Abstract
Porcine endogenous retroviruses (PERVs) are integrated in the genome of all pigs. PERV-A, PERV-B and PERV-C can be released as infectious virus particles and PERV-A and PERV-B can infect human cells in culture. PERV-C does not infect human cells, but high-titer recombinant PERV-A/C can infect them. Retroviruses are able to induce immunosuppression and/or tumors in the infected host. Numerous methods have been developed to study PERV in donor pigs. No PERV infections were observed in infection experiments as well as in preclinical and clinical xenotransplantation trials. Despite this, several strategies have been developed to prevent PERV infection of the recipient. PCR-based and immunological methods are required to screen xenotransplant recipients. Since the proviruses are integrated into the pig genome, PERV infection has to be distinguished from microchimerism, e.g., the presence of pig cells in the recipient, which is common in xenotransplantation. Sensitive PCR methods using pig short interspersed nuclear elements (SINE) sequences allow to detect pig cells easily. Virus infection can also be detected by an increase of viral genomic or mRNA in human cells. The method of choice, however, is to screen for specific antibodies against PERV using different recombinant PERV proteins, purified viruses or peptides.
Introduction
Xenotransplantation may be associated with the transmission of pathogenic pig viruses. The recent report on the first patient receiving a pig heart in Baltimore, Maryland, United States, underlined the importance of virus safety in the context of xenotransplantation. The porcine cytomegalovirus, which is correctly defined as a porcine roseolovirus (PCMV/PRV), was not detected in the xenotransplant due to inappropriate detection methods, but in the transplanted organ the virus was massively replicating due to the absence of the porcine immune system and this contributed to the cascade leading to the death of the patient [1]. Whereas PCMV/PRV and other viruses such as the zoonotic virus hepatitis E virus (HEV), which may induce a disease in the recipient, can be easily eliminated by selection of virus-negative animals, early weaning, colostrum-deprivation, Cesarean delivery or embryo transfer [2], this is not possible with PERVs, since their proviruses are integrated in the genome of all pigs. Therefore, PERVs pose a special risk for xenotransplantation using pig cells or organs: Many retroviruses are able to induce immunodeficiencies and tumors in the infected host. Not only the human immunodeficiency virus (HIV) induces severe immunodeficiencies, but also viruses closely related to PERV such as the murine leukemia virus (MuLV), the feline leukemia virus (FeLV) and the koala retrovirus (KoRV) induce immunodeficiencies in addition to leukemias and lymphomas in the infected host. Since there are excellent reviews available, describing the biology, the detection of PERV in donor pigs and the strategies how to prevent PERV transmission to the recipients [3–6], I will give only a short summary on these topics and concentrate on monitoring for PERV infection following xenotransplantation.
Biology of Pervs
Retroviruses are characterized by their ability to integrate their RNA genome using the viral enzymes reverse transcriptase and integrase as a DNA copy into the genome of the infected cell. This integrated DNA copy is called provirus. Endogenous retroviruses are the result of integration of proviruses into the oocyte or the sperm cell and consequently, they are present in all cells of the developing organism. PERV-A and PERV-B are present in the genome of all pigs, whereas PERV-C is present in most, but not all pigs. PERV-A and PERV-B are able to infect human cells in culture and therefore pose a risk for xenotransplantation, whereas PERV-C infects only pig cells [5]. However, PERV-C can recombine with PERV-A and acquire so the receptor binding site for the receptor of PERV-A. PERV-A/C can therefore infect human cells and replicate with higher titers compared to the paternal PERV-A. PERV-A and PERV-B infect mainly human tumor cells [7–9], reports concerning the infection of primary cells are rare [9, 10].
Detection in Donor Pigs
PCR and real-time PCR methods can be used to detect PERV proviruses in the genome of pigs. Droplet digital PCR (ddPCR) allows to quantify the average number of proviruses in the genome of one cell. The copy number depends on the pig breed. In European pigs the copy number is around 60, in Asian pigs the copy number is slightly lower (for review see [11, 12]). To analyze the expression of PERVs at the mRNA level, reverse transcriptase (RT) real-time PCR may be used. To detect the expression at the protein level, immunofluorescence, immunoperoxidase assay or immunohistochemistry using specific sera against viral proteins may be applied. Electron microscopy and measurement of RT activity can be applied to measure the release of virus particles. Infections assays can be used to detect infectious virus particles. PERVs able to infect pig cells will be detected if pig cells are used in these assays; PERVs able to infect human cells will be detected if human cells are used. Details of the mentioned detection methods are described in [13–21].
