Abstract
Despite the recognized clinical significance of vitamin D deficiency in other solid organ transplant recipients, its specific relevance in lung transplantation remains to be fully understood. In this study, we performed a retrospective observational study on the impact of vitamin D deficiency on clinical outcomes and prognosis in 125 lung transplant recipients (LTRs) from October 2014 to March 2020 at a university hospital in Seoul, South Korea. Among 125 LTRs, 51 patients (40.8%) were vitamin D deficient. LTRs in the vitamin D-deficient group exhibited a higher incidence of post-transplant pneumonia and overall mortality than those with normal vitamin D levels during the follow-up period. This trend persisted when subjects were stratified into vitamin D tertiles. Furthermore, post-transplant vitamin D levels and C-reactive protein (CRP) significantly impacted pneumonia incidence and survival outcomes. Prognosis also varied based on cumulative vitamin D supplementation after transplantation, with patients receiving higher cumulative supplementation demonstrating improved prognosis. Our findings underscore the importance of assessing and maintaining optimal vitamin D levels post-transplantation, suggesting a potential avenue for improving outcomes in lung transplant recipients, especially in mitigating infection risk and enhancing long-term survival. Further research into optimal vitamin D levels and supplementation strategies in this population is warranted.
Introduction
Beyond calcium homeostasis and bone metabolism, vitamin D deficiency is associated with numerous chronic diseases. Receptors and enzymes involved in vitamin D metabolism are broadly expressed in almost all tissues and cells in vivo, thus mediating various extraskeletal effects [1]. These include immunomodulatory and anti-infective properties, so vitamin D has been linked to major lung diseases and lung transplant status.
Vitamin D deficiency appears to be associated with the prognosis of various respiratory diseases, including chronic obstructive pulmonary disease (COPD) [2–4], bronchial asthma (BA) [5, 6], respiratory infections [7–9], and interstitial lung disease (ILD) [10, 11]. Vitamin D deficiency is frequently observed in solid organ transplant recipients as well. It has been reported to be related with an increased risk of acute rejection and infection, as well as overall survival in liver, kidney, and lung transplant recipients (LTRs) [12–14]. Studies have shown that solid organ transplant recipients who received vitamin D supplementation had a lower incidence of rejection than those who did not [12, 15].
However, high-dose vitamin D supplementation showed no significant difference from the placebo control group in chronic rejection and overall survival in a randomized controlled trial for LTRs [16]. It is unclear whether low vitamin D status in LTRs merely reflects the patient’s severity and poor health condition or is a risk factor independent of morbidity and mortality.
In this context, this study was performed with the aim of elucidating the clinical relevance of post-transplant vitamin D status in lung transplant recipients (LTR), with a specific focus on clinical outcomes and prognosis.
Materials and Methods
Study Participants
The study included adult patients who underwent lung transplantation at a tertiary hospital in Seoul, South Korea, between October 2014 and March 2020. Exclusions comprised cases of retransplantation, multi-organ transplantation, and patients with a survival duration of less than 1 month. Post-transplant vitamin D status was determined based on levels measured 3–9 months after lung transplantation. Thirty-six transplant recipients missing vitamin D level data at this specific point were excluded from the analysis (Figure 1).
FIGURE 1
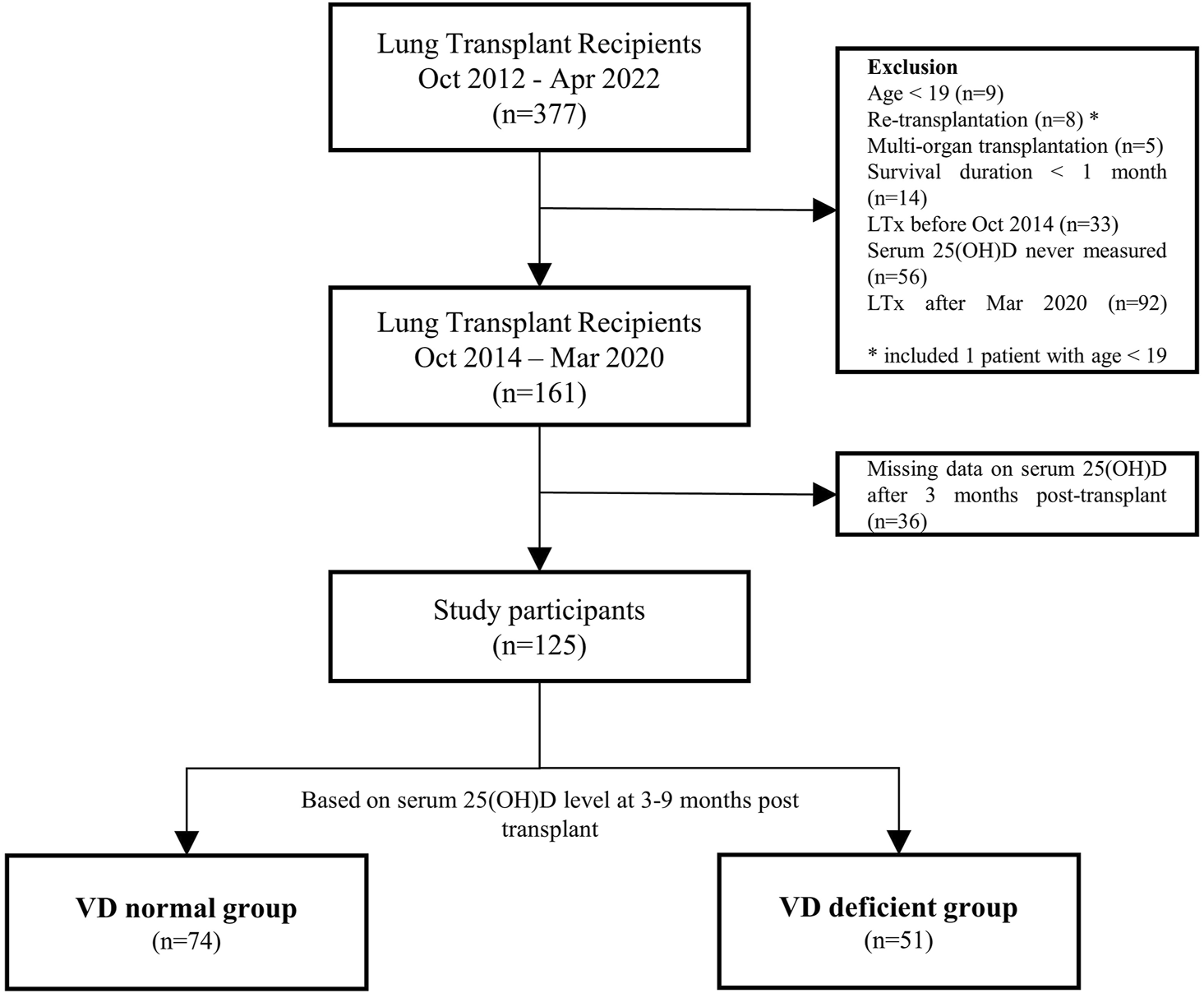
Flowchart of the study participants. LTx, lung transplantation; 25(OH)D, 25-hydroxyvitamin D.
Determination of Vitamin D Status
Vitamin D status was determined by measuring serum 25(OH)D according to the guidelines, and the measurement was performed using the radioimmunoassay instrument in our institution (Dream Gamma-10, Shin Jin Medics Inc., Goyang, South Korea) [17]. In the case of multiple vitamin D values, post-transplant vitamin D was determined as the average. According to the clinical practice guideline of the American Endocrinology Association, patients with serum 25(OH) < 20 ng/mL were assigned as the vitamin D deficient group, and those above that were classified as the vitamin D normal group [17]. Given the lack of an established reference for optimal vitamin D levels in lung transplant recipients, we stratified the study population into tertiles based on their vitamin D levels to examine sequential trends. The cut-off points for the vitamin D tertiles were 18.2 ng/mL and 24.5 ng/mL, with the groups named VD tertile 1, VD tertile 2, and VD tertile 3 in order of increasing levels (Supplementary Figure S1).
This research protocol was approved by the Institutional Review Board of Severance Hospital (IRB number: 4-2020-0228). The appropriate ethics review boards approved the study design, and informed consent was waived.
Data Collection
Clinical and demographic data, such as age, sex, preoperative body weight and body mass index (BMI), pre-transplant diagnosis, and comorbidities, were examined from electronic medical records. Transplant waiting time, preoperative intensive care unit (ICU) admission, ventilator care, and extracorporeal membrane oxygenation (ECMO) care were also checked.
