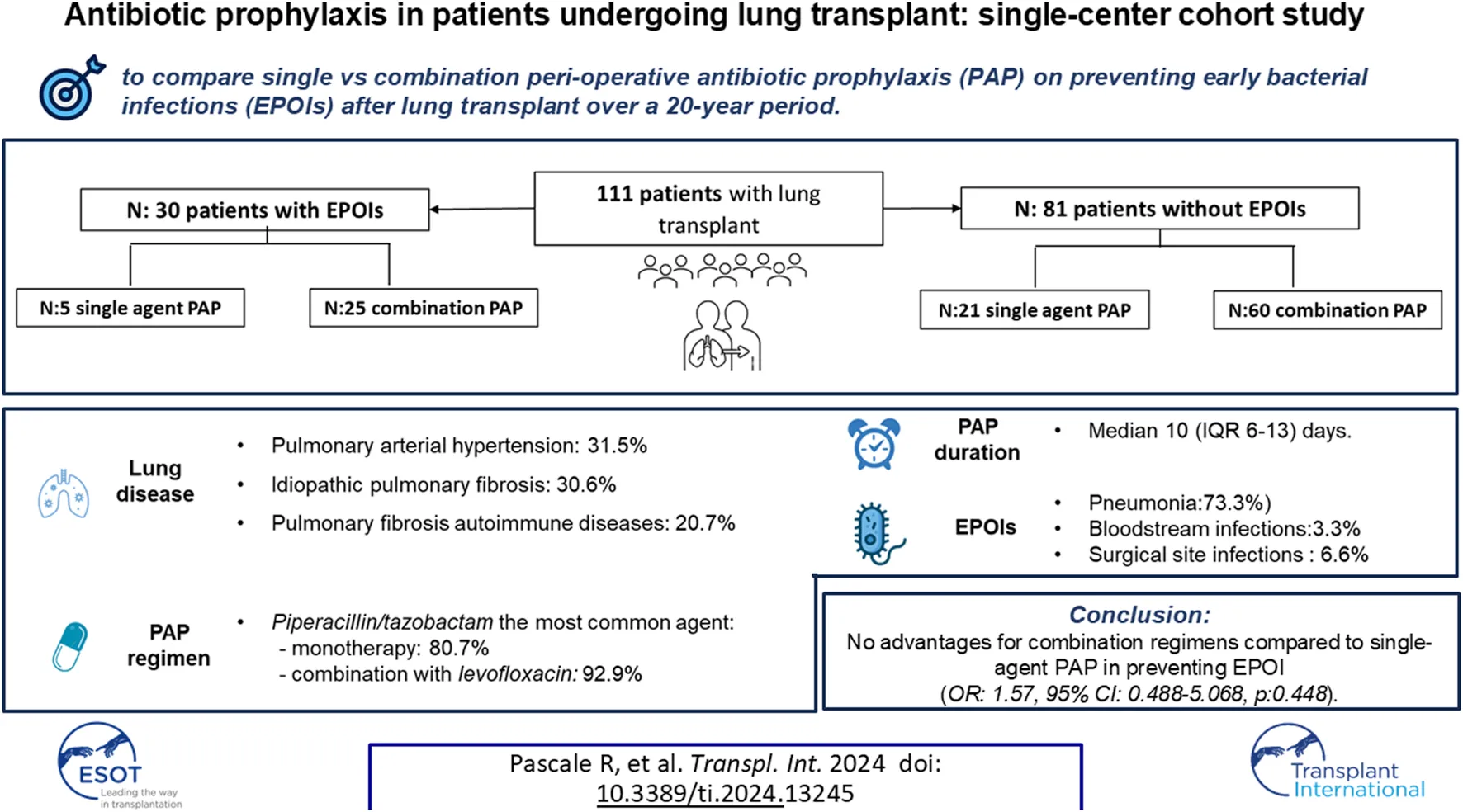
Abstract
Perioperative antibiotic prophylaxis (PAP) in lung transplant recipients (LuTRs) has high heterogeneity between centers. Our aim was to investigate retrospectively the approach to PAP in our center over a 20-year period (2002–2023), and its impact on early post-operative infections (EPOIs) after lung transplantation (LuT). Primary endpoint was diagnosis of EPOI, defined as any bacterial infection including donor-derived events diagnosed within 30 days from LuT. Main exposure variables were type of PAP (combination vs. monotherapy) and PAP duration. We enrolled 111 LuTRs. PAP consisted of single-agent or combination regimens in 26 (25.2%) and 85 (74.8%) LuTR. Median PAP duration was 10 days (IQR 6–13) days. Piperacillin/tazobactam was the most common agent used either as monotherapy (n = 21, 80.7%) or as combination with levofloxacin (n = 79, 92.9%). EPOIs were diagnosed in 30 (27%) patients. At multivariable analysis no advantages were found for combination regimens compared to single-agent PAP in preventing EPOI (OR: 1.57, 95% CI: 0.488–5.068, p:0.448). The impact of PAP duration on EPOIs development was investigated including duration of PAP ≤6 days as main exposure variables, without finding a significantly impact (OR:2.165, 95% CI: 0.596–7.863, p: 0.240). Our results suggest no advantages for combination regimens PAP in preventing EPOI in LuTR.
Introduction
Bacterial infections are clinically relevant complications in lung transplant recipients (LuTRs) causing chronic lung allograft dysfunction and high rates of mortality, especially within the first year after transplant [1, 2]. Infectious episodes occurring in the first 30 days following lung transplantation (LuT) are due to microorganisms deriving from the donor lung or pre-existing recipient flora [3]. Indeed, even though native lungs are removed, colonization of the grafts from recipients colonizing strains often rapidly occurs [4–6]. While some experts advise against the use of the organs with microorganism isolation or detection, others support it, if it is combined with at least 24 h of antibiotic therapy according to susceptibility patterns of recovered microorganisms [7]. This last approach is supported by retrospective studies showing no difference in overall survival of recipients of infected/colonized lungs, compared to recipients of uninfected lungs [8], even in case of multi-drug resistant organisms (MDRO). All this considered, antibiotic prophylaxis is routinely administered in LuTRs [8, 9]. International guidelines recommendations are generic and predominantly based on cardiac procedures and no formal recommendations to guide antimicrobial selection in LuT surgery are currently available [10]. Therefore, antibiotic regimens for peri-operative antibiotic prophylaxis (PAP) are based on clinical judgment, bacterial infection and/or colonization present in the donor and/or recipient and knowledge of the local epidemiology, inducing high heterogeneity, both for drug choice and treatment duration, in clinical practice between centers [7].
