- Department of Psychiatry, Shenzhen Longgang Center for Chronic Disease Control, Shenzhen, China
Depression is a common psychiatric disorder. Due to the disadvantages of current clinical drugs, including poor efficacy and unnecessary side effects, research has shifted to novel natural products with minimal or no adverse effects as therapeutic alternatives. The ocean is a vast ecological home, with a wide variety of organisms that can produce a large number of natural products with unique structures, some of which have neuroprotective effects and are a valuable source for the development of new drugs for depression. In this review, we analyzed preclinical and clinical studies of natural products derived from marine organisms with antidepressant potential, including the effects on the pathophysiology of depression, and the underlying mechanisms of these effects. It is expected to provide a reference for the development of new antidepressant drugs.
Introduction
As one of the most common mental illnesses, there are currently at least 50 million patients with depression in China (Huang et al., 2019). The incidence of depression is increasing year by year, and it is expected to become one of the major causes of disease burden in China by 2030 (Huang et al., 2019). Depression imposes a heavy burden on individuals, families and society due to its high incidence, high disability rate and high suicide rate (Cipriani et al., 2018). Selective serotonin reuptake inhibitors (SSRIs), norepinephrine reuptake inhibitors (NRIs), and dopamine reuptake inhibitors (DRIs) are the main first-line antidepressants used in clinical practice today (Harmer et al., 2017; Moragrega and Ríos, 2021). These medications either work on the neurotransmitter systems of serotonin (5-HT), Norepinephrine (NE), and dopamine (DA) or they suppress the action of the enzyme monoamine oxidase (MAO) to produce antidepressant effects (Harmer et al., 2017). However, even after adequate and sufficient antidepressant treatment, about one-third of patients still do not have a significant therapeutic effect. Patients often experience side effects such as gastrointestinal discomfort and loss of libido, in addition to poor treatment compliance. Therefore, it is of great significance to find more effective and safer antidepressant compounds from a wide range of natural products, and it can also provide new ideas for the development of antidepressant products.
The living environment of marine organisms is complex, resulting in a large number of marine natural products (MNPs). Studies have shown that MNPs have significant pharmacological activities, and the toxicity and side effects are significantly lower than those of synthetic compounds (Corona, 2018). These marine-derived active substances have played an important role in the prevention and treatment of many diseases, and their pharmacodynamic mechanisms are constantly being elucidated. In recent years, with the relentless exploration of researchers, the bioactive substances derived from MNPs have increased. The metabolites isolated from marine organisms have diverse chemical structures, including polyketides, terpenoids, alkaloids, macrolides, cyclic peptides, quinones, polyethers, sterols, polysaccharides, unsaturated fatty acids, and a wide range of pharmacological activities, including antibacterial, antiparasitic, enzyme inhibitor, antioxidant, cytotoxic activity, etc. The majority of marine drug research and development is focused on anti-tumor, anti-cardiovascular disease, and antibacterial agents (Russo et al., 2015; Lima and Medeiros, 2022). Many marine compounds have received clinical approval for use, including the analgesic ziconotide and the anti-cancer drug cytarabine (Jimenez et al., 2020; Yang et al., 2021). MNPs and compounds generated from MNPs are becoming more and more valuable due to their biological activities. Numerous studies have recently revealed that MNPs have antidepressant properties and may slow the course of depression (Subermaniam et al., 2021a). MNPs may be a useful resource for the development of brand-new antidepressant alternatives. This article examines preclinical and clinical studies on the antidepressant effects of MNPs and research on the neuroscience of depression.
Subsections relevant to the subject
Pathogenesis of depression
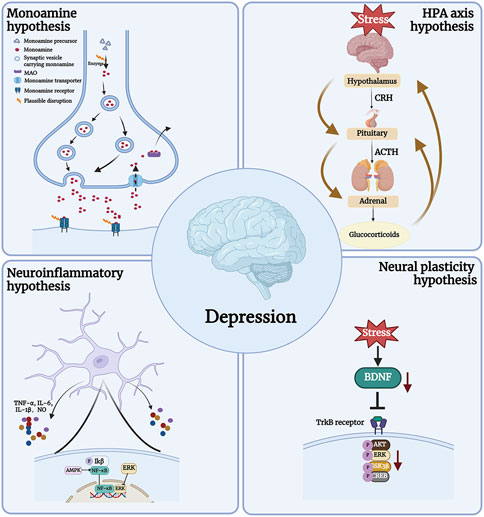
The pathogenesis of depression is complex and the biological mechanism is not fully understood (Jesulola et al., 2018). Currently, the widely accepted pathogenic hypotheses include the Monoamine hypothesis, the Neural plasticity hypothesis, the Neuroinflammatory hypothesis, and the Hypothalamic-pituitary-adrenal (HPA) axis (Figure 1) (Subermaniam et al., 2021a; Borbély et al., 2022). It should be mentioned that depression has a complex etiology and may be brought on by a confluence of various pathogenic variables. The search for drugs with multiple targets is thus a crucial area of research for the development of antidepressants.
Monoamine hypothesis
One of the primary causes of depression has been determined to be decreased levels and functional deficiencies of 5-HT, DA, and NE, which are typically present in the brains of depressed patients (Matraszek-Gawron et al., 2019; Wang et al., 2022). The monoamine hypothesis is strongly supported by the fact that 5-HT or NERI can alleviate depression (Locher et al., 2017; Rana et al., 2021). By improving neurotransmission in the central nervous system and increasing the amount of related monoamine neurotransmitters in the synaptic cleft, this class of medications reduces the symptoms of depression (Locher et al., 2017; Yohn et al., 2017).
Neural plasticity hypothesis
The pathogenesis of depression is significantly influenced by neuroplasticity and remodeling failure (Wang et al., 2021a). The neurotrophic family includes brain-derived neurotrophic factor (BDNF), which regulates neuronal plasticity (Castrén and Monteggia, 2021). On the one hand, it might have an impact on the development of synaptic structures, such as axons and dendrites, and their growth and remodeling. On the other hand, it might enhance the long-term synaptic transmission function of the hippocampus through pre- and post-synaptic pathways (Wardle and Poo, 2003). Decreased levels of BDNF have been found in brain samples from depressed patients (Chen et al., 2001; Dwivedi, 2009; Dwivedi et al., 2009; Carlino et al., 2013). In contrast, antidepressant treatment increases the expression of BDNF in the brains of depressed patients (Chen et al., 2001). Therefore, it shows great potential in the treatment of depression as it increases BDNF levels.
Neuroinflammatory hypothesis
Glial cells and cytokines play a role in the immune response known as neuroinflammation that occurs in the central nervous system (Zhou et al., 2022). Depression is an inflammation-related disease that worsens as inflammation increases and progresses (Yirmiya et al., 2015; Wang et al., 2021b). According to research, aberrant glial cell activation in the brains of depressed patients results in the release of pro-inflammatory cytokines such as interleukin-6 (IL-6), interleukin 1 beta (IL-1β), and tumor necrosis factor-alpha (TNF-α), which can lead to neuroinflammation and neuronal death (Matraszek-Gawron et al., 2019). In addition, glial cells produce nitric oxide synthase (NOS), and cyclooxygenase-2 (COX-2) could also induce neuroinflammation by promoting oxidative stress levels. Neuroinflammation has emerged as a novel target for the treatment of depression (Wang et al., 2021b; Zhou et al., 2022).
Hypothalamic-pituitary-adrenal (HPA) axis
The hypothalamus, pituitary, and adrenal glands work together as part of the HPA axis to govern the body’s reaction to physiological or psychological stimuli (Herman et al., 2016; Lew et al., 2020). Patients with depression have been discovered to have HPA dysfunction, resulting in high glucocorticoid (GC) levels, which in turn cause neuronal dysfunction and structural changes in the hippocampus (Dean and Keshavan, 2017). Clinical trials have established the antidepressant properties of glucocorticoid receptor (GR) antagonists and the viability of targeting HPA regulation in the treatment of depression (Dean and Keshavan, 2017).
