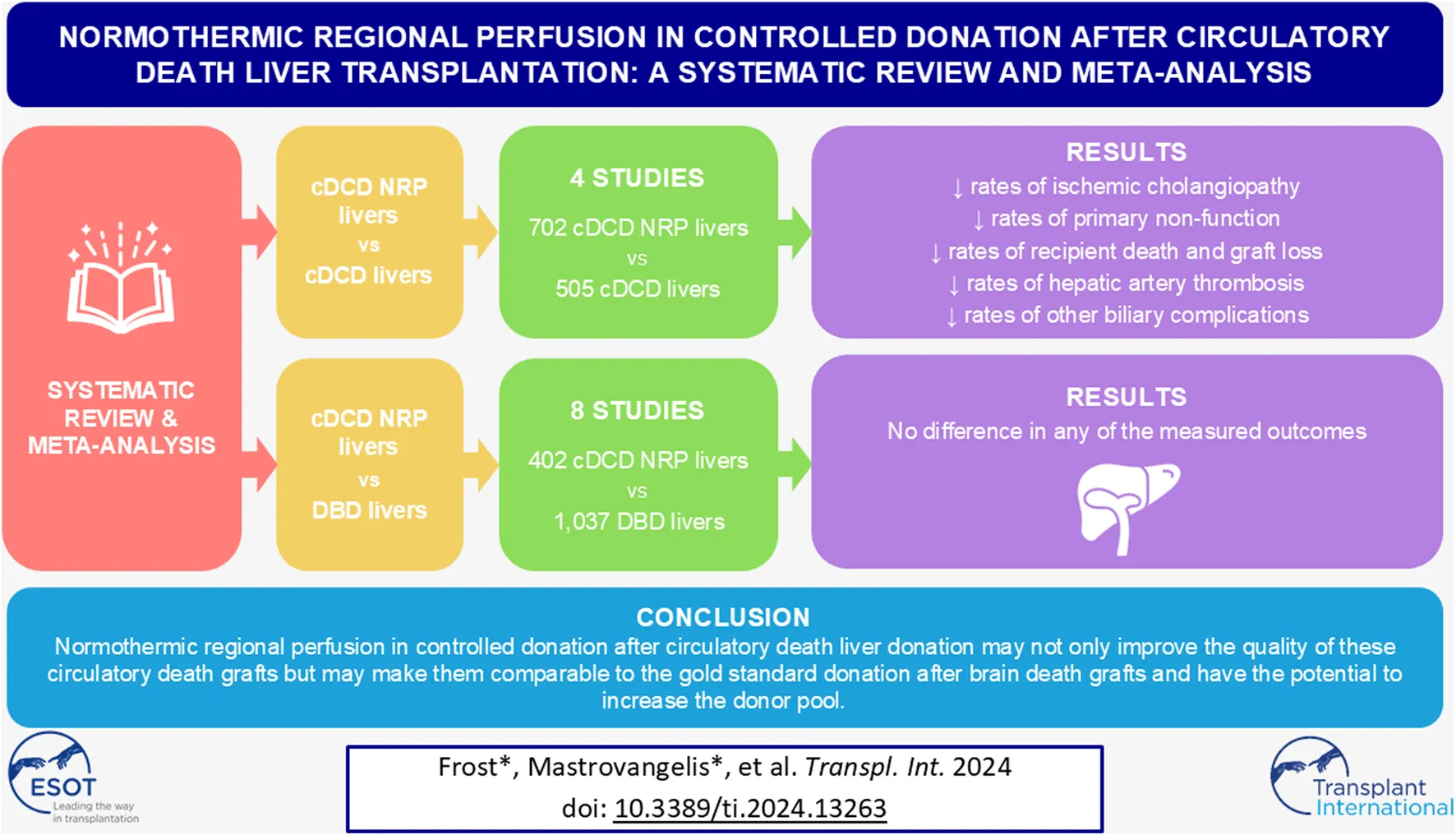
Abstract
Liver grafts from controlled donation after circulatory death (cDCD) donors have lower utilization rates due to inferior graft and patient survival rates, largely attributable to the increased incidence of ischemic cholangiopathy, when compared with grafts from brain dead donors (DBD). Normothermic regional perfusion (NRP) may improve the quality of cDCD livers to allow for expansion of the donor pool, helping to alleviate the shortage of transplantable grafts. A systematic review and metanalysis was conducted comparing NRP cDCD livers with both non-NRP cDCD livers and DBD livers. In comparison to non-NRP cDCD outcomes, NRP cDCD grafts had lower rates of ischemic cholangiopathy [RR = 0.23, 95% CI (0.11, 0.49), p = 0.0002], primary non-function [RR = 0.51, 95% CI (0.27, 0.97), p = 0.04], and recipient death [HR = 0.5, 95% CI (0.36, 0.69), p < 0.0001]. There was no difference in outcomes between NRP cDCD donation compared to DBD liver donation. In conclusion, NRP improved the quality of cDCD livers compared to their non-NRP counterparts. NRP cDCD livers had similar outcomes to DBD grafts. This provides further evidence supporting the continued use of NRP in cDCD liver transplantation and offers weight to proposals for its more widespread adoption.
Introduction
Due to a shortage of suitable donor livers, there is a need for expansion of the liver donor pool [1]. One proposed method of addressing this shortage has been to utilize livers from donation after circulatory death (DCD) donors. In these donors, declaration of death is made following cessation of circulation as determined by heartbeat, blood pressure, and/or electrocardiography [2]. This is followed by a super-rapid recovery procurement technique, during which the blood is flushed and the organ is cooled in situ prior to placement on ice. This is contrasted with donation after brain death (DBD) donors where, although the donor’s heart is still beating, brain death has been declared based on neurological criteria. DCD donors are commonly further classified as controlled (cDCD) or uncontrolled (uDCD) [3]. cDCD livers are generally considered less desirable than those recovered from DBD donors, as they are associated with higher rates of graft loss, ischemic cholangiopathy (IC), and inferior recipient survival [4, 5]. Therefore, there is significant interest in the development of novel organ procurement and preservation techniques to help improve outcomes associated with cDCD liver transplantation.