Strategies to Prevent Transmission
Trans-species transmissions of retroviruses in the sense of infection are well known [2, 22], and the AIDS pandemic is the most disastrous example of a transmission of a zoonotic retrovirus to humans [23]. PERV itself is the result of trans-species transmission of retroviral sequences from rock hyrax, lesser Egyptian jerboa and rodents into pigs [24, 25]. Until now, PERV infections were not observed in all preclinical trials transplanting pig organs into NHPs [26–33]. No PERV infection was observed in first clinical trials transplanting pig cells and organs into human patients, too [26, 34–40]. Furthermore, PERV infection was not observed in infection experiments with small animals as well as with non-human primates under strong immunosuppression [33]. Despite this, numerous strategies to prevent transmission including selection of PERV-C-free animals using PERV-C-specific detection methods to prevent recombination with PERV-A, antiretroviral drugs which are partially also used against (HIV [41, 42], and RNA interference using siRNA [43–47] were developed. Furthermore, vaccines on the basis of neutralizing antibodies against the transmembrane envelope protein p15E and the surface envelope protein gp70 of PERV were generated [48–50]. However, these vaccines could not be tested due the absence of an animal model of PERV infection. Therefore, similar vaccines were developed against the closely related FeLV and it was shown that the vaccine prevented leukemia outbreak in cats infected with FeLV after immunization [51]. Best results were obtained by genome editing. After the failure to inactivate all PERVs using zinc finger nuclease [52], the application of CRISPR/Cas resulted in successful inactivation of all integrated PERVs by deletions in the reverse transcriptase (RT) gene in vitro [53] and in vivo [54]. However, until now it is unclear whether this inactivation is necessary because - as described in the beginning of this chapter – there is no evidence of PERV transmission to date. Furthermore, there may be off target effects of CRISPR/Cas, and it will be difficult to breed these animals to large colonies. It was shown that CRISPR/Cas treated pig cells are still able to release intact virus particles [55], which, however, should contain viral genomic RNA with an inactivated RT. It is likely that these particles can perform entry into human cells because they carry functional envelope proteins in their envelope. However, due to the inactivated RT they cannot integrate into the genome of the target cell. Since some human cells express RT, either from LINE sequences [56] or from human endogenous retroviruses (HERVs) [57], it cannot be excluded that these RT rescue PERV facilitating reverse transcription and integration. However, it is unlikely that the inactivating mutation in the PERV RT can be repaired or that a recombination between LINE or HERV-RT and PERV-RT is taking place to rescue the virus completely.
Detection in the Recipient
To screen PERV in the recipient of pig xenotransplants, PCR-based and immunological methods can be used. The use of PCR methods to screen for PERV proviral sequences is difficult, because these sequences are part of the pig genome. Pig cells will be found in all organs of the recipient and will interfere with this testing. The presence of donor cells in the recipient, called microchimerism, is common in xenotransplantation as well as in allotransplantation and pregnancy (for review see [58]). To make sure that the detected proviruses are part of the genome of pig cells, a PCR was developed which detects pig sequences, the so called short interspersed nuclear elements (SINE) sequences [59]. These sequences are found more than 100,000 times in the genome of pigs and this high copy number makes it easy to detect pig cells (Figure 1). When we screened baboons for PERV, PERV sequences were found in most of the organs tested (there are around 60 PERV copies in the pig genome), GAPDH sequences were rarely found (there are only 2 copies in the pig genome), but SINE sequences (more than 100,000 copies per genome) were found in all baboon organs tested, indicating microchimerism (Figure 1) [59]. Another possibility is to screen for increasing amounts of viral genomic or viral mRNA using a RT real-time PCR or sequencing of RNA, indicating virus replication. Another very effective way of testing for PERV would be looking for spliced env mRNA, which is a prerequisite for the translation of the Env protein and particle release [60, 61]. However, there is the possibility that viral RNA will be produced in pig cells expressing PERV or even releasing PERV. To demonstrate an infection, viral RNA has to be localized in human cells.