MNPs have anti-depressant potential
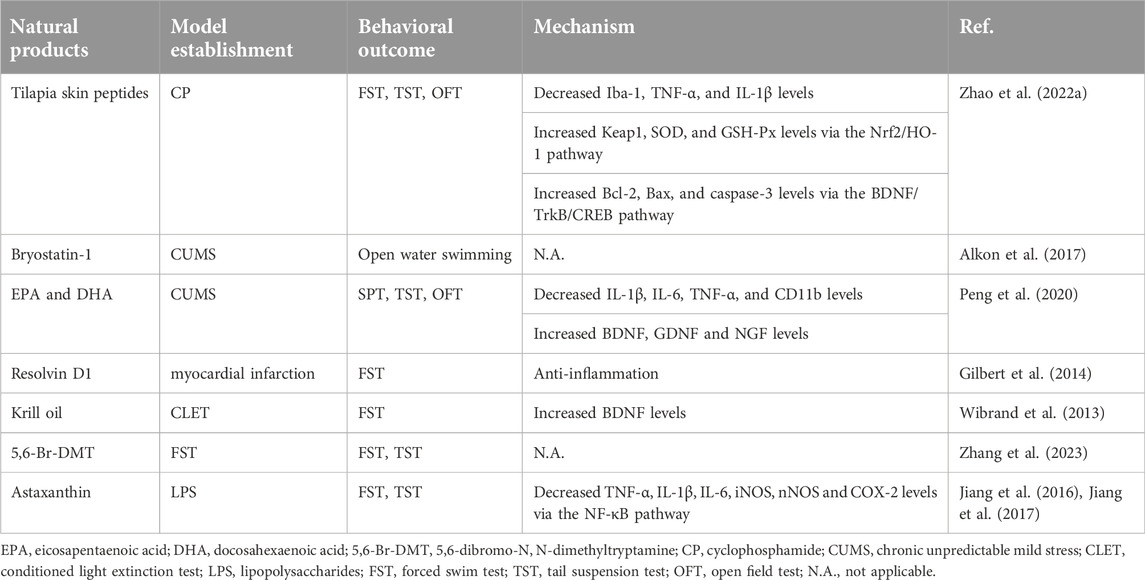
A significant number of MNPs are produced by the complex living environment of marine animals, and a novel entity structure and enormous diversity are provided by the chemical structure with strong biological activity (Zhao et al., 2022a). Some MNPs have participated in antidepressant preclinical or clinical trials and have proven to be great sources for novel and effective antidepressants (Tables 1, 2). The marine environment is a rich source of novel pharmaceuticals, many of the substances found there can regulate brain activity, reduce anxiety, and have potential therapeutic applications for disorders associated with anxiety and depression (Zhao et al., 2022a). At present, with the interaction between academia and the pharmaceutical sector, a large number of MNPs have been discovered and tested using current analytical methods.
Antidepressant natural products of marine animal origin
Tilapia skin peptides (TSP)
Tilapia skin peptides (TSP), derived from tilapia (Oreochromis mossambicus) scraps (Zhao et al., 2022a), have biological actions that are antioxidant, anti-inflammatory, anti-apoptotic and hypotensive (Ling et al., 2018; Zhao et al., 2022b; Song et al., 2022). Hippocampal neurons in mice that received an intraperitoneal infusion of cyclophosphamide (CP) experienced oxidative stress, neuroinflammation, and neuronal death (Iqubal et al., 2019). TSP [1,000 mg/kg/d, intragastrically (i.g.)] could improve CP-induced depressive-like behaviors such as Sucrose Preference Test (SPT), Forced Swim Test (FST), Tail Suspension Test (TST) and Open Field Test (OFT) in mice (Zhao et al., 2022a). Mechanistically, TSP may reduce CP-induced neuroinflammation by decreasing the expression of ionized calcium-binding adaptor molecule 1 (Iba-1), TNF-α, and IL-1β in the hippocampus of mice. TSP also attenuated CP-induced oxidative stress by increasing the Nrf2/HO-1 signaling pathway and increasing the levels of kelch-like ECH-associated protein 1 (Keap1), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) and malondialdehyde (MDA). In addition, TSP could also reduce neuronal apoptosis by increasing Bcl-2/Bax/Caspase-3 through the BDNF/TrkB/CREB signaling pathway. In conclusion, the antidepressant effect of the TSP may be involved in the regulation of synaptic plasticity and anti-inflammatory activity (Zhao et al., 2022a).
Bryostatin-1
Bugula neritina-derived bryostatin-1 can increase the expression of protein kinase C (PKC), induce PKC membrane translocation, and enhance synaptic plasticity (Nelson et al., 2017). PKC activity was reduced in the brains of depressed individuals (Pandey et al., 2021). In the rat model of depression generated by chronic unpredictable mild stress (CUMS), PKC expression was markedly reduced (Han et al., 2015). According to these investigations, PKC levels and the development of depression may be related. Furthermore, PKC has the ability to significantly alter synaptic transmission (Wierda et al., 2007; Shen et al., 2021). Bryostatin-1 [100 nmol/kg, intravenous (i.v.)] shortened the immobility time in the FST in rats. This antidepressant effect of Bryostatin-1 is largely abolished by 1-(5-isoquinolinylsulfonyl)-2-methylpiperazine (H-7), a PKC inhibitor, which suggests that Bryostatin-1 may have an antidepressant effect by enhancing synaptic plasticity through activation of PKC action (Alkon et al., 2017).
Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)
Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are mainly derived from the Omega-3 long-chain polyunsaturated fatty acids (n-3 PUFA) of deep-sea fish and shrimp, which play a key role in brain development (Andraka et al., 2020). Continuous oral administration (p.o.) of EPA or DHA for 45 days reduced the weight loss and depressive-like behavior caused by CUMS in SPT/OFT/FST. Mechanistically, EPA and DHA effectively reduced CUMS-induced expression of IL-1β, IL-6, TNF-α, microglial marker α M Integrin alpha M (CD11b), and increased expression of astrocyte marker glial fibrillary acidic protein (GFAP) by regulating the NF-κB/p38 signaling pathway (Peng et al., 2020). Additionally, EPA and DHA modulated the BDNF/TrkB signaling pathway to upregulate the production of BDNF, glial cell-derived neurotrophic factor (GDNF), nerve growth factor (NGF), and Bcl-2 and reduce the expression of Bax, reversing the effects of CUMS-induced neurotrophic factor deficiency and apoptosis (Peng et al., 2020). Moreover, EPA and DHA can also reduce the serum total cholesterol (STC) contents of serum total cholesterol and corticosterone (a glucocorticoid), and induce 5-HT and NE deficiency in the hippocampus, suggesting that EPA and DHA exert antidepressant activity by regulating HPA (Peng et al., 2020). Notably, EPA was more effective than DHA in reducing depressive-like behavior, which was also confirmed in clinical studies. Lipopolysaccharide (LPS) activates BV2 microglia, and docosapentaenoic acid (DPA, a PUFA) balances microglial M1 and M2 polarization, inhibiting NF-κB and p38 while activating neuronal BDNF/TrkB-PI3K/AKT pathways to protect neurons from neuroinflammatory damage (Liu et al., 2021). By increasing the expression of DA, decreasing the expression of NE and gamma-aminobutyric acid (GABA), and reducing the turnover rate of 5-HT in the mouse hippocampus, PUFAs may also enhance CUMS-induced depressive-like behaviors in the SPT, OFT, and FST (Yang et al., 2019). More importantly, a previous clinical trial has been carried out as a result of the positive safety and antidepressant characteristics of EPA and DHA (Su et al., 2014). Nobody left the study during the 2 weeks due to adverse events and, as determined by the investigators, the incidence of Interferon-alpha (IFN-α)-induced depression in patients with hepatitis C virus infection was significantly lower in those treated with EPA but not in those treated with DHA (Su et al., 2014). In a population-based study to prevent the risk of postpartum depression in Brazilian pregnant women, a daily intake of 1.8 g of PUFAs (1.08 g of EPA and 0.72 g of DHA) for 16 weeks starting at 22–24 weeks of gestation had no significant effect on early depressive symptoms during pregnancy or postpartum (Vaz et al., 2017). However, the Edinburgh Postnatal Depression Scale (EPDS) scores of women in the EPA/DHA group with a history of depression showed a greater decrease from the second trimester to the postpartum period. Additionally, there were no changes between the EPA/DHA groups and control groups in terms of gestational duration or birth weight (Vaz et al., 2017). According to a recent meta-analysis, both EPA and DHA have antidepressant effects, although EPA’s are more potent (Sublette et al., 2011).
Resolvin D1
Resolvin D1, a PUFA metabolite mostly found in deep-sea fish and shrimp, is effective in reducing inflammation by activating Akt and binding to 2 G-protein-coupled receptors (ALX and GPR32) (Serhan and Chiang, 2008; Nelson et al., 2014). Resolvin D1 reduces the depressive-like behavior seen in experimental models of myocardial infarction when administered before ischemia or 5 minutes after reperfusion (Gilbert et al., 2014). In the FST, there was a statistically significant relationship between infarct size, and immobility time (Gilbert et al., 2014). After myocardial infarction, inflammation is indeed well-documented, especially in the first hours of reperfusion (Sharma and Das, 1997; Nah and Rhee, 2009). Therefore, the anti-inflammatory effect may be the reason for its antidepressant-like function.