The current mainstay of organ preservation in liver transplantation is static cold storage (SCS) [6]. SCS in carefully selected DBD liver grafts have relatively low rates of known transplant complications such as early allograft dysfunction (EAD), primary non-function (PNF), and IC [6–9]. However, SCS alone in the cDCD context is associated with a higher incidence of graft complications and poorer recipient outcomes when compared with SCS in DBD livers [6]. IC is of particular concern with DCD livers (incidence of approximately 16% DCD vs. 3% DBD) [4, 10]. It has been postulated that warm ischemia (around the time of procurement) and vascular congestion contributes to microthrombus formation and subsequent biliary ischemia, leading to IC [5, 11, 12]. Compared with DBD livers, the PNF rate is greater in DCD livers (odds ratio of 3.6), as is the rate of total biliary complications (26% DCD vs. 16% DBD), and graft failure (odds ratio of 1.9) [4, 10]. These poorer outcomes contribute to higher rates of non-utilization of cDCD grafts for liver transplantation [13].
In normothermic regional perfusion (NRP) protocols, warm oxygenated perfusion with blood is restored in situ after declaration of circulatory death using an extracorporeal membrane oxygenation circuit. Although many technical variants exist, the circuit can be used to perfuse abdominal-only or all abdominal and thoracic organs simultaneously [13, 14]. Although the cellular mechanisms by which NRP works are not yet clear, it certainly allows for in situ assessment of organ function via macroscopic inspection, biopsy, and biochemical evaluation [13–16]. However, NRP does utilize more resources than super rapid recovery (SRR); including increased operating theatre time, disposables, and specifically trained perfusion staff [14].
The adoption of NRP varies significantly worldwide. It is policy to routinely use NRP in cDCD liver transplantation in Italy, France, and Norway, while also permitted for use in various other jurisdictions [13, 14, 17]. Some international centres combine NRP with additional ex-vivo machine perfusion technologies. The goal of NRP utilization is primarily to increase utilization of deceased donor organs and reduce mortality on the liver transplant waiting list. This systematic review and meta-analysis aims to compare outcomes from transplanted livers using NRP cDCD donors with non-NRP cDCD donors, as well comparing cDCD NRP outcomes with outcomes from DBD donation. We hypothesise that NRP improves the outcomes of cDCD livers and yields outcomes comparable to DBD livers.
Materials and Methods
Search Methods and Criteria
A literature search was conducted following the PRISMA 2020 Guidelines and was registered with PROSPERO (CRD42023432345) [18]. The databases searched included Medline, Embase and Scopus. The final search was conducted on 9th June 2023. Article screening, full text review, data extraction, and bias appraisal was conducted independently by Author 1 and Author 2. A third reviewer was used to resolve any conflicts. Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia) was used for title and abstract screening as well as full text review.
The search was restricted to human studies in the English language published after 1st January 2000. The search terms focused on capturing liver transplantation and NRP. Search terms defining the comparator groups were deliberately not included to prevent over-filtering otherwise eligible studies.
Studies eligible for inclusion were randomised controlled trials and cohort studies of adult recipients of cDCD livers that had undergone NRP. Comparator groups of cDCD livers with SRR ± non-NRP machine perfusion, or DBD livers with SCS ± non-NRP machine perfusion were eligible. All indications for transplant and all MELD scores were included.
Abstracts, case reports, and systematic reviews were excluded. Studies with <5 NRP livers transplanted, NRP livers from uncontrolled donation after circulatory death (uDCD) donors, and paediatric recipients (<18 years) were excluded. Studies specifying a no-touch-time ≥5 min or containing data from jurisdictions with mandatory no-touch-times ≥5 min were also excluded [19]. The studies included in the data extraction were assessed using the Newcastle-Ottawa Scale Risk of Bias for Cohort Studies tool. Full inclusion/exclusion criteria are available in the Supplementary Material, and appraisal results are available Table 1.
TABLE 1
| Study | Selection | Comparability | Outcome | Quality | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 1 | Median follow up | 1 | 2 | 3 | ||
| DeGoeij et al. [20] | ★ | ★ | ★ | ★ | - | 23 months | ★ | ★ | - | Poor |
| Gaurav et al. [8] | ★ | ★ | ★ | ★ | - | 38 months | ★ | ★ | - | Poor |
| Hessheimer et al. [21] | ★ | ★ | ★ | ★ | - | 31 months | ★ | ★ | - | Poor |
| Mohkam et al., [22], | ★ | - | ★ | ★ | ★ | 22 months | ★ | ★ | - | Good |
| Fernandez-delaVarga et al. [23] | ★ | ★ | ★ | ★ | ★ | 23.1 months | ★ | ★ | - | Good |
| Minambres et al. [24] | ★ | ★ | ★ | ★ | - | 6 months | ★ | - | - | Poor |
| Rodriguez et al. [25] | ★ | ★ | ★ | ★ | - | 22.7 months | ★ | ★ | - | Poor |
| Rodriguez-Sanjuan et al. [26] | ★ | ★ | ★ | ★ | - | 18 months | ★ | - | - | Poor |
| Ruiz et al. [27] | ★ | ★ | ★ | ★ | ★ | 36 months | ★ | ★ | - | Good |
| Savier et al. [28] | ★ | ★ | ★ | ★ | ★ | 34.8 months | ★ | ★ | - | Good |
| Viguera et al. [29] | ★ | ★ | ★ | ★ | - | >12 months | ★ | ★ | - | Poor |
Newcastle Ottawa Scale bias appraisal.
Bold values refer to scoring categories for Selection, Comparability, and Outcome domains.
Figure 1 is a PRISMA flow chart outlining the screening process undertaken in this review. Twelve studies were excluded from analysis due to containing duplicate data with other included studies. Preference for inclusion in these cases was given to studies published more recently and studies with higher participant numbers. Eleven studies were included in the final analysis.