FIGURE 1
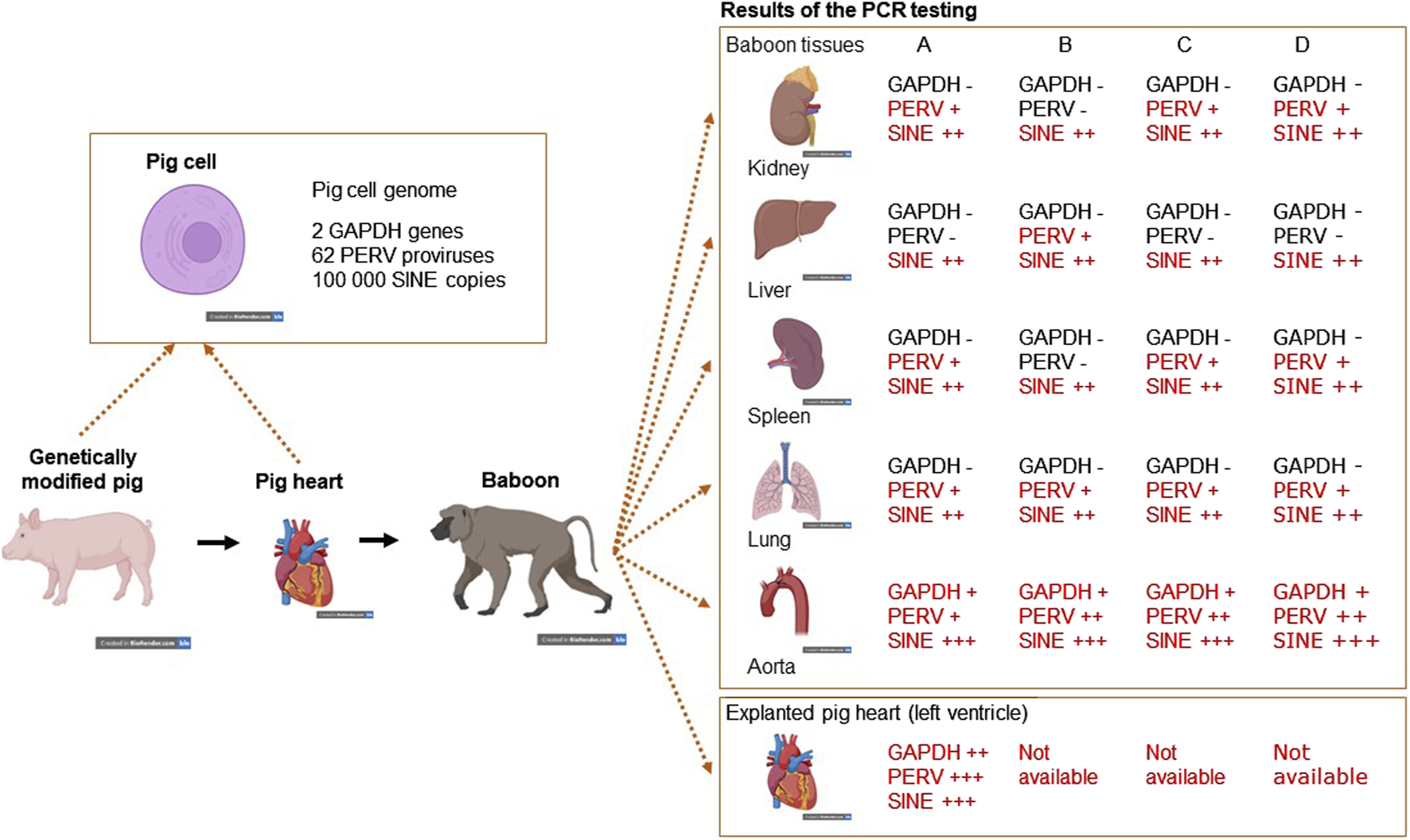
Detection of microchimerism in organs from four baboons (baboon A – survival time 195 days, baboon B – 194 days, baboon C – 26 days, and baboon D – 50 days) after transplantation of a heart from a genetically modified pig. DNA from kidney, liver, spleen, lung and aorta as well as from the explanted pig heart were screened for the presence of porcine GAPDH (2 copies in the pig genome), PERV (approximately 60 copies) and SINE (more than 100,000 copies) using real-time PCRs. Whereas GAPDH as pig marker was found only in the aorta, which is partially of pig origin and in the explanted heart, PERV was found in all organs with exception of the livers from baboons A. C and D. The SINE PCR detected pig cells in all organs analyzed, indicating the presence of microchimerism (This is the Graphical Abstract of publication [59]).
Having in mind these difficulties, the best method to detect PERV infection in the recipient is to detect antibodies against PERV as an indirect sign of virus infection. Antibody screening is a common method to detect retrovirus infection and is widely used to screen for an infection with HIV [62]. There are two main methods to detect PERV-specific antibodies, ELISA or Western blot analysis. Recombinant PERV proteins or purified virus particles can be used as antigens in Western blot analyses [19, 28, 34, 38] (Figures 2A, B). The advantage of Western blot analyses when using recombinant proteins is that the specific band can be accurately identified based on size, whereas false-positive reactions can be expected in ELISA when using recombinant proteins due to insufficient purity of the proteins produced in bacteria. Using lysates of highly purified virus preparations also allows to detect antibody responses against different viral proteins (Figure 2A). An ELISA can be performed using synthetic peptides corresponding to immunodominant epitopes of the viral proteins [19]. Such a immunodominant epitope was detected in the transmembrane envelope protein of several retroviruses [19]. It is very important to use two or three PERV proteins, for example the core protein p27Gag, the surface envelope protein gp70 and the transmembrane envelope protein p15E or corresponding synthetic peptides (Figure 2). It is not recommended to use p27Gag of PERV alone since there are a few human individuals who have antibodies against p27Gag of PERV, despite the fact that they are not infected [19]. The absence of antibodies against other PERV proteins indicates that they are not infected. It remains unclear whether the response against p27Gag in these few individuals is due to cross-reactive antibodies directed against an auto-antigen, a parasite-derived antigen or a related retrovirus [64–67]. False positive antibodies against the core protein p24 of HIV-1 were also common, for example in patients with systemic lupus erythematosus [68, 69].
FIGURE 2
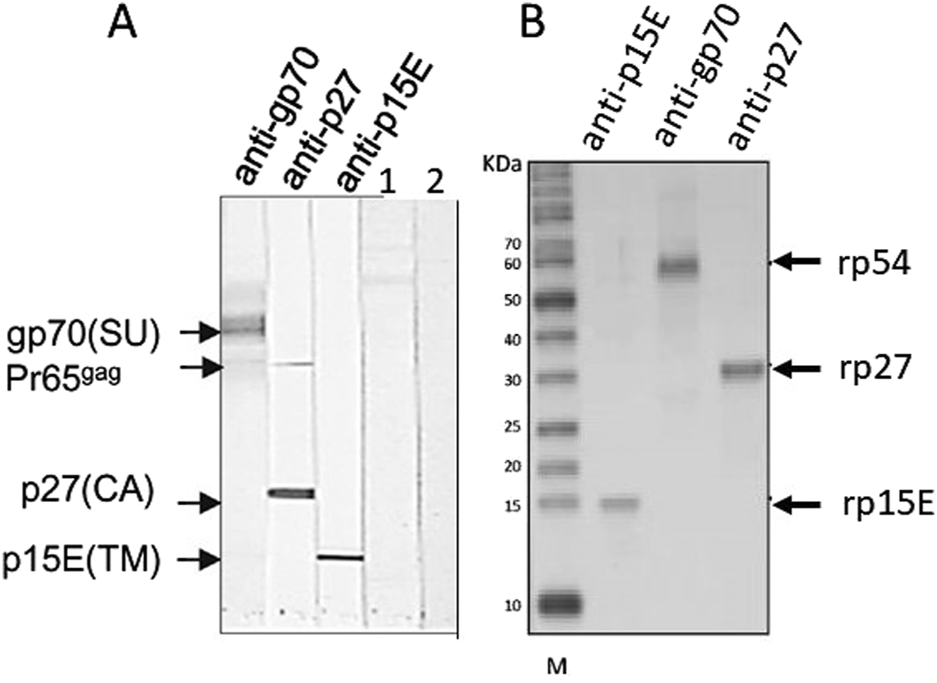
(A) Western blot analysis using gradient purified virus particles separated on an SDS-PAGE and goat sera raised against the recombinant surface envelope protein gp70 (SU) of PERV, recombinant capsid p27 (CA) and recombinant transmembrane envelope protein p15E (TM). The sera detect the corresponding viral proteins as well as the precursor molecule of Gag, Pr65gag. In the lanes 1 and 2 sera from patients receiving pig islet cells in the Argentina trial were tested negative (published in [34], with kind permission of Elsevier, 240606-011543). (B) Western blot analysis using recombinant rp54 as unglycosylated gp70, rp27Gag and rp15E, which is p15E without the membrane spanning region and the endodomain, and the goat sera as in A. M, marker. (published in [63], with kind permission of Elsevier).