We aim to carry-out a retrospective observational study to investigate different regimens of PAP used in our center over 20-year period, and their impact on preventing early bacterial infections and donor derived infection after LuT. The results obtained from our study will contribute to increasing knowledge about the prophylaxis regimens that can be adopted in hospitals with a similar clinical and microbiological epidemiology.
Materials and Methods
Study Design and Setting
Retrospective, monocentric observational cohort study including all adult patients who underwent LuT at IRCCS Azienda Ospedaliero-Universitaria di Bologna from 1st January 2002 to 31st August 2023. During the study period, LuTRs antibiotic prophylaxis regimens were established by Transplant Intensivists and Pneumologists who managed the patients in the immediate peri-transplant period, according to usual practice and internal guidelines.
All enrolled patients are followed from time of LuT to 30 days after (or until death, whichever occurred first). Data were retrospectively collected from medical charts and microbiology archives. Data were collected using a dedicated REDCap electronic case report form (eCRF) hosted by IRCCS Azienda Ospedaliero-Universitaria di Bologna [11]. The study was conducted according to declaration of Helsinki and Good Clinical Practice guidelines and approved by the local Ethics Committee (no 676/2023/Oss/AOUBo).
Population
All adult (≥18 years) patients who underwent LuT receiving PAP during the study period were screened for inclusion. Patients were excluded in case of lack of clinical and/or laboratory data regarding type of early postoperative bacterial infections (EPOIs).
Procedures
During the study period PAP regimen was composed by piperacillin-tazobactam administered with 9 g as loading dose followed by 4.5 g every 6 h in combination with levofloxacin 500 mg every 12 h [12–14]. This PAP regimen was chosen to provide two drugs with potential activity against Pseudomonas spp while awaiting donor/recipient culture results. PAP duration was continued until results of perioperative cultures. In case of positive recipient and/or donor cultures, PAP was tailored and extended for 10–14 days. In case of penicillin-drug allergy or intolerance, piperacillin/tazobactam was replaced by cefepime or meropenem. Levofloxacin was not administered in case of drug allergy, presence of a contraindication (history of epilepsy, connective disease, QT prolongation), or according with clinical judgement. When available, the pre-transplant recipient colonization status, at upper or lower respiratory tract, was used to target the antibiotic prophylaxis.
After transplantation, lower respiratory tract samples were taken when patients had clinical signs or symptoms of respiratory tract infection or rejection. During all the study period,in clinically stable patients, post-transplant bronchoscopy was performed after 48 h post-transplantation and subsequently every week for the first month. During each procedure, bronchoalveolar lavage and transbronchial biopsy were sent for microbiological cultures.
Variables and Definitions
Primary endpoint was diagnosis of EPOIs, defined as any bacterial infection diagnosed according to US Centers for Disease Control and Prevention (CDC) criteria [15] within 30 days from LuT. Among EPOIs, donor derived events were included and defined as any bacterial infection caused by the same microorganism isolated from the donor and the recipient [16].
PAP was defined as the antibiotic regimen administered at time of LuT before susceptibility report of donor cultures were available. Changes in antibiotic treatment according with recipient/donor cultures were recorded as well as the overall duration of PAP. PAP was mainly classified as single-agent or combination regimen.
The other variables were age and sex, co-morbidities summarized by the Charlson Comorbidity score [17], information on LuT (transplant date, graft Number; graft function during first 24 h). Immunosuppressive drugs used as induction and maintenance regimen were recorded. Donor and recipient colonization were inferred from respiratory samples. Infection etiology was also classified according to the causative species into Gram positives and Gram negative rods. According to the definitions of CDC [18] all strains from donors/recipients were categorized as Oxacillin-resistant (OxaR) or Vancomicin Resistant (VR) for Gram positive and Carbapenem resistance (CR), third generation cephalosporin resistance (ESCR), β-lactam/β-lactamase inhibitor (BL/BLIR) and fluroquinolone resistance (FQR) for Gram negatives. For the latter, Magiorakos criteria (non-MDR, MDR, XDR or PDR) and the new definition of “difficult to treat resistance” (DTR) were also used [19, 20].
Microbiology
Clinical samples collected during the study period were analysed following routine diagnostic workflow in the bacteriology laboratory, Unit of Microbiology. Since 2010, donor derived samples collected at the time of organ removal were referred to our center to be analyzed. Before that time, all donor cultures were performed at donor center and results subsequently provided at Transplant Unit. Results of any other microbiological samples previously analyzed at the donor center were provided to our center through the regional transplant coordination network.
Statistical Analysis
For descriptive analysis, categorical variables were presented as frequencies and percentages, continuous variables were presented as mean ± standard deviation (SD) or median and interquartile range (IQR) according to normal or non-normal distribution. The clinical and demographic characteristics of the two subgroups of the study population were described and compared using bivariate tests such as t-test or Mann-Whitney test for continuous variables, chi-square or Fisher’s exact test for categorical variables.
A multivariable binary logistic regression analysis was performed to investigate independent predictors of EPOIs considering type of PAP regimen (single-agent vs. combination) as main exposure variable along with all the other variables showing a p < 0.05 at univariable analysis, including male gender, Charlson Comorbidity Index, Tacrolimus as maintenance regimen, idiopatic pulmonary fibrosis as leading cause for lung transplant and primary graft non function. A second model to assess the impact of PAP duration on EPOIs development was done in which patients on PAP at the time of infection diagnosis were excluded. For this analysis we used the cutoff of PAP duration ≤6 vs. >6 days considering that in most cases cultures results of donor and recipient samples were available and communicated within 6 days of transplantation. SPSS 21.0 was used for all analyses, with significance level set at α = 0.05.