FIGURE 1
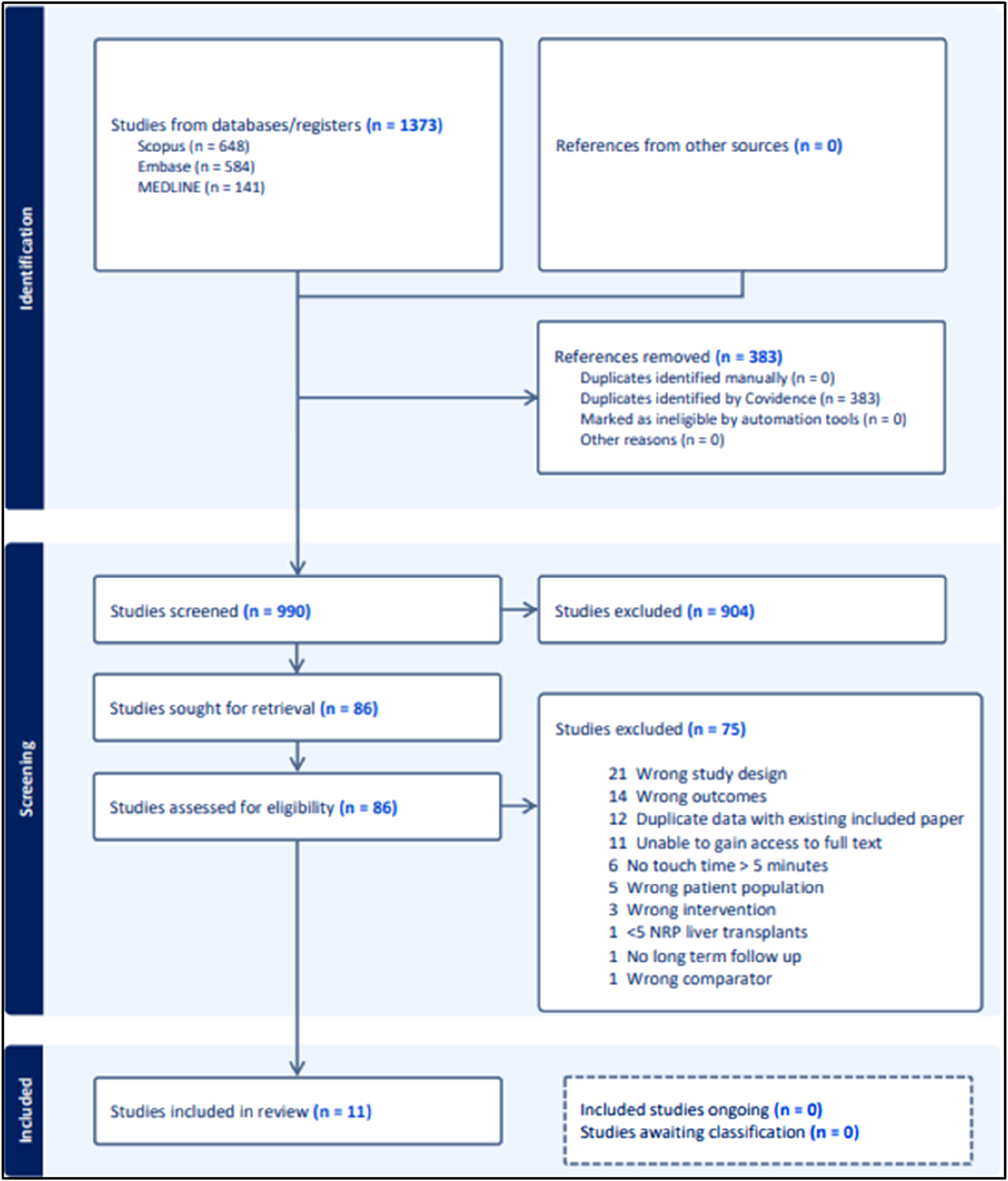
PRISMA chart.
Data Extraction
Data was independently extracted by Author 1 and Author 2 into a preformed template and cross-checked. Disparities were settled with discussion and repeated review. The data extracted included number of livers transplanted, recipient death, graft loss, ischemic cholangiopathy (IC), primary non-function (PNF), hepatic artery thrombosis (HAT), early allograft dysfunction (EAD), other biliary complications, intensive care unit (ICU) length of stay, and hospital length of stay.
The following outcomes were defined for the purpose of our analysis:
• IC: non-anastomotic strictures identified through appropriate imaging with a patent hepatic artery
• PNF: graft failure leading to urgent re-transplantation or death within 1-week post-surgery
• EAD: as per Olthoff criteria [30].
• HAT: thrombosis in the hepatic artery identified through relevant imaging
• Other biliary complications: defined as anastomotic strictures and leaks, and other biliary complications identified by the study excluding IC and HAT.
• The discard rate was defined as the rate of liver grafts which were not utilized post-procurement or NRP initialisation.
Statistical Analysis
Analysis was divided to make two separate comparisons: NRP vs. non-NRP for cDCD donation, and cDCD NRP vs. DBD donation. Further sub-group analysis was not possible due to study numbers.
Length of stay data underwent logarithmic transformation and subsequent conversion from median and interquartile range into mean and standard deviation as per Wan et al. [31] Patient death and graft loss data were analyzed by pooling hazard ratios (HR). If not reported, Kaplan-Meier plots were measured to estimate patient level survival data, which was then used to estimate hazard ratios by Cox regression. SPSS version 28.0.0.0 (IBM, United States) was used for this calculation.
Meta-analysis was performed using inverse variance random effects models. Risk ratios were calculated for dichotomous variables, mean difference was calculated for length of stay data, and hazard ratios calculated for survival data. For dichotomous variables, any study where zero events occurred in both arms was excluded. However, to ensure robustness of pooled effect, sensitivity analysis was performed by also estimating pooled effect size after continuity correction (factor of 0.5) for such studies [32]. The cut-off for statistically significant results and confidence intervals (CI) were defined as p < 0.05 and 95% respectively.
Pooled incidence of IC and PNF were estimated using the metaprop in Stata version 15.1 for Windows (StataCorp LLC, TX, United States) [33]. A random-effects model was used. As the incidence rates are at or close to zero for many studies, we enabled Freeman-Tukey double arsine transformation and used score confidence intervals for the individual studies. Heterogeneity was assessed using I2 values.
Results
Table 1 summarises the bias appraisal of each study as per the Newcastle Ottawa Scale. Four of the studies received an overall appraisal of “good,” and seven studies received an overall appraisal of “poor.” Of these seven studies, five studies received “poor” appraisal because they did not control for confounders between the two groups and hence failed to score points in the comparability domain. Two of the included studies received a “poor” appraisal in any of the other scoring domains.
Table 2 summarises the characteristics of each study included in the NRP vs. non-NRP for cDCD donation analysis. Three of the studies utilized NRP alone, and one study utilized NRP in combination with dual hypothermic oxygenated machine perfusion (D-HOPE) for some of the transplanted livers. The comparator groups are a mix of SCS alone and in combination with machine perfusion. The number of livers transplanted in the NRP and non-NRP groups totalled 702 and 505 respectively.
TABLE 2
| Author | Year | Location | Type | Comparison | NRP livers | Non-NRP livers |
|---|---|---|---|---|---|---|
| Hessheimer et al. [21] | 2022 | Spain | Multicentre | NRP vs. SCS | 545 | 258 |
| Mohkam et al. [22] | 2022 | France/Switzerland | Multicentre | NRP vs. NMP | 68 | 34 |
| Gaurav et al. [8] | 2022 | UK | Single centre | NRP vs. SCS/NMP | 69 | 164 |
| De Goeij et al. [20]a | 2022 | Netherlands | Multicentre | NRP ± DHOPE vs. SCS ± DHOPE | 20 | 49 |
| Total | 702 | 505 |
NRP vs. non-NRP for cDCD study characteristics.