There is one additional question: Are immunosuppressed individuals who received a pig organ able to mount an antibody response? Since immunosuppressed patients after allotransplantation are able to mount sufficient immune responses after vaccination, it is likely that xenotransplant patients can produce antibodies against PERV proteins [70, 71].
Conclusion
There are numerous assays available to screen for PERV infection in the recipient [72]. However, it is not easy since microchimerism, e.g., presence of pig cells in all organs of the recipient, complicates the proof. Therefore, it is important to distinguish between infection of human cells on one hand and proviruses in disseminated pig cells, which will always be present in xenotransplanted individuals, on the other hand. Highly sensitive SINE PCR will easily detect microchimerism. One further method is the detection of increasing amounts of viral genomic or mRNA indicating replication of PERV. However, to prove infection, it should be shown that this replication takes place in human and not in pig cells. The method of choice is the detection of antibodies against PERV using at least two or three recombinant viral proteins or corresponding synthetic peptides or purified virus particles in a Western blot assay or ELISA (Figure 3).
FIGURE 3
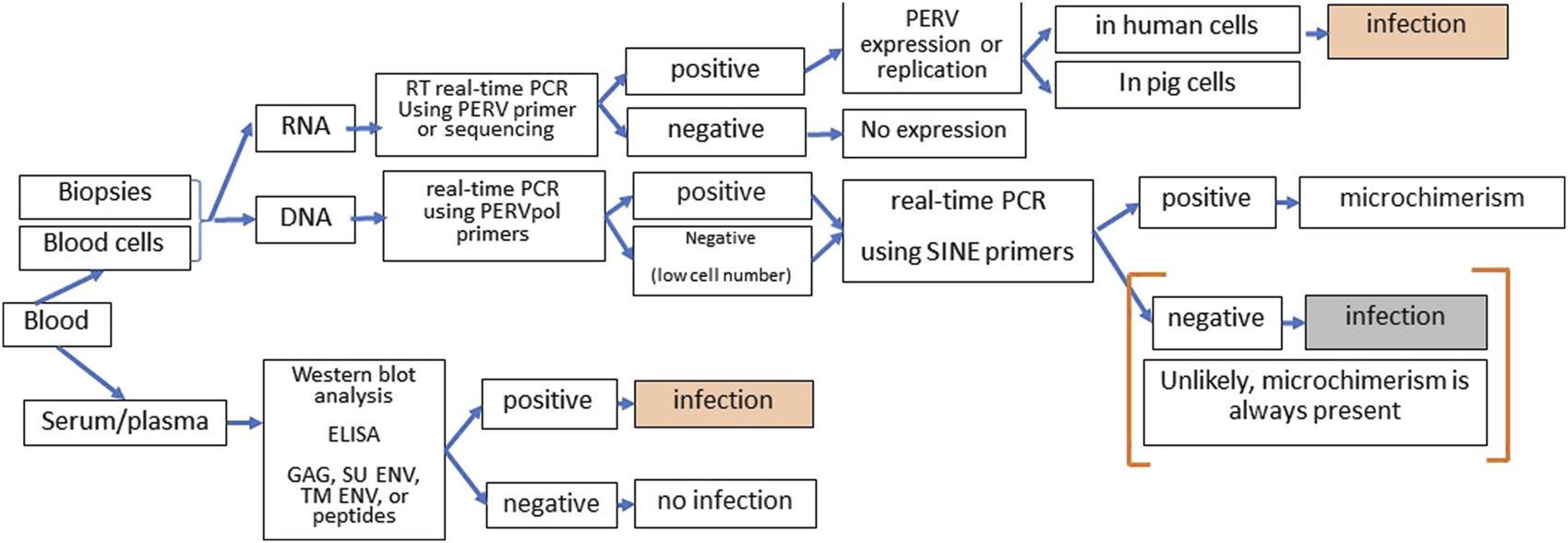
Strategy of screening for PERV infection in xenotransplantation recipients. DNA and RNA from blood cells or biopsies can be tested by PCR or RT-PCR for the presence of PERV proviruses and PERV expression. Since microchimerism is always present in xenotransplanted individuals, detection of PERV sequences in the DNA will always be associated with pig cell. When increasing amounts of PERV genomic and mRNA were observed, it has to be assured that virus replication takes part in human cells to diagnose PERV infection of the recipient. The presence of antibodies against two or more PERV antigens (GAG, capsid protein p27; SU ENV, surface envelope protein gp70, TM ENV, transmembrane envelope protein p15E, or corresponding peptides) using Western blot assays or ELISA clearly indicates an PERV infection. Absence of immune reaction means absence of infection approximately 3 to 4 weeks before.