Operative findings and postoperative complications that occurred within 1 month after lung transplantation were also investigated. The diagnosis of acute rejection was determined based on the International Society for Heart and Lung Transplant (ISHLT) standard guidelines [18]. As variables for postoperative complications, we investigated whether complications occurred by major organs after lung transplantation: respiratory complication [pneumonia, primary graft dysfunction (PGD), respiratory failure including re-intubation and tracheostomy], postoperative acute kidney injury (AKI), renal replacement therapy (RRT) use, bacteremia, infection (bacterial, viral or fungal), neurologic complication, cardiovascular complication, and gastrointestinal complication were examined. PGD was graded according to the International Society of Heart and Lung Transplant (ISHLT) Working Group criteria. The proposed standardized definition of PGD was based on diffuse pulmonary edema in an allograft on a chest radiograph and a PaO2/FiO2 (P/F) ratio [19]. Based on the kidney disease: improving global outcomes (KDIGO) guideline, AKI is defined as any of the following: an increase in serum creatinine by ≥ 0.3 mg/dL (≥26.5 μmol/L) within 48 h or an increase in serum creatinine to ≥1.5 times the baseline value, which is known or presumed to have occurred within the prior 7 days; or urine volume <0.5 mL/kg/h for 6 h) [20]. Neurologic, cardiovascular, gastrointestinal, and wound-related complications were defined as cases with appropriate intervention after discussion with the lung transplant team and in collaboration with relevant specialists.
We examined vitamin D supplementation (Cholecalciferol or Calcitriol) pre- and post-transplant, including cumulative dosages. The estimated daily vitamin D supplementation was calculated by dividing the total cumulative supplementation by the follow-up period post-transplantation. To assess prognosis based on vitamin D supplementation, subjects were divided into tertiles of cumulative supplementation. The cut-off points were 864,666.7 IU and 2,731,666.7 IU, labeled VD supplement tertiles 1, 2, and 3 in ascending order. Post-transplant tests were also reviewed, including serum C-reactive protein, pulmonary function tests (PFTs), and 6-minute walk tests (6MWT) measured 3–9 months after lung transplantation. According to relevant guidelines, PFT and 6MWT were performed if the patient’s condition was not limited [21, 22]. Among the study participants, LTRs with a survival period of 1 year or more were investigated for the development of bronchiolitis obliterans syndrome (BOS) based on the ISHLT diagnostic criteria published in 2019 [23].
Colonization by Pseudomonas and Aspergillus was determined based on the presence of these microorganisms in bronchial washing and bronchoalveolar lavage cultures. The cumulative incidence of post-transplant pneumonia episodes was established by identifying cases that fulfilled both criteria: 1) pneumonia detection on Chest computed tomography (CT) scan and 2) intravenous antibiotic administration within 1 week before and after the pneumonia-detected CT scan date.
All LTRs were initially given a triple immunosuppressive therapy that included a calcineurin inhibitor, an antimetabolite or purine synthesis inhibitor, and corticosteroids. Follow-up duration was defined as from the date of lung transplantation to the date of death or the last follow-up. The end date of the survival analysis was 1 September 2021.
Statistical Analysis
Continuous variables were summarized using means or medians, while categorical variables were represented by counts and percentages. The Student’s t-test or Mann-Whitney test was employed for continuous variables, and the Chi-squared test or Fisher’s exact test for categorical variables was used to compare the two groups. The survival was estimated using the Kaplan-Meier method, and the significance of the difference was assessed using a log-rank test. Univariable and multivariable analysis of overall survival was conducted using the Cox proportional hazard model to identify predictors of overall survival. In the multivariate logistic regression model, continuous variables, including age, estimated blood loss, operation time, total hospitalization, 6MWT distance, and FEV1, were categorized by their median values and incorporated into the analysis. Logistic regression analysis was conducted to determine if vitamin D deficiency significantly contributed to the development of pneumonia post-transplantation. P-values less than 0.05 were considered statistically significant. All statistical analyses were performed using R, version 4.1.1 (R Foundation for Statistical Computing).
Results
Among the 125 LTRs, there were 74 patients (59.2%) in the post-transplant vitamin D normal group (VD normal group) and 51 patients (40.8%) in the vitamin D deficient group (VD deficient group). The VD deficient group exhibited an older average age, a higher rate of male sex, and a higher prevalence of pre-transplant cardiovascular disease compared to the VD normal group. Operative findings revealed a higher proportion of recipients with pleural adhesion in the VD deficient group compared to the VD normal group, along with an increased estimated blood loss during lung transplantation. There was no statistically significant difference in the incidence of postoperative complications between the two groups. Total hospitalization periods for lung transplantation were more extended in the VD deficient group than in the VD normal group (Table 1).
TABLE 1
| VD normal group | VD deficient group | p-value | |
|---|---|---|---|
| (N = 74) | (N = 51) | ||
| Age | 52.6 ± 12.2 | 57.6 ± 11.4 | 0.021 |
| Male sex, n (%) | 42 (56.8%) | 43 (84.3%) | 0.002 |
| Body weight (kg) | 57.0 ± 11.2 | 59.4 ± 10.5 | 0.234 |
| BMI (kg/m2) | 21.2 ± 3.8 | 21.4 ± 3.5 | 0.697 |
| Pre-transplant diagnosis, n (%) | 0.443 | ||
| COPD and emphysema | 5 (6.8%) | 4 (7.8%) | |
| ILD | 52 (70.3%) | 40 (78.4%) | |
| Bronchiectasis | 9 (12.2%) | 2 (3.9%) | |
| Others | 8 (10.8%) | 5 (9.8%) | |
| Comorbidities, n (%) | |||
| DM | 10 (14.1%) | 13 (26.0%) | 0.159 |
| HTN | 8 (11.3%) | 8 (16.0%) | 0.628 |
| CV | 10 (14.1%) | 20 (40.0%) | 0.002 |
| CKD | 7 (9.9%) | 6 (12.0%) | 0.939 |
| Tuberculosis | 25 (34.7%) | 14 (28.0%) | 0.558 |
| Transplant waiting time (days) | 121.0 [41.0; 231.0] | 103.0 [44.5; 249.5] | 0.890 |
| Preoperative status, n (%) | |||
| Preop ICU admission | 29 (39.2%) | 23 (45.1%) | 0.635 |
| Preop ventilator care | 26 (35.1%) | 23 (45.1%) | 0.350 |
| Preop ECMO care | 22 (29.7%) | 20 (39.2%) | 0.362 |
| Operative findings, n (%) | |||
| Intraoperative ECMO weaning | 43 (65.2%) | 36 (70.6%) | 0.672 |
| Transplantation Type, Double | 66 (95.7%) | 50 (100.0%) | 0.368 |
| Size mismatch, Bronchus, or PA | 37 (56.1%) | 29 (59.2%) | 0.885 |
| Status of pleura, Adhesion | 39 (55.7%) | 37 (74.0%) | 0.063 |
| Estimated blood loss (mL) | 1800.0 [1050.0; 3000.0] | 2300.0 [1600.0; 3600.0] | 0.036 |
| ECMO time (min) | 300.0 [280.0; 360.0] | 300.0 [248.0; 390.0] | 0.825 |
| Operation time (min) | 380.9 ± 79.6 | 407.2 ± 71.5 | 0.090 |
| Anesthesia time (min) | 479.0 ± 84.8 | 496.8 ± 70.9 | 0.280 |
| Postop complications, n (%) | |||
| Acute rejection | 2 (2.7%) | 0 (0.0%) | 0.647 |
| Respiratorya | 30 (42.9%) | 28 (54.9%) | 0.260 |
| BPF | 2 (2.7%) | 3 (5.9%) | 0.669 |
| Pneumothorax, pleural effusion | 9 (12.3%) | 7 (13.7%) | 1.000 |
| Bronchial stenosis | 7 (9.5%) | 5 (9.8%) | 1.000 |
| PA stenosis | 3 (4.1%) | 3 (5.9%) | 0.965 |
| Postop AKI | 4 (5.6%) | 8 (15.7%) | 0.126 |
| Postop RRT use | 5 (6.8%) | 4 (7.8%) | 1.000 |
| Bacteremia | 1 (1.4%) | 3 (5.9%) | 0.393 |
| Infection | 5 (7.0%) | 7 (13.7%) | 0.360 |
| Neurologic | 2 (2.8%) | 2 (3.9%) | 1.000 |
| Cardiovascular | 2 (2.9%) | 3 (5.9%) | 0.717 |
| Gastrointestinal | 8 (11.1%) | 7 (13.7%) | 0.875 |
| Postop ICU stay (days) | 7.0 [5.0; 13.0] | 7.0 [4.5; 13.0] | 0.670 |
| Total hospitalization (days) | 53.5 [31.5; 91.0] | 86.0 [40.0; 136.0] | 0.011 |
Basic characteristics of lung transplant recipients according to vitamin D status.
Values are displayed as median (interquartile range), n (%), or mean ± standard error of the mean where appropriate. BMI, body mass index; COPD, chronic obstructive pulmonary disease; ILD, interstitial lung disease; DM, diabetes mellitus; HTN, hypertension; CV, cardiovascular; CKD, chronic kidney disease; ICU, intensive care unit; ECMO, extracorporeal membrane oxygenation; BPF, bronchopleural fistula; PA, pulmonary artery; AKI, acute kidney injury; RRT, renal replacement therapy.
Respiratory complications: pneumonia, primary graft dysfunction (PGD), respiratory failure including re-intubation and tracheostomy.
The VD normal group had higher post-transplant vitamin D supplementation rates and a greater cumulative dose of vitamin D supplementation. Post-transplant, the VD deficient group showed significantly higher CRP levels and a shorter 6MWT distance than the VD normal group (Table 2).