Results
Overall, 112 patients receiving LuT were screened for inclusion, 1 patient was excluded for lack of data and 111 were analysed (Figure 1). The main characteristics of study population are shown in Table 1. Of them, 64 (57.5%) were male, with a median age of 50 years (IQR 39–59), median Charlson Index was 2 (IQR:1–4). The most frequent underlying lung disease leading to LuT was pulmonary arterial hypertension (35, 31.5%), followed by idiopathic pulmonary fibrosis (IPF) (34, 30.6%), pulmonary fibrosis associated with autoimmune diseases (23, 20.7%) and emphysema and chronic obstructive pulmonary disease (COPD) (14, 12.6%). Patients with cystic fibrosis were absent in our cohort. Primary graft non function was experienced by 15 (14%) patients.
FIGURE 1
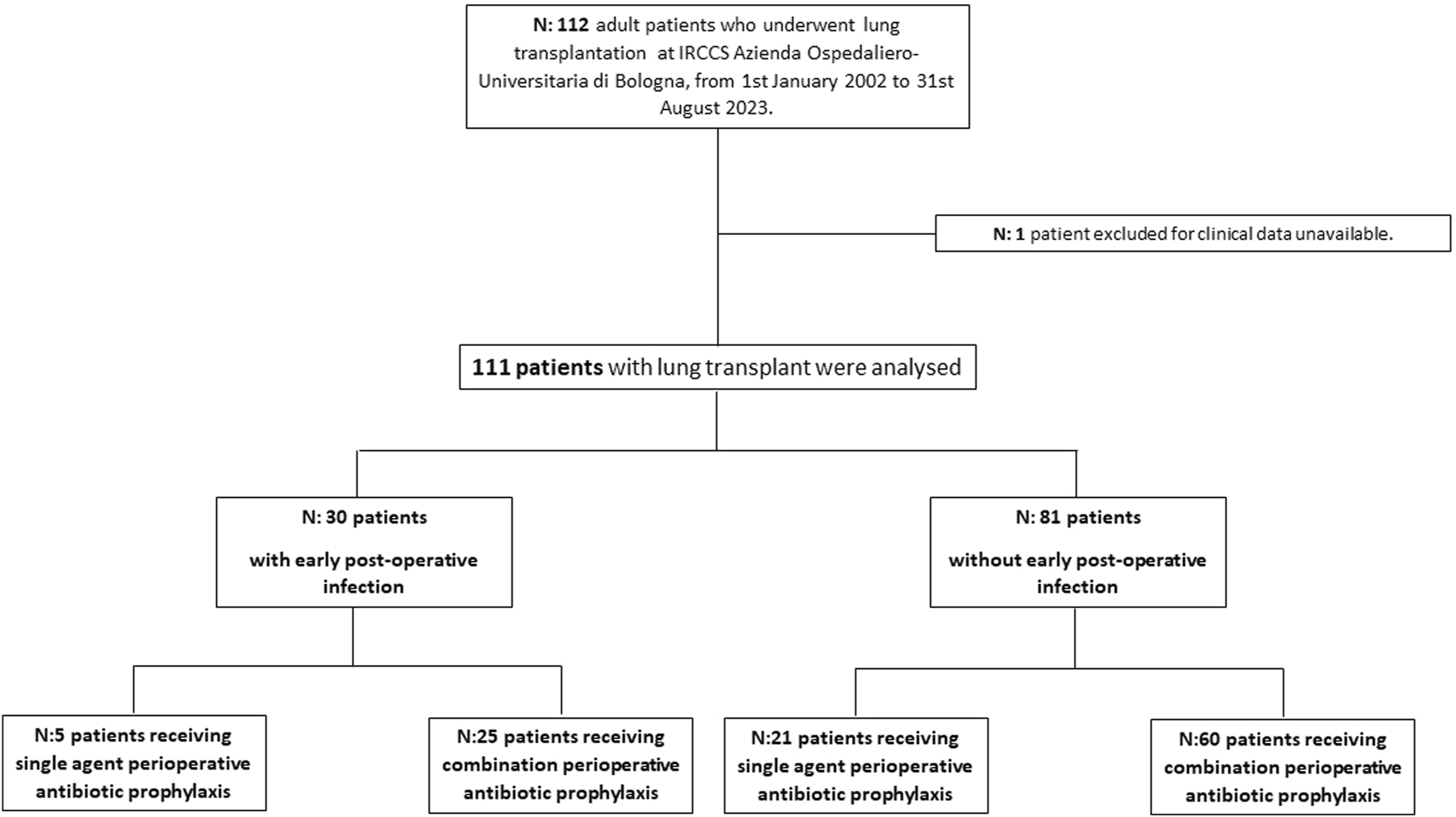
Study flow chart.