Statements
Author contributions
JD conceptualized and wrote the article.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
AIDS, acquired immune deficiency syndrome; CRISR/Cas, clustered regularly interspaced short palindromic repeats; CRISPR associated; ddPCR, droplet digital PCR; ELISA, enzyme-linked immunosorbent assay; FeLV, feline leukemia virus; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HERVs, human endogenous retroviruses; HEV, hepatitis E virus; HIV, human immunodeficiency virus; KoRV, koala retrovirus; LINE, long interspersed nuclear element; MuLV, murine leukemia virus; PCR, polymerase chain reaction; PERV, porcine endogenous retrovirus; PCMV/PRV, porcine cytomegalovirus/porcine roseolovirus; RT, reverse transcriptase; SINE, short interspersed nuclear elements.
References
1.
Mohiuddin MM Singh AK Scobie L Goerlich CE Grazioli A Saharia K et al Graft Dysfunction in Compassionate Use of Genetically Engineered Pig-To-Human Cardiac Xenotransplantation: A Case Report. Lancet (2023) 402(10399):397–410. 10.1016/S0140-6736(23)00775-4
2.
Denner J . Virus Safety of Xenotransplantation. Viruses (2022) 14(9):1926. 10.3390/v14091926
3.
Wilson CA . Porcine Endogenous Retroviruses and Xenotransplantation. Cell Mol Life Sci. (2008) 65(21):3399–412. 10.1007/s00018-008-8498-z
4.
Fishman JA . Risks of Infectious Disease in Xenotransplantation. N Engl J Med (2022) 387(24):2258–67. 10.1056/NEJMra2207462
5.
Denner J Tönjes RR . Infection Barriers to Successful Xenotransplantation Focusing on Porcine Endogenous Retroviruses. Clin Microbiol Rev (2012) 25(2):318–43. 10.1128/CMR.05011-11
6.
Denner J . Porcine Endogenous Retroviruses and Xenotransplantation, 2021. Viruses (2021) 13(11):2156. 10.3390/v13112156
7.
Patience C Takeuchi Y Weiss RA . Infection of Human Cells by an Endogenous Retrovirus of Pigs. Nat Med (1997) 3(3):282–6. 10.1038/nm0397-282
8.
Specke V Rubant S Denner J . Productive Infection of Human Primary Cells and Cell Lines with Porcine Endogenous Retroviruses. Virology (2001) 285(2):177–80. 10.1006/viro.2001.0934
9.
Martin U Winkler ME Id M Radeke H Arseniev L Takeuchi Y et al Productive Infection of Primary Human Endothelial Cells by Pig Endogenous Retrovirus (PERV). Xenotransplantation (2000) 7(2):138–42. 10.1034/j.1399-3089.2000.00052.x
10.
Denner J . Porcine Endogenous Retrovirus Infection of Human Peripheral Blood Mononuclear Cells. Xenotransplantation (2015) 22(2):151–2. 10.1111/xen.12150
11.
Denner J . How Active Are Porcine Endogenous Retroviruses (PERVs)?Viruses (2016) 8(8):215. 10.3390/v8080215
12.
Lu TF Sun B Yu TY Wu YJ Zhou J Wu SG . Porcine Endogenous Retroviruses: Quantification of the Viral Copy Number for the Four Miniature Pig Breeds in China. Front Microbiol (2022) 13:840347. 10.3389/fmicb.2022.840347
13.
Gola J Mazurek U . Detection of Porcine Endogenous Retrovirus in Xenotransplantation. Reprod Biol (2014) 14(1):68–73. 10.1016/j.repbio.2014.01.006
14.
Shah CA Böni J Bisset LR Seebach JD Schüpbach J . Ultra-Sensitive and Specific Detection of Porcine Endogenous Retrovirus (PERV) Using a Sequence-Capture Real-Time PCR Approach. J Virol Methods (2003) 109(2):209–16. 10.1016/s0166-0934(03)00073-9
15.
Denner J . Sensitive Detection Systems for Infectious Agents in Xenotransplantation. Xenotransplantation (2020) 18:e12594. 10.1111/xen.12594
16.
Godehardt AW Rodrigues Costa M Tönjes RR . Review on Porcine Endogenous Retrovirus Detection Assays--Impact on Quality and Safety of Xenotransplants. Xenotransplantation (2015) 22(2):95–101. 10.1111/xen.12154
17.
Moon HJ Park SJ Kim HK Ann SK Rho S Keum HO et al Simultaneous Detection and Subtyping of Porcine Endogenous Retroviruses Proviral DNA Using the Dual Priming Oligonucleotide System. J Vet Sci (2010) 11(3):269–71. 10.4142/jvs.2010.11.3.269
18.
Stephan O Schwendemann J Specke V Tacke SJ Boller K Denner J . Porcine Endogenous Retroviruses (PERVs): Generation of Specific Antibodies, Development of an Immunoperoxidase Assay (IPA) and Inhibition by AZT. Xenotransplantation (2001) 8(4):310–6. 10.1034/j.1399-3089.2001.00098.x
19.