TABLE 2
| VD normal group | VD deficient group | p-value | |
|---|---|---|---|
| (N = 74) | (N = 51) | ||
| Post-transplant 25(OH)D (ng/mL) | 26.5 ± 5.1 | 14.7 ± 3.5 | <0.001 |
| Post-transplant 25(OH)D (ng/mL) | 25.5 [22.3; 29.3] | 15.4 [12.1; 17.8] | <0.001 |
| Number of 25(OH)D measurements | 2.7 ± 1.1 | 2.3 ± 0.8 | 0.023 |
| Delta 25(OH)Da | 7.3 ± 7.1 | 0.5 ± 6.9 | <0.001 |
| Delta 25(OH)Da | 7.6 [2.6; 11.8] | 0.4 [−3.4; 6.4] | 0.001 |
| Preop VD supplementationb, n (%) | 67 (90.5%) | 40 (78.4%) | 0.102 |
| Preop VD cumulative dose (IU) | 437,800 [181,000; 688,600] | 183,000 [94,000; 458,400] | 0.008 |
| Postop VD supplementationb, n (%) | 73 (98.6%) | 44 (86.3%) | 0.016 |
| Postop VD cumulative dose (IU) | 2,713,200 [975,000; 3,766,000] | 760,800 [212,500; 1,854,500] | <0.001 |
| Post-transplant tests | |||
| CRP (mg/L) | 7.9 ± 14.3 | 22.5 ± 33.2 | 0.004 |
| CRP (mg/L) | 1.9 [0.7; 7.8] | 6.7 [1.4; 33.8] | 0.003 |
| FEV1, predicted % | 69.9 ± 20.0 | 68.4 ± 15.9 | 0.690 |
| FEV1, liter | 2.0 ± 0.7 | 2.0 ± 0.5 | 0.862 |
| FVC, predicted % | 62.9 ± 16.0 | 59.2 ± 16.0 | 0.262 |
| FVC, liter | 2.4 ± 0.8 | 2.4 ± 0.7 | 0.953 |
| DLCO, predicted % | 66.0 ± 21.4 | 63.0 ± 18.1 | 0.526 |
| 6MWT distance (m) | 384.5 ± 129.0 | 320.0 ± 149.0 | 0.027 |
Vitamin D measurements, supplementation and post-transplant test results according to vitamin D status.
Values are displayed as median (interquartile range), n (%), or mean ± standard error of the mean where appropriate. CRP, C-reactive protein; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; DLCO, diffusing capacity of the lungs for carbon monoxide; 6MWT, 6-minute walking test.
Delta 25(OH)D = post-transplant 25(OH)D - pre-transplant 25(OH)D.
VD supplementation: Cholecalciferol or Calcitriol.
The average follow-up period for the study participants was 35 months. During the follow-up period, the two groups had no statistically significant differences in the incidence of BOS, Pseudomonas, and Aspergillus colonization. However, the VD deficient group exhibited significantly higher rates of post-transplant pneumonia and a greater cumulative number of post-transplant pneumonia. The VD deficient group experienced a higher overall mortality rate during the follow-up duration compared to the VD normal group (20.3% vs. 51.0%, p =0.001), with infection identified as the primary cause of death in both groups (Table 3).
TABLE 3
| VD normal group | VD deficient group | p-value | |
|---|---|---|---|
| (N = 74) | (N = 51) | ||
| Follow-up duration, months | 46.1 ± 26.0 | 33.4 ± 22.9 | 0.006 |
| Follow-up duration, months | 41.5 [28.0; 68.0] | 32.0 [13.0; 42.5] | 0.008 |
| BOSa, n (%) | 18 (26.1%) | 8 (17.0%) | 0.356 |
| Pseudomonas colonization, n (%) | 20 (27.0%) | 14 (27.5%) | 1.000 |
| Aspergillus colonization, n (%) | 13 (17.6%) | 13 (25.5%) | 0.396 |
| Post-transplant pneumonia, n (%) | 31 (41.9%) | 37 (72.5%) | 0.001 |
| Cumulative episodes of post-transplant pneumonia | 0.0 [0.0; 2.0] | 1.0 [0.0; 3.5] | 0.001 |
| Cumulative episodes of post-transplant pneumonia | 1.1 ± 1.9 | 2.1 ± 2.6 | 0.014 |
| Overall mortality, n (%) | 15 (20.3%) | 26 (51.0%) | 0.001 |
| Cause of death, n (%) | 0.750 | ||
| Sepsis/Infection | 10 (66.7%) | 17 (65.4%) | |
| Neurologic | 0 (0.0%) | 1 (3.8%) | |
| Hematologic | 1 (6.7%) | 2 (7.7%) | |
| Cardiac | 1 (6.7%) | 1 (3.8%) | |
| GI | 1 (6.7%) | 0 (0.0%) | |
| Miscellaneous | 2 (13.3%) | 5 (19.2%) |
Overall mortality rate and incidence of infection/rejection of lung transplant recipients according to vitamin D status.
Values are displayed as median (interquartile range), n (%), or mean ± standard error of the mean where appropriate. BOS, bronchiolitis obliterans syndrome; GI, gastrointestinal.
Investigated among patients with a survival period of more than 1 year.
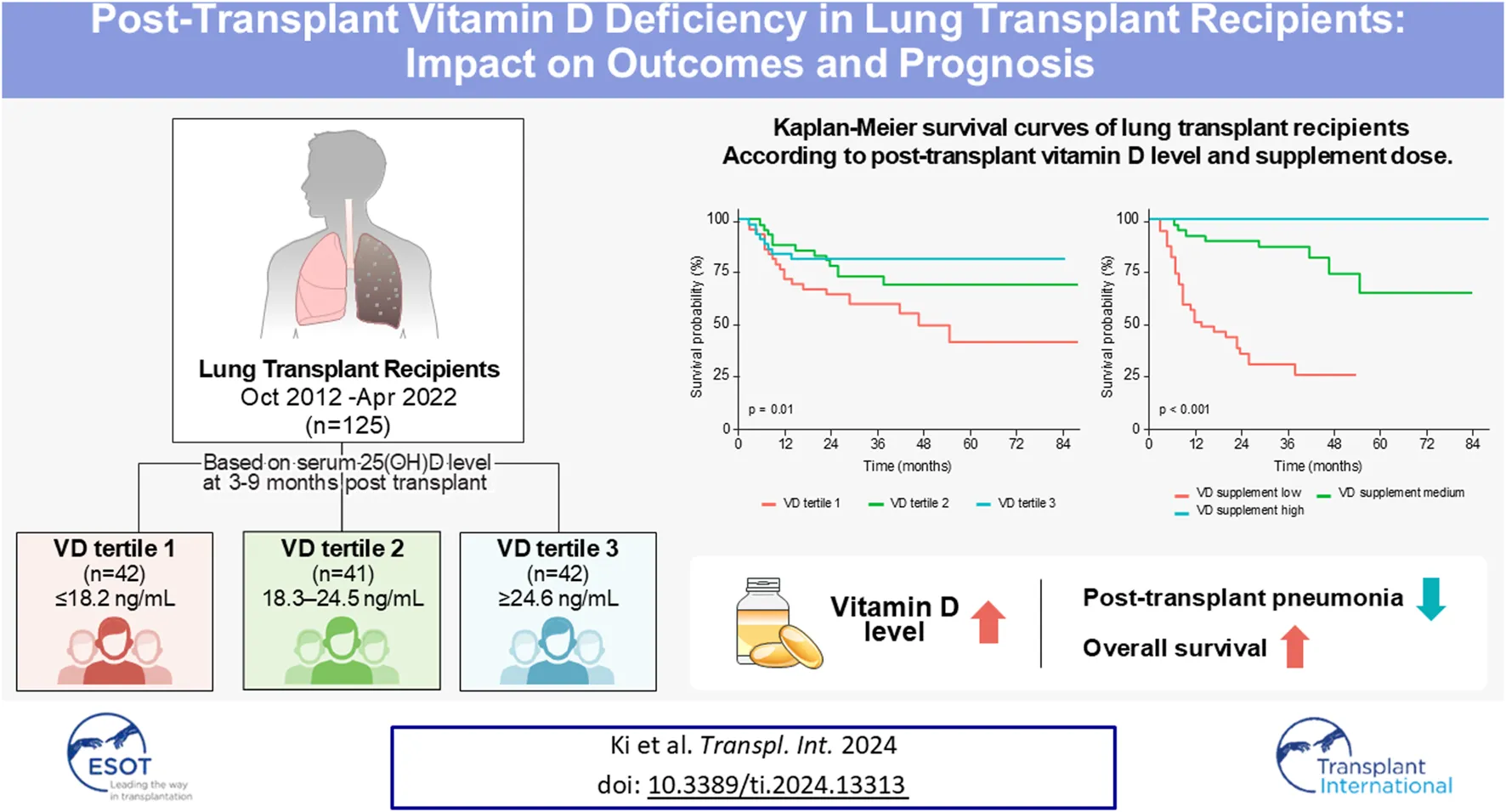
In the survival analysis, the VD deficient group showed a lower survival rate than the VD normal group (log-rank test, p < 0.001, Figure 2A). The univariate and multivariate Cox proportional hazard analyses were conducted, including post-transplant vitamin D status and covariates that showed statistically significant differences in the two groups. Variables with significant missing values (e.g., post-transplant FEV1, post-transplant 6MWT; 20 or more missing) or notable correlations (e.g., total hospitalization, estimated blood loss) were selected for inclusion in the Cox proportional hazards regression model. Following the multivariate analysis, post-transplant VD deficiency [adjusted hazard ratio (aHR) 2.22, 95% confidential interval (CI) 1.05–4.69, p = 0.036] and higher CRP level (aHR 9.38, 95% CI 3.61–24.4, p < 0.001) emerged as factors significantly related with the prognosis of lung transplant recipients (Table 4).