TABLE 1
| Cases with available data | Total of patient 111 (%) |
Patients without bacterial infection 81 (%) |
Patients with bacterial infection 30 (%) |
p-value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (years), median (IQR) | 111 | 50 (39–59) | 48 (36–59) | 54 (46–63) | 0.029 |
| Gender (male) | 111 | 64 (57.5) | 42 (51.9) | 22 (73.3) | 0.042 |
| Underlying Lung Disease | |||||
| Idiopatic pulmonary fibrosis | 111 | 34 (30.6) | 20 (24.7) | 14 (46.7) | 0.026 |
| Pulmonary fibrosis associated with autoimmune diseases | 111 | 23 (20.7) | 17 (21.0) | 6 (20.0) | 0.909 |
| Emphysema/COPD | 111 | 14 (12.6) | 11 (13.6) | 3 (10.0) | 0.614 |
| Pulmonary arterial hypertension | 111 | 35 (31.5) | 27 (33.3) | 8 (26.7) | 0.502 |
| Chronic Thromboembolic Pulmonary Hypertension | 111 | 1 (0.9) | 1 (1.2) | 0 (0.0) | 0.541 |
| Other | 111 | 10 (9.0) | 9 (11.1) | 1 (3.3) | 0.204 |
| Underlying diseases | |||||
| Myocardial infarction | 111 | 6 (5.4) | 4 (4.9) | 2 (6.7) | |
| Congestive heart failure | 111 | 45 (40.5) | 28 (34.6) | 17 (56.7) | 0.035 |
| Peripheral vascular disease | 111 | 7 (6.3) | 4 (4.9) | 3 (10.0) | 0.330 |
| Cerebrovascular disease | 111 | 3 (2.7) | 2 (2.5) | 1 (3.3) | 0.803 |
| Connective tissue disease | 111 | 13 (11.7) | 12 (14.8) | 1 (3.3) | 0.095 |
| Diabetes mellitus | 111 | 13 (11.9) | 7 (8.9) | 6 (20.0) | 0.109 |
| Charlson index, median (IQR) | 111 | 2 (1–4) | 2 (1–3) | 3 (2–4) | 0.006 |
| Donor information | |||||
| Age at the time of donation median (IQR) | 111 | 48 (31–55) | 48 (31–56) | 48 (34–55) | 0.750 |
| Donation after circulatory death (DCD) | 111 | 3 (2.7) | 1 (1.2) | 2 (6.7) | 0.117 |
| Donation after brain death (DBD) | 111 | 108 (96.4) | 79 (97.5) | 28 (93.3) | 0.292 |
| Infectious donor risk | 0.423 | ||||
| Standard | 103 | 81 (78.6) | 62 (80.5) | 19 (73.1) | |
| Non standard | 103 | 22 (21.4) | 15 (19.5) | 7 (26.9) | |
| Cause of donor death | 0.362 | ||||
| Trauma | 111 | 34 (30.6) | 26 (32.1) | 8 (26.7) | |
| Cerebrovascular | 111 | 69 (62.2) | 49 (60.5) | 20 (66.7) | |
| Other | 111 | 8 (7.2) | 6 (7.4) | 2 (6.7) | |
| Transplant information | |||||
| Days from inclusion list to transplant, median (IQR) | 111 | 235 (83–508) | 237 (83–534) | 154 (76–349) | 0.307 |
| Single-lung | 111 | 16 (14.4) | 9 (11.1) | 7 (23.3) | 0.103 |
| Double-lung | 111 | 95 (85.6) | 72 (88.9) | 23 (76.7) | 0.103 |
| Heart + Lung | 111 | 4 (3.7) | 3 (3.8) | 1 (3.4) | 0.941 |
| Ischemia Time (hours) median (IQR) | 85 | 5.4 (4.35–6.1) | 5.1 (4.3–6.4) | 5.8 (4.8–6.1) | 0.188 |
| Primary graft non function | 111 | 15 (14.0) | 12 (15.2) | 3 (10.7) | 0.558 |
| Delayed graft function | 111 | 1 (0.9) | 1 (1.3) | 0 (0.0) | 0.550 |
| Induction regimen | |||||
| Bolus of steroids | 111 | 104 (93.7) | 75 (92.6) | 29 (96.7) | 0.433 |
| Antylimphocyte globulin | 111 | 11 (9.9) | 7 (8.6) | 4 (13.3) | 0.463 |
| Basiliximab | 111 | 56 (50.5) | 41 (50.6) | 15 (50.0) | 0.954 |
| Maintenance regimen | |||||
| Cyclosporine | 111 | 11 (9.9) | 6 (7.4) | 5 (16.7) | 0.147 |
| Azathioprine | 111 | 13 (11.7) | 9 (11.1) | 4 (13.3) | 0.746 |
| Tacrolimus | 111 | 73 (65.8) | 59 (72.8) | 14 (46.7) | 0.010 |
| Mycophenolate | 111 | 37 (33.3) | 29 (35.8) | 8 (26.7) | 0.365 |
| Everolimus | 111 | 1 (0.9) | 1 (1.2) | 0 (0.0) | 0.541 |
| Steroids | 111 | 84 (75.7) | 64 (79.0) | 20 (66.7) | 0.178 |
| Etanercept | 111 | 28 (25.2) | 23 (28.4) | 5 (16.7) | 0.206 |
| Positive Donor Derived Samples | 111 | 59 (53.2) | |||
| BSI | 59 | 3 (10.1) | 1 (1.2) | 2 (6.7) | 0.117 |
| BAL | 59 | 56 (94.9) | 41 (50.6) | 15 (50.0) | 0.954 |
| Recipient colonization | 111 | 17 (77.3) | |||
| CPE Rectal colonization | 16 | 1 (0.9) | 0 (0.0) | 1 (3.3) | 0.099 |
| BAL | 16 | 16 (14.4) | 12 (14.8) | 4 (13.3) | 0.844 |
| Perioperative Antibiotic prophylaxis | |||||
| Mono - regimen | 111 | 26 (23.4) | 21 (25.9) | 5 (16.6) | 0.306 |
| Piperacillin/tazobactam | 111 | 21 (80.7) | 17 (80.9) | 4 (80) | 0.181 |
| Levofloxacin | 111 | 2 (7.7) | 1 (4.7) | 1 (20) | 0.250 |
| Meropenem | 111 | 2 (7.7) | 2 (9.5) | (0.0) | 0.473 |
| Cefepime | 111 | 1 (3.8) | 1 (4.7) | 0 (0.0) | 0.619 |
| Combo - regimen | 111 | 85 (76.6) | 60 (74.1) | 25 (8.3) | 0.306 |
| Vancomicin -Cefepime | 111 | 5 (5.9) | 2 (3.3) | 3 (12.0) | 0.099 |
| Piperacillina /tazobactam_levofloxacin | 111 | 79 (92.9) | 58 (96.6) | 21 (84.0) | 0.099 |
| Meropenem_levofloxacin | 111 | 1 (1.2) | 0 (0.0) | 1 (4) | 0.099 |
| Total Duration of PAP (median, IQR) | 110 | 10 (6–13) | 10 (6–14) | 9 (6–10) | 0.138 |
| Duration of PAP ≤6 days | 110 | 29 (27.1) | 20 (25.6) | 9 (31.0) | 0.577 |
| Recipient colonization after lung transplant | 110 | 25 (22.7) | 13 (16.2) | 12 (40.0) | 0.008 |
| BAL | 25 | 20 (18.0) | 9 (11.1) | 11 (36.7) | 0.002 |
| Rectal (CPE) | 25 | 4(3.6) | 3 (3.7) | 1 (3.3) | 0.926 |
| Urinary | 25 | 4 (3.6) | 3 (3.7) | 1 (3.3) | 0.504 |
| Time of colonization from Tx | 25 | 15 (6–41) | 17 (6–42) | 10 (4–36) | 0.695 |
| Outcome | |||||
| ICU Readmission | 111 | 15 (13.5) | 6 (7.4) | 9 (30.0) | 0.002 |
| ICU LOS | 109 | 15 (6–26) | 11 (6–24) | 21 (15–33) | 0.001 |
| Duration of MV | 110 | 6 (3–12) | 4 (2–9) | 9 (7–17) | <0.001 |
| Renal Replacement Therapy | 111 | 41 (36.9) | 22 (27.2) | 19 (63.3) | <0.001 |
| Continous Renal Replacement Therapy | 111 | 38 (34.2) | 21 (25.9) | 17 (56.7) | 0.002 |
| Days of Continous Renal Replacement Therapy median (IQR) | 36 | 12 (8–21) | 10 (5–20) | 16 (11–21) | 0.002 |
| Re-IOT | 106 | 17 (15.3) | 10 (12.8) | 7 (25.0) | 0.132 |
| Re-hospitalization | 94 | 5 (4.5) | 3 (4.5) | 2 (7.4) | 0.567 |
| Death 30 days | 111 | 18 (16.2) | 14 (17.3) | 4 (13.3) | 0.616 |
Characteristics of patients receiving lung transplant and comparison of patients with and without bacterial infection after lung-transplant.