Includes 2 uDCD donations.
Table 3 summarises the characteristics of studies included in the comparison of cDCD with NRP vs. DBD donation. Two studies utilized NRP in combination with ex-vivo machine perfusion, whilst six studies utilize NRP alone. The comparator groups all utilized standard DBD techniques, except for one which utilized D-HOPE for some DBD transplants. The number of transplants in the cDCD with NRP and DBD groups totalled 402 and 1,037 respectively.
TABLE 3
| Author | Year | Location | Type | Comparison | cDCD livers with NRP | DBD livers |
|---|---|---|---|---|---|---|
| Rodriguez et al. [25] | 2020 | Spain | Single centre | NRP vs. DBD | 39 | 78 |
| Rodríguez-Sanjuán et al. [26] | 2019 | Spain | Single centre | NRP vs. DBD | 11 | 51 |
| Ruiz et al. [27] | 2021 | Spain | Single centre | NRP + DHOPE vs. DBD | 100 | 200 |
| Savier et al. [28] | 2020 | France | Multicentre | NRP vs. DBD | 50 | 100 |
| Viguera et al. [29] | 2021 | Spain | Multicentre | NRP vs. DBD | 144 | 447 |
| De Goeij et al. [20]a | 2022 | Netherlands | Multicentre | NRP ± DHOPE vs. DBD ± DHOPE | 20 | 81 |
| Fernandez-de la Varga et al. [23] | 2022 | Spain | Single centre | NRP vs. DBD | 22 | 51 |
| Minambres et al. [24] | 2019 | Spain | Multicentre | NRP Vs. DBD | 16 | 29 |
| Total | 402 | 1,037 |
cDCD with NRP vs. DBD study characteristics.
Includes 2 uDCD donations.
cDCD NRP vs. Non-NRP
Figure 2 summarises the analysis of IC, PNF, and recipient death for the NRP vs. non-NRP comparison. These demonstrated statistically significant results favouring the NRP group [IC: RR = 0.23, 95% CI (0.11, 0.49), p = 0.0002, PNF: RR = 0.51, 95% CI (0.27, 0.97), p = 0.04, recipient death: HR = 0.5, 95% CI (0.36, 0.69), p < 0.0001]. Overall incidence of IC in the NRP group was 2.6% [95% CI (0.13%–6.9%)], and 13.2% [95% CI (7.3%–21%)] in the non-NRP group. The incidence of PNF was 1.4% [95% CI (0.28%–3.0%)] in the NRP group and 3.5% [95% CI (1.7%–6.0%)] in the non-NRP group. NRP was associated with lower rates of graft loss, HAT, and other biliary complications [Graft loss: HR = 0.44, 95% CI (0.33, 0.58), p < 0.00001, HAT: RR = 0.53, 95% CI (0.31, 0.92), p = 0.02, other biliary complications: RR = 0.61, 95% CI (0.44, 0.84), p = 0.003]. There was no difference in the rate of EAD [RR = 0.78, 95% CI (0.51, 1.21), p = 0.27]. The discard rate for the NRP and non-NRP groups was 30% and 31% respectively.
FIGURE 2
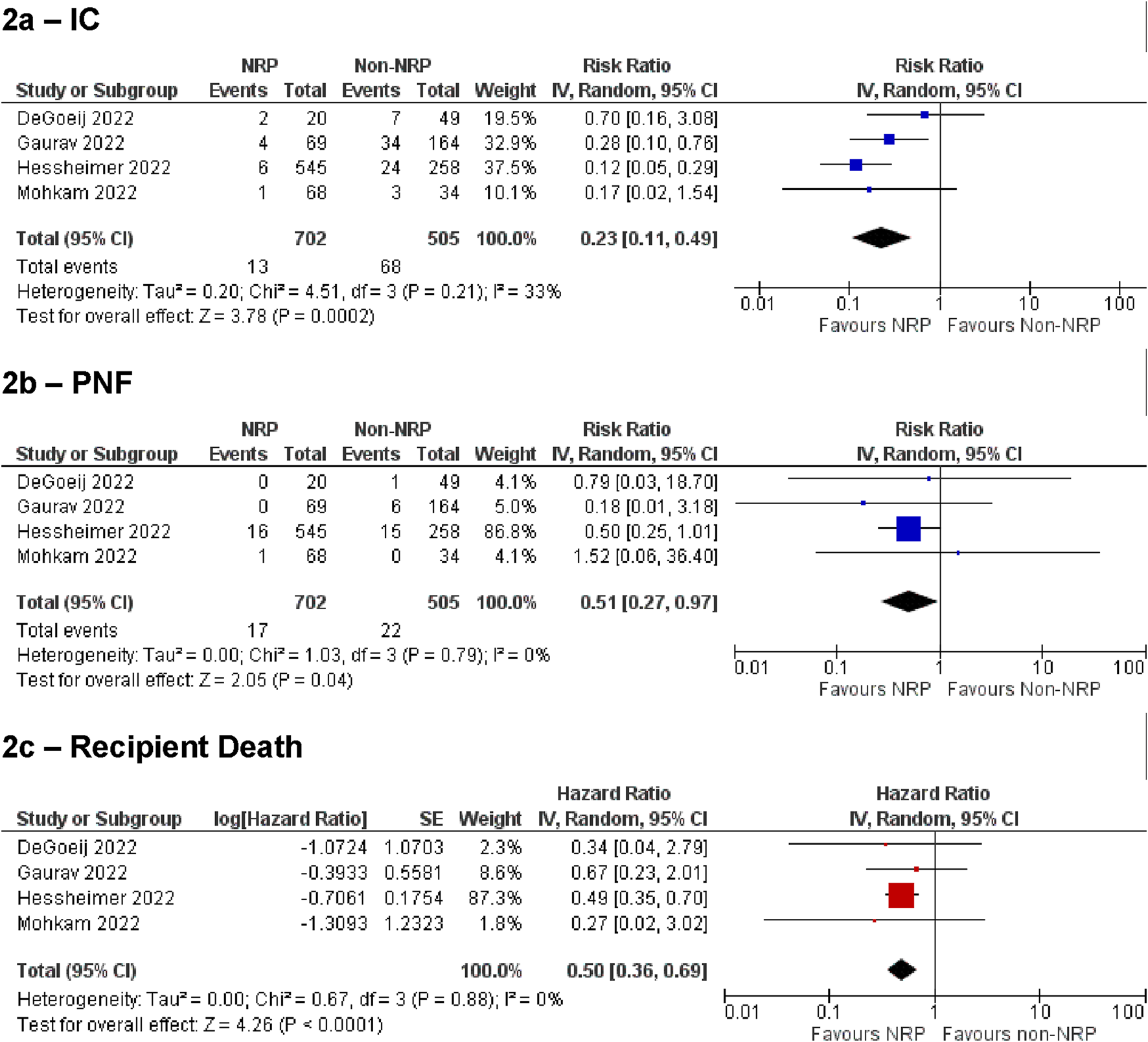
Summary of primary outcomes for NRP vs. non-NRP for cDCD. (A) ischemic cholangiopathy, (B) primary non-function, (C) recipient death.
