- Department of Viral Immunology, Biomedical Research Center SAS, Bratislava, Slovakia
Tick-borne encephalitis virus (TBEV, Flaviviridae), a small enveloped flavivirus with an unsegmented positive-stranded RNA genome, is the most prominent member of the mammalian group of tick-borne flaviviruses. TBEV, originally isolated in 1937, is identified as Orthoflavivirus encephalitidis now. TBEV causes the most important arboviral disease of the human central nervous system (CNS) in Europe and Northeast Asia. It is transmitted to hosts primarily by ticks of the genus Ixodes and Dermacentor, but can also be acquired by ingestion of infected unpasteurized dairy products. Approximately one-third of all human TBEV infections are associated with severe clinical neurological disease. The remaining two-thirds are asymptomatic or present with mild clinical symptoms. In hosts, TBEV tend to induce different types of immune effector mechanisms. Components of innate immunity - natural killer cells, complement proteins, macrophages and dendritic cells usually provide rapid and intense protection in the acute phase of infectious diseases. In turn, cell-mediated immunity provided by T and B lymphocytes plays an important role in virus clearance and protective immunity, and thus influences the outcome of disease. The virus-host relationship is not passive. Therefore, viruses themselves respond actively to host immune defence activities. This is made possible by a number of mechanisms that ensure their escape from the host’s immune surveillance. The aim of this review is to summarize the history of the last 50 years as well as advances in research on the immunology of TBEV, specifically in the Central European area.
Introduction
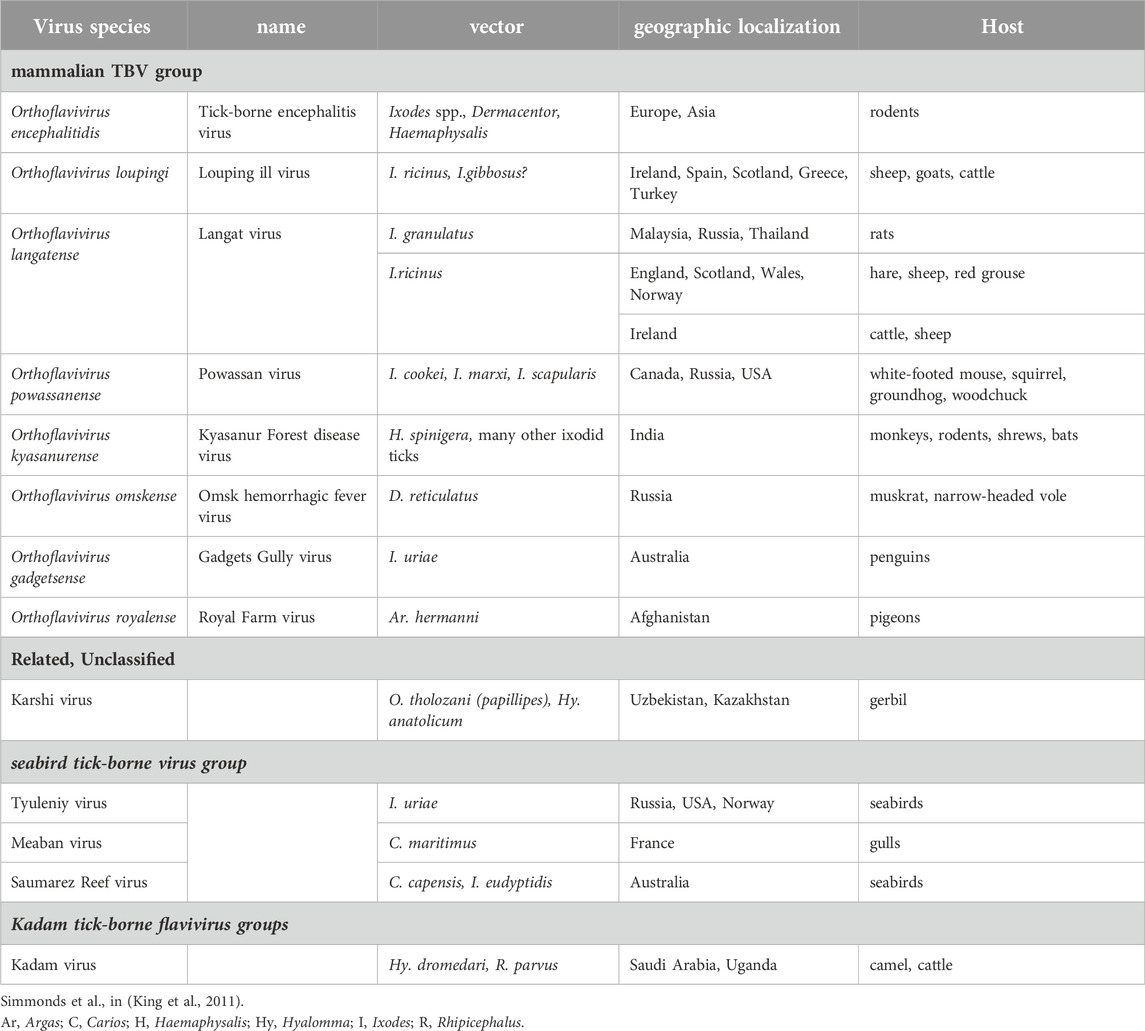
The family Flaviviridae represents a diverse group of small enveloped viruses classified into four genera: Orthoflavivirus, Pestivirus, Hepacivirus and Pegivirus. Only genus Orthoflavivirus comprises arboviruses, of which about 28% are tick-borne (Simmonds et al., 2017). By a phylogenetic analysis, tick-borne flaviviruses (TBFVs) are divided into three distinct groups, i.e., a group associated with mammals and sea-birds, respectively, and the Kadam virus that forms a third evolutionary lineage (Table 1) (Gaunt et al., 2001; Grard et al., 2007). TBFVs persist in nature through circulation between vector ticks and vertebrate hosts. To survive, TBFVs have adapted to two entirely dissimilar inner environments, invertebrate vector and vertebrate hosts, and are capable to infect and multiply in both of them (Nuttall, 2009). Of the viruses transmitted by ticks, the mammalian TBFVs, involving serologically and genetically related viruses, are considered the most important affecting human health globally. These viruses cause serious neurological illnesses and hemorrhagic fevers and pose a significant public health problem due to re-emergence and spread to new geographic areas, the growing number of human outbreaks and high rates of morbidity and mortality. The number of identified members of mammalian TBFVs has increased in recent years (Carpio et al., 2023).
Of the mammalian TBFVs, of particular concern in the Central Europe is tick-borne encephalitis virus (TBEV), causing tick-borne encephalitis (TBE), the disease affecting the central nervous system (CNS).
Since this review is dedicated to a special issue “Virology in Central Europe: Past, Present, and Future” we performed a search of different database MEDLINE/PubMed, CDC, ECDC, eLibrary, institutional library and archives using key words – “TBEV AND innate immunity;” “TBEV AND adaptive immunity;” “TBEV AND cell immunity;” “TBEV AND dendritic cells;” TBEV AND NK cells;” TBEV AND T-cells;” TBEV AND antibodies.” We provide an inclusive compilation of studies focused on the European subtype of this virus (TBEV-Eu) and conducted mainly in laboratories of Central European countries involving Austria, Czech Republic, Germany, Hungary, Poland, Slovenia, Slovakia and Switzerland or with collaborations (European affiliation included). Moreover, despite a key role of ticks in life cycle of TBEV, mutual interactions between vector and virus in context of tick immunity are beyond the scope of this paper.
The study is devided on several chapters; the first is focused on a brief characterization of TBEV and associated disease, on virus circulation in nature through vectors and hosts. In the following three chapters, which represents the essential part of this study, the facts known so far about the antiviral mechanisms of both innate and acquired immunity, including vaccination, are processed and summarized. In the section on innate immune reactions, we focus on both cellular (dendritic and NK cells, macrophages, neutrophils, etc.) and soluble (interferons, cytokines/chemokines) components and their role in defense against TBEV.
Tick-borne encephalitis virus (TBEV) – background, epidemiology, vectors, hosts, pathogenesis
Tick-borne encephalitis virus (TBEV), is a small enveloped flavivirus, approximately 50 nm in diameter. TBEV has ∼11 kb long unsegmented single-stranded positive RNA genome, encoding a single polyprotein that is processed co- and post-translational into three structural (C, prM, E) and seven non-structural (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) proteins. Based on phylogenetic analysis, the TBEV has been divided into three main subtypes, European (Eu), Siberian (Sib) and Far Eastern (FE) that are transmitted mainly by Ixodes ricinus (TBEV-Eu) or I. persulcatus ticks (TBEV-Sib, -FE), respectively (Labuda and Randolph, 1999; Kovalev and Mukhacheva, 2017). In addition to the three main TBEV subtypes, other subtypes, i.e., the Baikalian (TBEV-Bkl) (Dai et al., 2018), the Himalayan (TBEV-Him) (Tkachev et al., 2017), the TBEV2871 (Obskaja), have been described recently (Deviatkin et al., 2020). TBEV-Eu is prevalent across Europe, from Ural to France (east-west) from Scandinavia to Adriatic see (north-south), while TBEV-Sib and TBEV-FE extend from Finland and the Baltic countries through Central and Eastern Asia to Japan (Im et al., 2020; Shah et al., 2023). However, the geographical distribution of TBEV has been constantly expanding due to lack of efficient control measures and changes in global socio-economic and climatic conditions leading to a wide spread of tick vectors. TBEV, the most prominent member of the mammalian group of TBFVs, is the most prevalent arbovirus in Europe and Asia causing tick-borne encephalitis (TBE), the disease affecting CNS and diagnosed in ∼12,000 people per year. The fatality rate of TBEV-Eu is approxim. 1%–3% with neuroinvasive case (>40%) resulting in long-term neurological sequelae.