FIGURE 2
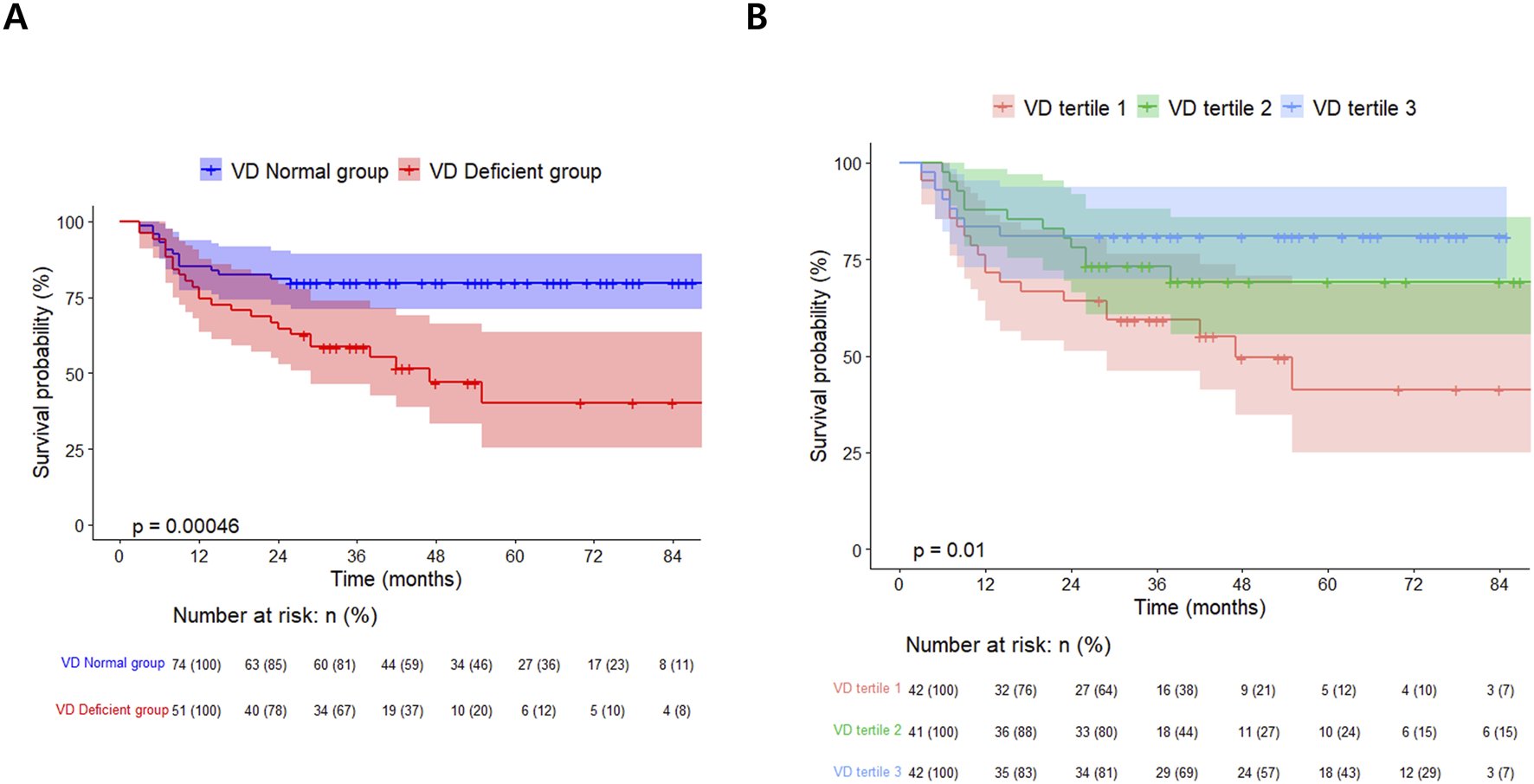
Kaplan-Meier survival curves for lung transplant recipients: (A) by post-transplant vitamin D status and (B) stratified by Vitamin D tertiles.
TABLE 4
| Variables | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | aHR | 95% CI | p-value | |
| Age ≥ 58 (vs. < 58) | 2.5 | 1.29–4.85 | 0.007 | 2.33 | 1.13–4.82 | 0.022 |
| Female (vs. male) | 1.09 | 0.57–2.08 | 0.793 | 1.96 | 0.89–4.30 | 0.094 |
| BMI (kg/m2) | 1.01 | 0.93–1.10 | 0.784 | |||
| Cardiovascular disease: Presence (vs. Absence) | 1.09 | 0.53–2.23 | 0.823 | |||
| Status of pleura: Adhesion (vs. Normal) | 1.19 | 0.62–2.29 | 0.597 | |||
| Estimated Blood loss (mL) ≥ 2,000 (vs. < 2,000) | 3.58 | 1.78–7.18 | <0.001 | 1 | 1.00–1.00 | 0.002 |
| Operation time (min) ≥ 377 (vs. < 377) | 1.97 | 0.93–4.16 | 0.077 | |||
| Total Hospitalization (days) ≥ 61 (vs. < 61) | 4.05 | 1.95–8.38 | <0.001 | |||
| Post-transplant VD: Deficient (vs. Normal) | 2.96 | 1.56–5.62 | 0.001 | 2.22 | 1.05–4.69 | 0.036 |
| CRP (mg/L) > 3.1 (vs. < 3.1) | 1.06 | 1.04–1.07 | <0.001 | 9.38 | 3.61–24.4 | <0.001 |
| 6MWT distance (m) < 375 (vs. ≥ 375) | 3.77 | 1.35–10.53 | 0.011 | |||
| FEV1, predicted (%) < 70 (vs. ≥ 70) | 3.21 | 1.26–8.22 | 0.015 | |||
| Post-transplant pneumonia: Presence (vs. Absence) | 1.9 | 0.98–3.67 | 0.057 | 0.64 | 0.29–1.42 | 0.273 |
| Cumulative episodes of post-transplant pneumonia | 1.09 | 0.98–1.20 | 0.108 | |||
Cox proportional hazard analysis for lung transplant recipients’ survival.
HR, hazard ratio; aHR, adjusted hazard ratio; CI, confidence interval; BMI, body mass index; CRP, C-reactive protein; 6MWT, 6-minute walk test; FEV1, forced expiratory volume in 1 s.
Comparisons of the Vitamin D Tertiles
The results of the comparison divided into vitamin D level tertiles also showed that the lower the vitamin D level, the higher the age, the higher the male ratio, and the higher the rate of cardiovascular disease. Otherwise, there were no significant differences between vitamin D tertiles in the remaining baseline characteristics (Supplementary Table S1). The differences in vitamin D supplementation and post-transplant test results among the vitamin D tertiles mirrored those observed in the VD deficient/normal group. The estimated daily vitamin D supplementation doses for tertiles 1, 2, and 3 were approximately 871 IU, 1685 IU, and 1884 IU, respectively (Supplementary Table S2).
In the vitamin D tertiles, lower vitamin D levels were linked to a higher incidence of post-transplant pneumonia over a shorter follow-up period. Additionally, a significant difference in the overall mortality rate was observed, demonstrating a sequential trend related to vitamin D levels [VD tertile 1: 21/42 (50.0%), VD tertile 2: 12/41 (29.3%), VD tertile 3: 8/42 (19.0%); p = 0.009, Supplementary Table S3]. Among vitamin D tertiles 1, 2, and 3, a poorer survival curve was observed at lower vitamin D levels (log-rank test, p = 0.01, Figure 2B). Multivariate Cox proportional hazards analysis using vitamin D tertiles indicated that VD tertile 1 demonstrated a marginally significant hazard ratio in comparison to VD tertile 3 (aHR 2.45, 95% CI 0.92–6.54, p = 0.074, Supplementary Table S4). Logistic regression analysis of post-transplant pneumonia occurrence indicated that lower post-transplant vitamin D levels and higher post-transplant CRP levels were significant covariates (Supplementary Table S5; Figure 3).