cDCD With NRP vs. DBD
Figure 3 Summarises the analysis of IC, PNF and recipient death for the NRP vs. DBD comparison. These demonstrated no difference between the groups [IC: RR = 1.73, 95% CI (0.48, 6.24), p = 0.4, PNF: RR = 2.0, 95% CI (0.48, 8.37), p = 0.34, recipient death: HR = 0.74, 95% CI (0.39, 1.41), p = 0.36]. Sensitivity analysis by including studies with zero events on both arms (by continuity correction) confirmed these findings to be robust [IC: 1.93, 95% CI (0.66 to 5.65), p = 0.23; PNF: 2.16, 95% CI (0.62–7.52)]. The estimated overall incidence of IC was 0.13% [95% CI (0.0%–1.9%)] in the cDCD with NRP group, and 0.37% [95% CI (0.0%–2.0%)] in the DBD group. The incidence of PNF was 1.1% [95% CI (0.0%–6.2%)] in the cDCD with NRP group, and 0.69% [95% CI (0.02%–1.9%)] in the DBD group.
FIGURE 3
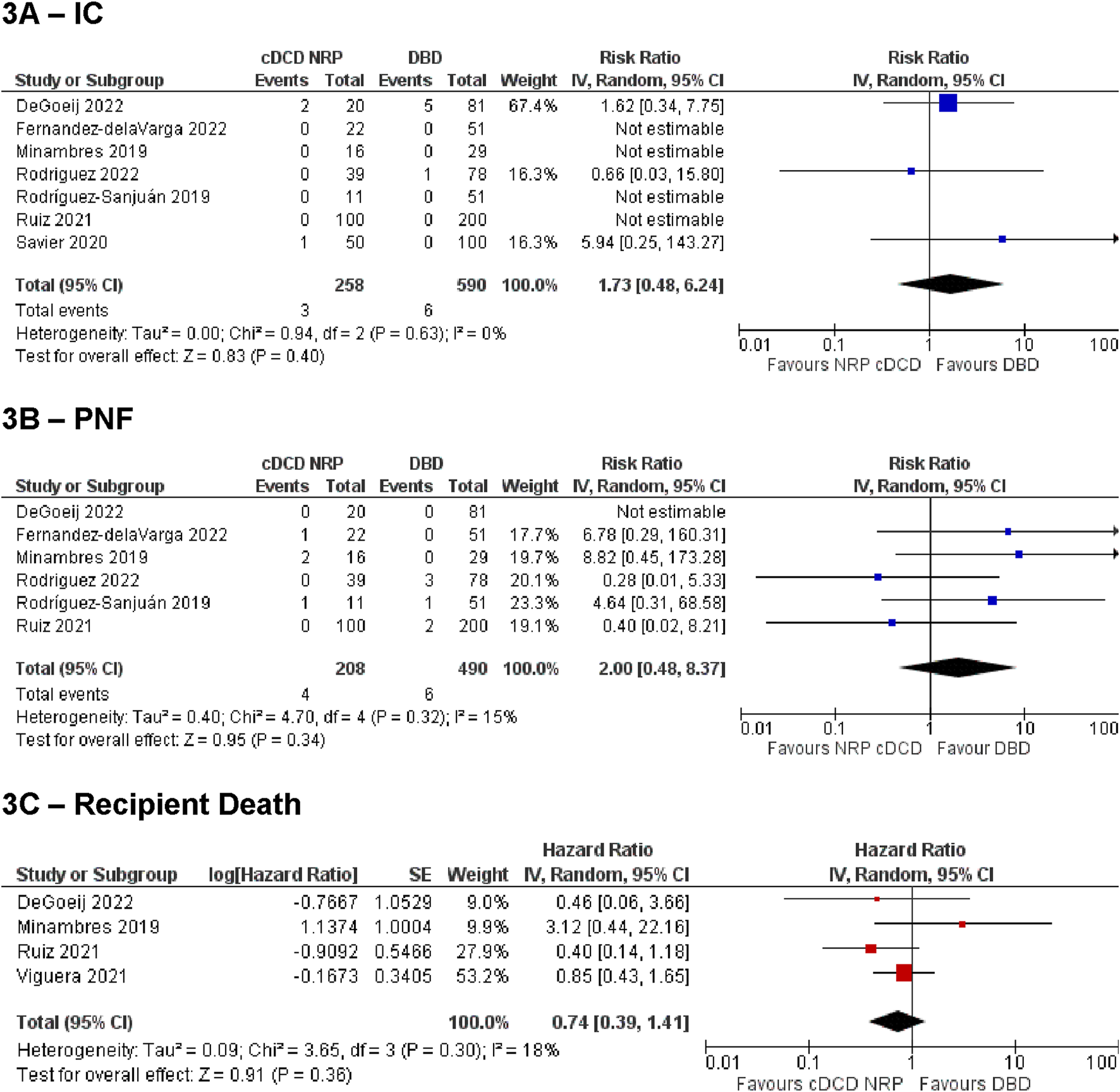
Summary of primary outcomes for cDCD with NRP vs DBD. (A) ischemic cholagniopathy, (B) primary non-function, (C) recipient death.
Statistical analysis of secondary outcomes demonstrated no difference between the two groups for any outcome [graft loss: HR = 0.75, 95% CI (0.47, 1.20), p = 0.23, HAT: RR = 0.64, 95% CI (0.24, 1.73), p = 0.38, EAD: RR = 0.94, 95% CI (0.64, 1.39), p = 0.77, other biliary complications: RR = 0.99, 95% CI (0.64, 1.53), p = 0.96, ICU stay length: MD = −0.03, 95% CI (−0.08, 0.03), p = 0.34, hospital stay length: MD = −0.07, 95% CI (−0.15, 0.02), p = 0.12].