Tacke SJ Bodusch K Berg A Denner J . Sensitive and Specific Immunological Detection Methods for Porcine Endogenous Retroviruses Applicable to Experimental and Clinical Xenotransplantation. Xenotransplantation (2001) 8(2):125–35. 10.1034/j.1399-3089.2001.00080.x-i1
20.
Switzer WM Shanmugam V Chapman L Heneine W . Polymerase Chain Reaction Assays for the Diagnosis of Infection with the Porcine Endogenous Retrovirus and the Detection of Pig Cells in Human and Nonhuman Recipients of Pig Xenografts. Transplantation (1999) 68(2):183–8. 10.1097/00007890-199907270-00003
21.
Bittmann I Mihica D Plesker R Joachim Denner J . Expression of Porcine Endogenous Retroviruses (PERV) in Different Organs of a Pig. Virology (2012) 433(2):329–36. 10.1016/j.virol.2012.08.030
22.
Denner J . Transspecies Transmissions of Retroviruses: New Cases. Virology (2007) 369:229–33. 10.1016/j.virol.2007.07.026
23.
Hahn BH Shaw GM De Cock KM Sharp PM . AIDS as a Zoonosis: Scientific and Public Health Implications. Science (2000) 287:607–14. 10.1126/science.287.5453.607
24.
Chen Y Chen X Duan X Cui J . Ancient Origin and Complex Evolution of Porcine Endogenous Retroviruses. Biosaf Health (2020) 2:142–51. 10.1016/j.bsheal.2020.03.003
25.
Denner J . The Origin of Porcine Endogenous Retroviruses (PERVs). Arch Virol (2021) 166:1007–13. 10.1007/s00705-020-04925-8
26.
Denner J . Why Was PERV Not Transmitted During Preclinical and Clinical Xenotransplantation Trials and after Inoculation of Animals?Retrovirology (2018) 15(1):28. 10.1186/s12977-018-0411-8
27.
Li A Zhang Y Liu Y Pan Z . Corneal Xenotransplantation from Pig to Rhesus Monkey: No Signs of Transmission of Endogenous Porcine Retroviruses. Transpl Proc (2017) 49(9):2209–14. 10.1016/j.transproceed.2017.07.018
28.
Morozov VA Ludwig S Ludwig B Rotem A Barkai U Bornstein SR et al Islet Cell Transplantation From Göttingen Minipigs to Cynomolgus Monkeys: Analysis of Virus Safety. Xenotransplantation (2016) 23(4):320–7. 10.1111/xen.12252
29.
Plotzki E Wolf-van Buerck L Knauf Y Becker T Maetz-Rensing K Schuster M et al Virus Safety of Islet Cell Transplantation From Transgenic Pigs to Marmosets. Virus Res (2015) 204:95–102. 10.1016/j.virusres.2015.04.016
30.
Garkavenko O Dieckhoff B Wynyard S Denner J Elliott RB Tan PL et al Absence of Transmission of Potentially Xenotic Viruses in a Prospective Pig to Primate Islet Xenotransplantation Study. J Med Virol (2008) 80(11):2046–52. 10.1002/jmv.21272
31.
Gazda LS Vinerean HV Laramore MA Hall RD Carraway JW Smith BH . No Evidence of Viral Transmission Following Long-Term Implantation of Agarose Encapsulated Porcine Islets in Diabetic Dogs. J Diabetes Res (2014) 2014:727483. 10.1155/2014/727483
32.
Moscoso I Hermida-Prieto M Mañez R Lopez-Pelaez E Centeno A Diaz TM et al Lack of Cross-Species Transmission of Porcine Endogenous Retrovirus in Pig-To-Baboon Xenotransplantation With Sustained Depletion of Anti-Alphagal Antibodies. Transplantation (2005) 79(7):777–82. 10.1097/01.tp.0000152662.55720.83
33.
Specke V Schuurman HJ Plesker R Coulibaly C Ozel M Langford G et al Virus Safety in Xenotransplantation: First Exploratory In Vivo Studies in Small Laboratory Animals and Non-human Primates. Transpl Immunol (2002) 9(2-4):281–8. 10.1016/s0966-3274(02)00039-4
34.
Morozov VA Wynyard S Matsumoto S Abalovich A Denner J Elliott R . No PERV Transmission during a Clinical Trial of Pig Islet Cell Transplantation. Virus Res (2017) 227:34–40. 10.1016/j.virusres.2016.08.012
35.
Hermida-Prieto M Domenech N Moscoso I Diaz T Ishii J Salomon DR et al Lack of Cross-Species Transmission of Porcine Endogenous Retrovirus (PERV) to Transplant Recipients and Abattoir Workers in Contact With Pigs. Transplantation (2007) 84(4):548–50. 10.1097/01.tp.0000275203.91841.23
36.
Paradis K Langford G Long Z Heneine W Sandstrom P Switzer WM et al Search for Cross-Species Transmission of Porcine Endogenous Retrovirus in Patients Treated With Living Pig Tissue. The XEN 111 Study Group. Science (1999) 285(5431):1236–41. 10.1126/science.285.5431.1236
37.
Wynyard S Nathu D Garkavenko O Denner J Elliott R . Microbiological Safety of the First Clinical Pig Islet Xenotransplantation Trial in New Zealand. Xenotransplantation (2014) 21(4):309–23. 10.1111/xen.12102
38.