Krill oil
Antarctic krill (Euphausia superba), a zooplankton that resembles shrimp and is rich in EPA, DHA, and astaxanthin, is used to produce krill oil (Wibrand et al., 2013). The conditioned Light Extinction Test (CLET) - induced depressive-like behavior in the FST was reduced in rats after 7 weeks of krill oil administration. Additionally, krill oil reduced depressive-like behaviors by modifying the expression levels of synaptic plasticity-related genes in the prefrontal cortex and hippocampus (Wibrand et al., 2013). Moreover, krill oil supplementation in mice ameliorated chronic unpredictable mild stress (CUMS)-induced depressive-like behaviors by prompting the metabolism of glycerophospholipids and sphingolipids through regulation of differentially expressed genes mainly enriched in the membrane structures and neuroactive ligand-receptor interaction pathway (Zhang et al., 2023). Additionally, Krill oil facilitated fear extinction and reduced depressive-like behaviors by increasing hippocampal calcineurin A levels in mice (Alvarez-Ricartes et al., 2018).
5,6-dibromo-N, N-dimethyltryptamine (5,6-Br-DMT)
5,6-dibromo-N, N-dimethyltryptamine (5,6-Br-DMT) was isolated as a pale light yellow crystal. The precise mechanism underlying how 5,6-Br-DMT [20 mg/kg, intraperitoneally, (i.p.)] ameliorated depressive-like behaviors in the FST and TST in mice has not been determined. Indole alkaloids related to 5,6-Br-DMT have been found to have a strong affinity for 5-HT2 receptors, indicating that their antidepressant effects may be caused by inhibition of 5-HT reuptake (Hu et al., 2002).
Astaxanthin
The red carotenoid pigment astaxanthin is abundant in microalgae, salmon, trout, and marine invertebrates (Ávila-Román et al., 2021). It has numerous pharmacological properties, such as anti-inflammatory and antioxidant activities (Wu et al., 2015; Balietti et al., 2016). Trans-astaxanthin (20–80 mg/kg, p.o.) for 7 days prevented mice from displaying depressive-like symptoms after being exposed to LPS (Jiang et al., 2016). Neurochemical analysis showed that trans-astaxanthin could also reverse LPS-induced overexpression of IL-1β, IL-6, and TNF-α, and reduce the expression of inducible nitric oxide synthase (iNOS), neuronal nitric oxide synthase (nNOS), and COX-2 by modulating the NF-κB pathway (Jiang et al., 2016). In conclusion, trans-astaxanthin may produce antidepressant effects through its potent anti-inflammatory properties (Jiang et al., 2016). Similarly, administration of astaxanthin (20–80 mg/kg, i.g.) to mice improved their depressive-like behavior and reduced immobility time during the FST and TST (Jiang et al., 2017). Pretreatment with para-chlorophenylalanine (PCPA) (a 5-HT synthesis inhibitor) abolished the anti-immobility effect of Astaxanthin in FST and TST, suggesting that the mechanism of the antidepressant-like effects of Astaxanthin may involve the 5-HT system (Jiang et al., 2017). More importantly, a clinical trial investigated the effects of Astaxanthin on 28 adults diagnosed with depression and fatigue. The study also recruited healthy, active, and non-depressed adults. Subjects who received 12 mg of Astaxanthin daily for 8 weeks significantly reduced depression and fatigue, compared to the group who received a matching placebo (Talbott et al., 2019).
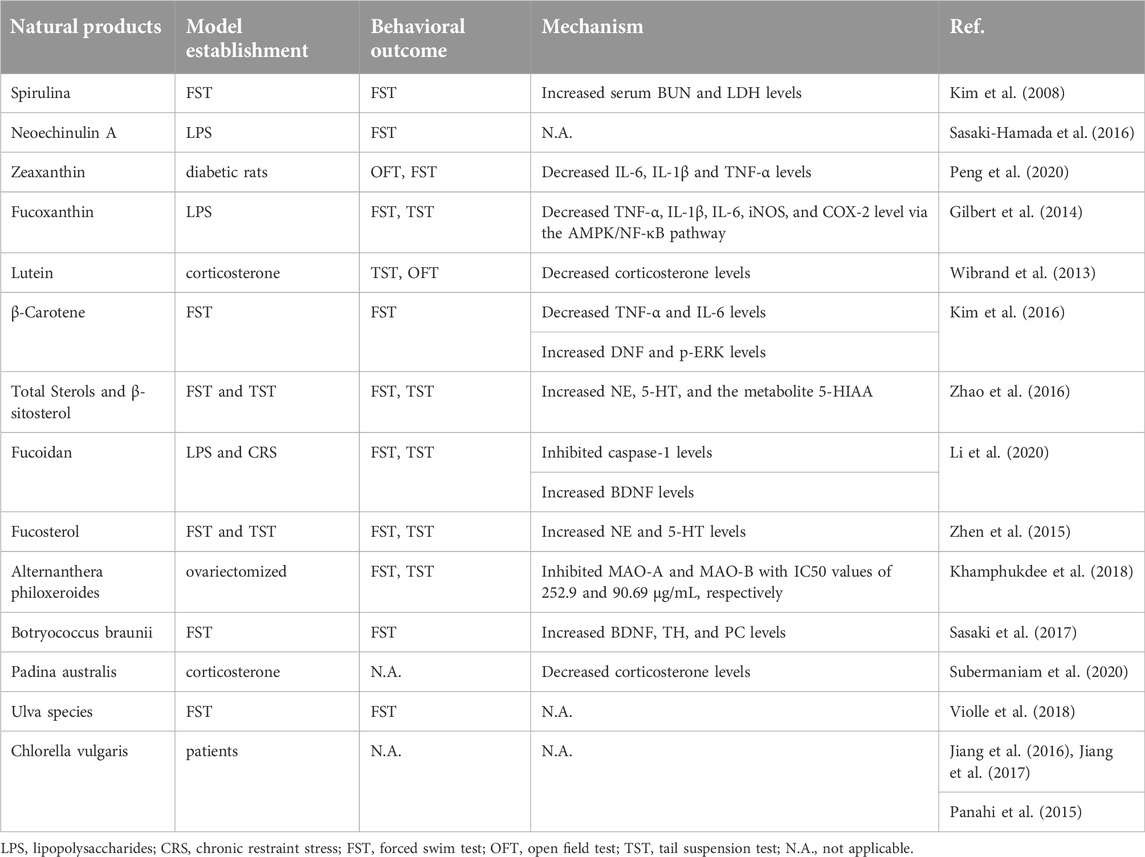
Natural antidepressants derived from marine plants
Spirulina
Spirulina is a kind of true filamentous spiral cyanobacteria protoplasm that has the biological activities of enhancing immunity, antioxidation, reducing cholesterol levels, and relieving hyperlipidemia (Kim et al., 2008; Subermaniam et al., 2021b). Hydrolyzed Spirulina by malted barley reduces immobility time on FST in mice and increases serum blood urea nitrogen (BUN) and LDH levels (Kim et al., 2008). Moreover, Spirulina improved adolescent stress-induced anxiety and depressive-like symptoms via oxidative stress and alterations in prefrontal cortex BDNF and 5HT-3 receptors in female rats (Moradi-Kor et al., 2020). The specific mechanism needs to be further explored.
Neoechinulin A
Aspergillus amstelodami yielded Neoechinulin A, an isoprenyl indole alkaloid with antioxidant, anti-tumor, and anti-apoptotic properties from Aspergillus fumigatus MR2012 from the Red Sea. Neoechinulin A [300 ng/kg, Intracerebroventricularly, (i.c.v.)] significantly ameliorated memory decline caused by LPS and restored immobility time in the FST in mice. This effect may be due to modulation of the 5-HT system by direct or indirect action on the 5-HT1A receptor (Sasaki-Hamada et al., 2016).