Discussion
The outcomes examined in this systematic review were chosen because of their clinical importance and their past association of these outcomes with DCD liver transplantation. In the comparison of the NRP and non-NRP groups for cDCD livers, NRP is unanimously associated with lower rates of IC, PNF, HAT, and other biliary complications in conjunction with lower rates of recipient death and graft loss. The discard rate in each group was comparable, suggesting that the improved outcomes seen with NRP were not due to selection bias in the discard of organs in the NRP group. The analysis of utilization is potentially confounded by the fact that the comparison is performed after a decision to proceed to donation is already made. NRP utilization is often associated with more liberal organ acceptance criteria in terms of donor age, agonal time, and graft steatosis. Grafts of poorer quality that would not commonly be utilized as part of the non-NRP denominator are compared with some livers that were considered appropriate for procurement only because NRP was available. Hence, NRP is associated with a greater overall utilization, but a similar non-utilization rate from the point of intended recovery. This parameter is not captured in the reported data, but the advantage may be inferred. Ideally, further meta-analysis on donor and recipient factors such as degree of steatosis, MELD scores, BMI, and specific NRP protocols would have been included; however, the included articles did not consistently provide this data, and the articles that did were notably heterogenous with varied graft management options in addition to NRP.
Although the heterogeneity of interventions of the included studies is recognized, we considered that this was outweighed by the benefit of such analysis in a practical sense; cDCD NRP vs. non-NRP liver transplantation studies with strictly no additional perfusion technologies are limited and are unlikely to become available in the future as it would be unethical to withhold treatment from these organs when global standards permit their use and the emerging evidence supports their effectiveness. In the clinical setting, it is also more practical to compare cDCD NRP vs. non-NRP livers that may have been treated with other perfusion technologies as this is more reflective of current practice.
In the comparison of NRP cDCD vs. DBD, NRP cDCD livers perform equally well as DBD livers, exhibiting comparable complication and survival rates. A previous systematic review by De Beule et al. reported an overview of NRP for liver and kidney transplantation, including cDCD transplantation [34]. That review compared outcomes of NRP against SCS for cDCD livers. Although the authors reported lower rates of IC, EAD, biliary strictures (of any type), and anastomotic biliary strictures, there was no difference in PNF, 1 year patient survival or HAT. Additionally, no comparison could be made for cDCD NRP vs. DBD livers at that time. The conclusion made by the authors was that NRP could possibly provide benefits for reducing biliary complications for cDCD donation. In our review, the rate of discard with NRP DCD was comparable to DCD liver programs around the world. Haque et al. describes a 30% discard rate for all DCD liver donation within the US, and Oniscu et al. describes a 29.6% discard rate for non-NRP DCD donation in the UK. [17, 35]. Oniscu et al. did however describe a lower discard rate of 18.3% for NRP DCD donation in the UK. The same study also reported a higher overall utilization rate when using NRP for liver grafts. This was attributed to two main factors; the ability for functional evaluation of organs in situ, and a higher acceptance rate of the initial graft offer when NRP is known to be utilized. This review has focussed on liver transplantation, however, previous analyses have shown improved post-transplant outcomes and organ utilization for other abdominal organs, such as the kidneys, when employing NRP compared to standard DCD techniques [36, 37]. All studies included in our cDCD NRP vs. non-NRP analysis reported liver transplant outcomes only, and no studies were found meeting the inclusion criteria which reported multiple organ donation outcomes. NRP circuits may be configured in a manner that allows simultaneous perfusion to multiple other abdominal and thoracic organs, allowing the potential benefits of NRP to be extended to other transplanted grafts. Further studies looking at the outcomes of multiple grafts from the same NRP donor may be beneficial.
A notable limitation of our study is that all included studies were observational, as no randomised controlled trials satisfied the inclusion criteria. The need for randomised trials to provide high quality evidence of the benefit of NRP has been previously outlined, although conducting such studies is now arguably unethical in the context of the results demonstrated above [38]. Additionally, more than half of the included studies are classified as “poor” according to the Newcastle Ottawa Scale due to the nature of the scoring system of the scale. Any paper that does not score in the comparability domain receives an automatic “poor” designation, although they may score well in all other respects. Importantly, the majority did specify that there was no statistically significant difference between the donor and recipient groups in a variety of metrics, however this demonstration is not considered sufficient to score points for comparability on the Newcastle Ottawa Scale. Another limitation is that although this review examines the use of NRP compared to non-NRP, we were unable to make any direct comparison of NRP vs. SCS, HOPE, or NMP. Hence, the outcome may be slightly confounded by livers receiving a combination of NRP, HOPE, and NMP in addition to NRP. The number of studies currently published is insufficient to facilitate direct comparisons between each technology combination. Ideally, the effect of NRP on recipient outcomes would be isolated from the effects of other ex-vivo perfusion technologies, however this is not currently possible with the available data. The control groups in each comparison (non-NRP cDCD and DBD groups) also contain liver grafts treated with HOPE or NMP in addition to standard SCS. The inclusion of these technologies in the control groups may lead to an underestimate of NRP effect. One included study contained 2 uDCD livers which could not be separated from our cDCD with NRP vs. DBD analysis. The decision was made to include this study even with the increased risk of bias, as the effect of only 2 livers in the sample size was highly unlikely to alter the results in any meaningful way and their inclusion allowed for the inclusion of 49 additional cDCD livers to increase the power of our analysis. As uDCD livers are of poorer quality, the inclusion of these livers would more likely disadvantage the NRP analysis than advantage it, making the positive effect of NRP results even more persuasive.
The most important future analysis should focus on the effect of NRP to increase utilization from the point of organ offer due to the more liberal acceptance criteria (principally on account of acceptance of more advanced donor age, longer agonal times, and higher rates of steatosis). Direct comparisons of NRP with ex-vivo machine perfusion may also be useful. It is certainly possible that some combination of NRP, HOPE, and NMP will provide the optimal combination of maximal utilization and acceptable recipient outcomes, but this will be challenging to investigate robustly on account of the possible number of combinations [13]. It should be noted that a randomised controlled trial examining NMP vs. SCS for liver transplantation demonstrated no change in biliary complication rate, graft survival, or patient survival rates whilst increasing the number of transplantable grafts by 20% [39].
In summary, this review demonstrates that the use of NRP in cDCD liver transplantation is associated with lower rates of many significant post operative complications as well as improved graft and patient survival. NRP cDCD outcomes were comparable to DBD outcomes. The use of NRP appears to also increase the utilization of cDCD livers for transplantation, although non-utilization rates of recovered DCD livers are similar between NRP and standard techniques following donation. NRP has the potential to allow for the expansion of the donor pool and improvement of outcomes so reducing the mortality for those patients needing liver transplantation.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
All authors participated in the review of the manuscript. CM and CF conducted the review, participated in the statistical analysis and wrote the manuscript. AH and HP supervised the project and edited the manuscript. TP conducted the statistical analysis, and JL edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/ti.2024.13263/full#supplementary-material
Abbreviations
cDCD, controlled donation after circulatory death; D-HOPE, dual hypothermic oxygenated machine perfusion; DBD, donation after brain death; DCD, donation after circulatory death; EAD, early allograft dysfunction; HAT, hepatic artery thrombosis; IC, ischemic cholangiopathy; ICU, intensive care unit; NMP, normothermic machine perfusion; NRP, normothermic regional perfusion; PNF, primary non-function; SCS, static cold storage; SRR, super rapid recovery; uDCD, uncontrolled donation after circulatory death; WLST, withdrawal of life sustaining treatment.