FIGURE 3
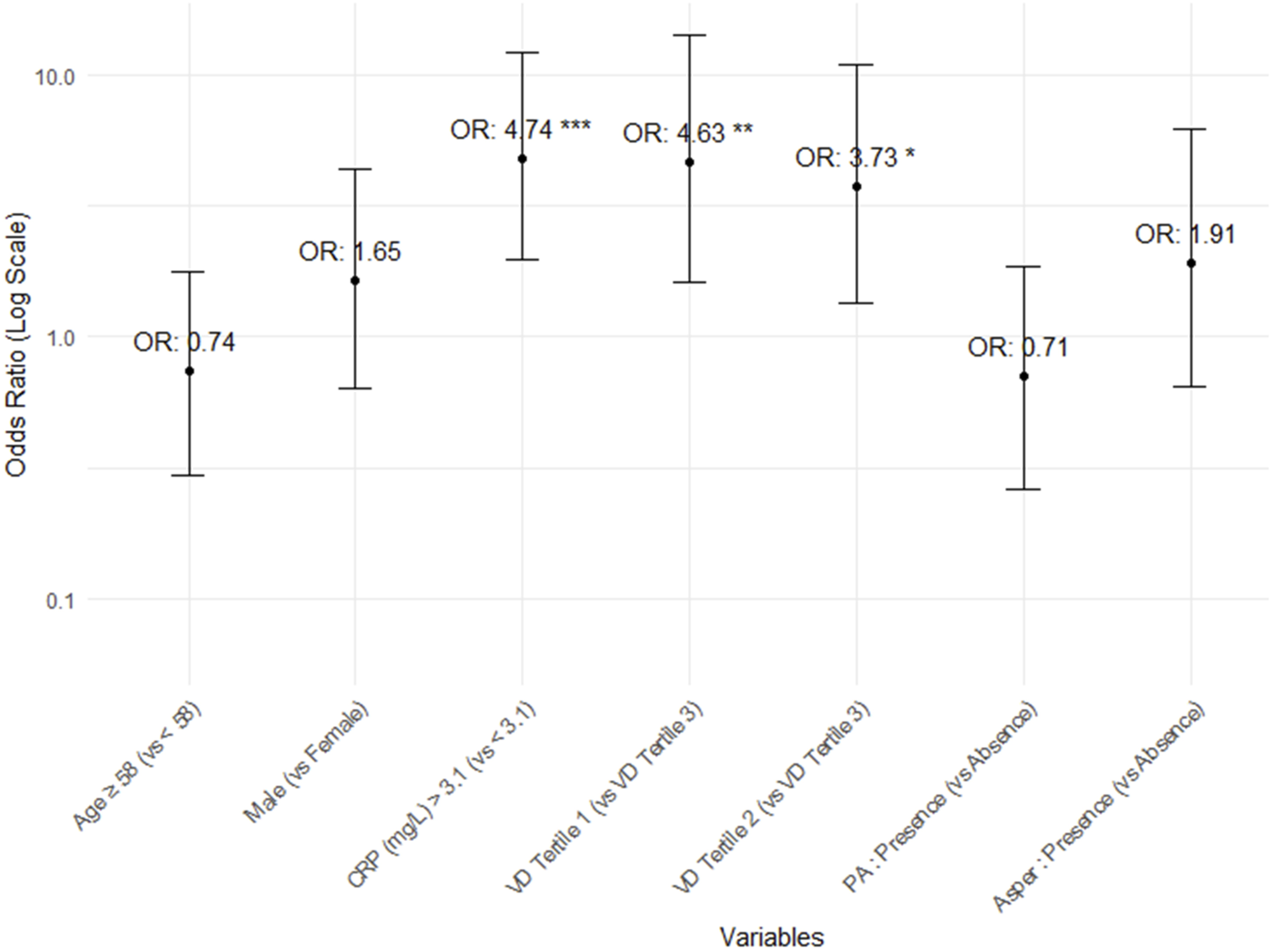
Odds ratios and 95% confidence intervals for the occurrence of post-transplant pneumonia.
Comparisons of the Vitamin D Supplement Tertiles
The 125 lung transplant recipients were categorized into three tertiles of cumulative vitamin D supplementation after lung transplantation (VD supplement tertile 1, VD supplement tertile 2, and VD supplement tertile 3), with higher cumulative doses resulting in greater vitamin D levels. These tertiles’ estimated daily supplementation doses were 747 IU, 1735 IU, and 1934 IU, respectively (Table 5). The group receiving higher vitamin D supplementation demonstrated improved post-transplant lung function, a reduced incidence of pneumonia, and lower mortality rates. However, the frequency of BOS exhibited an opposite trend (Table 5). The survival curves of lung transplant recipients (LTRs) who received less vitamin D supplementation were inferior to those of LTRs who received higher doses of vitamin D (log-rank test, p < 0.001,Figure 4).
TABLE 5
| VD supplement tertile 1 | VD supplement tertile 2 | VD supplement tertile 3 | p-value | |
|---|---|---|---|---|
| (N = 39) | (N = 39) | (N = 39) | ||
| Post-transplant 25(OH)D (ng/mL) | 18.9 [15.8; 22.6] | 21.3 [17.1; 26.6] | 25.5 [21.7; 29.0] | <0.001 |
| Number of 25(OH)D measurements | 2.3 ± 1.0 | 2.6 ± 1.0 | 2.8 ± 1.0 | 0.101 |
| Postop VD cumulative dose (IU) | 269,000 [181,500; 639,000] | 1,820,000 [1,394,000; 2,369,200] | 3,821,000 [3,179,500; 4,599,600] | <0.001 |
| Estimated daily VD supplement dose (IU) | 747.2 [251.6; 982.3] | 1,734.5 [1,160.5; 2,120.9] | 1,933.7 [1,565.1; 2,643.2] | <0.001 |
| Post-transplant tests | ||||
| CRP (mg/L) | 14.2 [4.3; 36.2] | 2.4 [1.2; 7.3] | 1.2 [0.5; 3.8] | <0.001 |
| FEV1, predicted % | 63.2 ± 19.4 | 64.5 ± 16.9 | 76.3 ± 18.1 | 0.006 |
| FEV1, liter | 1.8 ± 0.6 | 1.9 ± 0.7 | 2.1 ± 0.7 | 0.119 |
| FVC, predicted % | 56.0 ± 18.2 | 56.4 ± 15.0 | 67.8 ± 13.2 | 0.002 |
| FVC, liter | 2.2 ± 0.8 | 2.2 ± 0.8 | 2.6 ± 0.7 | 0.075 |
| DLCO, predicted % | 61.5 ± 18.0 | 54.5 ± 20.9 | 73.0 ± 18.9 | 0.001 |
| 6MWT distance (m) | 327.4 ± 162.8 | 344.9 ± 139.5 | 386.7 ± 129.6 | 0.238 |
| Follow up duration, months | 14.0 [7.5; 33.0] | 35.0 [29.0; 47.5] | 68.0 [54.0; 81.5] | <0.001 |
| BOS, n (%)a | 5 (13.9%) | 5 (13.5%) | 16 (44.4%) | 0.002 |
| Pseudomonas colonization, n (%) | 12 (30.8%) | 10 (25.6%) | 11 (28.2%) | 0.881 |
| Aspergillus colonization, n (%) | 12 (30.8%) | 6 (15.4%) | 7 (17.9%) | 0.207 |
| Post-transplant pneumonia, n (%) | 28 (71.8%) | 20 (51.3%) | 16 (41.0%) | 0.021 |
| Cumulative episodes of post-transplant pneumonia | 2.0 [0.0; 2.0] | 1.0 [0.0; 2.0] | 0.0 [0.0; 1.0] | 0.040 |
| Cumulative episodes of post-transplant pneumonia | 1.8 ± 2.1 | 1.8 ± 2.8 | 1.1 ± 2.1 | 0.352 |
| 1-year mortality, n (%) | 17 (43.6%) | 3 (7.7%) | 0 (0.0%) | <0.001 |
| 3-year mortality, n (%) | 27 (69.2%) | 5 (12.8%) | 0 (0.0%) | <0.001 |
| Overall mortality, n (%) | 28 (71.8%) | 8 (20.5%) | 1 (2.6%) | <0.001 |
Clinical outcomes of lung transplant recipients by vitamin D supplementation tertiles.
Cut-off points for Vitamin D supplementation were 864,666.7 IU and 2,731,666.7 IU, creating VD supplement tertiles 1 (≤864,666.7 IU), 2 (864,666.8–2,731,666.7 IU), and 3 (≥2,731,666.8 IU). Estimated daily supplementation doses were 747 IU, 1,735 IU, and 1,934 IU for tertiles 1, 2, and 3, respectively.
Values are displayed as median (interquartile range), n (%), or mean ± standard error of the mean where appropriate. CRP, C-reactive protein; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; DLCO, diffusing capacity of the lungs for carbon monoxide; 6MWT, 6-minute walking test; 25(OH)D, 25-hydroxyvitamin D; IU, international unit; BOS, bronchiolitis obliterans syndrome; GI, gastrointestinal.
Investigated among patients with a survival period of more than 1 year.