Abbreviations: BAL, bronchoalveolar lavage; BSI, bloodstream infection; COPD, chronic obstructive pulmonary disease; CPE, carabapenem producing enterobacterales; DBD, donation after brain death; DCD, donation after circulatory death; ICU, intensive care unit; IQR, interquartile range; LOS, length of stay; MV, mechanical ventilation; OI, orotracheal intubation; PAP, Perioperative antibiotic prophylaxis.
Recipient BAL colonization was found in 16 (14.4%) patients, data shown in Supplementary Table S1. Donor characteristics are summarized in Table 1: median age at the time of donation was 48 years (IQR: 31–55). Mainly were donation after brain death (DBD) (107, 96.4%). Infectious risk was considered as “non-standard” in 22 (21.4%) donations and ≥1 positive sample from BAL and blood was obtained from 59 (53.2%) donors. Data about donor/recipient BAL colonization are shown in Supplementary Table S1.
PAP consisted of single-agent or combination regimens in 26 (25.2%) and 85 (74.8%) LuTR, respectively (Figure 2).
FIGURE 2
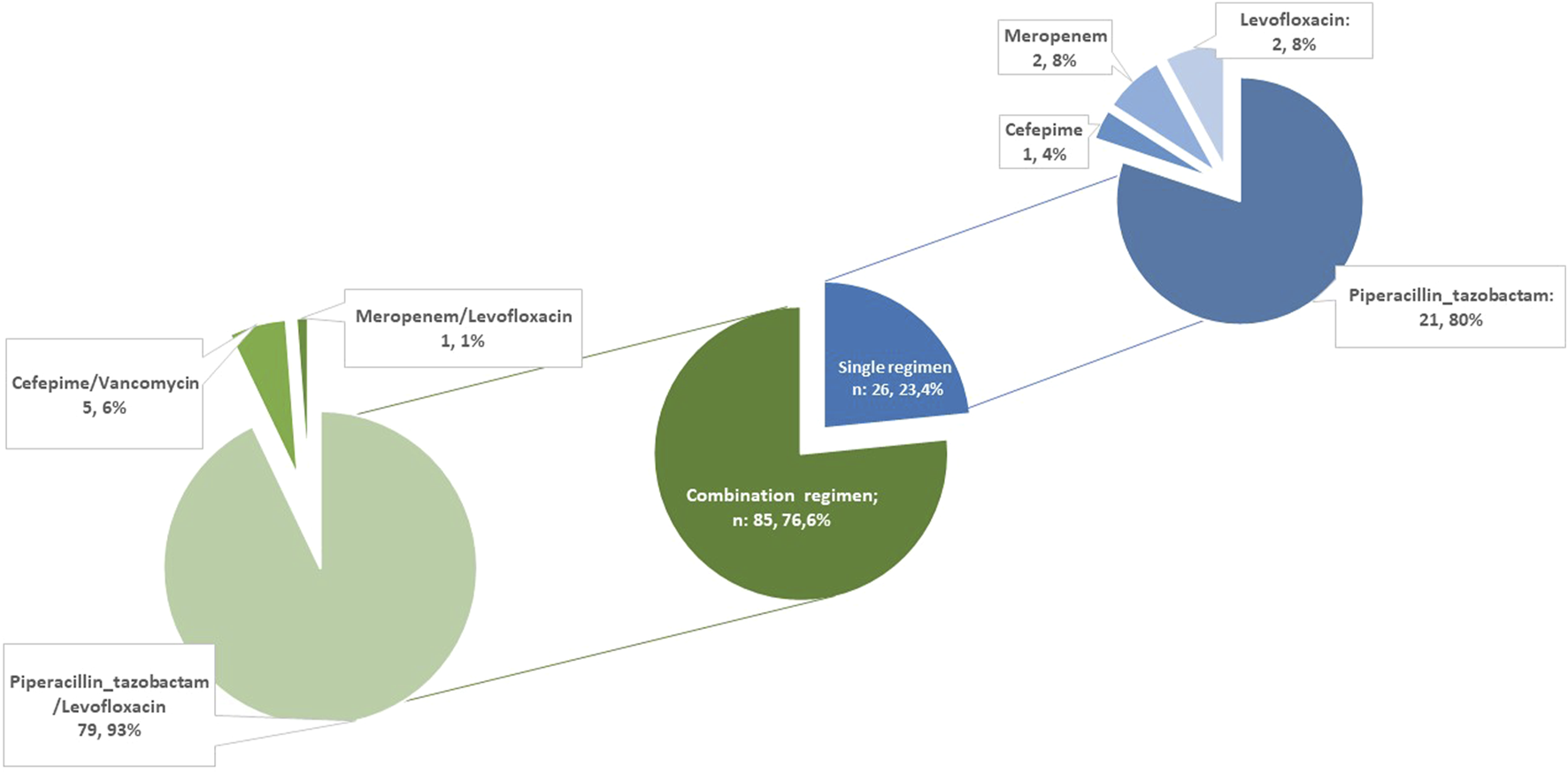
Perioperative antibiotic prophylaxis regimens.