References
1.
Melandro F Basta G Torri F Biancofiore G Del Turco S Orlando F et al Normothermic Regional Perfusion in Liver Transplantation from Donation after Cardiocirculatory Death: Technical, Biochemical, and Regulatory Aspects and Review of Literature. Artif Organs (2022) 46(9):1727–40. 10.1111/aor.14330
2.
Detry O Le Dinh H Noterdaeme T De Roover A Honoré P Squifflet JP et al Categories of Donation After Cardiocirculatory Death. Transplant Proc (2012) 44(5):1189–95. 10.1016/j.transproceed.2012.05.001
3.
Park HA-O Jung EA-O Oh JA-O Lee YA-OX Lee JA-O . Organ Donation After Controlled Circulatory Death (Maastricht Classification Iii) Following the Withdrawal of Life-Sustaining Treatment in Korea: A Suggested Guideline. Korean J Transpl (2021) 35:2671–6. 10.4285/kjt.21.0004
4.
O'Neill S Roebuck A Khoo E Wigmore SJ Harrison EM . A Meta-Analysis and Meta-Regression of Outcomes Including Biliary Complications in Donation After Cardiac Death Liver Transplantation. Transpl Int (2014) 27(11):1159–74. 10.1111/tri.12403
5.
Watson CJE MacDonald S Bridgeman C Brais R Upponi SS Foukaneli T et al D-Dimer Release FROM Livers During Ex Situ Normothermic Perfusion and After In Situ Normothermic Regional Perfusion: Evidence for Occult Fibrin Burden Associated WITH Adverse Transplant Outcomes and Cholangiopathy. Transplantation (2023) 107(6):1311–21. 10.1097/TP.0000000000004475
6.
Tchilikidi KY . Liver Graft Preservation Methods During COLD Ischemia Phase and Normothermic Machine Perfusion. World J Gastrointest Surg (2019) 11(3):126–42. 10.4240/wjgs.v11.i3.126
7.
von Horn C Luer B Malkus L Minor T . Comparison Between Terminal or Preterminal Conditioning of Donor Livers by Ex Situ Machine Perfusion. Transplantation (2023) 107(6):1286–90. 10.1097/TP.0000000000004568
8.
Gaurav R Butler AJ Kosmoliaptsis V Mumford L Fear C Swift L et al Liver Transplantation Outcomes FROM Controlled Circulatory Death Donors: Scs vs In Situ Nrp vs Ex Situ Nmp. Ann Surg (2022) 275(6):1156–64. 10.1097/SLA.0000000000005428
9.
Craig EV Heller MT . Complications of Liver Transplant. Abdom Radiol (2021) 46(1):43–67. 10.1007/s00261-019-02340-5
10.
Jay CL Lyuksemburg V Ladner DP Wang E Caicedo JC Holl JL et al Ischemic Cholangiopathy After Controlled Donation After Cardiac Death Liver Transplantation: A Meta-Analysis. Ann Surg (2011) 253(2):259–64. 10.1097/SLA.0b013e318204e658
11.
Hessheimer AJ Cárdenas A García‐Valdecasas JC Fondevila C . Can We Prevent Ischemic‐TYPE Biliary Lesions in Donation After Circulatory Determination of Death Liver Transplantation?Liver Transplant (2016) 22(7):1025–33. 10.1002/lt.24460
12.
Coffey JC Wanis KN Monbaliu D Gilbo N Selzner M Vachharajani N et al The Influence of Functional WARM Ischemia TIME on Dcd Liver Transplant Recipients’ Outcomes. Clin Transplant (2017) 31(10):e13068. 10.1111/ctr.13068
13.
De Carlis R Paolo M Taner B . Donation After Circulatory Death: Novel Strategies to Improve the Liver Transplant Outcome. J Hepatol (2023) 78(6):1169–80. 10.1016/j.jhep.2023.04.008
14.
Schlegel A Mergental H Fondevila C Porte RJ Friend PJ Dutkowski P . Machine Perfusion of the Liver and Bioengineering. J Hepatol (2023) 78(6):1181–98. 10.1016/j.jhep.2023.02.009
15.
Watson C . Uk Protocol for Normothermic Regional Perfusion (Nrp) in Controlled Donation after Circulatory Determination of Death (2021).
16.
Schurink IJ van de Leemkolk FEM Fondevila C De Carlis R Savier E Oniscu GC et al Donor Eligibility Criteria and Liver Graft Acceptance Criteria during Normothermic Regional Perfusion: A Systematic Review. Liver Transplant (2022) 28(10):1563–75. 10.1002/lt.26512
17.
Oniscu GC Mehew J Butler AJ Sutherland A Gaurav R Hogg R et al Improved Organ Utilization and Better Transplant Outcomes WITH In Situ Normothermic Regional Perfusion in Controlled Donation After Circulatory Death. Transplantation (2023) 107(2):438–48. 10.1097/TP.0000000000004280
18.
Page MJ McKenzie JE Bossuyt PM Boutron I Hoffmann TC Mulrow CD et al The Prisma 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Int J Surg (2021) 88:105906. 10.1016/j.ijsu.2021.105906
19.
Lomero M Gardiner D Coll E Haase-Kromwijk B Procaccio F Immer F et al Donation After Circulatory Death Today: An Updated Overview of the European Landscape. Transpl Int (2020) 33(1):76–88. 10.1111/tri.13506
20.
De Goeij FHC Schurink IJ Habets LJM Van De Leemkolk FEM Van Dun CAA Oniscu GC et al Salvage of Declined Extended Criteria Dcd Livers Using Abdominal Normothermic Regional Perfusion (Anrp). Transplantation (2022) 106(8):121. 10.1097/SLA.0000000000005611
21.
Hessheimer AJ de la Rosa G Gastaca M Ruiz P Otero A Gomez M et al Abdominal Normothermic Regional Perfusion in Controlled Donation After Circulatory Determination of Death Liver Transplantation: Outcomes and RISK Factors for Graft LOSS. Am J Transplant : official J Am Soc Transplant Am Soc Transpl Surgeons (2022) 22(4):1169–81. 10.1111/ajt.16899
22.