Generally, infections with the TBEV-Eu subtype range from asymptomatic course through influenza-like uncomplicated meningitis to life-threatening meningoencephalitis and myelitis (Pikelj et al., 1995; Bogovič et al., 2018). In 72%–87% of patients infected with TBEV-Eu, the infection usually has a two-phase course. A short incubation period (7–14 days, in extreme cases 4–28 days) is followed by a first (viremic) phase with an atypical flu-like illness lasting 2–4 days (range: 1–8 days) with fever. Common symptoms are malaise, headache, myalgia, gastrointestinal symptoms, leukocytopenia, thrombocytopenia and elevated liver enzymes. Often, before the second stage, there is an asymptomatic period (about 1-week, general range: 1–33 days). Seroconversion without significant morbidity is also common. In 20%–30% of infected patients, the disease can go into the second stage of TBE, which is characterized by four clinical features of varying severity – meningitis (≈50% of adult patients), meningoencephalitis (≈40%), meningoencephalomyelitis (≈10%) or meningoencephaloradiculitis (rev. in Kaiser, 2008; Kaiser, 2012; Ruzek et al., 2010). The appearance of specific antibodies in serum and cerebrospinal fluid (CSF) is typical. The mortality rate of adult patients is less than 2%. Factors determining clinical variability of TBEV infection remain controversial and outcome unpredictable, but animal model data suggest that CNS pathology is largely driven by live host immune response. These assumptions are supported by studies of Saksida et al. (2005), Saksida et al. (2018) on human patients who lacked detectable TBEV in CSF, showing only a weak correlation in the local incidence of TBEV antigen and inflammation of the cerebral parenchyma (Gelpi et al., 2005). The inflammatory response in the CNS has pathological effects (Ruzek et al., 2009a). However, in some cases, Slovenian researchers have found that the severity of TBE was independent of the initial viremia (Saksida et al., 2018).
Over the past decades, tick-borne encephalitis (TBE) has become a growing public health concern and is the most important viral tick-borne disease in Europe (Beauté et al., 2018), since 1931, when an outbreak of an “acute epidemic serious meningitis” was reported in south-eastern Austria (Amicizia et al., 2013). The first European TBEV (TBEV-Eu) was isolated in Czechoslovakia after the Second World War in 1948 (Feal, 1949; Krejcí, 1949). Since then, the scientific community in Central European countries has been intensively engaged in several areas related to TBEV research. The research objectives (ecology, epidemiology, prevention, diagnosis, management of TBEV, immunology and others) and scientific approaches were mainly determined by the available methodologies (Pospíšil et al., 1954; Nosek et al., 1970; Radda, 1973; Minár, 1995; Gresíková and Sekeyová, 1978; Kunz, 1992; Palanova et al., 1992; Gresikova and Kaluzova, 1997; Grešíková, 1999; Pazdiora et al., 2000; Daniel et al., 2006; 2011; Svobodova et al., 2010; Kříž et al., 2009; Kříž et al., 2013; rev. in Ruzek et al., 2019; Hubálek, 2021).
In 2001, TBE became a notifiable disease in Germany (Süss et al., 2004; Hellenbrand et al., 2019) and at the EU level in 2012 (European Centre for Disease Prevention and Control, 2022). Updated standardized TBE incidence maps are provided regularly by the European Center for Disease Prevention and Control (ECDC). Since 1998, the International Scientific Working Group for Tick-borne Encephalitis (ISW-TBE, 2016) officially began to operate. Scientists from several fields - virology, epidemiology, medicine from more than 30 different European countries cooperate in the basic and applied research of TBE. Amongst others things, the main goals are a raising awareness of TBE in endemic and non-endemic countries, increasing vaccination coverage in different countries, recognizing TBE as a travel risk, and building contacts with the ECDC.
Currently, there has been an alarming increase in TBE cases in countries such as Sweden, France, the Czech Republic, Denmark and Slovakia. In 2022, 3,650 cases of tick-borne encephalitis (TBE) were reported, 96.3% of which were confirmed; and more frequently among men and in the age group 45–64 years. In 2023, from the countries of Central Europe, the most cases were reported in the Czech Republic, closely followed by Germany and Poland. Most cases of TBE are reported during the period of peak tick activity, which in Central Europe lasts from April to November (Satapathy et al., 2023).
The circulation and persistence of TBEV in nature is provided by biological transmission through a competent tick vector and by broad range of hosts from terrestrial vertebrates - amphibians, reptiles, birds and mammals (Figure 1) (Süss, 2003) while some group of mammals (rodents, insectivores, carnivores, etc.) comprise natural reservoirs of the virus (Michelitsch et al., 2019; Kwasnik et al., 2023). Humans are an accidental and a dead-end host. The relationships between ticks and the transmitted viruses are highly specific, only approximately 10% of the 900 known tick species have been proved to be vectors (Labuda and Nuttall, 2008). Due to its long lifespan, the tick is the main reservoir of the virus (Řeháček, 1965; Řeháček, 1960; Slovák et al., 2014). The principal vectors of TBEV-Eu are the castor bean tick, I. ricinus (Süss et al., 1996; Süss et al., 2006) and ornate dog tick D. reticulatus (Nosek et al., 1984; Nosek and Kozuch, 1985; Karbowiak and Kiewra, 2010; Karbowiak et al., 2015; Wójcik-Fatla et al., 2011; Biernat et al., 2014; Karbowiak, 2014; Földvári et al., 2016; Chitimia-Dobler et al., 2019; Ličková et al., 2020). TBEV was also isolated from the I. ricinus tick at the same time as its first recorded appearance in Europe, suggesting its role as a vector of the disease (Rampas and Gallia, 1949). The medical importance of D. reticulatus in Central Europe is lower, because it may transmit TBEV in endemic areas (Chitimia-Dobler et al., 2019). Unlike a single occasional occurrence in humans, the number of D. reticulatus tick bites exceed ixodid ticks on large domestic and game animals, leading to a potential further circulatory cycle of TBEV (Földvári et al., 2016; Biernat et al., 2014). Another tick species of hard tick, Rhipicephalus appendiculatus, Haemaphysalis spp., are also involved in transmission of TBEV. Due their local distribution and low abundance (Kazimírová, 2022; rev. in Stanko et al., 2022), their medical and veterinary impact is lower too.
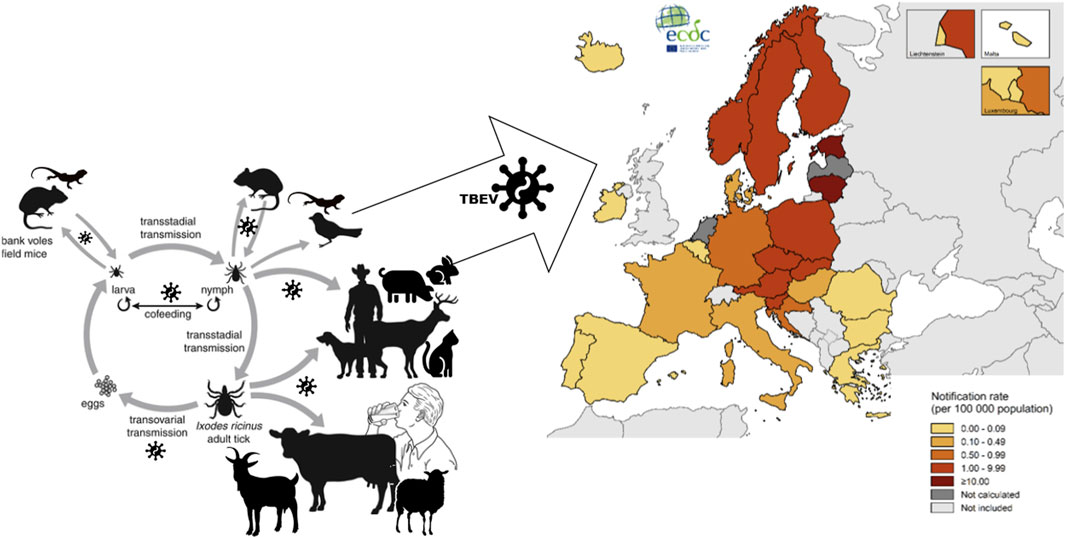
Figure 1. In nature, TBEV circulates and persists by biological transmission through a competent tick vector and by broad range of hosts. Ticks themselves as well as some group of mammals (rodents, insectivores, carnivores, etc.) comprise natural reservoirs of the virus. Humans are an accidental and a dead-end host. Tick-borne encephalitis (TBE), TBEV associated disease of central nervous system, has become a growing public health concern. TBE incidence maps are provided regularly by the European Center for Disease Prevention and Control (ECDC). The map showed TBE confirmed cases per 100 000 population by country of EU/EEA in 2022.