Zeaxanthin
Zeaxanthin, a yellow-orange xanthophyll, has been extracted from the cyanobacteria Synechocystis sp. and Microcystis aeruginosa and the microalgae Nannochloropsis oculate (Lee et al., 2006; Wojtasiewicz and Stoń-Egiert, 2016). Daily oral zeaxanthin administration from weeks 6–19 could reduce depressive-like behaviors in the OFT and FST of diabetic rats. Zeaxanthin administration could also reduce IL-6, IL-1, and TNF-α overproduction, indicating that it has anti-inflammatory characteristics that help minimize depressive-like behaviors in diabetic rats (Zhou et al., 2018a).
Fucoxanthin
Fucoxanthin, a natural carotenoid, is abundant in edible brown seaweed and has been shown to have excellent antioxidant, anti-inflammatory, and anti-diabetic effects (Méresse et al., 2020; Bustamam et al., 2021). In the FST and TST of mice, fucoxanthin (200 mg/kg, i.g.) significantly reversed LPS-induced depressive-like behaviors (Jiang et al., 2019). Biochemical analysis showed that Fucoxanthin could inhibit LPS-induced overexpression of IL-1β, IL-6, TNF-α, iNOS, and COX-2 in the hippocampus, frontal cortex, and hypothalamus by regulating the AMPK-NF-κB signaling pathway (Jiang et al., 2019).
Lutein
Lutein, orange-yellow, is mainly found in microalgae and Chlorella vulgaris (Jalali Jivan and Abbasi, 2019; Wang et al., 2020), and has neuroprotective effects (Stringham et al., 2019). In the TST, OFT, and Splash test (ST), lutein (10 mg/kg, p.o.) administered once daily for 7 or 21 days significantly reversed corticosterone-induced depressive-like behaviors. This suggests that Lutein may regulate the HPA to exert neuroprotective effects by reducing the level of glucocorticoids (Zeni et al., 2019).
β-Carotene
β-Carotene has been extracted mainly from the microalga Dunaliella salina (Han et al., 2019). β-Carotene has also been shown to be a potent inhibitor of oxidative stress and inflammation (Zhou et al., 2018b). Oral administration of β-carotene once daily for 28 days significantly reduced immobility time during the FST in mice (Kim et al., 2016). When compared to the control group, β-Carotene significantly reduced the levels of TNF-α and IL-6, and increased the levels of BDNF and pERK (Kim et al., 2016).
Total sterols and β-sitosterol
Total Sterols and β-sitosterol have been extracted from Sargassum horneri, a brown seaweed found in the Northwestern Pacific Ocean and adjacent seas of Korea, Japan, and China (Zhao et al., 2016). Total steroids and β-sitosterol have been used to treat scrofula, gall, goiter, and edema (Shao et al., 2014; Shao et al., 2015). In both the FST and TST, mice who received total sterols (100–200 mg/kg, p.o.) and β-sitosterol (10–30 mg/kg, i.p.) had significantly shorter immobility times. Additionally, NE, 5-HT, and the metabolite of 5-Hydroxyindoleacetic acid (5-HIAA) were all considerably elevated by total sterols and β-sitosterol in the mouse brain, suggesting that these neurotransmitters may be involved in mediating the antidepressant-like function (Zhao et al., 2016).
Fucoidan
Fucoidan is a bioactive sulfated polysaccharide abundant in brown seaweed with anti-inflammatory activity (Li et al., 2017). Fucoidan (50–100 mg/kg, p.o.) significantly attenuated LPS and chronic restraint stress (CRS) induced depressive-like behaviors in the TST and FST in mice (Li et al., 2020). Fucoidan also reduced the downregulation of BDNF-dependent synaptic plasticity in the mouse hippocampus and decreased caspase-1-mediated inflammation (Li et al., 2020). Furthermore, blocking BDNF abolished the antidepressant-like effects of fucoidan in mice, indicating that fucoidan ameliorates depression by inhibiting inflammation and modulating synaptic plasticity (Li et al., 2020).
Fucosterol
Fucosterol is a bioactive compound belonging to the sterol group that can be isolated from algae, seaweed and diatoms (Meinita et al., 2021). Fucosterol exhibits various biological activities including anticancer, anti-inflammatory, anti-neurological, and antioxidant characteristics (Lee et al., 2003; Jung et al., 2013; Gan et al., 2019). Fucosterol (10–40 mg/kg, i.p.) significantly shortened the immobility time in the FST and TST of mice. The expression of NE and 5-HT was strongly upregulated by fucosterol in the mouse brain, suggesting that fucosterol may act via these neurotransmitters (Zhen et al., 2015).
Alternanthera philoxeroides
Alternanthera philoxeroides is a true puree of filamentous, spiral-shaped, blue-green freshwater microalgae (Kim et al., 2008). The crude ethanolic extract of A. philoxeroides (250–500 mg/kg, p.o. once daily for 8 weeks) significantly ameliorated antidepressant-like behaviors in the FST and TST of ovariectomized mice (Khamphukdee et al., 2018). Additionally, it was discovered that the crude extract controlled the levels of BDNF in the frontal cortex and hippocampus. In addition, the crude ethanol extract of A. philoxeroides was found to inhibit both MAO-A and MAO-B with IC50 values of 252.9 and 90.69 μg/mL, respectively. These findings suggest that the antidepressant effect of the A. philoxeroides extract may be involved in regulating synaptic plasticity and inhibiting MAO activity (Khamphukdee et al., 2018).
Botryococcus braunii
Botryococcus braunii is a pyramid-shaped green colonial microalga that contains triterpenes (Cheng et al., 2019). Daily administration of B. braunii ethanol extract (100 mg/kg, for 14 days, p.o.) ameliorated depressive-like behaviors with decreased immobility in the FST (Sasaki et al., 2017). The administration of B. braunii ethanol extract induced upregulation of gene expression associated with energy metabolism (polyribonucleotide nucleotidyltransferase 1/PNPT1), dopamine production (arginine/serine-rich coiled-coil 1/SRC1), and neurogenesis (short stature homeobox 2/SHOX2, paired-like homeodomain transcription factor 2/PITX2, teashirt zinc finger family member 1/TSHZ1, LIM homeobox 9/LHX9). In addition, the expression of BDNF, tyrosine 3-monooxygenase (TH), and pyruvate carboxylase (PC) was also upregulated (Sasaki et al., 2017). The antidepressant effect of B. braunii in animal models of depression is mediated by enhancing energy promotion, neurogenesis, and dopamine synthesis in the brain.
Padina australis
Padina australis is a species of brown macroalgae belonging to the class Phaeophyceae (Subermaniam et al., 2021a). P. australis has been reported to possess numerous biological activities including antioxidant, anti-neuroinflammatory, and anti-acetylcholinesterase properties (Gany et al., 2014). pretreatment with P. australis (0.25 mg/mL) attenuated high-dose corticosterone-mediated oxidative damage in a PC12 cell model mimicking depression (Subermaniam et al., 2020). P. australis reversed the effects of corticosterone, which decreased cell viability, glutathione levels, aconitase activity, and mitochondrial membrane potential while increasing the release of lactate dehydrogenase. This finding indicates that P. australis could be developed as a mitochondria-targeted antioxidant to mitigate antidepressant-like effects (Subermaniam et al., 2020).
Ulva species
Ulva species are green macroalgae found in marine, fresh, and brackish waters. U. species are widely distributed throughout the world with 18 species identified in Japan (Shimada et al., 2008). Acute and subchronic oral toxicity studies showed that 10–40 mg/kg body weight/day of hydrophilic extract of U. species for 14 days significantly reduced the immobility time in the FST in rats (Violle et al., 2018). U. species have the potential to be a useful supplement or replacement for currently prescribed antidepressants. Further studies are necessary to confirm the mechanism of action of MSP and its modulation of brain function (Violle et al., 2018).
Chlorella vulgaris
Chlorella vulgaris is a unicellular green microalgae with many pharmacological properties that include antioxidant, anti-inflammatory, antihypertensive, detoxifying, and anti-atherosclerotic effects (Panahi et al., 2012a; Panahi et al., 2012b). A clinical trial investigated the effects of C. vulgaris Beijerinck on 92 patients with major depression. 42 patients were assigned to adjuvant therapy with C. vulgaris, while 50 patients received standard antidepressant therapy. Participants in the C. vulgaris intervention group received six 300 mg tablets per day for 6 weeks, and the intervention group showed improvements in somatic and cognitive symptoms of depression and anxiety (Panahi et al., 2015).
