2):E108-E110. doi:10.5489/cuaj.7304
12. Bollen, J, Snoeijs, M, Ten Hoopen, R, Shaw, D, van Mook, W, van Heurn, E, et al. Promising Results of Kidney Transplantation From Donors Following Euthanasia. Transplantation (2020) 104(S294):S394–1193. doi:10.1097/01.tp.0000700584.87933.96
13. Lim, WH, Chapman, JR, and Wong, G. Peak Panel Reactive Antibody, Cancer, Graft, and Patient Outcomes in Kidney Transplant Recipients. Transplantation (2015) 99(5):1043–50. doi:10.1097/TP.0000000000000469
14. Nwakanma, LU, Williams, JA, Weiss, ES, Russell, SD, Baumgartner, WA, and Conte, JV. Influence of Pretransplant Panel-Reactive Antibody on Outcomes in 8,160 Heart Transplant Recipients in Recent Era. The Ann Thorac Surg (2007) 84(5):1556–62. doi:10.1016/j.athoracsur.2007.05.095
15. Halazun, KJ, Al-Mukhtar, A, Aldouri, A, Willis, S, and Ahmad, N. Warm Ischemia in Transplantation: Search for a Consensus Definition. Transpl Proc (2007) 39(5):1329–31. doi:10.1016/j.transproceed.2007.02.061
16. Levey, AS, Coresh, J, Fau - Greene, T, Greene, T, Fau - Stevens, LA, Stevens La Fau - Zhang, YL, et al. Using Standardized Serum Creatinine Values in the Modification of Diet in Renal Disease Study Equation for Estimating Glomerular Filtration Rate. Ann Intern Med (2006) 145(4):247–54. doi:10.7326/0003-4819-145-4-200608150-00004
17. von Elm, E, Altman, DG, Egger, M, Pocock, SJ, Gøtzsche, PC, Vandenbroucke, JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. The Lancet (2007) 370(9596):1453–7. doi:10.1016/S0140-6736(07)61602-X
18. Hyun, J, Park, K, Fau - Yoo, Y, Yoo, Y, Fau - Lee, B, Fau - Han By, LB, et al. Effects of Different Sensitization Events on HLA Alloimmunization in Solid Organ Transplantation Patients. Transpl Proc. (2012) 44(1):222–5. doi:10.1016/j.transproceed.2011.12.049
19. Purnell, TS, Auguste, P, Crews, DC, Lamprea-Montealegre, J, Olufade, T, Greer, R, et al. Comparison of Life Participation Activities Among Adults Treated by Hemodialysis, Peritoneal Dialysis, and Kidney Transplantation: A Systematic Review. Am J Kidney Dis (2013) 62(5):953–73. doi:10.1053/j.ajkd.2013.03.022
20. Wolfe, RA, Ashby, VB, Milford, EL, Ojo, AO, Ettenger, RE, Agodoa, LY, et al. Comparison of Mortality in All Patients on Dialysis, Patients on Dialysis Awaiting Transplantation, and Recipients of a First Cadaveric Transplant. N Engl J Med (1999) 341(23):1725–30. doi:10.1056/NEJM199912023412303
21. Azegami, T, Kounoue, N, Sofue, T, Yazawa, M, Tsujita, M, Masutani, K, et al. Efficacy of Pre-emptive Kidney Transplantation for Adults With End-Stage Kidney Disease: A Systematic Review and Meta-Analysis. Ren Fail (2023) 45(1):2169618. doi:10.1080/0886022X.2023.2169618
22. Tullius, SG, and Rabb, H. Improving the Supply and Quality of Deceased-Donor Organs for Transplantation. N Engl J Med (2018) 378(20):1920–9. doi:10.1056/NEJMra1507080
23. Snoeijs, MG, Schaubel, DE, Hene, R, Hoitsma, AJ, Idu, MM, Ijzermans, JN, et al. Kidneys from Donors after Cardiac Death Provide Survival Benefit. J Am Soc Nephrol (2010) 21(6):1015–21. doi:10.1681/ASN.2009121203
24. Active Kidney Waiting List in 2023 2024. Available from: https://statistics.eurotransplant.org/index.php?search_type=waiting+list&search_organ=&search_region=All+ET&search_period=by+year&search_characteristic=&search_text. (Accessed April 3, 2024).
25. Waiting List Mortality in 2023. Available from: https://statistics.eurotransplant.org/index.php?search_type=WL+removals&search_organ=kidney&search_region=All+ET&search_period=2023&search_characteristic=&search_text=&search_collection=. (Accessed April 3, 2024).