Piperacillin/tazobactam was the most common agent used either as monotherapy (n = 21, 80.7%) as combination with levofloxacin (n = 79, 92.9%). Among all, 11 patients did not receive piperacillin/tazobactam as backbone of peri-operative antibiotic prophylaxis (PAP), 8 due to drug allergy/intolerance (of which 2, 1 and 5 of them received meropenem, levofloxacin alone and cefepime, respectively) and 3 due to surgeon decision. Levofloxacin was not administered in 8 patients (in 3 cases due to reported allergy to fluoroquinolones and in 5 patients due to other contraindications - history of epilepsy n = 2, QT prolongation n = 2, connective tissue disease n = 1). Vancomycin was administered as part of the PAP regimen in two recipients due to MRSA colonization of the respiratory samples in the pre-transplant period. The median duration of antibiotic prophylaxis was 10 days (IQR 6–13). No differences were found in PAP duration according to donor sample and recipient colonization (Supplementary Table S2).
MDRO colonization after LuT is reported in Supplementary Table S3. No differences were found regarding MDRO colonization in patients with PAP ≤6 days or >6 days (9, 32.1% vs. 15, 19.2%, p = 0.161). No Clostridioides difficile infection was found in the entire patient cohort.
EPOIs were diagnosed in 30 (27%) patients: 22 (73.3%) pneumonia, 1 (3.3%) bloodstream infections (BSI) and 2 (6.6%) surgical site infections. The median time to EPOIs was 10 days (IQR 6–23) from LuT. Overall, 13 patients with EPOIs were still on PAP at the time of infection diagnosis and therefore the antibiotic treatment was changed and targeted based on antimicrobial susceptibility of the pathogens. Enterobacterales were the main pathogens, none had a DTR profile. Two EPOIs were considered as donor derived events, data shown in Supplementary Table S4. Trend of LuT performed and related EPOIs devolopment during the study period is showed in Supplementary Figure S1. Comparison of patients with and without EPOIs is shown in Table 1. Patients with EPOIs were more frequently male (22, 73.3% vs. 42, 51.9%, p = 0.042) with older age (54, IQR: 46–63 vs. 48, IQR: 36–59, p = 0.029) with more frequently IPF (14, 46.7% vs. 20, 24.7%, p = 0.026) as underlying lung disease. No differences were found as regard single or combination PAP regimens administered. There were no differences in 30-day mortality (4, 13.3% vs. 14, 17.3%, p 0.616). However, patients with EPOIs had more longer length of stay (LOS) in ICU (15 days, IQR:6–26 vs. 11 days, IQR: 6–24, p:0.001) and ICU readmission rates (9, 30% vs. 6, 7.4%, p = 0.002), longer duration of mechanical ventilation (9 days, IQR: 7–17 vs. 4 days, IQR: 2–9, p < 0.001) and more frequently need of renal replacement therapy (19, 63.3% vs. 22, 27.2%, p < 0.001).
The multivariable analysis of risk factors for EPOIs is showed in Table 2, panel A. No advantages were found for combination regimens compared to single-agent PAP in preventing EPOI (OR: 1.57, 95% CI: 0.488–5.068, p:0.448). The model was adjusted for male gender, Charlson Comorbidity Index, Tacrolimus as maintenance immunosuppresive regimen, idiopatic pulmonary fibrosis as leading cause for lung transplant and primary graft non function. To investigate the impact of PAP duration of EPOIs development, we excluded from analysis patients with ongoing antibiotic prophylaxis at infection diagnosis (13 of 30, 43%). The remaining 17 patients developing EPOIs were included in the model. PAP duration ≤6 days was used as main exposure variable. We didn’t find a significantly impact of PAP duration (OR:2.165, 95% CI: 0.596–7.863, p: 0.240) (Table 2, panel B). The multivariable analysis of risk factors for EPOIs was repeated by selecting only patients treated with piperacillin/tazobactam in monotherapy and in association with levofloxacin confirming no advantages for combination regimen compared to single-agent PAP in preventing EPOI and neither significantly impact of PAP duration (Supplementary Table S5, panel A and B).
TABLE 2
| Panel a | OR | IC 95% | P |
|---|---|---|---|
| Male gender | 0.736 | 0.241–2.244 | 0.590 |
| Idiopatic pulmonary fibrosis as leading cause for lung transplant | 1.436 | 0.517–3.984 | 0.487 |
| Primary graft non function | 0.304 | 0.062–1.488 | 0.142 |
| Charlson comorbidity index | 1.236 | 0.916–1.667 | 0.167 |
| Tacrolimus as mantainance regimen | 0.295 | 0.095–0.918 | 0.035 |
| PAP combination regimens | 1.573 | 0.488–5.068 | 0.448 |
| Panel b | OR | IC 95% | P |
| Male gender | 0.854 | 0.194–3.756 | 0.834 |
| Idiopatic pulmonary fibrosis as leading cause for lung transplant | 0.466 | 0.111–1.955 | 0.297 |
| Primary graft non function | 0.149 | 0.013–1.671 | 0.123 |
| Charlson comorbidity index | 1.391 | 0.937–2.064 | 0.102 |
| Tacrolimus as mantainance regimen | 0.324 | 0.076–1.376 | 0.127 |
| PAP combination regimens | 5.606 | 0.643–48.901 | 0.119 |
| Duration of PAP ≤6 days | 2.165 | 0.596–7.863 | 0.240 |
Multivariable binary logistic regression of: total EPOIs development at 30 days after lung transplantation (Panel a); EPOIs in patients without PAP at the time of infection diagnosis (Panel b).
Abbreviations: OR, odds ratio; IC, confidence intervals; PAP, perioperative antibiotic prophylaxis.