Current regulatory situation and commercialization of MNPs
MNPs are the source of modern marine pharmaceuticals. The study of MNPs, which originated in the 1930 s, can be regarded as the starting point of modern marine drug research. So far, about 33,200 new MNPs have been reported. Based on these new MNPs, the FDA has approved eight marine drugs, i.e., Cefalotin, Alexan, Zikonotide, Omega-3 fatty acid ethyl ester, Ericline mesylate, Brentuximab vedotin, and Trabectedin. Research on MNPs in China began in the 1970 s. In China, the first Marine Pharmaceutical Symposium was held in 1979. In 1982, the journal “Chinese Marine Drugs” was founded. In 1985, the first marine polysaccharide new drug, alginate diester sodium (for cardiovascular disease), was successfully developed and approved for marketing in China in 1990. In view of the unique structure and significant activity of MNPs, the Ministry of Science and Technology launched the Marine “863” Science and Technology Project (“863” Marine Biotechnology Research Program) in 1996. The National Natural Science Foundation of China also separated marine drugs from medicinal chemistry and funded them separately in 2008. These initiatives have greatly promoted the development of marine natural products in China and trained a group of excellent marine drug researchers. So far, about 6,700 new MNPs have been found in China, accounting for approximately 20% of the world’s new MNPs.
Discussion
With the continuous development of modern society, the incidence of depression is increasing, but the existing antidepressant drugs are not effective enough to meet the clinical needs. Therefore, the need for novel, effective antidepressant treatments is critical. In total, 95% of biodiversity and 71% of the Earth’s surface are in the oceans (Haefner, 2003; Sagar et al., 2010). The physical and chemical conditions of the ocean provide marine organisms with unique active compounds that offer new possibilities for the development of new drugs. The data presented in this review shows the great value of MNPs and their derivatives in the prevention and treatment of depression, demonstrating the potential of MNPs as a promising source of antidepressant drugs. Through a variety of processes, such as the modulation of neurotransmitter systems, synaptic plasticity, anti-inflammatory qualities, and the modulation of HPA function, these MNPs exhibit antidepressant properties. However, most of the current efficacy of MNPs and derivatives in the treatment of depression is based on data from in vitro and in vivo studies, and a large number of clinical studies are still needed to prove their safety and efficacy, which will help to develop promising new medicines. With the in-depth exploration of marine organisms by mankind, an increasing number of new compounds will be continuously extracted and isolated from marine organisms, which will bring new impetus to the treatment of depression, a disease that plagues the world.
Author contributions
Conceptualization: XW and CW; literature collection and summarization: XZ, CY, and WL; data curation: XW and CY; writing–original draft preparation: XW and CY; writing–review and editing: XW and CW. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Zhejiang Province Medical and Health Technology Project (2024KY503) and Special Fund for Economic and Technological Development of Longgang District, Shenzhen (LGWJ2021-124).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank all authors for their contributions to the article.
References
Alkon, D. L., Hongpaisan, J., and Sun, M. K. (2017). Effects of chronic bryostatin-1 on treatment-resistant depression in rats. Eur. J. Pharmacol. 807, 71–74. doi:10.1016/j.ejphar.2017.05.001
Alvarez-Ricartes, N., Oliveros-Matus, P., Mendoza, C., Perez-Urrutia, N., Echeverria, F., Iarkov, A., et al. (2018). Intranasal cotinine plus krill oil facilitates fear extinction, decreases depressive-like behavior, and increases hippocampal calcineurin A levels in mice. Mol. Neurobiol. 55 (10), 7949–7960. doi:10.1007/s12035-018-0916-0
Andraka, J. M., Sharma, N., and Marchalant, Y. (2020). Can krill oil be of use for counteracting neuroinflammatory processes induced by high fat diet and aging? Neurosci. Res. 157, 1–14. doi:10.1016/j.neures.2019.08.001
Ávila-Román, J., García-Gil, S., Rodríguez-Luna, A., Motilva, V., and Talero, E. (2021). Anti-inflammatory and anticancer effects of microalgal carotenoids. Mar. Drugs 19 (10), 531. doi:10.3390/md19100531
Balietti, M., Giannubilo, S. R., Giorgetti, B., Solazzi, M., Turi, A., Casoli, T., et al. (2016). The effect of astaxanthin on the aging rat brain: Gender-related differences in modulating inflammation. J. Sci. Food Agric. 96 (2), 4295–4618. doi:10.1002/jsfa.7865
Borbély, E., Simon, M., Fuchs, E., Wiborg, O., Czéh, B., and Helyes, Z. (2022). Novel drug developmental strategies for treatment-resistant depression. Br. J. Pharmacol. 179 (6), 1146–1186. doi:10.1111/bph.15753
Bustamam, M. S. A., Pantami, H. A., Azizan, A., Shaari, K., Min, C. C., Abas, F., et al. (2021). Complementary analytical platforms of NMR spectroscopy and LCMS analysis in the metabolite profiling of isochrysis galbana. Mar. Drugs 19 (3), 139. doi:10.3390/md19030139
Carlino, D., De Vanna, M., and Tongiorgi, E. (2013). Is altered BDNF biosynthesis a general feature in patients with cognitive dysfunctions? Neuroscientist 19 (4), 345–353. doi:10.1177/1073858412469444
Castrén, E., and Monteggia, L. M. (2021). Brain-derived neurotrophic factor signaling in depression and antidepressant action. Biol. Psychiatry 90 (2), 128–136. doi:10.1016/j.biopsych.2021.05.008
Chen, B., Dowlatshahi, D., MacQueen, G. M., Wang, J. F., and Young, L. (2001). Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol. Psychiatry 50 (4), 260–265. doi:10.1016/s0006-3223(01)01083-6
Cheng, P., Okada, S., Zhou, C., Chen, P., Huo, S., Li, K., et al. (2019). High-value chemicals from Botryococcus braunii and their current applications - a review. Bioresour. Technol. 291, 121911. doi:10.1016/j.biortech.2019.121911
Cipriani, A., Furukawa, T. A., Salanti, G., Chaimani, A., Atkinson, L. Z., Ogawa, Y., et al. (2018). Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Focus Am Psychiatr. Publ. 16 (4), 420–429. doi:10.1176/appi.focus.16407
Corona, J. C. (2018). Natural compounds for the management of Parkinson's disease and attention-deficit/hyperactivity disorder. BioMed Res. Int. 2018, 1–12. doi:10.1155/2018/4067597
Dean, J., and Keshavan, M. (2017). The neurobiology of depression: an integrated view. Asian J. Psychiatry 27, 101–111. doi:10.1016/j.ajp.2017.01.025
Dwivedi, Y. (2009). Brain-derived neurotrophic factor: Role in depression and suicide. Neuropsychiatric Dis. Treat. 5, 433–449. doi:10.2147/ndt.s5700
Dwivedi, Y., Rizavi, H. S., Zhang, H., Mondal, A. C., Roberts, R. C., Conley, R. R., et al. (2009). Neurotrophin receptor activation and expression in human postmortem brain: Effect of suicide. Biol. Psychiatry 65 (4), 319–328. doi:10.1016/j.biopsych.2008.08.035
Gan, S. Y., Wong, L. Z., Wong, J. W., and Tan, E. L. (2019). Fucosterol exerts protection against amyloid β-induced neurotoxicity, reduces intracellular levels of amyloid β and enhances the mRNA expression of neuroglobin in amyloid β-induced SH-SY5Y cells. Int. J. Biol. Macromol. 121, 207–213. doi:10.1016/j.ijbiomac.2018.10.021
Gany, S. A., Tan, S. C., and Gan, S. Y. (2014). Antioxidative, anticholinesterase and anti-neuroinflammatory properties of Malaysian Brown and green seaweeds. World academy of science, engineering and Technology. Int. J. Pharmacol. Pharm. Sci. 8 (11), 1269.