26. Ceulemans, LJ, Vanluyten, C, Monbaliu, D, Schotsmans, P, Fieuws, S, Vandervelde, CM, et al. Lung Transplant Outcome Following Donation After Euthanasia. J Heart Lung Transpl (2022) 41(6):745–54. doi:10.1016/j.healun.2022.01.1375
27. Watanabe, T, Kawashima, M, Kohno, M, Yeung, J, Downar, J, Healey, A, et al. Outcomes of Lung Transplantation from Organ Donation after Medical Assistance in Dying: First North American Experience. Am J Transpl (2022) 22(6):1637–45. doi:10.1111/ajt.16971
28. Healey, A, Cypel, M, Pyle, H, Mills, C, Heffren, J, Katz, D, et al. Lung Donation After Medical Assistance in Dying at Home. Am J Transpl (2021) 21(1):415–8. doi:10.1111/ajt.16267
29. van Reeven, M, Gilbo, N, Monbaliu, D, van Leeuwen, OB, Porte, RJ, Ysebaert, D, et al. Evaluation of Liver Graft Donation After Euthanasia. JAMA Surg (2020) 155(10):917–24. doi:10.1001/jamasurg.2020.2479
30. Ray, S, Torres-Hernandez, A, Bleszynski, MS, Parmentier, C, McGilvray, I, Sayed, BA, et al. Medical Assistance in Dying (MAiD) as a Source of Liver Grafts: Honouring the Ultimate Gift. Ann Surg (2023) 277(5):713–8. doi:10.1097/SLA.0000000000005775
31. Glinka, J, Sachar, Y, Tang, E, Brahmania, M, Hwang, J, Waugh, E, et al. Liver Transplantation With Donation After Medical Assistance in Dying: Case Series and Systematic Review of the Literature. Liver Transpl (2023) 29(6):618–25. doi:10.1097/LVT.0000000000000100
32. Tchana-Sato, V, Hans, G, Brouckaert, J, Detry, O, Van Cleemput, J, Rex, S, et al. Successful Heart Transplantation From Donation after Euthanasia with Distant Procurement Using Normothermic Regional Perfusion and Cold Storage. Am J Transpl (2022) 22(12):3146–9. doi:10.1111/ajt.17204
33. Bollen, J, van Smaalen, T, Ten Hoopen, R, van Heurn, E, Ysebaert, D, and van Mook, W. Potential Number of Organ Donors After Euthanasia in Belgium. JAMA (2017) 317(14):1476–7. doi:10.1001/jama.2017.0729
34. van Dijk, N, Starcke, P, de Jongh, W, Jansen, N, Shaw, D, Bollen, J, et al. Organ Donation After Euthanasia in Patients Suffering from Psychiatric Disorders: 10-Years of Preliminary Experiences in the Netherlands. Transpl Int (2023) 36:10934. doi:10.3389/ti.2023.10934
35. Domínguez-Gil, BA-O, Ascher, N, Capron, AM, Gardiner, D, Manara, AR, Bernat, JL, et al. Expanding Controlled Donation After the Circulatory Determination of Death: Statement from an International Collaborative. Intensive Care Med (2021) 47(3):265–281. doi:10.1007/s00134-020-06341-7
36. Hyun, SA-O, and Haam, SA-O. Donation After Circulatory Death in Lung Transplantation. J Chest Surg (2022) 55(4):283–287. doi:10.5090/jcs.22.060
37. Morrissey, PE, and Monaco, AP. Donation After Circulatory Death: Current Practices, Ongoing Challenges, and Potential Improvements. Transplantation (2014) 97(3):258–64. doi:10.1097/01.TP.0000437178.48174.db
38. Debout, A, Foucher, Y, Trébern-Launay, K, Legendre, C, Kreis, H, Mourad, G, et al. Each Additional Hour of Cold Ischemia Time Significantly Increases the Risk of Graft Failure and Mortality Following Renal Transplantation. Kidney Int (2015) 87(2):343–9. doi:10.1038/ki.2014.304
39. Foley, MA-O, Vinson, AA-O, Skinner, TAA, Kiberd, BA, and Tennankore, KA-O. The Impact of Combined Warm and Cold Ischemia Time on Post-transplant Outcomes. Can J Kidney Health Dis (2023) 10:20543581231178960. doi:10.1177/20543581231178960
40. Tennankore, KK, Kim, SJ, Alwayn, IP, and Kiberd, BA. Prolonged Warm Ischemia Time Is Associated with Graft Failure and Mortality After Kidney Transplantation. Kidney Int (2016) 89(3):648–58. doi:10.1016/j.kint.2015.09.002
41. Fu, YC, Xu, ZL, Zhao, MY, and Xu, K. The Association Between Smoking and Renal Function in People Over 20 Years Old. Front Med (Lausanne) (2022) 9:2296–858X. (Print). doi:10.3389/fmed.2022.870278
42. Eid, HA-OX, Moazen, EA-O, Elhussini, M, Shoman, H, Hassan, AA-O, Elsheikh, AA-O, et al. The Influence of Smoking on Renal Functions Among Apparently Healthy Smokers. J Multidiscip Healthc (2022) 15:2969–2978. doi:10.2147/JMDH.S392848
43. Wet op de Orgaandonatie 2022. Available from: https://wetten.overheid.nl/BWBR0008066/2022-01-01/#Hoofdstuk3. (Accessed March 23, 2024).
Keywords: organ donation, euthanasia, donation after circulatory death, donation after brain death, kidney transplantation, organdonation after euthanasia, medical assistance in dying, physician assisted death
Citation: Susanna C, van Dijk N, de Jongh W, Verberght H, van Mook W, Bollen J and van Bussel B (2024) Promising Results of Kidney Transplantation From Donors Following Euthanasia During 10-Year Follow-Up: A Nationwide Cohort Study. Transpl Int 37:13142. doi: 10.3389/ti.2024.13142
Received: 16 April 2024; Accepted: 23 September 2024;
Published: 18 October 2024.
Copyright © 2024 Susanna, van Dijk, de Jongh, Verberght, van Mook, Bollen and van Bussel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nathalie van Dijk, bi52YW4uZGlqa0BtdW1jLm5s
†These authors have contributed equally to this work and share first authorship
 Charlotte Susanna
Charlotte Susanna Nathalie van Dijk
Nathalie van Dijk Wim de Jongh2
Wim de Jongh2 Walther van Mook
Walther van Mook Jan Bollen
Jan Bollen