FIGURE 4
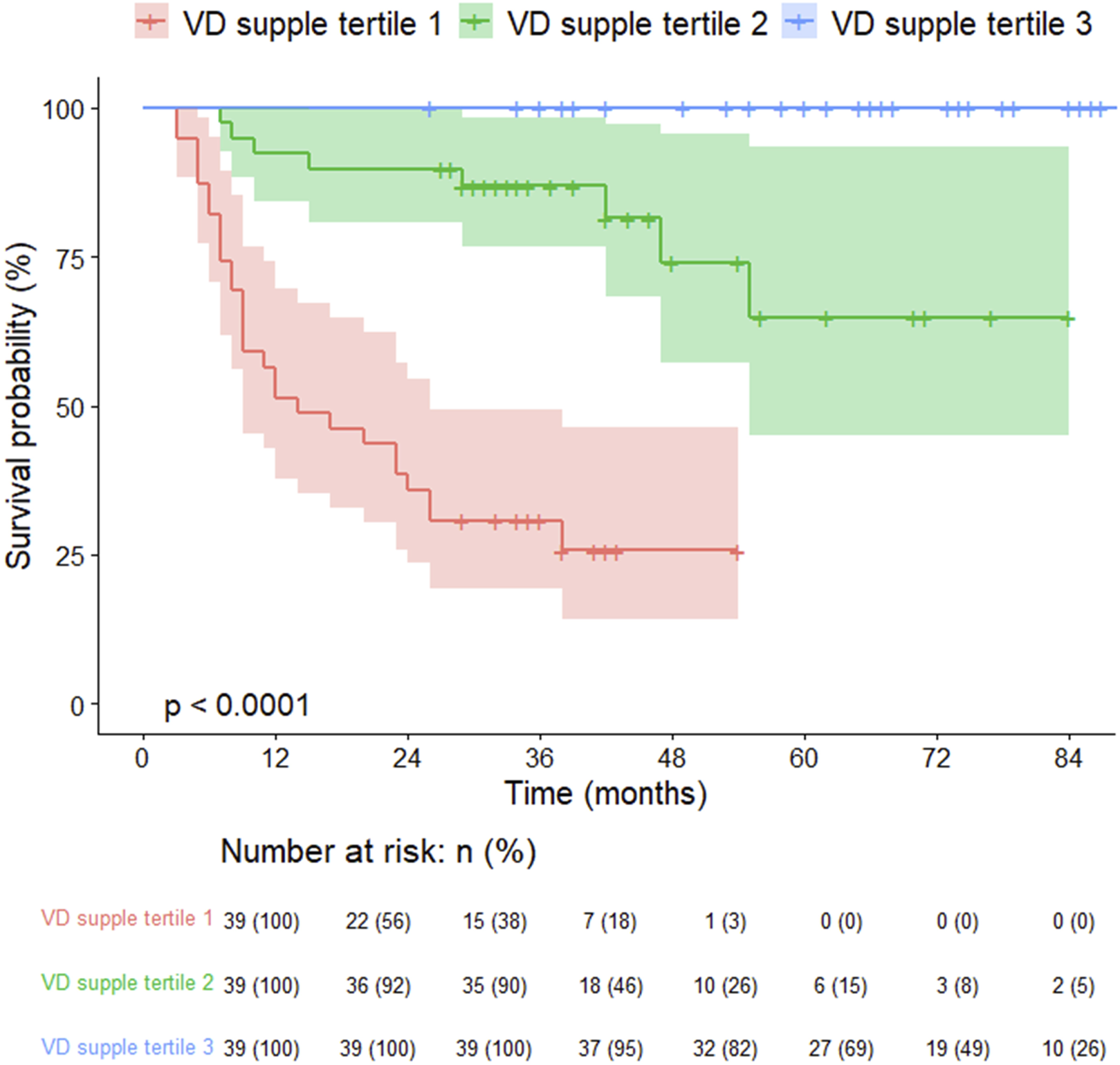
Kaplan-Meier survival curves for lung transplant recipients by vitamin D supplementation tertiles.
Discussion
In this study, deficiencies in vitamin D levels were observed in a significant number of lung transplant recipients despite high rates of vitamin D supplementation, with differences in frequency of post-transplant pneumonia, overall mortality and survival rates based on post-transplant vitamin D status. Stratifying subjects into vitamin D tertiles revealed a sequential trend in outcomes based on vitamin D levels. Post-transplant vitamin D levels and CRP significantly influenced pneumonia incidence and survival. Additionally, the prognosis varied with the cumulative vitamin D supplementation after transplantation.
Vitamin D is a fat-soluble vitamin absorbed by the body through food (20%) or synthesized in the skin (80%) from 7-dihydrocholesterol after ultraviolet B-ray exposure [24]. Vitamin D from food and skin is hydroxylated in the liver and converted to 25-hydroxyvitamin D [25(OH)D], which has a long half-life and is used to measure and evaluate the vitamin D status of patients. 25(OH)D is metabolized once more in the kidneys to the fully active form of 1,25-dihydroxyvitamin D [1,25(OH)D], which is closely controlled by blood parathyroid hormone and calcium/phosphate levels [25, 26]. Physiologically activated vitamin D mediates various physiological functions by acting on bone, immune cells, and target cells of various organs [27].
Low vitamin D levels have been observed in various disease groups, including end-stage lung diseases and LTRs. Vitamin D deficiency has been reported in 20%–50% of patients with advanced lung disease and in up to two-thirds of patients waiting for lung transplantation [28–31]. In these individuals, inadequate vitamin D levels are associated with lower fat mass, obstructive pulmonary disease, insufficient dietary vitamin D intake, and limited sunlight exposure and have been investigated as predictors of reduced walking distance [31, 32]. Poor health conditions after transplantation, changes in vitamin D metabolism due to glucocorticoid use, and limited sun exposure due to increased risk of skin cancer may further lower vitamin D levels in lung transplant recipients [33, 34].
This study identified post-transplant vitamin D status as an independent variable related to survival. Studies of liver and kidney transplant recipients have also identified post-transplant vitamin D status as an independent factor associated with prognosis [12, 35]. Vitamin D levels after solid organ transplantation appear to reflect the patient’s clinical course, and more needs to be discovered to determine its relationship to prognosis.
In this study, the LTRs with post-transplant vitamin D deficiency had poorer baseline characteristics, including older age, comorbidities such as cardiovascular disease, and surgical findings like pleural adhesion, longer operation times, and extended hospitalization periods. Additionally, they experienced more pneumonia episodes during follow-up.
Lung transplant prognosis is influenced by pre-transplant characteristics and intraoperative factors, with their impact evolving during the post-transplant period [36]. The key prognostic factors include: recipient factors such as age, sex, BMI, pre-transplant diagnosis, ECMO or ventilator use, hospitalization, pulmonary hypertension, and malnutrition; donor factors, including donor age and donor-recipient weight/height mismatch; procedural factors such as ischemic time, severe bleeding, and pleural adhesions; and post-transplant factors, including ECMO requirement, infection, PGD, BOS/chronic lung allograft dysfunction (CLAD), and immunosuppression levels [37]. Previous studies have also shown that prolonged ischemic time, massive bleeding due to pleural adhesions, and other factors are associated with poor prognosis in lung transplant patients [37]. Despite these factors indicating potentially poorer functional status and lower survival rates, low vitamin D status emerged as a significant prognostic factor alongside CRP in multivariate analysis, highlighting its independent impact on outcomes.
In this study, the group with higher vitamin D levels exhibited a greater frequency of BOS, though this was not statistically significant. Additionally, a higher frequency of BOS was observed in those receiving greater cumulative supplementation doses. This trend may be attributed to the longer follow-up period, which could lead to increased BOS diagnoses among patients with higher vitamin D levels and supplementation. Our study had a median follow-up period of 35 months, and according to the ISHLT report, about 65% of transplant recipients did not develop BOS at this time [38]. Considering the complex mechanism and diagnostic process of BOS [23, 39], it seems necessary to figure out the link between vitamin D status and the development of BOS through a sufficiently extended follow-up period.
Similar to the study by Lowery et al., the LTRs with low vitamin D had more episodes of pneumonia after lung transplantation in this study [14]. Considering that the majority of patients who died in this study were due to infection, frequent cases of infection may have contributed to the poor prognosis of the LTRs. Infection is the most common cause of death within the first year after lung transplantation and the second most common cause of death between one and 5 years after transplantation [40].
The association between vitamin D levels and prognosis within 5 years after lung transplantation can primarily be attributed to vitamin D’s protective effects against infections. The activated form of vitamin D, 1,25(OH)D, produced by CYP27B1 (the 25-hydroxyvitamin D 1α-hydroxylase), in various peripheral tissues, initiates signaling pathways that regulate both innate and adaptive immune responses [41]. This signaling enhances the expression of genes crucial for innate immune defense, including those coding for cytokines, chemokines, antimicrobial peptides, and pattern recognition receptors [41]. Additionally, 1,25(OH)D promotes bacterial killing and viral clearance through autophagy, playing a vital role in human defense mechanisms beyond skeletal health [41].
Epidemiological studies and randomized controlled trials have highlighted vitamin D’s protective effects against respiratory infections [42, 43], which may be especially significant for lung transplant recipients immunocompromised due to medications, prolonged hospitalization, and malnutrition. Given that infections are a leading cause of mortality in the years following transplantation [40], vitamin D deficiency could lead to increased infection rates and poorer outcomes in these patients.