Gilbert, K., Bernier, J., Godbout, R., and Rousseau, G. (2014). Resolvin D1, a metabolite of omega-3 polyunsaturated fatty acid, decreases post-myocardial infarct depression. Mar. drugs 12 (11), 5396–5407. doi:10.3390/md12115396
Haefner, B. (2003). Drugs from the deep: Marine natural products as drug candidates. Drug Discov. Today 8 (12), 536–544. doi:10.1016/s1359-6446(03)02713-2
Han, J., Wang, L. U., Bian, H., Zhou, X., and Ruan, C. (2015). Effects of paroxetine on spatial memory function and protein kinase C expression in a rat model of depression. Exp. Ther. Med. 10 (4), 1489–1492. doi:10.3892/etm.2015.2663
Han, S. I., Kim, S., Lee, C., and Choi, Y. E. (2019). Blue-Red LED wavelength shifting strategy for enhancing beta-carotene production from halotolerant microalga, Dunaliella salina. J. Microbiol. 57 (2), 101–106. doi:10.1007/s12275-019-8420-4
Harmer, C. J., Duman, R. S., and Cowen, P. J. (2017). How do antidepressants work? New perspectives for refining future treatment approaches. lancet Psychiatry 4 (5), 409–418. doi:10.1016/s2215-0366(17)30015-9
Herman, J. P., McKlveen, J. M., Ghosal, S., Kopp, B., Wulsin, A., Makinson, R., et al. (2016). Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr. Physiol. 6 (2), 603–621. doi:10.1002/cphy.c150015
Hu, J. F., Schetz, J. A., Kelly, M., Peng, J. N., Ang, K. K. H., Flotow, H., et al. (2002). New antiinfective and human 5-HT2 receptor binding natural and semisynthetic compounds from the Jamaican sponge Smenospongia aurea. J. Nat. Prod. 65 (4), 476–480. doi:10.1021/np010471e
Huang, Y., Wang, Y., Wang, H., Liu, Z., Yu, X., Yan, J., et al. (2019). Prevalence of mental disorders in China: A cross-sectional epidemiological study. Lancet Psychiatry 6 (3), 211–224. doi:10.1016/s2215-0366(18)30511-x
Iqubal, A., Sharma, S., Najmi, A. K., Syed, M. A., Ali, J., Alam, M. M., et al. (2019). Nerolidol ameliorates cyclophosphamide-induced oxidative stress, neuroinflammation and cognitive dysfunction: plausible role of Nrf2 and NF- κB. Life Sci. 236, 116867. doi:10.1016/j.lfs.2019.116867
Jalali Jivan, M., and Abbasi, S. (2019). Nano based lutein extraction from marigold petals: Optimization using different surfactants and co-surfactants. Heliyon 5 (4), e01572. doi:10.1016/j.heliyon.2019.e01572
Jesulola, E., Micalos, P., and Baguley, I. J. (2018). Understanding the pathophysiology of depression: from monoamines to the neurogenesis hypothesis model - are we there yet? Behav. Brain Res. 341, 79–90. doi:10.1016/j.bbr.2017.12.025
Jiang, X., Chen, L., Shen, L., Chen, Z., Xu, L., Zhang, J., et al. (2016). Trans-astaxanthin attenuates lipopolysaccharide-induced neuroinflammation and depressive-like behavior in mice. Brain Res. 1649 (Pt A), 30–37. doi:10.1016/j.brainres.2016.08.029
Jiang, X., Wang, G., Lin, Q., Tang, Z., Yan, Q., and Yu, X. (2019). Fucoxanthin prevents lipopolysaccharide-induced depressive-like behavior in mice via AMPK- NF-κB pathway. Metab. Brain Dis. 34 (2), 431–442. doi:10.1007/s11011-018-0368-2
Jiang, X., Zhu, K., Xu, Q., Wang, G., Zhang, J., Cao, R., et al. (2017). The antidepressant-like effect of trans-astaxanthin involves the serotonergic system. Oncotarget 8 (15), 25552–25563. doi:10.18632/oncotarget.16069
Jimenez, P. C., Wilke, D. V., Branco, P. C., Bauermeister, A., Rezende-Teixeira, P., Gaudêncio, S. P., et al. (2020). Enriching cancer pharmacology with drugs of marine origin. Br. J. Pharmacol. 177 (1), 3–27. doi:10.1111/bph.14876
Jung, H. A., Jin, S. E., Ahn, B. R., Lee, C. M., and Choi, J. S. (2013). Anti-inflammatory activity of edible brown alga Eisenia bicyclis and its constituents fucosterol and phlorotannins in LPS-stimulated RAW264.7 macrophages. Food Chem. Toxicol. 59, 199–206. doi:10.1016/j.fct.2013.05.061
Khamphukdee, C., Monthakantirat, O., Chulikhit, Y., Buttachon, S., Lee, M., Silva, A., et al. (2018). Chemical constituents and antidepressant-like effects in ovariectomized mice of the ethanol extract of Alternanthera philoxeroides. Molecules 23 (9), 2202. doi:10.3390/molecules23092202
Kim, N. H., Jeong, H. J., Lee, J. Y., Go, H., Ko, S. G., Hong, S. H., et al. (2008). The effect of hydrolyzed Spirulina by malted barley on forced swimming test in ICR mice. Int. J. Neurosci. 118 (11), 1523–1533. doi:10.1080/00207450802325603
Kim, N. R., Kim, H. Y., Kim, M. H., Kim, H. M., and Jeong, H. J. (2016). Improvement of depressive behavior by Sweetme Sweet Pumpkin™ and its active compound, β-carotene. Life Sci. 147, 39–45. doi:10.1016/j.lfs.2016.01.036
Lee, M. Y., Min, B. S., Chang, C. S., and Jin, E. (2006). Isolation and characterization of a xanthophyll aberrant mutant of the green alga Nannochloropsis oculata. Mar. Biotechnol. (NY) 8 (3), 238–245. doi:10.1007/s10126-006-5078-9
Lee, S., Lee, Y. S., Jung, S. H., Kang, S. S., and Shin, K. H. (2003). Anti-oxidant activities of fucosterol from the marine algae Pelvetia siliquosa. Arch. Pharm. Res. 26 (9), 719–722. doi:10.1007/bf02976680
Lew, S. Y., Teoh, S. L., Lim, S. H., Lim, L. W., and Wong, K. H. (2020). Discovering the potentials of medicinal mushrooms in combating depression - a review. Mini-Reviews Med. Chem. 20 (15), 1518–1531. doi:10.2174/1389557520666200526125534
Li, J., Zhang, Q., Li, S., Dai, W., Feng, J., Wu, L., et al. (2017). The natural product fucoidan ameliorates hepatic ischemia-reperfusion injury in mice. Biomed. Pharmacother. 94, 687–696. doi:10.1016/j.biopha.2017.07.109
Li, M., Sun, X., Li, Q., Li, Y., Luo, C., Huang, H., et al. (2020). Fucoidan exerts antidepressant-like effects in mice via regulating the stability of surface AMPARs. Biochem. Biophysical Res. Commun. 521 (2), 318–325. doi:10.1016/j.bbrc.2019.10.043
Lima, E., and Medeiros, J. (2022). Marine organisms as alkaloid biosynthesizers of potential anti-alzheimer agents. Mar. Drugs 20 (1), 75. doi:10.3390/md20010075
Ling, Y., Liping, S., and Yongliang, Z. (2018). Preparation and identification of novel inhibitory angiotensin-I-converting enzyme peptides from tilapia skin gelatin hydrolysates: Inhibition kinetics and molecular docking. Food & Funct. 9 (10), 5251–5259. doi:10.1039/c8fo00569a
Liu, B., Zhang, Y., Yang, Z., Liu, M., Zhang, C., Zhao, Y., et al. (2021). ω-3 DPA protected neurons from neuroinflammation by balancing microglia M1/M2 polarizations through inhibiting NF-κB/MAPK p38 signaling and activating neuron-BDNF-PI3K/AKT pathways. Mar. Drugs 19 (11), 587. doi:10.3390/md19110587
Locher, C., Koechlin, H., Zion, S. R., Werner, C., Pine, D. S., Kirsch, I., et al. (2017). Efficacy and safety of selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, and placebo for common psychiatric disorders among children and adolescents: a systematic review and meta-analysis. JAMA psychiatry 74 (10), 1011–1020. doi:10.1001/jamapsychiatry.2017.2432
Matraszek-Gawron, R., Chwil, M., Terlecka, P., and Skoczylas, M. M. (2019). Recent studies on anti-depressant bioactive substances in selected species from the genera hemerocallis and gladiolus: a systematic review. Pharm. (Basel) 12 (4), 172. doi:10.3390/ph12040172