Discussion
We analysed a large cohort of LuTRs to evaluate different PAP regimens used to prevent EPOIs, mainly with piperacillin/tazobactam as backbone. No differences were found as regard EPOIs development between combination or single agent PAP regimens. In addition, we observed a prolonged PAP not justified by donor/recipient culture results underlying the need of ad hoc strategies to limit the use of broad spectrum and unnecessary prolonged regimens.
The knowledge of the patient’s infectious risk is crucial for an appropriate management of LuTRs in the perioperative period. It may be helpful to consider targeted PAP for patients who are colonized with MDROs and, conversely, to limit the use of high microbiological impact antibiotics (i.e., carbapenems) if alternatives available. Regarding this aspect, characteristics of patients in our cohort are peculiar. The most frequent lung diseases requiring transplantation, COPD/emphysema and cystic fibrosis, are poor represented in our study [21]. Patients with COPD and cystic fibrosis suffer frequently of bacterial infections with consequently prolonged broad-spectrum antibiotics exposition and higher risk of MDROs colonization [3, 9]. Conversely, patients with interstitial lung disease show low rates of bacterial complication with a reduced antibiotic exposure and MDROs colonization [22]. Indeed, in our cohort, the rate of MDROs recipient colonization and infection was very low and PAP regimen was almost always effective on antimicrobial susceptibility profiles of donor/recipient isolates. We noted that, among all interstitial lung diseases collected in our center, patients with IPF appeared to have the highest risk of developing infections. Further studies are needed to confirm this finding.
The choice of single or combination PAP regimens is left to referral clinicians. If drugs with activity against MDR Gram negative rods are almost universally used, a second antibiotic with activity against Staphylococcus aureus could be added, according with local epidemiological data. In a large survey on perioperative antibiotic therapy in LuT involving 99 centers worldwide, most of the participants reported PAP regimens covering Gram negative rods with activity against Pseudomonas aeruginosa. Only one-third of the centres targeted S. aureus, almost exclusively from the USA and against methicillin resistant S.aureus (MRSA) [7], with vancomycin as preferred drug. The low S. aureus rate and the absence of a clear benefit from using a combination regimen in our cohort, support the need to set PAP according with local epidemiology.
Finally, duration of PAP is another matter of debate. Ideally, PAP should be stopped as soon as cultures of the donor and the recipients are reported as negative to reduce the risk of MDROs selection and/or C. difficile infections [23]. However, it has been shown that PAP duration among transplant centers is very heterogeneous [7]. In our study, PAP duration was unacceptably too prolonged even in cases without donor/recipient positive cultures, deviating from internal guidelines. Although with few cases, our analysis shows that a prolonged PAP is not protective for EPOIs development, thus supporting the opportunity of shortening PAP duration. In this regard, it seems desirable to design and/or standardize ad hoc antimicrobial stewardship strategies to avoid unnecessary prolonged PAP in lung transplant recipients in our center [23, 24].
There are several limitations in this study. First, we collected data from a single-center cohort of LuTR over a 20-year study period, however PAP regimens and approach to EPOI diagnosis did not change across years. Furthermore, our patients suffered mainly from interstitial lung disease and cystic fibrosis was not represented. Both these limitations could limit the generalizability of our results. However, our findings could add evidence supporting prophylaxis with a single drug in LuTRs with non-cystic fibrosis/COPD as underlying disease. Moreover, the rate of donor derived events could have been be underestimated due to the lack of respiratory sample in around half of the donors. In addition, the limited sample size and the heterogeneity of PAP administration did not allow to infer any conclusion about the impact of prophylaxis duration on EPOIs development. Finally, the retrospective design of the study could have reduced the accuracy of data collection. However, we attempted to reduce this limitation by thorough data quality control.
Despite these limitations, our results suggest no advantages for combination regimens over a single-agent regimen in preventing EPOIs in LuTRs with interstitial lung diseases as underlying disease. However, further studies are needed to confirm this hypothesis.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the CE AREA VASTA EMILIA ROMAGNA 676/2023/Oss/AOUBo. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
RP, BT, and AA contributed to conceptualisation and design of the study; AA, ES, GD, FA, MB, SP, and SA contributed to acquisition of data; RP and MG performed the analysis; RP contributed to writing the original draft; MG, MP, and PV supervised the work. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was funded by the Italian Ministry of Health, RC-2024-2789955.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/ti.2024.13245/full#supplementary-material
References
1.
Yusen RD Edwards LB Kucheryavaya AY Benden C Dipchand AI Goldfarb SB et al The Registry of the International Society for Heart and LUNG Transplantation: Thirty-Second Official Adult LUNG and Heart-Lung Transplantation Report--2015; Focus Theme: Early Graft Failure. J Heart Lung Transpl (2015) 34(10):1264–77. 10.1016/j.healun.2015.08.014
2.
Verleden SE Sacreas A Vos R Vanaudenaerde BM Verleden GM . Advances in Understanding Bronchiolitis Obliterans After LUNG Transplantation. Chest (2016) 150(1):219–25. 10.1016/j.chest.2016.04.014
3.
Aguilar-Guisado M Givaldá J Ussetti P Ramos A Morales P Blanes M et al Pneumonia After LUNG Transplantation in the RESITRA Cohort: A Multicenter Prospective Study. Am J Transpl (2007) 7(8):1989–96. 10.1111/j.1600-6143.2007.01882.x
4.
Beaume M Köhler T Greub G Manuel O Aubert JD Baerlocher L et al Rapid Adaptation Drives Invasion of Airway Donor Microbiota by Pseudomonas After LUNG Transplantation. Sci Rep (2017) 7:40309. 10.1038/srep40309
5.
Vital D Hofer M Benden C Holzmann D Boehler A . Impact of Sinus Surgery on Pseudomonal Airway Colonization, Bronchiolitis Obliterans Syndrome and Survival in Cystic Fibrosis LUNG Transplant Recipients. Respiration (2013) 86(1):25–31. 10.1159/000339627
6.
Syed SA Whelan FJ Waddell B Rabin HR Parkins MD Surette MG . Reemergence of Lower-Airway Microbiota in LUNG Transplant Patients WITH Cystic Fibrosis. Ann Am Thorac Soc (2016) 13(12):2132–42. 10.1513/AnnalsATS.201606-431OC
7.