Mohkam K Nasralla D Mergental H Muller X Butler A Jassem W et al In Situ Normothermic Regional Perfusion Versus Ex Situ Normothermic Machine Perfusion in Liver Transplantation FROM Donation After Circulatory Death. Liver Transplant (2022) 28(11):1716–25. 10.1002/lt.26522
23.
Fernandez-de la Varga M del Pozo-del Valle P Bejar-Serrano S Lopez-Andujar R Berenguer M Prieto M et al Good Post-Transplant Outcomes Using Liver Donors After Circulatory Death WHEN Applying Strict Selection Criteria: A Propensity-Score Matched-Cohort Study. Ann Hepatol (2022) 27(5):100724. 10.1016/j.aohep.2022.100724
24.
Miñambres E Ruiz P Ballesteros MA Álvarez C Cifrián JM Atutxa L et al Combined LUNG and Liver Procurement in Controlled Donation After Circulatory Death Using Normothermic Abdominal Perfusion. Initial Experience in Two Spanish Centers. Am J Transpl (2020) 20(1):231–40. 10.1111/ajt.15520
25.
Rodriguez RP Perez BS Daga JAP Diaz FJL Aguilar JLF Munoz MAS et al Outcome of Liver Transplants Using Donors After Cardiac Death WITH Normothermic Regional Perfusion. Transplant Proc (2022) 54(1):37–40. 10.1016/j.transproceed.2021.10.007
26.
Rodriguez-Sanjuan JC Ruiz N Minambres E Toledo E Gonzalez-Noriega M Fernandez-Santiago R et al Liver Transplant FROM Controlled Cardiac Death Donors Using Normothermic Regional Perfusion: Comparison WITH Liver Transplants FROM Brain DEAD Donors. Transplant Proc (2019) 51(1):12–9. 10.1016/j.transproceed.2018.04.067
27.
Ruiz P Valdivieso A Palomares I Prieto M Ventoso A Salvador P et al Similar Results in Liver Transplantation FROM Controlled Donation After Circulatory Death Donors WITH Normothermic Regional Perfusion and Donation After Brain Death Donors: A Case-Matched Single-Center Study. Liver Transplant (2021) 27(12):1747–57. 10.1002/lt.26281
28.
Savier E Lim C Rayar M Orlando F Boudjema K Mohkam K et al Favorable Outcomes of Liver Transplantation From Controlled Circulatory Death Donors Using Normothermic Regional Perfusion Compared to Brain Death Donors. Transplantation (2020) 104(9):1943–51. 10.1097/TP.0000000000003372
29.
Viguera L Blasi A Reverter E Arjona B Caballero M Chocron I et al Liver Transplant WITH Controlled Donors After Circulatory Death WITH Normothermic Regional Perfusion and Brain DEAD Donors: A Multicenter Cohort Study of Transfusion, One-Year Graft Survival and Mortality. Int J Surg (2021) 96:106169. 10.1016/j.ijsu.2021.106169
30.
Olthoff KM Kulik L Samstein B Kaminski M Abecassis M Emond J et al Validation of a Current Definition of Early Allograft Dysfunction in Liver Transplant Recipients and Analysis of RISK Factors. Liver Transplant (2010) 16(8):943–9. 10.1002/lt.22091
31.
Wan X Wang W Liu J Tong T . Estimating the Sample MEAN and Standard Deviation FROM the Sample SIZE, Median, Range And/or Interquartile Range. BMC Med Res Methodol (2014) 14(1):135. 10.1186/1471-2288-14-135
32.
Cheng J Pullenayegum E Marshall JK Iorio A Thabane L . Impact of Including or Excluding Both-Armed Zero-Event Studies on Using Standard Meta-Analysis Methods for RARE Event Outcome: A Simulation Study. BMJ Open (2016) 6(8):e010983. 10.1136/bmjopen-2015-010983
33.
Nyaga VN Arbyn M Aerts M . Metaprop: A Stata Command to Perform Meta-Analysis of Binomial DATA. Arch Public Health (2014) 72(1):39. 10.1186/2049-3258-72-39
34.
De Beule J Vandendriessche K Pengel LHM Bellini MI Dark JH Hessheimer AJ et al A Systematic Review and Meta-Analyses of Regional Perfusion in Donation After Circulatory Death Solid Organ Transplantation. Transpl Int (2021) 34(11):2046–60. 10.1111/tri.14121
35.
Haque O Yuan Q Uygun K Markmann JF . Evolving Utilization of Donation After Circulatory Death Livers in Liver Transplantation: The Day of Dcd Has COME. Clin Transpl (2021) 35(3):e14211. 10.1111/ctr.14211
36.
De Carlis R Centonze L Migliorini M Pitoni L Cerchione R Lauterio A et al Abdominal Normothermic Regional Perfusion in Donation After Circulatory Death: Organ Viability or Organ Preservation? European. J Transplant (2023) 113–20. 10.57603/EJT-013
37.
Padilla M Coll E Fernández-Pérez C Pont T Ruiz Á Pérez-Redondo M et al Improved Short-Term Outcomes of Kidney Transplants in Controlled Donation After the Circulatory Determination of Death WITH the Use of Normothermic Regional Perfusion. Am J Transplant (2021) 21(11):3618–28. 10.1111/ajt.16622
38.
Weissenbacher A Vrakas G Nasralla D Ceresa CDL . The Future of Organ Perfusion and Re-Conditioning. Transpl Int (2019) 32(6):586–97. 10.1111/tri.13441
39.
Nasralla D Coussios CC Mergental H Akhtar MZ Butler AJ Ceresa CDL et al A Randomized Trial of Normothermic Preservation in Liver Transplantation. Nature (2018) 557(7703):50–6. 10.1038/s41586-018-0047-9
Summary
Keywords
liver transplantation, donation after circulatory death, normothermic regional perfusion, cDCD, NRP
Citation
Mastrovangelis C, Frost C, Hort A, Laurence J, Pang T and Pleass H (2024) Normothermic Regional Perfusion in Controlled Donation After Circulatory Death Liver Transplantation: A Systematic Review and Meta-Analysis. Transpl Int 37:13263. doi: 10.3389/ti.2024.13263
Received
16 May 2024
Accepted
13 August 2024
Published
23 August 2024
Volume
37 - 2024
Updates
Copyright
© 2024 Mastrovangelis, Frost, Hort, Laurence, Pang and Pleass.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carly Mastrovangelis, c.mastrovangelis@outlook.com
†These authors share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.