Meinita, M. D. N., Harwanto, D., Tirtawijaya, G., Negara, BFSP, Sohn, J. H., Kim, J. S., et al. (2021). Fucosterol of marine macroalgae: bioactivity, safety and toxicity on organism. Mar. Drugs 19 (10), 545. doi:10.3390/md19100545
Méresse, S., Fodil, M., Fleury, F., and Chénais, B. (2020). Fucoxanthin, a marine-derived carotenoid from Brown seaweeds and microalgae: a promising bioactive compound for cancer therapy. Int. J. Mol. Sci. 21 (23), 9273. doi:10.3390/ijms21239273
Moradi-Kor, N., Dadkhah, M., Ghanbari, A., Rashidipour, H., Bandegi, A. R., Barati, M., et al. (2020). <p>Protective effects of <em>Spirulina platensis</em>, voluntary exercise and environmental interventions against adolescent stress-induced anxiety and depressive-like symptoms, oxidative stress and alterations of BDNF and 5HT-3 receptors of the prefrontal cortex in female rats</p>. Neuropsychiatric Dis. Treat. 16, 1777–1794. doi:10.2147/ndt.s247599
Moragrega, I., and Ríos, J. L. (2021). Medicinal plants in the treatment of depression: evidence from preclinical studies. Planta. Medica. 87 (9), 656–685. doi:10.1055/a-1338-1011
Nah, D. Y., and Rhee, M. Y. (2009). The inflammatory response and cardiac repair after myocardial infarction. Korean Circ. J. 39 (10), 393–398. doi:10.4070/kcj.2009.39.10.393
Nelson, J. W., Leigh, N. J., Mellas, R. E., McCall, A. D., Aguirre, A., and Baker, O. J. (2014). ALX/FPR2 receptor for RvD1 is expressed and functional in salivary glands. Am. J. Physiology-Cell Physiology 306 (2), C178–C185. doi:10.1152/ajpcell.00284.2013
Nelson, T. J., Sun, M. K., Lim, C., Sen, A., Khan, T., Chirila, F. V., et al. (2017). Bryostatin effects on cognitive function and PKCɛ in alzheimer's disease phase IIa and expanded access trials. J. Alzheimer's Dis. 58 (2), 521–535. doi:10.3233/jad-170161
Panahi, Y., Badeli, R., Karami, G. R., Badeli, Z., and Sahebkar, A. (2015). A randomized controlled trial of 6-week Chlorella vulgaris supplementation in patients with major depressive disorder. Complementary Ther. Med. 23 (4), 598–602. doi:10.1016/j.ctim.2015.06.010
Panahi, Y., Ghamarchehreh, M. E., Beiraghdar, F., Zare, R., Jalalian, H. R., and Sahebkar, A. (2012b). Investigation of the effects of Chlorella vulgaris supplementation in patients with non-alcoholic fatty liver disease: A randomized clinical trial. Hepato-gastroenterology 59 (119), 2099–2103. doi:10.5754/hge10860
Panahi, Y., Tavana, S., Sahebkar, A., Masoudi, H., and Madanchi, N. (2012a). Impact of adjunctive therapy with chlorellav ulgaris extract on antioxidant status, pulmonary function, and clinical symptoms of patients with obstructive pulmonary diseases. Sci. Pharm. 80 (3), 719–730. doi:10.3797/scipharm.1202-06
Pandey, G. N., Sharma, A., Rizavi, H. S., and Ren, X. (2021). Dysregulation of protein kinase C in adult depression and suicide: evidence from postmortem brain studies. Int. J. Neuropsychopharmacol. 24 (5), 400–408. doi:10.1093/ijnp/pyab003
Peng, Z., Zhang, C., Yan, L., Zhang, Y., Yang, Z., Wang, J., et al. (2020). EPA is more effective than DHA to improve depression-like behavior, glia cell dysfunction and hippcampal apoptosis signaling in a chronic stress-induced rat model of depression. Int. J. Mol. Sci. 21 (5), 1769. doi:10.3390/ijms21051769
Rana, T., Behl, T., Sehgal, A., Mehta, V., Singh, S., Sharma, N., et al. (2021). Elucidating the possible role of FoxO in depression. Neurochem. Res. 46 (11), 2761–2775. doi:10.1007/s11064-021-03364-4
Russo, P., Del Bufalo, A., and Fini, M. (2015). Deep sea as a source of novel-anticancer drugs: Update on discovery and preclinical/clinical evaluation in a systems medicine perspective. EXCLI J. 14, 228–236. doi:10.17179/excli2015-632
Sagar, S., Kaur, M., and Minneman, K. P. (2010). Antiviral lead compounds from marine sponges. Mar. Drugs 8 (10), 2619–2638. doi:10.3390/md8102619
Sasaki, K., Othman, M. B., Demura, M., Watanabe, M., and Isoda, H. (2017). Modulation of neurogenesis through the promotion of energy production activity is behind the antidepressant-like effect of colonial green alga, Botryococcus braunii. Front. Physiol. 8, 900. doi:10.3389/fphys.2017.00900
Sasaki-Hamada, S., Hoshi, M., Niwa, Y., Ueda, Y., Kokaji, A., Kamisuki, S., et al. (2016). Neoechinulin A induced memory improvements and antidepressant-like effects in mice. Prog. Neuro-Psychopharmacology Biol. Psychiatry 71, 155–161. doi:10.1016/j.pnpbp.2016.08.002
Serhan, C. N., and Chiang, N. (2008). Endogenous pro-resolving and anti-inflammatory lipid mediators: A new pharmacologic genus. Br. J. Pharmacol. 153 (Suppl. 1), S200–S215. doi:10.1038/sj.bjp.0707489
Shao, P., Chen, X., and Sun, P. (2014). Chemical characterization, antioxidant and antitumor activity of sulfated polysaccharide from Sargassum horneri. Carbohydr. Polym. 105, 260–269. doi:10.1016/j.carbpol.2014.01.073
Shao, P., Liu, J., Chen, X., Fang, Z., and Sun, P. (2015). Structural features and antitumor activity of a purified polysaccharide extracted from Sargassum horneri. Int. J. Biol. Macromol. 73, 124–130. doi:10.1016/j.ijbiomac.2014.10.056
Sharma, H. S., and Das, D. K. (1997). Role of cytokines in myocardial ischemia and reperfusion. Mediat. Inflamm. 6 (3), 175–183. doi:10.1080/09629359791668
Shen, C., Cao, K., Cui, S., Cui, Y., Mo, H., Wen, W., et al. (2021). Corrigendum to “SiNiSan ameliorates depression-like behavior in rats by enhancing synaptic plasticity via the CaSR-PKC-ERK signaling pathway” [Biomed. Pharmacother. 124 (2020) 109787]. Biomed. Pharmacother. 133, 110892. doi:10.1016/j.biopha.2020.110892
Shimada, S., Yokoyama, N., Arai, S., and Hiraoka, M. (2008). Phylogeography of the genus Ulva (Ulvophyceae, Chlorophyta), with special reference to the Japanese freshwater and brackish taxa. J. Appl. Phycol. 20 (5), 979–989. doi:10.1007/s10811-007-9296-y
Song, X., Li, Z., Li, Y., and Hou, H. (2022). Typical structure, biocompatibility, and cell proliferation bioactivity of collagen from Tilapia and Pacific cod. Colloids Surfaces B Biointerfaces 210, 112238. doi:10.1016/j.colsurfb.2021.112238
Stringham, N. T., Holmes, P. V., and Stringham, J. M. (2019). Effects of macular xanthophyll supplementation on brain-derived neurotrophic factor, pro-inflammatory cytokines, and cognitive performance. Physiology Behav. 211, 112650. doi:10.1016/j.physbeh.2019.112650
Su, K. P., Lai, H. C., Yang, H. T., Su, W. P., Peng, C. Y., Chang, J. P. C., et al. (2014). Omega-3 fatty acids in the prevention of interferon-alpha-induced depression: Results from a randomized, controlled trial. Biol. Psychiatry 76 (7), 559–566. doi:10.1016/j.biopsych.2014.01.008
Subermaniam, K., Teoh, S. L., Yow, Y. Y., Tang, Y. Q., Lim, L. W., and Wong, K. H. (2021a). Marine algae as emerging therapeutic alternatives for depression: a review. Iran. J. basic Med. Sci. 24 (8), 997–1013. doi:10.22038/ijbms.2021.54800.12291
Subermaniam, K., Teoh, S. L., Yow, Y. Y., Tang, Y. Q., Lim, L. W., and Wong, K. H. (2021b). Marine algae as emerging therapeutic alternatives for depression: a review. Iran. J. Basic Med. Sci. 24 (8), 997–1013. doi:10.22038/ijbms.2021.54800.12291