Generally, the hosts are divided into three groups: reservoir, indicator and accidental hosts. Terrestrial mammals participate in ecological cycle of TBEV. They have been used to monitor TBEV and define risk areas, as almost all of them had a confirmed virus or virus-specific antibodies (Klaus et al., 2010). In Europe, the main vertebrate reservoir hosts, especially for larvae and nymphs, are rodents of the genera Mus, Microtus, Micromys, Pitymys, Arvicola, Glis, Sciurus, Citellus, Apodemus, Myodes glareolus, insectivores (Sorex, Talpa and Erinaceus) and carnivores (Vulpes and Mustela) (Ernek et al., 1963; Kožuch et al., 1963; Kožuch et al., 1976; Kožuch et al., 1981; Kožuch et al., 1990; Heigl and Zeipel, 1966; Blaškovič, Nosek, 1972; Černý, 1975; Smetana and Malkova, 1976; Labuda et al., 1993a; Labuda et al., 1993b; Suss, 2003; Weidmann et al., 2006; Achazi et al., 2011; Knap et al., 2012; Pintér et al., 2014; Zöldi et al., 2015; Tonteri et al., 2013; Egyed et al., 2015; rev. in Michelitsch et al., 2019; Kwasnik et al., 2023). Humans, as well as large animals such as goats, cows, muflons, horses, sheep, roe deer, dogs, foxes and pigs, and bison are accidental hosts of TBEV (Rosický, 1953; Radda et al., 1968a; Radda et al., 1968b; Gresiková and Rehacek, 1959; Nosek, 1971; Černý, 1972; Nosek, 1972; Nosek et al., 1972; Gresiková et al., 1975; Hubálek et al., 1986; Gresiková and Calisher, 1988; Rieger et al., 1999; Wurm et al., 2000; Klimes et al., 2001; Bagó et al., 2002; Sikutova et al., 2009; Pfeffer, Dobler, 2011; Süss, 2011; Klaus, et al., 2012; Klaus et al., 2013; Kříž et al., 2014; Böhm et al., 2017; Rieille et al., 2017; de Heus et al., 2021; de Heus et al., 2023; Krzysiak et al., 2021; Gothe et al., 2023).
Many wild and domestic animals are tick hosts and can develop measurable anti-TBEV antibody titers upon natural infection and have been investigated in the past as potential new indicators of risk of TBE infection (Kožuch et al., 1963; Kožuch et al., 1981; Süss et al., 2008; Pfeffer and Dobler, 2011; Klaus et al., 2011; Klaus et al., 2013; Burri et al., 2012; Balling et al., 2014; Balling et al., 2015; Csank et al., 2018; Grzybek et al., 2018; Rockstroh et al., 2019; Haut et al., 2020; Krzysiak et al., 2021; Kvapil et al., 2021; Salat et al., 2022; Brandenburg et al., 2023; Bauer et al., 2023; Gothe et al., 2023; Topp et al., 2023). They may develop disease with viremia, but in nature they do not participate in viral circulation and are therefore the dead end of the natural TBEV cycle. Seroprevalence in human and large vertebrates can be an indirect means of measuring the intensity of TBEV transmission within a geographical region and make them valuable indicators for assessing epidemiological risk.
The examination of goat and sheep sera could be a helpful additional tool for analyzing the risk of getting infected with TBEV by tick bite (Klaus et al., 2011; Klaus et al., 2012; Klaus et al., 2013; Klaus et al., 2014). Moreover, the investigation of animals used for milk production has been of special interest. They may be the source of alimentary TBEV infections. Probably about 1% of all TBE cases are caused by foodborne TBEV, the number of cases can vary widely in different regions. The infection is caused by the consumption of unpasteurized milk (goat, sheep and cow) and dairy products containing TBEV (Kríž et al., 2009). Transmission of TBEV by milk of goats, sheep and cows is known and was observed in recent decades mainly in Central and Eastern European countries (Gresikova, 1958a; Gresikova, 1958b, Grešíková, 1972; Grešíková, 1999; Gresikova and Rehacek, 1959; Gresikova, 1960; Gresikova, 1975; Jezyna, 1976; Kohl et al., 1996; Matuszczyk et al., 1997; Rieger et al., 1998; Labuda et al., 2002; Daniel et al., 2011; Holzmann et al., 2009; Balogh et al., 2010; Cisak et al., 2010; Caini et al., 2012; Hudopisk et al., 2013; Zöldi et al., 2013; Markovinović et al., 2016; Rieille et al., 2017; Brockmann et al., 2018; Dorko et al., 2018; Kerlik et al., 2018; Król et al., 2019; Rónai and Egyed, 2020; Ličková et al., 2022; rev. in Elbaz et al., 2022; Paraličova et al., 2022). Among European countries, Slovakia has the highest rate of food-borne infections due to the presence of the virus in the milk of infected goats, sheep or cows, with an upward trend since 2007 (Kerlik et al., 2018).
Although lizards and birds are the natural hosts of I. ricinus, but do not probably participate in the natural circulation of TBEV. However, it should be mentioned that Grešíková-Kohútová and Albrecht (1959) as well as Sekeyová et al. (1970) detected clinical signs of infection and production of antibody against the virus in the laboratory. Already about 60 years ago, several scientific teams in Central European countries focused on importance determination of birds and small mammals different species in the spread of different strains of TBEV and on their immune responses to different methods of infection or vaccination. The then diagnostic technologies very rarely, if ever, detected viremia and clinical signs in the great tit, red squirrel, sparrow, pheasant and peregrine falcon, coot and chicken, as well as the production of neutralizing antibodies (Blaškovič, 1961; Ernek, 1962; Ernek et al., 1967; Ernek et al., 1968; Ernek et al., 1975; Grešíková et al., 1962; Nosek, et al., 1962; Řeháček et al., 1963; Ernek and Lichard, 1964; Grešíková and Ernek, 1965; Ernek et al., 1968; Ernek et al., 1969a; Ernek et al., 1969b; Grešíková, 1972). Later, in wild and domestic ducks infected with different strains of TBEV, not only several days of viremia but also seroconversion was found (Ernek, 1962; Ernek et al., 1969a; Ernek et al., 1969b). As serological studies, viral isolations as well as sequential similarities suggest, migratory birds migrating north from Central Europe are also likely to play a role in the transmission of TBEV to new Europe areas (Süss et al., 2008; Lommano et al., 2014; Michel et al., 2021). In 2022, Penazziová with team performed a serosurvey for several tick-borne virus infections, including TBEV-Eu in tick-infested or uninfested birds. By screening microtiter, TBEV neutralizing antibodies (NAb) were also detected (Peňazziová et al., 2022).
Antiviral immune response against TBEV
All nuclear cells respond to virus infection by innate immune responses. Both innate responses intracellular mediated by type I interferon (IFN I) and extracellular mediated by specialized immune cells (natural killer, antigen presenting cells) are activated. This rapid, nonspecific innate immune response is necessary for subsequent activation of specific adaptive immune responses, represented by both antibody-associated humoral and T-cell-mediated immune responses. By combining of these two reactions, long-term immune memory is provided. Together, they can lead to clearance of TBEV infection. The immune response of a mammal hosts to TBEV infection depends on the route of infection: tick bites, alimentary way or inhalation of infected aerosols. Recently, several cases of TBEV infection associated with solid organ transplantation have been reported in Poland (Lipowski et al., 2017).
The transmission pathway of TBEV to the host affect different tissues and organs - skin, olfactory or gastrointestinal tract - involvement in the immune response as well the crossing of the blood-brain barrier (BBB), and thus the success rate of infecting the brain as a target organ (Dörrbecker et al., 2010).
The most widespread mode of TBEV transmission is via saliva of infected ticks within minutes of the tick-bite (Figure 2A) (Lindquist and Vapalahti, 2008). After transmission of TBEV by a tick, virus replicates locally in the subcutaneous tissue, namely in Langerhans cells (LC), subset of myeloid dendritic cells in skin, and neutrophils. In a mouse model, Labuda and team (1996) found TBEV replication at the tick bite site even in keratinocytes and dermal macrophages (Figure 2B). Although macrophages together with DCs are critical early reactors in host defense against tick-borne viral infections, they do not always function as a protective barrier. DCs, the most potent antigen-presenting cells, were identified by Labuda’s team (1996) as TBEV vectors during local intrastadially transmission of the virus in the skin during tick co-feeding. Migrated monocytes and macrophages were found to be vectors for the transmission of viral particles to draining lymph nodes (Figure 2C). Subsequent virus replication in the nodes results in virus spread into the bloodstream and induction of viremia resulting in infection of various peripheral organs and tissues Figure 2D). At this stage, the infection can be successfully cured and seroconversion occurs without obvious clinical symptoms (Prokopowicz et al., 1995). The immune response induced by a viral infection is the second major barrier that a virus encounters, and if overcome, it can spread and cause viremia. In some cases, viral penetration into the CNS (secondary viremia) is followed by the development of a second neurological phase of the disease (Figures 2E, F). Exactly how TBEV crosses the BBB is not entirely clear. It is most likely a combination of mechanisms of enter the CNS without its disrupting (Ruzek et al., 2011), or by direct infection of the microvascular endothelial cells (Palus et al., 2017). The olfactory route of infection was confirmed using Langat virus (LGTV), naturaly atenuated strain TBEV serocomplex (Kurhade et al., 2016).