This study’s logistic regression analysis demonstrated that low vitamin D levels were associated with higher instances of pneumonia post-transplant. Frequent pneumonia hospitalizations correlate with adverse outcomes, including reduced lung function, diminished quality of life, and increased mortality. Thus, vitamin D deficiency likely exacerbates lung transplant recipients’ already compromised infection defense mechanisms.
Although vitamin D supplementation has not shown overall health benefits in clinical studies for various chronic diseases, it has been reported to result in some extraskeletal benefits, such as reduced infections and increased lung function, in patients with profound vitamin D deficiency [43, 44]. Several clinical trials have been conducted in solid organ transplant recipients to investigate the clinical benefits of correcting vitamin D deficiency [16, 44, 45]. In a clinical trial targeting LTRs, high-dose vitamin D supplementation failed to prove a clinical benefit in chronic lung allograft dysfunction prevalence, overall survival, pulmonary function, acute rejection, and respiratory infections [16]. In the previous trial, the placebo group also received a standard-dose vitamin D supplementation and maintained serum 25(OH)D levels above 30 ng/mL 1 year after lung transplantation, limiting the interpretation of the clinical significance of the much lower vitamin D levels [16]. Considering vitamin D’s impact on the immune system and inflammatory cascade [46, 47], maintaining adequate vitamin D levels after lung transplantation may be necessary in reducing the risk of infection and improving prognosis. Further exploration into how vitamin D deficiency intertwines with infections and prognosis in the intricate immune context of lung transplant recipients is warranted. Additionally, research into optimal vitamin D levels and supplementation dosages in LTRs holds clinical promise.
Most LTRs in this study received vitamin D supplementation, but doses varied widely. Given the lack of clear guidelines on appropriate supplementation doses, we compared prognoses based on these doses. The tertile of LTRs receiving the highest supplementation had an estimated daily intake exceeding the recommended 1,000 IU and achieved serum 25(OH)D levels in the mid-20s ng/mL [48].
A trend of increased pneumonia and poorer prognosis was noted at vitamin D levels below 10–20 ng/mL. Drawing from previous randomized controlled trials [16] and research in other fields [49], further investigation is needed to determine the optimal vitamin D levels for effective infection defense in lung transplant recipients. This research would enable tailored supplementation and management strategies in vitamin D deficiency. Larger studies focusing on the prognosis of lung transplant recipients with vitamin D deficiency could also enhance predictions and outcomes in this population.
This study has several limitations. It examined only 125 lung transplant recipients (LTRs) from a single center, which limits its generalizability and applicability to broader populations. Additionally, the relatively short follow-up period restricts our ability to assess the relationship between low vitamin D levels and chronic rejection. Variability in the timing of vitamin D measurements among patients and the reliance on prescription history rather than actual dosing for vitamin D supplementation further complicate the findings. Despite these limitations, this study highlights the clinical significance of vitamin D deficiency in relation to short-term outcomes after lung transplantation. It also suggests a potential supplementation dose that could serve as a foundation for future large-scale studies to determine optimal vitamin D levels and supplementation strategies.
Conclusion
This study highlights the significant impact of vitamin D deficiency on clinical outcomes in lung transplant recipients, emphasizing the need for further exploration of its role, optimal levels, and supplementation strategies in this population.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Institutional Review Board of Severance Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because of the nature of a retrospective observational study using electronic medical records.
Author contributions
MK and NK participated in the writing of the manuscript and data analysis; AW, SK, MP, and YK participated in research design, data acquisition, data analysis, and reviewing of the manuscript; HK, JL, and HP participated in data acquisition. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
MID (Medical Illustration and Design), as a member of the Medical Research Support Services of Yonsei University College of Medicine, providing excellent support with medical illustration.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/ti.2024.13313/full#supplementary-material
Supplementary Figure 1Flow diagram of study participants by vitamin D tertiles. Cut-off points for Vitamin D tertiles were 18.2 ng/mL and 24.5 ng/mL, classifying participants into VD tertile 1 (≤18.2 ng/mL), VD tertile 2 (18.3–24.5 ng/mL), and VD tertile 3 (≥24.6 ng/mL).
Abbreviations
25(OH)D, 25-hydroxyvitamin; 6MWT, six-minute walk test; AKI, acute kidney injury; BOS, bronchiolitis obliterans syndrome; BPF, bronchopleural fistula; ECMO, extracorporeal membrane oxygenation; FEV1, forced expiratory volume in 1 sec; ICU, intensive care unit; LTRs, lung transplant recipients; PFT, pulmonary function test; PGD, primary graft dysfunction; RRT, renal replacement therapy.
References
1.
Bikle DD . Vitamin D Metabolism, Mechanism of Action, and Clinical Applications. Chem Biol (2014) 21(3):319–29. 10.1016/j.chembiol.2013.12.016
2.
Herr C Greulich T Koczulla RA Meyer S Zakharkina T Branscheidt M et al The Role of Vitamin D in Pulmonary Disease: COPD, Asthma, Infection, and Cancer. Respir Res (2011) 12:31–9. 10.1186/1465-9921-12-31
3.
Janssens W Bouillon R Claes B Carremans C Lehouck A Buysschaert I et al Vitamin D Deficiency Is Highly Prevalent in COPD and Correlates With Variants in the Vitamin D-Binding Gene. Thorax (2010) 65(3):215–20. 10.1136/thx.2009.120659
4.
Maes K Serré J Mathyssen C Janssens W Gayan-Ramirez G . Targeting Vitamin D Deficiency to Limit Exacerbations in Respiratory Diseases: Utopia or Strategy With Potential?Calcified Tissue Int (2020) 106(1):76–87. 10.1007/s00223-019-00591-4
5.
Sutherland ER Goleva E Jackson LP Stevens AD Leung DY . Vitamin D Levels, Lung Function, and Steroid Response in Adult Asthma. Am J Respir Crit Care Med (2010) 181(7):699–704. 10.1164/rccm.200911-1710OC
6.
Paul G Brehm JM Alcorn JF Holguín F Aujla SJ Celedón JC . Vitamin D and Asthma. Am J Respir Crit Care Med (2012) 185(2):124–32. 10.1164/rccm.201108-1502CI
7.
Joo MH Han MA Park SM Shin HH . Vitamin D Deficiency Among Adults With History of Pulmonary Tuberculosis in Korea Based on a Nationwide Survey. Int J Environ Res Public Health (2017) 14(4):399. 10.3390/ijerph14040399
8.
Ganmaa D Enkhmaa D Nasantogtokh E Sukhbaatar S Tumur‐Ochir KE Manson J . Vitamin D, Respiratory Infections, and Chronic Disease: Review of Meta‐Analyses and Randomized Clinical Trials. J Intern Med (2022) 291(2):141–64. 10.1111/joim.13399
9.
Grant WB . Variations in Vitamin D Production Could Possibly Explain the Seasonality of Childhood Respiratory Infections in Hawaii. Pediatr Infect Dis J (2008) 27(9):853. 10.1097/INF.0b013e3181817bc1
10.
Hagaman JT Panos RJ McCormack FX Thakar CV Wikenheiser-Brokamp KA Shipley RT et al Vitamin D Deficiency and Reduced Lung Function in Connective Tissue-Associated Interstitial Lung Diseases. Chest (2011) 139(2):353–60. 10.1378/chest.10-0968
11.
Gao Y Zhao Q Qiu X Zhuang Y Yu M Dai J et al Vitamin D Levels Are Prognostic Factors for Connective Tissue Disease Associated Interstitial Lung Disease (CTD-ILD). Aging (Albany NY) (2020) 12(5):4371–8. 10.18632/aging.102890
12.
Doi J Moro A Fujiki M Eghtesad B Quintini C Menon KN et al Nutrition Support in Liver Transplantation and Postoperative Recovery: The Effects of Vitamin D Level and Vitamin D Supplementation in Liver Transplantation. Nutrients (2020) 12(12):3677. 10.3390/nu12123677
13.
Yin S Wang X Li L Huang Z Fan Y Song T et al Prevalence of Vitamin D Deficiency and Impact on Clinical Outcomes After Kidney Transplantation: A Systematic Review and Meta-Analysis. Nutr Rev (2022) 80(4):950–61. 10.1093/nutrit/nuab058
14.
Lowery EM Bemiss B Cascino T Durazo-Arvizu RA Forsythe SM Alex C et al Low Vitamin D Levels Are Associated With Increased Rejection and Infections After Lung Transplantation. J Heart Lung Transplant (2012) 31(7):700–7. 10.1016/j.healun.2012.02.012
15.
Caballero-Velázquez T Montero I Sánchez-Guijo F Parody R Saldana R Valcarcel D et al Immunomodulatory Effect of Vitamin D After Allogeneic Stem Cell Transplantation: Results of a Prospective Multicenter Clinical Trial. Clin Cancer Res (2016) 22(23):5673–81. 10.1158/1078-0432.ccr-16-0238
16.