Subermaniam, K., Yow, Y. Y., Lim, S. H., Koh, O. H., and Wong, K. H. (2020). Malaysian macroalga Padina australis Hauck attenuates high dose corticosterone-mediated oxidative damage in PC12 cells mimicking the effects of depression. Saudi J. Biol. Sci. 27 (6), 1435–1445. doi:10.1016/j.sjbs.2020.04.042
Sublette, M. E., Ellis, S. P., Geant, A. L., and Mann, J. J. (2011). Meta-analysis of the effects of eicosapentaenoic acid (EPA) in clinical trials in depression. J. Clin. Psychiatry 72 (12), 1577–1584. doi:10.4088/jcp.10m06634
Talbott, S. M., Hantla, D., Capelli, B., Ding, L., Li, Y., and Artaria, C. (2019). Effect of astaxanthin supplementation on psychophysiological heart-brain Axis dynamics in healthy subjects. Funct. Foods Health Dis. 9 (8), 521–531. doi:10.31989/ffhd.v9i8.636
Vaz, J. D. S., Farias, D. R., Adegboye, A. R. A., Nardi, A. E., and Kac, G. (2017). Omega-3 supplementation from pregnancy to postpartum to prevent depressive symptoms: A randomized placebo-controlled trial. BMC Pregnancy Childbirth 17 (1), 180. doi:10.1186/s12884-017-1365-x
Violle, N., Rozan, P., Demais, H., Nyvall Collen, P., and Bisson, J. F. (2018). Evaluation of the antidepressant- and anxiolytic-like effects of a hydrophilic extract from the green seaweed Ulva sp. in rats. Nutr. Neurosci. 21 (4), 248–256. doi:10.1080/1028415x.2016.1276704
Wang, H., He, Y., Sun, Z., Ren, S., Liu, M., Wang, G., et al. (2022). Microglia in depression: An overview of microglia in the pathogenesis and treatment of depression. J. Neuroinflammation 19 (1), 132. doi:10.1186/s12974-022-02492-0
Wang, H., Yang, Y., Yang, S., Ren, S., Feng, J., Liu, Y., et al. (2021b). Ginsenoside Rg1 ameliorates neuroinflammation via suppression of Connexin43 ubiquitination to attenuate depression. Front. Pharmacol. 12, 709019. doi:10.3389/fphar.2021.709019
Wang, X., Zhang, M. M., Sun, Z., Liu, S. F., Qin, Z. H., Mou, J. H., et al. (2020). Sustainable lipid and lutein production from Chlorella mixotrophic fermentation by food waste hydrolysate. J. Hazard. Mater. 400, 123258. doi:10.1016/j.jhazmat.2020.123258
Wang, Y. T., Wang, X. L., Feng, S. T., Chen, N. H., Wang, Z. Z., and Zhang, Y. (2021a). Novel rapid-acting glutamatergic modulators: targeting the synaptic plasticity in depression. Pharmacol. Res. 171, 105761. doi:10.1016/j.phrs.2021.105761
Wardle, R. A., and Poo, M. M. (2003). Brain-derived neurotrophic factor modulation of GABAergic synapses by postsynaptic regulation of chloride transport. J. Neurosci. 23 (25), 8722–8732. doi:10.1523/jneurosci.23-25-08722.2003
Wibrand, K., Berge, K., Messaoudi, M., Duffaud, A., Panja, D., Bramham, C. R., et al. (2013). Enhanced cognitive function and antidepressant-like effects after krill oil supplementation in rats. Lipids health Dis. 12, 6. doi:10.1186/1476-511x-12-6
Wierda, K. D., Toonen, R. F., de Wit, H., Brussaard, A. B., and Verhage, M. (2007). Interdependence of PKC-dependent and PKC-independent pathways for presynaptic plasticity. Neuron 54 (2), 275–290. doi:10.1016/j.neuron.2007.04.001
Wojtasiewicz, B., and Stoń-Egiert, J. (2016). Bio-optical characterization of selected cyanobacteria strains present in marine and freshwater ecosystems. J. Appl. Phycol. 28, 2299–2314. doi:10.1007/s10811-015-0774-3
Wu, H., Niu, H., Shao, A., Wu, C., Dixon, B., Zhang, J., et al. (2015). Astaxanthin as a potential neuroprotective agent for neurological diseases. Mar. Drugs 13 (9), 5750–5766. doi:10.3390/md13095750
Yang, M., Xuan, Z., Wang, Q., Yan, S., Zhou, D., Naman, C. B., et al. (2021). Fucoxanthin has potential for therapeutic efficacy in neurodegenerative disorders by acting on multiple targets. Nutr. Neurosci. 25, 2167–2180. doi:10.1080/1028415x.2021.1926140
Yang, R., Zhang, M. Q., Xue, Y., Yang, R., and Tang, M. M. (2019). Dietary of n-3 polyunsaturated fatty acids influence neurotransmitter systems of rats exposed to unpredictable chronic mild stress. Behav. Brain Res. 376, 112172. doi:10.1016/j.bbr.2019.112172
Yirmiya, R., Rimmerman, N., and Reshef, R. (2015). Depression as a microglial disease. Trends Neurosci. 38 (10), 637–658. doi:10.1016/j.tins.2015.08.001
Yohn, C. N., Gergues, M. M., and Samuels, B. A. (2017). The role of 5-HT receptors in depression. Mol. Brain 10 (1), 28. doi:10.1186/s13041-017-0306-y
Zeni, A. L. B., Camargo, A., and Dalmagro, A. P. (2019). Lutein prevents corticosterone-induced depressive-like behavior in mice with the involvement of antioxidant and neuroprotective activities. Pharmacol. Biochem. Behav. 179, 63–72. doi:10.1016/j.pbb.2019.02.004
Zhang, H., Liu, X., Li, B., Zhang, Y., Gao, H., Zhao, X., et al. (2023). Krill oil treatment ameliorates lipid metabolism imbalance in chronic unpredicted mild stress-induced depression-like behavior in mice. Front. Cell Dev. Biol. 11, 1180483. doi:10.3389/fcell.2023.1180483
Zhao, D., Zheng, L., Qi, L., Wang, S., Guan, L., Xia, Y., et al. (2016). Structural features and potent antidepressant effects of total sterols and β-sitosterol extracted from Sargassum horneri. Mar. Drugs 14 (7), 123. doi:10.3390/md14070123
Zhao, Y. T., Yin, H., Hu, C., Zeng, J., Shi, X., Chen, S., et al. (2022b). Tilapia skin peptides restore cyclophosphamide-induced premature ovarian failure via inhibiting oxidative stress and apoptosis in mice. Food & Funct. 13 (3), 1668–1679. doi:10.1039/d1fo04239d
Zhao, Y. T., Yin, H., Hu, C., Zeng, J., Zhang, S., Chen, S., et al. (2022a). Tilapia skin peptides ameliorate cyclophosphamide-induced anxiety- and depression-like behavior via improving oxidative stress, neuroinflammation, neuron apoptosis, and neurogenesis in mice. Front. Nutr. 9, 882175. doi:10.3389/fnut.2022.882175
Zhen, X. H., Quan, Y. C., Jiang, H. Y., Wen, Z. S., Qu, Y. L., and Guan, L. P. (2015). Fucosterol, a sterol extracted from Sargassum fusiforme, shows antidepressant and anticonvulsant effects. Eur. J. Pharmacol. 768, 131–138. doi:10.1016/j.ejphar.2015.10.041
Zhou, L., Ouyang, L., Lin, S., Chen, S., Liu, Y., Zhou, W., et al. (2018b). Protective role of β-carotene against oxidative stress and neuroinflammation in a rat model of spinal cord injury. Int. Immunopharmacol. 61, 92–99. doi:10.1016/j.intimp.2018.05.022
Zhou, S., Chen, R., She, Y., Liu, X., Zhao, H., Li, C., et al. (2022). A new perspective on depression and neuroinflammation: non-coding RNA. J. Psychiatric Res. 148, 293–306. doi:10.1016/j.jpsychires.2022.02.007
Keywords: depression, marine natural products, neurotransmitter systems, synaptic plasticity, anti-inflammatory
Citation: Wang X, Yang C, Zhang X, Ye C, Liu W and Wang C (2024) Marine natural products: potential agents for depression treatment. Acta Biochim. Pol 71:12569. doi: 10.3389/abp.2024.12569
Received: 15 December 2023; Accepted: 08 March 2024;
Published: 30 April 2024.
Edited by:
Grzegorz Wegrzyn, University of Gdansk, PolandReviewed by:
Mohamad Taufik Hidayat Baharuldin, National Defence University of Malaysia, MalaysiaŁukasz Grabowski, University of Gdansk, Poland
Copyright © 2024 Wang, Yang, Zhang, Ye, Liu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chengmin Wang, Y2hlbmdtaW53YW5nQHRvbS5jb20=
†These authors have contributed equally to this work
 Xunqiang Wang†
Xunqiang Wang† Chengmin Wang
Chengmin Wang