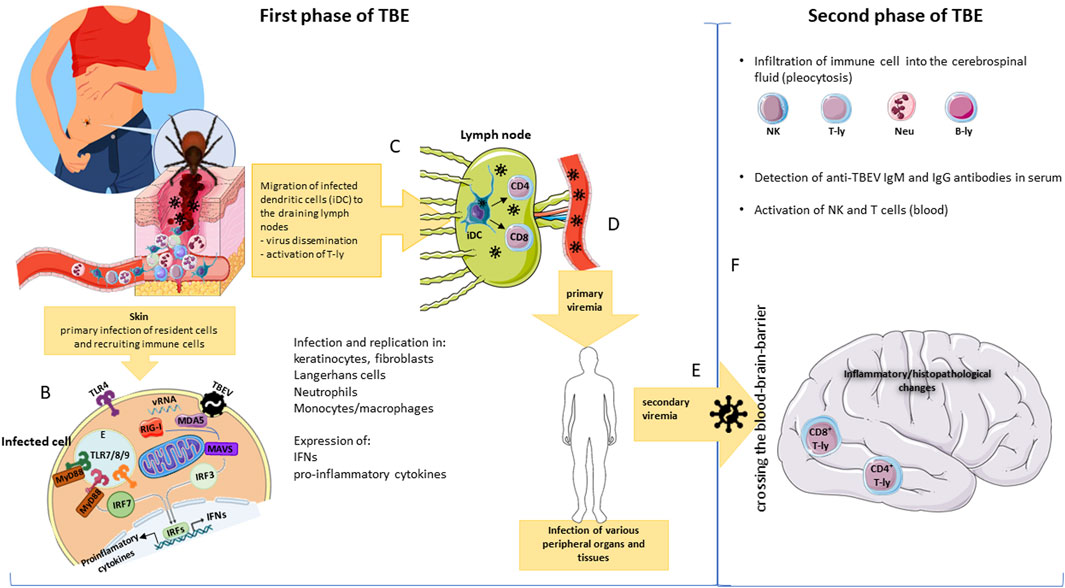
Figure 2. Of the ways to be TBEV infected, the most common mode is via saliva of infected ticks (A). After a tick bite, TBEV is transmitted into the skin where it infects local (keratinocytes, fibroblasts, dendritic cells) as well as infiltrating immune cells. In mammalian host cells, many pattern recognition receptors (PRRs), including Toll-like receptors (TLRs), RIG-I and MDA5, directly recognize viral genome (vRNA) and their activation leads to the expression of either interferons (IFNs) or inflammatory cytokines (B). Antigen presenting cells (dendritic cells, monocytes/macrophages) transport virus particles to the draining lymph nodes and contribute to virus dissemination, yet they can initiate T cell responses (C). Viral replication in the nodes leads to spread into the bloodstream and to primary viremia. During primary viremia, the virus infects various peripheral organs and tissues (D); infection of these cells results in secondary viremia (E). At this time, the virus crosses the blood-brain barrier (by several mechanisms) and initiates infection in the brain (F). Second neuropathological phase is characteristic by pleocytosis (infiltration of immune cells into the cerebrospinal fluid); by presence of activated T and NK cells in peripheral blood and anti-TBEV IgM and IgG antibodies (F). E, endosome; vRNA, viral RNA.
Innate immunity vs. TBEV
In mammalian host cells, many pattern recognition receptors (PRRs) directly recognize viral nucleic acids as a type of pathogen-associated molecular pattern (PAMP). In the case of RNA viruses, the most important PRRs that recognize viral RNAs are Toll-like receptors (TLRs) located in endosomes and the plasma membrane (Figure 2B), or cytoplasmic receptors similar to retinoic acid-induced gene I (RIG-I) (RLR); involving RIG-I and melanoma differentiation-associated gene 5 (MDA5) (Kawai and Akira, 2006). Depending on their subcellular localization, PRR/TLR activation leads to the expression of either interferons (IFNs) or inflammatory cytokines (Figure 2B) (rev. in Lester and Li, 2014). Through IFN secretion, these antiviral responses can be amplified and spread to surrounding uninfected skin cells and through activated Janus kinases (Jak1 and Tyk2) and signal transducers of transcription (STAT1 and STAT2), leading to the activation and translocation of interferon-stimulated gene factor 3 (ISGF3) into the nucleus and subsequently to the activation of hundreds of IFN-stimulated genes (ISGs). Proteins encoded by activated ISGs can either enhance the IFN response (e.g., PRR and IRF), modulate it (e.g., suppressor of cytokine signaling [SOCS]), or directly target the invading pathogen (e.g., antiviral effector proteins) (MacMicking, 2012; Schneider et al., 2014). However, the detection of TBEV after entering the cell is particular difficult due to TBEV-induced host membrane rearrangements in such a way to create compartmentalized viral factories that probably protect the viral RNA from the host’s defenses. The unavailability of dsRNA to cytoplasmic PRRs delays the activation of interferon regulatory factor 3 (IRF-3), a key transcriptional regulator of the type I IFN response, and subsequently IFN production, allowing TBEV to replicate unimpeded (Overby et al., 2010; Overby and Weber, 2011; Bílý et al., 2015).
For TBEV, it is not clear which PRRs are dominant. The importance of TLRs in TBEV infection is very little known. For example, upregulation of TLR3 during inflammation in different types of glial cells in the CNS was confirmed. According to several Swedish or Russian studies of TLR3 polymorphisms, functional TLR3 is a risk factor for severe TBE (Kindberg et al., 2011; Barkhash et al., 2013; Mickiene et al., 2014). TLR7, investigated by Americans in the context of LGTV infection in a mouse model, might be important for regulating neuroinflammation (Baker et al., 2013). German researchers also contributed to identifying the importance of TLR7/8 in relation to TBEV infection. They co-discovered the role of a subpopulation of plasmacytoid dendritic cells (pDC) specialized to produce type I IFN in the immune response to TBEV. They detected TBEV sensing via the TLR7/8 pathway, leading to robust IFN-α production. They identified the critical influence of interleukin 6 (IL6) and the BAFF molecule on the differentiation of B lymphocytes into plasmablasts by dendritic cells (Etna et al., 2021). During the validation of TLR2 as a biomarker for the differentiation of neuroborreliosis (NB) from TBE in serum or CSF, the Polish team of Moniusko-Malinowska (2019) confirmed the involvement of TLR 2 in the development of the inflammatory process in the CNS. Regarding the role of RLR (MDA5/RIG-I), German researchers in collaboration with Sweden pointed to the role of IFN-β promoter stimulator 1 (IPS1, also known as MAVS), which is crucial for the upregulation of IFN-β in vivo and in vitro. After binding of the RNA ligand to MDA5/RIG-I, its conformational change leads to the activation of transcription factors–IFN regulatory factor 3 (IRF3), IRF7 and nuclear factor kappa B (NF-κB). Phosphorylated homo- and heterodimers of IRF3 and IRF7 translocate to the nucleus to activate IFN-β transcription (Kurhade et al., 2016; Overby et al., 2010). The importance of IPS expression in the CNS for virus spread and replication control was indicated by Kurhade and his team (2016). Deficiency of IPS1 leading to inability to induce IFN-β transcription in response to TBEV, rendering cells more susceptible to infection (Overby et al., 2010), IPS1-deficient mice reported lower systemic levels of IFN-α and higher viral loads, and consequently succumbed earlier to LGTV and TBEV infection (Kurhade et al., 2016). Selinger et al. (2017) also demonstrated that TBEV infection in the CNS triggers innate immune signaling by interacting with RIG-I/MDA5, which promotes IRF-3 translocation to the nucleus. In human medulloblastoma cells derived from cerebellar neurons (DAOY), they revealed a broad panel of ISGs, including interferon-induced protein with tetratricopeptide repeats (IFIT) 1, IFIT2, IFIT3, radical S-adenosylmethionine domain (RSAD) 2 containing 2′-5′-Oligoadenylate Synthetase Like (OASL), 2′,5′-oligoadenylate synthetase 2 (OAS2), ISG15 and ISG20 and pro-inflammatory cytokines (see section Inflammation vs. TBEV) however without an inhibitory effect on TBEV titers. Results indicated IFN- or IRF3-independent transcriptional induction of IRF1 and IFN-independent ISG induction pathways. Upregulated expression of type III, but not type I or II IFNs activated after TBEV infection has also been demonstrated and probably reflected a defect in recognition of viral RNA by RIG-I/MDA-5.
Polish researchers found the highest levels of IFN-γ in the CSF of patients with severe TBE with impaired consciousness. In patients with milder clinical manifestations, they found higher expression of IL10, IFN-β and IFN-λ3, associated with genentic polymorphisms (Kondrusik et al., 2005; Grygorczuk et al., 2015). Several European researchers have demonstrated the induction of type I IFN expression in host cells after TBEV infection and the very sensitive response of the virus to pre-treatment of the host cell with IFN (Stancek and Vilcek, 1965; Vilcek, 1960; Hofmann et al., 1971; Kopecký et al., 1991a; Overby et al., 2010; Weber et al., 2014; Kurhade et al., 2016; Lindquist et al., 2016).
The innate immune system includes natural killer (NK) cells. Activated NK cells, like T cells, are involved in immunopathological responses. They kill infected cells either directly or indirectly via cytokines or chemokines. They can also recruit inflammatory cells into tissues. Not much is known about NK cells in the context of TBEV. NK cells appear to be activated at the beginning of the second phase of TBE. Their involvement in TBEV infection was noted by Tomazic, who detected NK cells in peripheral blood of TBEV patients (Tomazic and Ihan, 1997). Toczylowski et al. (2020) found their relative depletion in the lymphoid population in the CSF of TBE patients compared to blood. TBE infection differs from other human viral infections in the characteristics of the NK cell response. Cytotoxic granule release early in NK cell activation may contribute to TBEV pathogenesis.