Irgang M Sauer IM Karlas A Zeilinger K Gerlach JC Kurth R et al Porcine Endogenous Retroviruses: No Infection in Patients Treated With a Bioreactor Based on Porcine Liver Cells. J Clin Virol (2003) 28(2):141–54. 10.1016/s1386-6532(02)00275-5
39.
Heneine W Tibell A Switzer WM Sandstrom P Rosales GV Mathews A et al No Evidence of Infection With Porcine Endogenous Retrovirus in Recipients of Porcine Islet-Cell Xenografts. Lancet (1998) 352(9129):695–9. 10.1016/S0140-6736(98)07145-1
40.
Han B Shi XL Zhang Y Gu ZZ Yuan XW Ren HZ et al No Transmission of Porcine Endogenous Retrovirus in an Acute Liver Failure Model Treated by a Novel Hybrid Bioartificial Liver Containing Porcine Hepatocytes. Hepatobiliary Pancreat Dis Int (2015) 14(5):492–501. 10.1016/s1499-3872(15)60401-5
41.
Argaw T Colon-Moran W Wilson C . Susceptibility of Porcine Endogenous Retrovirus to Anti-Retroviral Inhibitors. Xenotransplantation (2016) 23(2):151–8. 10.1111/xen.12230
42.
Denner J . Can Antiretroviral Drugs Be Used to Treat Porcine Endogenous Retrovirus (PERV) Infection After Xenotransplantation?Viruses (2017) 9(8):213. 10.3390/v9080213
43.
Miyagawa S Nakatsu S Nakagawa T Kondo A Matsunami K Hazama K et al Prevention of PERV Infections in Pig to Human Xenotransplantation by the RNA Interference Silences Gene. J Biochem (2005) 137(4):503–8. 10.1093/jb/mvi059
44.
Karlas A Kurth R Denner J . Inhibition of Porcine Endogenous Retroviruses by RNA Interference: Increasing the Safety of Xenotransplantation. Virology (2004) 325(1):18–23. 10.1016/j.virol.2004.04.022
45.
Dieckhoff B Petersen B Kues WA Kurth R Niemann H Denner J . Knockdown of Porcine Endogenous Retrovirus (PERV) Expression by PERV-specific shRNA in Transgenic Pigs. Xenotransplantation (2008) 15(1):36–45. 10.1111/j.1399-3089.2008.00442.x
46.
Dieckhoff B Karlas A Hofmann A Kues WA Petersen B Pfeifer A et al Inhibition of Porcine Endogenous Retroviruses (PERVs) in Primary Porcine Cells by RNA Interference Using Lentiviral Vectors. Arch Virol (2007) 152(3):629–34. 10.1007/s00705-006-0868-y
47.
Ramsoondar J Vaught T Ball S Mendicino M Monahan J Jobst P et al Production of Transgenic Pigs That Express Porcine Endogenous Retrovirus Small Interfering RNAs. Xenotransplantation. (2009) 16(3):164–80. 10.1111/j.1399-3089.2009.00525.x
48.
Fiebig U Stephan O Kurth R Denner J . Neutralizing Antibodies Against Conserved Domains of p15E of Porcine Endogenous Retroviruses: Basis for a Vaccine for Xenotransplantation?Virology (2003) 307(2):406–13. 10.1016/s0042-6822(02)00140-x
49.
Denner J Mihica D Kaulitz D Schmidt CM . Increased Titers of Neutralizing Antibodies After Immunization With Both Envelope Proteins of the Porcine Endogenous Retroviruses (PERVs). Virol J (2012) 9:260. 10.1186/1743-422X-9-260
50.
Waechter A Denner J . Novel Neutralising Antibodies Targeting the N-Terminal Helical Region of the Transmembrane Envelope Protein p15E of the Porcine Endogenous Retrovirus (PERV). Immunol Res (2014) 58(1):9–19. 10.1007/s12026-013-8430-y
51.
Langhammer S Hübner J Jarrett O Kurth R Denner J . Immunization With the Transmembrane Protein of a Retrovirus, Feline Leukemia Virus: Absence of Antigenemia Following Challenge. Antivir Res (2011) 89(1):119–23. 10.1016/j.antiviral.2010.11.011
52.
Semaan M Ivanusic D Denner J . Cytotoxic Effects During Knock Out of Multiple Porcine Endogenous Retrovirus (PERV) Sequences in the Pig Genome by Zinc Finger Nucleases (ZFN). PLoS One (2015) 10(4):e0122059. 10.1371/journal.pone.0122059
53.
Yang L Güell M Niu D George H Lesha E Grishin D et al Genome-Wide Inactivation of Porcine Endogenous Retroviruses (PERVs). Science (2015) 350(6264):1101–4. 10.1126/science.aad1191
54.
Niu D Wei HJ Lin L George H Wang T Lee IH et al Inactivation of Porcine Endogenous Retrovirus in Pigs Using CRISPR-Cas9. Science (2017) 357(6357):1303–7. 10.1126/science.aan4187
55.
Godehardt AW Fischer N Rauch P Gulich B Boller K Church GM et al Characterization of Porcine Endogenous Retrovirus Particles Released by the CRISPR/Cas9 Inactivated Cell Line PK15 Clone 15. Xenotransplantation (2020) 27(2):e12563. 10.1111/xen.12563
56.