Vos R Ruttens D Verleden SE Vandermeulen E Bellon H Van Herck A et al High-Dose Vitamin D After Lung Transplantation: A Randomized Trial. J Heart Lung Transplant (2017) 36(8):897–905. 10.1016/j.healun.2017.03.008
17.
Holick MF Binkley NC Bischoff-Ferrari HA Gordon CM Hanley DA Heaney RP et al Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab (2011) 96(7):1911–30. 10.1210/jc.2011-0385
18.
Stewart S Fishbein MC Snell GI Berry GJ Boehler A Burke MM et al Revision of the 1996 Working Formulation for the Standardization of Nomenclature in the Diagnosis of Lung Rejection. J Heart Lung Transplant (2007) 26(12):1229–42. 10.1016/j.healun.2007.10.017
19.
Christie JD Carby M Bag R Corris P Hertz M Weill D . Report of the ISHLT Working Group on Primary Lung Graft Dysfunction Part II: Definition. A Consensus Statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant (2005) 24(10):1454–9. 10.1016/j.healun.2004.11.049
20.
Kellum JA Lameire N Aspelin P Barsoum RS Burdmann EA Goldstein SL et al Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int supplements (2012) 2(1):1–138.
21.
Society AT . Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS Statement: Guidelines Six-Minute Walk Test Am J Respir Crit Care Med (2002) 166(1):111–7. 10.1164/ajrccm.166.1.at1102
22.
Graham BL Steenbruggen I Miller MR Barjaktarevic IZ Cooper BG Hall GL et al Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med (2019) 200(8):e70–e88. 10.1164/rccm.201908-1590ST
23.
Verleden GM Glanville AR Lease ED Fisher AJ Calabrese F Corris PA et al Chronic Lung Allograft Dysfunction: Definition, Diagnostic Criteria, and Approaches to treatment―A Consensus Report From the Pulmonary Council of the ISHLT. J Heart Lung Transplant (2019) 38(5):493–503. 10.1016/j.healun.2019.03.009
24.
Sassi F Tamone C D’Amelio P . Vitamin D: Nutrient, Hormone, and Immunomodulator. Nutrients (2018) 10(11):1656. 10.3390/nu10111656
25.
Holick MF . Vitamin D Deficiency. New Engl J Med (2007) 357(3):266–81. 10.1056/nejmra070553
26.
DeLuca HF . Overview of General Physiologic Features and Functions of Vitamin D. Am J Clin Nutr (2004) 80(6):1689S-96S–96S. 10.1093/ajcn/80.6.1689S
27.
Bouillon R Marcocci C Carmeliet G Bikle D White JH Dawson-Hughes B et al Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions. Endocr Rev (2019) 40(4):1109–51. 10.1210/er.2018-00126
28.
Shane E Silverberg SJ Donovan D Papadopoulos A Staron RB Addesso V et al Osteoporosis in Lung Transplantation Candidates With End-Stage Pulmonary Disease. Am J Med (1996) 101(3):262–9. 10.1016/S0002-9343(96)00155-6
29.
Tschopp O Boehler A Speich R Weder W Seifert B Russi EW et al Osteoporosis Before Lung Transplantation: Association With Low Body Mass Index, But Not With Underlying Disease. Am J Transplant (2002) 2(2):167–72. 10.1034/j.1600-6143.2002.020208.x
30.
Tschopp O Schmid C Speich R Seifert B Russi EW Boehler A . Pretransplantation Bone Disease in Patients With Primary Pulmonary Hypertension. Chest (2006) 129(4):1002–8. 10.1378/chest.129.4.1002
31.
Førli L Halse J Haug E Bjørtuft Ø Vatn M Kofstad J et al Vitamin D Deficiency, Bone Mineral Density and Weight in Patients With Advanced Pulmonary Disease. J Intern Med (2004) 256(1):56–62. 10.1111/j.1365-2796.2004.01337.x
32.
Stein EM Shane E . Vitamin D in Organ Transplantation. Osteoporos Int (2011) 22:2107–18. 10.1007/s00198-010-1523-8
33.
Reichrath J . Dermatologic Management, Sun Avoidance and Vitamin D Status in Organ Transplant Recipients (OTR). J Photochem Photobiol B: Biol (2010) 101(2):150–9. 10.1016/j.jphotobiol.2010.04.001
34.
Manelli F Giustina A . Glucocorticoid-Induced Osteoporosis. Trends Endocrinol Metab (2000) 11(3):79–85. 10.1016/s1043-2760(00)00234-4
35.
Lee JR Dadhania D August P Lee JB Suthanthiran M Muthukumar T . Circulating Levels of 25-Hydroxyvitamin D and Acute Cellular Rejection in Kidney Allograft Recipients. Transplantation (2014) 98(3):292–9. 10.1097/TP.0000000000000055
36.
Singh TP Cherikh WS Hsich E Lewis A Perch M Kian S et al Graft Survival in Primary Thoracic Organ Transplant Recipients: A Special Report From the International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant (2023) 42(10):1321–33. 10.1016/j.healun.2023.07.017
37.
Foroutan F Malik A Clark KE Buchan TA Yang H Cheong GHL et al Predictors of 1-Year Mortality After Adult Lung Transplantation: Systematic Review and Meta-Analyses. J Heart Lung Transplant (2022) 41(7):937–51. 10.1016/j.healun.2022.03.017
38.
Chambers DC Perch M Zuckermann A Cherikh WS Harhay MO Hayes JD et al The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-Eighth Adult Lung Transplantation Report—2021; Focus on Recipient Characteristics. J Heart Lung Transplant (2021) 40(10):1060–72. 10.1016/j.healun.2021.07.021
39.
Thomas PA . Commentary: Challenging the Final Frontier of Lung Transplantation. J Thorac Cardiovasc Surg (2023) 165(2):e38–e9. 10.1016/j.jtcvs.2022.04.003
40.
Perch M Hayes D Cherikh WS Zuckermann A Harhay MO Hsich E et al The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-Ninth Adult Lung Transplantation Report—2022; Focus on Lung Transplant Recipients With Chronic Obstructive Pulmonary Disease. J Heart Lung Transplant (2022) 41(10):1335–47. 10.1016/j.healun.2022.08.007
41.
Ismailova A White JH . Vitamin D, Infections and Immunity. In: Reviews in Endocrine and Metabolic Disorders (2022). p. 1–13.
42.
Jolliffe DA Camargo CA Sluyter JD Aglipay M Aloia JF Ganmaa D et al Vitamin D Supplementation to Prevent Acute Respiratory Infections: A Systematic Review and Meta-Analysis of Aggregate Data From Randomised Controlled Trials. Lancet Diabetes Endocrinol (2021) 9(5):276–92. 10.1016/s2213-8587(21)00051-6
43.
Lucas A Wolf M . Vitamin D and Health Outcomes: Then Came the Randomized Clinical Trials. JAMA (2019) 322(19):1866–8. 10.1001/jama.2019.17302
44.
Bouillon R Manousaki D Rosen C Trajanoska K Rivadeneira F Richards JB . The Health Effects of Vitamin D Supplementation: Evidence From Human Studies. Nat Rev Endocrinol (2022) 18(2):96–110. 10.1038/s41574-021-00593-z
45.
Obi Y Ichimaru N Sakaguchi Y Iwadoh K Ishii D Sakai K et al Correcting Anemia and Native Vitamin D Supplementation in Kidney Transplant Recipients: A Multicenter, 2× 2 Factorial. Open‐Label, Randomized Clinical Trial Transpl Int (2021) 34(7):1212–25. 10.1111/tri.13885
46.
Ghaseminejad-Raeini A Ghaderi A Sharafi A Nematollahi-Sani B Moossavi M Derakhshani A et al Immunomodulatory Actions of Vitamin D in Various Immune-Related Disorders: A Comprehensive Review. Front Immunol (2023) 14:950465. 10.3389/fimmu.2023.950465
47.
de Castro Kroner J Sommer A Fabri M . Vitamin D Every Day to Keep the Infection Away?Nutrients (2015) 7(6):4170–88. 10.3390/nu7064170
48.
Jomphe V Lands LC Mailhot G . Nutritional Requirements of Lung Transplant Recipients: Challenges and Considerations. Nutrients (2018) 10(6):790. 10.3390/nu10060790
49.
Demay MB Pittas AG Bikle DD Diab DL Kiely ME Lazaretti-Castro M et al Vitamin D for the Prevention of Disease: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol & Metab (2024) 109(8):1907–47. 10.1210/clinem/dgae290
Summary
Keywords
vitamin D deficiency, lung transplantation, survival, prognosis, pneumonia
Citation
Ki MS, Kim NE, Woo A, Kim SY, Kim YS, Kim HE, Lee JG, Paik HC and Park MS (2024) Post-Transplant Vitamin D Deficiency in Lung Transplant Recipients: Impact on Outcomes and Prognosis. Transpl Int 37:13313. doi: 10.3389/ti.2024.13313
Received
29 May 2024
Accepted
15 October 2024
Published
25 October 2024
Volume
37 - 2024
Updates
Copyright
© 2024 Ki, Kim, Woo, Kim, Kim, Kim, Lee, Paik and Park.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Moo Suk Park, pms70@yuhs.ac
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.