Monocytes/macrophages play an important role in CNS inflammation, but their pathogenetic role and migratory mechanisms in human flavivirus encephalitis are not well understood. Because TBEV targets macrophages (Figure 2), in laboratory conditions, the role of macrophages in experimental TBEV infection was described by Khozinsky et al. (1985). They demonstrated that reversible massive blockade of macrophage phagocytic activity (PAM) in vivo during acute TBEV infection significantly increased acute TBEV lethality. PAM suppression did not affect macrofages interactions with B- and T-lymphocytes during the first 48 hpi. In the presence of anti-TBEV antibodies, peritoneal macrophages (PM) from both infected and uninfected donors acquired the ability to kill TBEV-infected target cells. Antibody-dependent macrophage cytotoxicity (ADC) was associated with a phagocytic cell population. Kreil’s team showed that TBEV infection of macrophages profoundly alters the requirements for inducing nitric oxide (NO) synthesis. They demonstrated that IFN-γ, plus TNF-α-stimulated production of NO was down-modulated in macrophages infected with TBEV. They hypothesized that NO production is probably antagonized by TBEV-induced IFN α/β (Kreil and Eibl, 1995). Later, they demonstrated spontaneous NO production in macrophages isolated from TBEV-infected mice. High levels of NO production did not inhibit TBEV replication in vitro, but suppression of macrophage NO production in infected mice resulted in increased survival, suggesting that NO production may even contribute to viral pathogenesis in vivo (Kreil and Eibl, 1996). Another study contributed to clarifying the role of macrophages in the pathogenesis of TBEV. A retrospective analysis of blood monocyte counts in the CSF of TBE patients with meningitis, meningoencephalitis, or meningoencephalomyelitis revealed a correlation of CSF monocyte counts with other CSF inflammatory parameters, but not with peripheral monocytosis, which was related to their active recruitment to the CNS. Counts did not correlate with clinical image (Grygorczuk et al., 2021). Using macrophages, Beránková et al. (2022) investigated the subtle and complex involvement of autophagy at the level of virus entry and IFN-β production in the control of TBEV infection. They found that autophagy is the limiting factor for IFN-β production by TBEV-infected macrophages. Recently, they investigated the interplay between TBEV infection and stress pathways in the mouse macrophage cell line PMJ2-R. They found changes in redox balance and defenses against cellular stress triggered by TBEV infection, including antioxidant responses and the IRE1 UPR pathway. Their experimements showed negative effect of stress-induced events on TBEV replication and only a marginal effect of tick saliva on stress cellular pathways (Beránková et al., 2024).
Dendritic cells (DCs) are one of the first cells that TBEV contacts when it invades the host (Figure 2). They are permissive to TBEV. This may contribute to the subsequent spread of the virus and the initiation of adaptive immune responses (Labuda et al., 1996). They express many types of PRRs on their surface or in their cytoplasm, allowing them to recognise different pathogens. In the early stages of viral infection, these cells act as antigen presenters, costimulators and cytokine producers (including type I IFNs). They bridge the innate and adaptive immune systems (Labuda et al., 1996). Dörrbecker with colleagues (2010) proposed that LC/DCs are first subset of antigen presenting cells that present TBEV to T-lymphocytes, activate their differenciation to CD4+ type 1, 2 and CD8+ T-lymphocytes (CTL). Despite their role or importance in the innate antiviral immune response, their performance during TBEV infection has long been poorly understood. Information originating from European research about the role of DCs in TBEV infection comes from the studies of tick saliva effects on the transmission of different pathogens, including TBEV, the production of antiviral or pro-inflammatory cytokines after infection, or on apoptosis or migration (Skallova et al., 2008; Fialova et al., 2010; Lieskovská and Kopecký, 2012; Kotál et al., 2015). Skallova and her team showed that tick saliva inhibits DCs maturation after ligation of TLR3, TLR7, TLR9, or CD40, which may maintain a less mature DCs phenotype. These could then remain permissive for the virus. Fialova et al. (2010) detected significant amounts of TNF-α and IFN-β and high amounts of IL-6, minimal IL-12p70 and IL-10 in TBEV- infected DCs. Effects were virus strain dependent. Because the random cells retained an immature phenotype (low expression of B7-2 and MHC class II molecules), they hypothesized that cytokines were likely produced by infected rather than random DCs. They also identified increased apopoptotic effect of TBEV infection on DCs. In Lieskovská et al. (2018) did find activation of the PI3K/Akt antiapoptotic pathway in TBEV-infected DCs. However, they did not confirm activation of STAT1 signaling or phosphorylation of NF-κB, nor detectable levels of IFN-β in TBEV-infected DCs. Marušic et al. (2023) investigated DCs activation and maturation and cytokine production after in vitro TBEV stimulation of peripheral blood mononuclear cells (PBMCs) obtained from vaccine breakthrough (VBT) and unvaccinated TBE patients. Their results showed upregulation of HLA-DR and CD86 expression on DCs to a similar extent in both group of TBE patients, but differed in cytokine production after TBEV challenge. PBMCs from VBT TBE patients responded to TBEV challenge by lower levels of IFN-α and pro-inflammatory cytokinesin vitro, which likely facilitated viral replication and affected the development of cell-mediated immunity 48 h.p.i. Significantly higher levels of IL-6 and TNF-α measured after 6 days of in vitro stimulation of PBMCs could promote disruption of the blood-brain barrier and influx of viral and immune cells into the CNS, leading to more severe disease in VBT TBE patients.
The main target organ for TBEV is the host brain, where the virus primarily infects neurons (Bílý et al., 2015). However, Slovenian and Czech scientists have shown that other nerve cells, such as astrocytes, are also likely targets for TBEV infection (Palus et al., 2014a; Potokar et al., 2014). Because of the neurotropism of TBEV and also the fact that TBEV replicates 10,000-fold more frequently in human neuronal cells compared to epithelial cells (Ruzek et al., 2009b), most of the information on the immune response of cells to TBEV infection comes from experiments following infection of neuronal cells or the brain. Within the CNS, TBEV productively infects neurons and astrocytes, which leads to the development of neuropathology (Bílý et al., 2015; Palus et al., 2014b; Ruzek et al., 2009a). In 2014, Weber and colleagues showed that type I IFNs protect and control TBEV- and Langat virus (LGTV, a naturally attenuated member of the TBEV serogroup)-induced inflammation and encephalitis by limiting systemic LGTV replication, CNS dissemination, and associated immunopathology. They also demonstrated the importance of IFNAR, a key receptor molecule in the type I IFN response (Weber et al., 2014). Deficiency of IFNAR led to uncontrollable multiplication of LGTV and TBEV, with mice dying very quickly. Its controlling role in LGTV replication has been demonstrated in all cell types including hematopoietic, stromal and neuroectodermal cells plus cells in the periphery.
The very early type I IFN antiviral response in TBEVinfected astrocytes in vitro was also proven by experiments by Swedish, American and German scientists, pointing out that these cells thus limit the replication and spread of the virus (Lindquist et al., 2016). Just recently, Ghita et al. (2021) identified astrocytes as major IFN-β producers upon in vitro TBEV infection. Astrocytes and microglia were less permissive to TBEV and produced significantly higher levels of IFN-β than neurons. In TBEV-infected astrocytes, MAVS signaling drove early innate responses, and restriction of TBEV replication and induction of early IFN and ISG responses depended on it. They demonstrated that IFN-β induction in infected astrocytes depends on MAVS and MyD88/TRIF sequential signaling. Important host effector molecules in the type I IFN response to viruses are the interferon-induced transmembrane (IFITM) protein families. Polish researchers investigated the role of IFITM1, IFITM2 and IFITM3 in the inhibition of TBEV infection and virus-induced cell death. Their results showed that IFN- and IFITM-mediated immunity did not significantly affect intercellular spread of TBEV (Chmielewska et al., 2022).
There are evidence growing that susceptibility to TBEV disease and severity of the disease course are related not only to viral factors, but also to host factors - age, neurological symptoms at baseline, and low early IgM response in CSF. A detailed study of the potential role of the genetic background of TBE conducted in detail, especially in the Russian population, was followed by Czech scientists. In TBE animal models, they have identified several candidate genes that influence survival after TBEV infection, including Oas1b, Cd33, Klk1b22, Siglece, Klk1b16, Fut2, Grwd1, Abcc6, Otog, and Mkrn3 (Palus et al., 2013; Palus et al., 2018). Recently, Fortova et al. (2023a), Fortova et al. (2023b) investigated the contribution of selected single nucleotide polymorphisms (SNPs) in innate immunity genes to the predisposition to TBE in humans. Impact of selected single nucleotide polymorphisms (SNPs) in the genes IFIT1, IFIT2, RIG-I and DDX58 on the outcome of TBEV infection was investigated. Their analysis showed an association of IFIT1 rs304478 SNP and DDX58 rs3739674 and rs17217280 SNPs with predisposition to TBE in the Czech population. They also identified correlation of serum MMP-9 levels with clinical course, especially in patients heterozygous for the single-nucleotide polymorphism rs17576 (A/G; Gln279Arg) in the MMP-9 gene. Probably, these SNPs present novel risk factors for clinical TBE but not for disease severity.
Inflammation during TBE
Inflammation upon acute infections is an important part of the immune response essential for the elimination of pathogens. On the other hand, excessive inflammation may be harmful to the host. Recruitment and activation of immune cells in inflamed tissues is mediated by chemokines. Data on soluble factor/cytokine/chemokines response in TBEV infection are limited. Determination of cytokine composition in different clinical samples of patients with TBE (CSF, urine, serum) may reveal a complex and distinct network of cytokines of early innate immune response, CD4+ Th1, Th2, Th9, Th22, and Th17 subpupulations and anti-inflammatory cytokines.
Beside DCs and macrophages, neutrophils are recruited to the site of TBEV infection (Labuda et al., 1996). Neutrophils are highly phagocytic cells, after activation they are also able to produce inflammatory cytokines. Like other immune competent cells, they likely play a role in complementing the cytokine and chemokine response during the first period after TBEV infection. They are attracted to the bite site during tick feeding by high levels of CXCL8 and can also be infected by TBEV (Labuda et al., 1996).