Coiffard B Prud’Homme E Hraiech S Cassir N Le Pavec J Kessler R et al Worldwide Clinical Practices in Perioperative Antibiotic Therapy for LUNG Transplantation. BMC Pulm Med (2020) 20(1):109. 10.1186/s12890-020-1151-9
8.
Ruiz I Gavaldà J Monforte V Len O Román A Bravo C et al Donor-To-Host Transmission of Bacterial and Fungal Infections in LUNG Transplantation. Am J Transpl (2006) 6(1):178–82. 10.1111/j.1600-6143.2005.01145.x
9.
Corris PA . LUNG Transplantation for Cystic Fibrosis and Bronchiectasis. Semin Respir Crit Care Med (2013) 34(3):297–304. 10.1055/s-0033-1348469
10.
Bratzler DW Dellinger EP Olsen KM Perl TM Auwaerter PG Bolon MK et al Clinical Practice Guidelines for Antimicrobial Prophylaxis in Surgery. Am J Health Syst Pharm (2013) 70(3):195–283. 10.2146/ajhp120568
11.
Harris PA Taylor R Minor BL Elliott V Fernandez M O'Neal L et al The REDCap Consortium: Building an International Community of Software Platform Partners. J Biomed Inform (2019) 95:103208. 10.1016/j.jbi.2019.103208
12.
Saravolatz LD Pea F Viale P . The Antimicrobial Therapy Puzzle: Could Pharmacokinetic-Pharmacodynamic Relationships Be Helpful in Addressing the Issue of Appropriate Pneumonia Treatment in Critically Ill Patients?Clinical Infectious Dis (2006) 42(12):1764–71. 10.1086/504383
13.
Sistanizad M Hassanpour R Pourheidar E . Are Antibiotics Appropriately Dosed in Critically Ill Patients WITH Augmented Renal Clearance? A Narrative Review. Int J Clinical Pract (2022) 2022:1867674–17. Saad K. 10.1155/2022/1867674
14.
Yang H Zhang C Zhou Q Wang Y Chen L . Clinical Outcomes WITH Alternative Dosing Strategies for Piperacillin/Tazobactam: A Systematic Review and Meta-Analysis. PLoS ONE (2015) 10(1):e0116769. Cameron DW. 10.1371/journal.pone.0116769
15.
Horan TC Andrus M Dudeck MA . CDC/NHSN Surveillance Definition of Health Care-Associated Infection and Criteria for Specific Types of Infections in the Acute CARE Setting. Am J Infect Control (2008) 36(5):309–32. 10.1016/j.ajic.2008.03.002
16.
Wolfe CR Ison MG, AST Infectious Diseases Community of Practice. Donor-Derived Infections: Guidelines FROM the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transpl (2019) 33(9):e13547. 10.1111/ctr.13547
17.
Charlson ME Pompei P Ales KL MacKenzie CR . A New Method of Classifying Prognostic Comorbidity in Longitudinal Studies: Development and Validation. J Chronic Dis (1987) 40(5):373–83. 10.1016/0021-9681(87)90171-8
18.
Garner JS Jarvis WR Emori TG Horan TC Hughes JM . CDC Definitions for Nosocomial Infections, 1988. Am J Infect Control (1988) 16(3):128–40. 10.1016/0196-6553(88)90053-3
19.
Kadri SS Adjemian J Lai YL Spaulding AB Ricotta E Prevots DR et al Difficult-To-Treat Resistance in Gram-Negative Bacteremia at 173 US Hospitals: Retrospective Cohort Analysis of Prevalence, Predictors, and Outcome of Resistance to All First-Line Agents. Clin Infect Dis (2018) 67(12):1803–14. 10.1093/cid/ciy378
20.
Magiorakos AP Srinivasan A Carey RB Carmeli Y Falagas ME Giske CG et al Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin Microbiol Infect (2012) 18(3):268–81. 10.1111/j.1469-0691.2011.03570.x
21.
Leard LE Holm AM Valapour M Glanville AR Attawar S Aversa M et al Consensus Document for the Selection of LUNG Transplant Candidates: An Update FROM the International Society for Heart and LUNG Transplantation. J Heart Lung Transpl (2021) 40(11):1349–79. 10.1016/j.healun.2021.07.005
22.
Azadeh N Limper AH Carmona EM Ryu JH . The ROLE of Infection in Interstitial LUNG Diseases: A Review. Chest (2017) 152(4):842–52. 10.1016/j.chest.2017.03.033
23.
Whiddon AR Dawson KL Fuentes A Perez KK Peterson LE Kaleekal T . Postoperative Antimicrobials After LUNG Transplantation and the Development of Multidrug-Resistant Bacterial and Clostridium Difficile Infections: An Analysis of 500 Non-Cystic Fibrosis LUNG Transplant Patients. Clin Transpl (2016) 30(7):767–73. 10.1111/ctr.12746
24.
McCort M MacKenzie E Pursell K Pitrak D . Bacterial Infections in LUNG Transplantation. J Thorac Dis (2021) 13(11):6654–72. 10.21037/jtd-2021-12
Summary
Keywords
lung transplant, antibiotic prophylaxis, bacterial infection, donor derived infections, idiopatic pulmonary fibrosis
Citation
Pascale R, Tazza B, Amicucci A, Salvaterra E, Dolci G, Antonacci F, Baiocchi M, Pastore S, Ambretti S, Peghin M, Viale P and Giannella M (2024) Antibiotic Prophylaxis in Patients Undergoing Lung Transplant: Single-Center Cohort Study. Transpl Int 37:13245. doi: 10.3389/ti.2024.13245
Received
12 May 2024
Accepted
08 August 2024
Published
16 August 2024
Volume
37 - 2024
Updates
Copyright
© 2024 Pascale, Tazza, Amicucci, Salvaterra, Dolci, Antonacci, Baiocchi, Pastore, Ambretti, Peghin, Viale and Giannella.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Renato Pascale, renato.pascale2@unibo.it
ORCID: Renato Pascale, orcid.org/0000-0003-2687-4235
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.