Spadafora C . A Reverse Transcriptase-Dependent Mechanism Plays Central Roles in Fundamental Biological Processes. Syst Biol Reprod Med (2008) 54(1):11–21. 10.1080/19396360701876815
57.
Baldwin ET Götte M Tchesnokov EP Arnold E Hagel M Nichols C et al Human Endogenous Retrovirus-K (HERV-K) Reverse Transcriptase (RT) Structure and Biochemistry Reveals Remarkable Similarities to HIV-1 RT and Opportunities for HERV-K-Specific Inhibition. Proc Natl Acad Sci U S A (2022) 119(27):e2200260119. 10.1073/pnas.2200260119
58.
Denner J . Microchimerism, PERV and Xenotransplantation. Viruses (2023) 15(1):190. 10.3390/v15010190
59.
Jhelum H Bender M Reichart B Mokelke M Radan J Neumann E et al Evidence for Microchimerism in Baboon Recipients of Pig Hearts. Viruses (2023) 15(7):1618. 10.3390/v15071618
60.
Karlas A Irgang M Votteler J Specke V Ozel M Kurth R et al Characterisation of a Human Cell-Adapted Porcine Endogenous Retrovirus PERV-A/C. Ann Transpl (2010) 15(2):45–54.
61.
Denner J Scobie L . Are There Better Assays to Evaluate the Risk of Transmission of Porcine Endogenous Retroviruses (PERVs) to Human Cells?Xenotransplantation (2019) 26(4):e12510. 10.1111/xen.12510
62.
Hashida S Hashinaka K Ishikawa S Ishikawa E . More Reliable Diagnosis of Infection With Human Immunodeficiency Virus Type 1 (HIV-1) by Detection of Antibody IgGs to Pol and Gag Proteins of HIV-1 and P24 Antigen of HIV-1 in Urine, Saliva, And/or Serum With Highly Sensitive and Specific Enzyme Immunoassay (Immune Complex Transfer Enzyme Immunoassay): A Review. J Clin Lab Anal (1997) 11(5):267–86. 10.1002/(SICI)1098-2825(1997)11:5<267::AID-JCLA5>3.0.CO;2-4
63.
Denner J Petersen B Niemann H . Tolerance and Immune Response to the Porcine Endogenous Retrovirus in German Landrace Pigs Immunised With Viral Proteins. Virus Res (2015) 208:39–43. 10.1016/j.virusres.2015.05.019
64.
Sayre KR Dodd RY Tegtmeier G Layug L Alexander SS Busch MP . False-Positive Human Immunodeficiency Virus Type 1 Western Blot Tests in Noninfected Blood Donors. Transfusion (1996) 36(1):45–52. 10.1046/j.1537-2995.1996.36196190514.x
65.
Biggar RJ Gigase PL Melbye M Kestens L Sarin PS Bodner AJ et al ELISA HTLV Retrovirus Antibody Reactivity Associated With Malaria and Immune Complexes in Healthy Africans. Lancet (1985) 2(8454):520–3. 10.1016/s0140-6736(85)90461-1
66.
Elm J Desowitz R Diwan A . Serological Cross-Reactivities Between the Retroviruses HIV and HTLV-1 and the Malaria Parasite Plasmodium Falciparum. P N G Med J (1998) 41(1):15–22.
67.
Greenberg AE Schable CA Sulzer AJ Collins WE Nguyen-Dinh P . Evaluation of Serological Cross-Reactivity Between Antibodies to Plasmodium and HTLV-III/LAV. Lancet (1986) 2(8501):247–9. 10.1016/s0140-6736(86)92071-4
68.
Herrmann M Baur A Nebel-Schickel H Vornhagen R Jahn G Krapf FE et al Antibodies Against P24 of HIV-1 in Patients With Systemic Lupus Erythematosus? Viral Immunol (1992) 5(3):229–31. 10.1089/vim.1992.5.229
69.
Gül A Inanç M Yilmaz G Ocal L Koniçe M Aral O et al Antibodies Reactive With HIV-1 Antigens in Systemic Lupus Erythematosus. Lupus (1996) 5(2):120–2. 10.1177/096120339600500206
70.
Danziger-Isakov L Kumar D , AST ID Community of Practice. Vaccination of Solid Organ Transplant Candidates and Recipients: Guidelines From the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transpl (2019) 33(9):e13563. 10.1111/ctr.13563
71.
De Bouver C Bouziotis J Wijtvliet VPWM Ariën KK Mariën J Heyndrickx L et al Humoral Immunity to SARS-CoV-2 in Kidney Transplant Recipients and Dialysis Patients: IgA and IgG Patterns Unraveled after SARS-CoV-2 Infection and Vaccination. Virol J (2024) 21(1):138. 10.1186/s12985-024-02410-1
72.
Denner J . Infectious Risk in Xenotransplantation – What Post-Transplant Screening for the Human Recipient?Xenotransplantation (2011) 18:151–7. 10.1111/j.1399-3089.2011.00636.x
Summary
Keywords
virus safety of xenotransplantation, retroviruses, porcine endogenous retroviruses (PERVs), PERV-C, vaccine
Citation
Denner J (2024) Monitoring for PERV Following Xenotransplantation. Transpl Int 37:13491. doi: 10.3389/ti.2024.13491
Received
04 July 2024
Accepted
16 September 2024
Published
07 October 2024
Volume
37 - 2024
Updates
Copyright
© 2024 Denner.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joachim Denner, joachim.denner@fu-berlin.de
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.