Numerous studies investigated the levels of cytokines and chemokines in serum and CSF of TBE patients (Kondrusik et al., 1998; Kondrusik et al., 2001; Grygorczuk et al., 2006a; Grygorczuk et al., 2015; Grygorczuk et al., 2017; Grygorczuk et al., 2018a; Grygorczuk et al., 2018b; Lepej et al., 2007; Zajkowska et al., 2011; Bogovič et al., 2019; Bogovič et al., 2022; Zidovec-Lepej et al., 2022), yet there are limited data on the role of inflammation in the pathogenesis of TBE. Zajkowska et al. (2006) investigated the role of sCD40 and sCD40L in TBEV-induced intrathecal inflammation. The CD40-CD40L costimulatory pathway plays a key role in the production of cytokines, including interleukin (IL)-10 and IL-12, by monocytes and macrophages. In addition to various functions, these cytokines also modulate the activity of T-lymhocytes. The interaction between CD40 and CD40L is essential in generating an immunological response even intrathecally. The detected increased concentration of sCD40L in the inflammatory CSF indicated the persistence of peripheral activation of the immune system after the end of treatment and after clinical recovery. Kondrusik et al. (1998) found significantly higher levels of TNF-α and IL-1β in patients with tick-borne encephalitis before and after treatment and normalization of CSF parameters. In order to evaluate the efficacy of treatment and resolution of infection, the authors proposed and used measurement of these cytokines in patients with tick-borne encephalitis. Although they did not confirm the significance of serum IL-1β determination, due to the decrease in TNF-α concentration after treatment, they assume that measuring its concentration may be useful in evaluating the effectiveness of asseptic CNS treatment (Kondrusik et al., 2001). In 2005, Zajkowska with team evaluated the concentrations of the soluble forms of intercellular adhesion molecule (ICAM) - ICAM-1, ICAM-2, ICAM-3 in the serum and CSF of TBE patients. By detection of sICAM-2 increased concentration in CSF, they confirmed its important role in inflammatory process of viral origin (Zajkowska et al., 2005). A year later, the participation of intercellular ICAM-1, ICAM-2, ICAM-3 in the TBEV pathogenesis was confirmed by Pietruzuk et al. (2006).
Polish researchers found increased levels of CXCL9 (Koper et al., 2018) and CXCL10 and CXCL11 (Lepej et al., 2007) in the CSF of patients with TBEV. Zajkowská and colleagues (2011) investigated differences in the concentration levels of several CXCL chemokines (CXCL10, 11, 12, and 13) in the serum and CSF of TBE patients before and after infection. They proposed their possible use as biomarkers of CNS inflammation caused by TBEV, mainly the detection of CXCL10 in CSF and CXCL13 in serum as indicators of patient recovery. They detected the involvement of CXCL13 in the recruitment of CXCR3-expressing T-lymphocytes to the CNS. Grygorczuk et al. (2006a), Grygorczuk et al. (2006b), Grygorczuk et al. (2015), Grygorczuk et al. (2016) also confirmed increased levels of CCL3, CCL5, CXCL11, CXCL12, CXCL13 and IFN-λ3, IFN-β, IL-10 in the CSF of TBE patients. The increased level of CCL5 in the CSF correlated with the activation of CD4+ T cells in the CSF, which expressed a high level of CCR5, CXCL1 and CXCL2 attracted neutrophil here. In Moniuszko-Malinowska et al. (2018) confirmed high mobility group protein box 1 (HMGB-1) in the serum and CSF of TBE patients. HMGB1, a multifunctional cytokine (depending on its subcellular localization), serves as a biomarker of inflammation. The pathological mechanisms of which it is a component underlie the complications associated with CNS diseases (Moniuszko-Malinowska et al., 2018). Bogovič et al. (2019), who analyzed the expression profiles of cytokines/chemokines associated with innate and adaptive immune responses of serum T and B lymphocytes and CSF, showed similar levels in the acute phase, but with the onset of postencephalitic syndrome (PES), they noted increased expression of Th1 (IFN-γ, IL-12P40, IL-12P70, CXCL10, CXCL9, and CCL19), B (CXCL12, CXCL13) mediators, but decreased expression of Th17 (IL-17F, IL-17A, IL-22, IL-21, IL-23, IL-25, and IL-27). Comparing laboratory and immunological findings in the early and second (meningoencephalitic) stages of TBE, Bogovič et al. (2022) found the same low number of leukocytes and platelets in both stages of TBE, but very different patterns of clustering of immune mediators. They found the involvement of different immune processes depending on the stage of TBE and the compartment studied. In the serum, the expression of CXCL1, CXCL13, BAFF, IL-4, and IL-27, associated with innate immunity, but also the activity of B lymphocytes and growth factors associated with angiogenesis (Growth regulated peptide (GRO)-α and Vascular Endothelial Growth Factor (VEGF)-A) dominated. In the second phase, the typical immune response was represented by innate and Th1 mediators in serum and CSF.
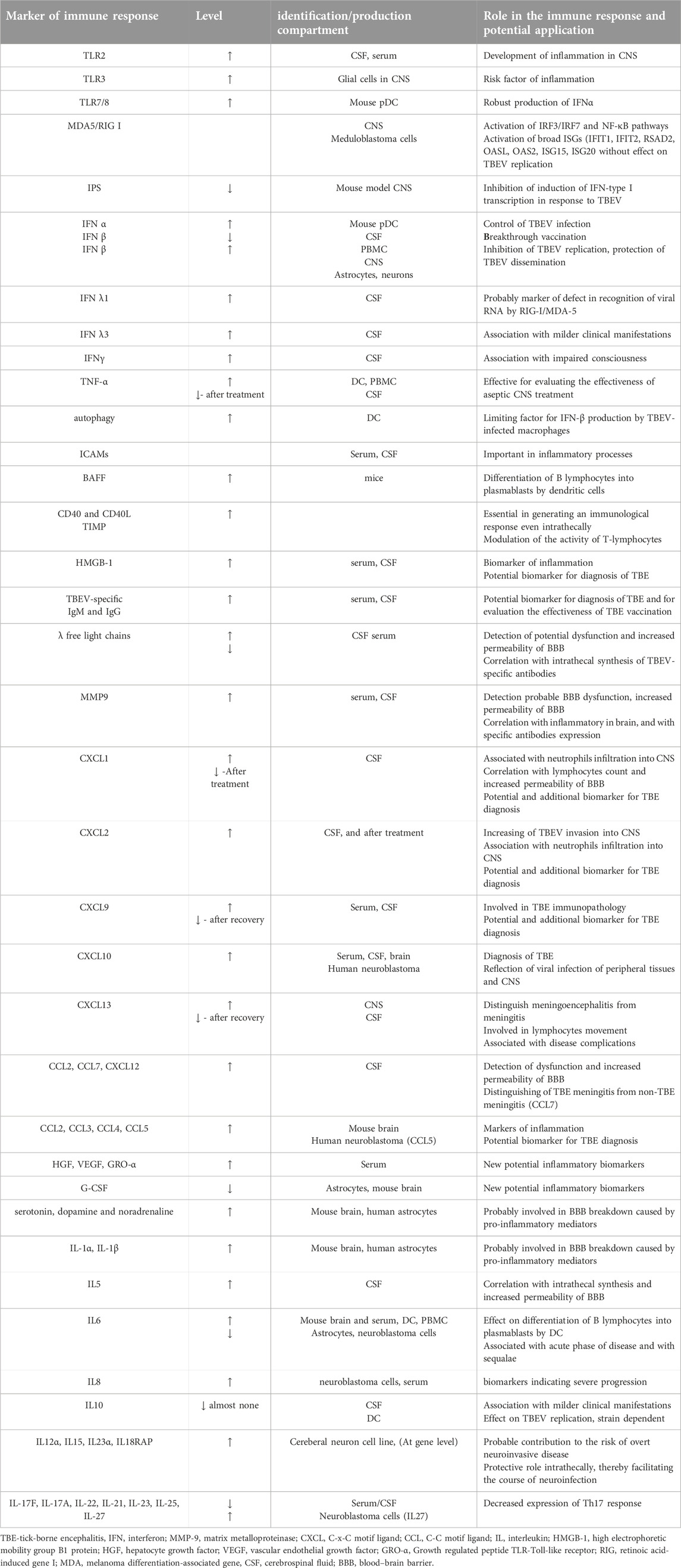
The target organ of TBEV is the CNS and a critical component of the CNS is the BBB. The effect of the virus on the CNS as well as the tissue-specific response associated with infection depends on the types of inflammatory cells, the humoral response, as well as the cellular components and chemokines/cytokines involved by the host (Dörrbecker et al., 2010). Entry of TBEV into the CNS is probably facilitated by a peripheral inflammatory response to the virus. The main features of TBEV pathogenesis are neuroinflammations. Dominant histological inflammatory reactions are lymphocytic meningeal and perivascular infiltrates, microglial nodules and neuronophagy (Tomazic and Ihan, 1997). Ruzek et al. (2011) demonstrated in a mouse model the disruption of BBB integrity in TBEV-infected mice. Since BBB permeability was increased in the later stages of TBE infection when a high viral load is present in the brain, its disruption was likely a consequence of TBEV infection in the brain. CD8+ T cells were not involved in BBB breakdown; its disruption was probably caused by pro-inflammatory mediators such as cytokines, chemokines and matrix metallopeptidase (MMP), especially MMP-9, expressed in infected brain tissue. Palus and his team confirmed the research of Russian and Swedish scientists (Atrasheuskaya et al., 2003; Blom et al., 2016), who revealed significantly increased levels of pro-inflammatory and inflammatory cytokines at the beginning of the second phase of TBE in patient serum samples. Palus' team revealed that TBEV infection of mouse brain and human astrocytes resulted in inflammatory responses that included increased production of cytokines (IL-1α, IL-1β, IL-6, IL-8, IFN-α, and IFN-γ) and chemokines (CCL2, CCL3, CCL4, CCL5, and CXCL10) and later supplemented the spectrum with new potential inflammatory markers, MMP-9, monoamine neurotransmitters serotonin, dopamine and noradrenaline, as well as hepatocyte growth factor (HGF) and VEGF (Palus et al., 2013; Palus et al., 2014a; Palus et al., 2015). Grykorczuk and colleagues (2017) confirmed, as a hallmark of inflammation facilitating viral passage across the BBB, the activation of a signaling pathway involving TLR3 and the inflammatory cytokines, macrophage migration inhibitory factor (MIF), TNF-α and IL-1β. In vitro experiments of Selinger et al. (2017) with TBEV-infected human medulloblastoma cells derived from DAOY cerebellar neurons revealed activation of a panel of genes encoding similar cytokines after TBEV infection as detected by Palus et al. (2013)–Palus et al. (2015), supplemented by five additional genes encoding cytokines (IL-6 and TNF-α, CXCL2, CXCL11, IL-12α, IL-15, and IL-23α) and the IL-18 receptor protein (IL18RAP). Although on the one hand it contributes to the risk of overt neuroinvasive disease, it also plays a protective role intrathecally, thereby facilitating the course of neuroinfection. Individual variability in the risk of clinical manifestation of TBE may be due to genetically determined variability in the peripheral and intrathecal expression of mediators of the inflammatory response. They revealed the intrathecal expression of cytokines IL-5, Th17 and CXCR2-binding chemokines and contributed to the elucidation of their pathogenetic role in TBE (Grygorczuk et al., 2018a; Grygorczuk et al., 2018b). They identified elevated serum levels of the pro-inflammatory cytokines IL-6, IL-8, IL-12, IL-17A, and IL-17F and the chemokines CCL3, CXCL2, CXCL10, and CXCL13 as possible biomarkers indicating severe progression. Later, intrathecal and CSF findings revealed an increase in the mean concentration of CCL7 and CXCL12 with no apparent concentration gradients towards the CSF (Grygorczuk et al., 2021). Investigating differences in cytokine/chemokine communication during TBEV infection in naive and TBEV-infected mice and human neuronal cells and astrocytes within the innate immune response, Pokorna Formanova et al. (2019) found time-dependent concentrations of CXCL10 as well as CXCL1, granulocyte colony stimulating factor G-CSF, IL-6 in mouse serum or brain. In human neuroblastoma cells they found increased production of CXCL8 and IL-27, IL-4, BAFF and downregulation of CCL2 and HGF, in human astrocytes mainly IL-6, CXCL8, CXCL10, CCL5 and G-CSF) and decreased expression of VEGF. They identified both neurons and astrocytes as potential sources of pro-inflammatory cytokines in TBEV-infected brain tissue. Induced cytokines and chemokines stimulated the innate immune response of neurons, which reduced viral replication and increased the survival of infected neurons. Several of these papers offer many of the cytokines, chemokines or growth factors identified by them as potential biomarkers of the immune response to TBEV, but given the non-specificity of TBEV, as complementary (see Table 2). Recent studies have identified several promising biomarkers for tick-borne encephalitis virus (TBEV) that could be useful in clinical trials. These biomarkers include metabolites and lipids (Du et al., 2021), immunoglobulins and free light chains, and cytokines and chemokines (see above). Considering the limitation of the reviewed scientific articles to Central Europe, based on the findings of Palus and Fortova, we prefer MMP-9 as a potential marker of the immune response to TBE.
Adaptive immunity against TBEV
Adaptive immune responses are provided by antibodies (humoral responses) in collaboration with cell-mediated responses involving T cells. This provides long-term immune memory. All these reactions together can eliminate virus infection. Infection with TBEV triggers a humoral as well as a cell-mediated immune response (Figure 2). In contrast to the well-characterized humoral antibody-mediated response, the cell-mediated immune responses elicited to natural TBEV-infection have been poorly characterized until recently.
Humoral immunity against TBEV
Many viral infections as well as vaccines against them induce long-lasting protective immunity consisting of pathogen-specific antibodies and memory B cells. TBEV infection also elicits an effective B cell response. The detection of TBEV in both natural and accidental hosts of ticks went hand in hand with the detection of the immune response, including antibodies against TBEV. It mainly served to identify and monitor not only the virus, but also the prevalence of infection in ticks and hosts, as well as the seroprevalence in natural deposits. The virus was isolated from ticks (Grešíková, 1972; Labuda et al., 2002), and in the case of different types of hosts from the brain and other organs caught during the monitoring of TBE foci. The sera of these animals contained antibodies against TBEV (Ernek et al., 1963; Bárdoš, 1965; Kožuch et al., 1967; Kožuch et al., 1990; Kožuch et al., 1995; Nosek et al., 1970; Nosek et al., 1982; Trávniček et al., 1999; Labuda et al., 2002; Sláviková et al., 2019). A number of studies have focused on the correlation of the humoral response to TBE infection with clinical outcome as well as with TBE vaccine development. The antibody response to TBEV is targeted primarily against the E and NS1 proteins and is critically important in controlling and clearing the infection. Nonstructural protein 1 (NS1) is secreted into circulation by infected cells and serum levels correlate with disease severity. Both TBEV neutralizing (mainly against E) and partially non-neutralizing antibodies (against NS1 or prM) can prevent disease development.
In laboratory experimental condition, production of neutralizing antibodies was identified by Rada et al. (1968a), Hofmann´s team Hofmann et al. (1978); Hofmann and Popow-Kraupp (1982), Labuda ‘s team (1997). In the natural host, Kožuch et al. (1981) and Kopecký et al. (1991a) have been monitoring the development of viremia and the production of specific antibodies during the observation period. By comparing the immune response in TBEV-infected Apodemus sylvaticus natural hosts and TBEV-infected laboratory ICR mice, Kopecký et al. (1991b) identified an earlier and more intense infection-induced antibody response in the natural host compared to laboratory mice. Klaus et al. (2019) tried to fullfil gaps in the knowledge about immunity in animals, for example with regard to the longevity of TBEV immunity. They investigated the potential risk of TBEV infection through serological studies in dairy goat and sheep herds from Germany using TBEV-VNT and found long-lasting antibody titres in goats and sheep after exposure to TBEV, albeit at low levels.
The protective effect of passive immunization with human or mouse monoclonal or polyclonal antibodies specific for TBEV (against protein E) in mice against an otherwise highly lethal TBEV attack was proven by Austrian and Czech scientists (Kreil and Eibl, 1997; Kreil et al., 1998a; Elsterova et al., 2017). Kreil et al. (1997) showed that passive immunisation by transferring anti-TBEV glycoprotein E antibodies into mice could provide immune protection against the spread of TBEV from skin monocytes/macrophages, prior to subsequent vaccination with free TBEV particles. However, despite passive protection by applied antibodies they found short-term replication of the virus at a low level, (Kreil et al., 1998a). Recently, Kubinsky with team (Kubinski et al., 2023) investigated the impact of adoption strategies for transferring serum or T cells from LGTV-infected mice to previously untreated recipient mice on the development of severe disease induced by lethal dose of TBEV. They confirmed effective protection of this strategy against the development of severe disease. Kreil et al. (1998b) identified a predominantly NS1-specific antibody response generated by mice vaccinated with a fully killed TBEV vaccine and challenged with a lethal dose of TBEV. They also showed that TBEV-neutralizing antibodies protected against disease but did not prevent localized infections in the host. Austrian researchers Kreil and Eibl (1997) while investigating potential antibody-dependent enhancement (ADE) of TBEV infection during pre- or post-exposure to passive immunoglobulin found that antibodies that enhance TBEV replication in mouse peritoneal macrophages in vitro protect against lethality of TBEV infection in mice. Haslwanter et al. (2017) found that non-neutralizing but protective antibodies targeting the NS1 protein bound to TBEV particles can mediate attachment and endocytosis of these complexes by Fcγ receptor-bearing cells such as macrophages. They hypothesized that this could lead to a subsequent increase in ADE antibody levels.
Dogs are frequently infected with TBEV. But diagnosis is complicated by the cross-reactivity of antibodies between different flavivirus species. Using a self-developed multispecies luciferase immunoprecipitation system to detection of antibodies against several different antigens from TBEV and West Nile virus (WNV), Könenkamp and his team (2022) identified the NS1 protein as a suitable antigen to differentiate specific TBEV and WNV antibodies in dogs. Clinical manifestation, as well as immune response of dogs to subcutaneous injection of TBEV was assessed by Czech scientists. They detected almost no changes for haematology and blood biochemistry parameters. Hovewer, a robust immune response was developed in all cases, in terms of neutralizing antibodies (Salat et al., 2021). Due to the absence of a TBEV vaccine for dogs, Salat and his team tested experimental candidates for a veterinary vaccine, not only in dogs, but also in the mouse system. By detection of specific anti-TBEV antibodies in sera of mice and dog, they confirmed developed vaccine candidate as a promising to protect dogs from severe TBEV infections (Salat et al., 2023).
The detection of TBEV-specific IgM and IgG antibodies in the patient’s serum and CSFseveral days after the onset of TBEV infection and the appearance of symptoms helps to establish a specific diagnosis. In Frańková et al., 1978 with team developed an indirect IF technique using suspensions of PS cells uninfected and infected with the TBE virus as an antigen-containing substrate, as a rapid and practical test allowing the detection of specific serum antibodies of the IgM class at the early stage of clinically manifest TBE. Later, Grubhoffer et al. (1988) presented the IgM-capture EIA system assembled from Czechoslovak immunopreparations (SEVAC) for rapid serological diagnosis of tick-borne encephalitis (TBE), which they tested on clinical material. The detection of IgM antibodies using pig antiserum and the selection of specific IgM antibodies performed with the TBE antigen followed by an indirect method of detection with this system proved to be sensitive and fully correspond to the clinical picture of the disease. In 1995, Slovenian scientists pointed out that the diagnosis of TBEV is made difficult by non-specific test results in the first phase of the disease, e.g., leukopenia, thrombocytopenia or liver tests (Lotrič-Furlan and Strle, 1995). Because the diagnosis of TBEV exposure relies on serologic testing, but there is also cross-reactivity between different flavivirus genera (Orlinger et al., 2011) and the detection of TBEV-specific antibodies may be biased, several investigators have attempted to validate various ELISA, EIA test systems for the detection of TBEV IgG antibodies (Niedrig et al., 2001; Niedrig et al., 2007; Weissbach and Hirsch, 2015; Ackermann-Gäumann et al., 2018; Ackermann-Gäumann et al., 2019). In 2014, an Austrian team of scientists studied the specifics of antibodies in peripheral blood induced by natural TBE infection, as well as vaccination with an inactivated TBE vaccine. To study the individual-specific variation of human antibody responses, they developed immunoassays with recombinant antigens representing viral surface protein domains and domain combinations. They found that individual and specific factors strongly influence the immunodominance of domains on the E-protein (Jarmer et al., 2014). For differentiating infection versus vaccination antibody responses, Girl et al. (2020) developed a novel immunosorbent assay, based on detection specific IgG antibodies against NS1 as an exclusively diagnostic marker of viral replication in natural infection, based on enzyme-linked immunosorbent assay (ELISA). Their assay has demonstrated a sensitivity of >94% and specificity of >93% in widely cross-reactive sera from patients with flavivirus vaccination or single or multiple flavivirus infections. For the study of TBEV, Czech scientists in cooperation with Japanese scientists have developed a powerful quantitative tool to facilitate the identification of potential antiviral agents and to measure the levels of neutralizing antibodies in human and animal serum. They created the mCherry-TBEV recombinant reporter virus as a powerful tool to facilitate the identification of potential antiviral agents and to measure neutralizing antibody levels in human and animal sera (Haviernik et al., 2021).
In humans, TBEV-specific IgM and IgG are found in serum and CSF. Günther et al. (1997) found that the intensity and duration of antibody production in serum and CSF did not correlate with disease severity. They observed wide variations in the kinetics of the antibody response. Kaiser and Holzmann (2000) and Holzmann (2003) verified Günther’s findings but did not confirm the correlation between persistence of antibodies in serum and CSF and disease severity. Later, Holzmann (2003) pointed out that TBEV-specific IgM and often IgG are present in the serum at the time of the first CNS symptoms, and IgG levels, slightly elevated during CNS infection, persisted after the onset of neurological symptoms. Bogovič et al. (2021) analyzed the severity of TBEV disease in relation to the level of serum IgG antibodies against the TBEV. By comparing IgG levels to TBEV of patients during the meningoencephalitic phase of TBE and with the laboratory parameters of patients during the acute illness and with the presence of postencephalitic syndrome (PES), they identified antibody levels, not cut-off values, correlated with clinical parameters, including the likelihood of PES. Low serum IgG antibody responses to TBEV at the onset of neurological involvement are associated with a more severe clinical course and adverse long-term outcomes, posing diagnostic and clinical challenges. Because CNS inflammation caused by a viral infection can be associated with increased immunoglobulin synthesis, Gudowska-Sawczuk et al. (2021) evaluated free light chain (FLC) kappa (κ) and lambda (λ) concentrations in the CSF) and serum of TBE patients. They were the first to demonstrate statistically significant differences in λFLC concentrations in serum and CSF, as well as in λIgG-index, κFLC-index and λIgG-index values before and after TBE treatment. The observed differences probably reflected intrathecal synthesis of immunoglobulins and increased BBB permeability in TBE patients. The obtained data could provide a basis for the development of new therapeutic strategies.
Together with drugs that interfere with TBEV replication, mAbs represent a promising approach against TBE. The effect of commercially available neutralizing IgG antibodies on the treatment of lethal TBEV infection in mouse model was investigated by Elsterova et al. (2017). They demonstrated more than 100-fold differences in virus-neutralizing capacity between batches of antibodies and no antibody-dependent increase in TBEV infectivity in vitro or in vivo. The main target of flavivirus-neutralizing antibodies is the envelope (E) protein, which mediates virus entry into host cells, particularly its receptor-binding domain EDIII, which is the main target of the most potent neutralizing antibodies. Czech scientists recently investigated the molecular mechanisms of TBEV neutralization using the mouse monoclonal IgG antibody, which, due to the absence of cross-reactivity with other flaviviruses, but at the same time the ability to neutralize several strains of TBEV, has significant therapeutic potential. This antibody blocked low pH-induced structural changes in the E-protein, its reorganization into a trimer, required for fusion between the viral and endosomal membranes during virus entry. As a result, the penetration of viral nucleocapsids into the cytoplasm of target cells was delayed or even prevented (Füzik et al., 2018). German and Czech scientists succeeded in isolating expanding clones of memory B cells from donors naturally infected with TBEV or vaccinated against TBEV, which produced monoclonal antibodies against TBE virus envelope domain III (EDIII). At the same time, the production of the strongest and broadly neutralizing antibodies was found in donors who overcame the natural infection. Both groups of volunteers also syntetized antibodies that neutralized other tick-borne flaviviruses, including Langat, louping ill, Omsk hemorrhagic fever, Kyasanur forest disease and Powassan viruses.They also confirmed the effectiveness of prophylactic or early therapeutic administration of antibodies even at low doses in mice that were lethally infected with TBEV (Agudelo et al., 2021). The Svoboda et al. (2023), in collaboration with colleagues from the USA and Switzerland, investigated TBEV escape from two potent human mAbs that target EDIII: T025 and T028. The result was the emergence of virus variants with reduced pathogenicity. The resistance of the resulting virus variants was made possible by characteristically different sets of amino acid changes in EDII and EDIII, necessary for conferring resistance. They found that the combination of mAbs prevented virus escape and improved neutralization of TBEV.
The investigation of immune responses to TBEV is also closely related to the development of vaccines against TBEV. Vaccination against TBE is generally safe with rare serious adverse events. TBE vaccines are immunogenic in terms of antibody response (Klimes et al., 2001). Several studies have been conducted by German and Austrian scientists to test the efficacy of the vaccines, because are based on closely genetically related Austrian and German TBEV strains. They revealed good tolerability and high immunogenicity, mild and transient side effects, as well as a strong relationship between the amount of antigen administered and the antibody response (Bock et al., 1990; Klockmann et al., 1991; Harabacz et al., 1992; Kunz, 2003; Kunz et al., 1980). However, age affects effectiveness, especially in priming; age is a key factor in the duration of protection (rev. in Rampa et al., 2020). Antibody responses to vaccination with a vaccine based on the European subtype of TBEV have been extensively studied and a degree of cross-neutralization between individual TBEV subtypes has been demonstrated by German scientists (Klockmann et al., 1991). Field studies to demonstrate the protective efficacy of TBEV vaccines were conducted in Austria in 2007 and 2013, in which approximately 85% of the population received at least 1 dose of TBE vaccine demonstrated 95%–99% efficacy (Heinz et al., 2007; Heinz et al., 2013). Orlinger et al. (2011) developed an assay platform that allows analysis of viral neutralization in an environment that balances viral replication and infectivity, while preserving individual viral strain surface characteristics that determine neutralization.
Although effective TBEV vaccination has been developed, VBT rarely occurs (Stiasny et al., 2009; Lotrič-Furlan et al., 2017; Dobler et al., 2020; Stiasny et al., 2021). Already in 2009, Stiasny and his team investigated the characteristics of antibody reactions in such VBT compared to reactions in unvaccinated patients with TBE. They found that the majority of VBT showed a delayed IgM antibody response, high avidity and strong neutralizing antibodies in contrast to unvaccinated controls. Janik et al. (2020) assessed the prevalence of seronegative rate for anti-TBEV antibodies and risk factors and showed that both a longer time since vaccination and a lower number of booster doses consistently increased the risk of lost anti-TBEV antibodies. They identified the extension of the interval of booster immunization is risky and suggested a more frequent monitoring of serum antibodies by personalized schedule to adjust the frequency of subsequent doses of booster vaccination.
Due to NS1 targeting of the anti-TBEV antibody response, TBEV vaccines also contain traces of NS1. However, there have been some inconsistencies regarding the production of NS1-specific antibodies after vaccination. While Salat and colleagues (2016) found NS1-specific antibodies (albeit mostly at low levels) in post-vaccination sera, the Girl et al. (2020) did not find a single sample positive for NS1 antibodies in post-vaccination sera, which was also confirmed by Stiasny with colleagues (2021). Whether vaccination induces TBEV NS1-specific antibodies in humans was also investigated by the Ackermann-Gäumann team (2023). Beicht et al. (2023) supported the inclusion of NS1 as a vaccine component in next-generation TBEV vaccines. Using recombinant TBEV viral vectors, they induced NS1-specific antibodies and T cell responses. Heterologous prime/boost regimens conferred partial protection against lethal TBEV infection in mice. Kubinski et al. (2024) evaluated TBEV’s NS3 as a potential vaccine target to induce protective immunity. Although immunization of mice with recombinant MVA-NS3 induced NS3-specific immune responses, particularly T cell responses, mice were not protected against subsequent challenge with a lethal dose of TBEV strain Neudoerfl.
The contribution of B-lymphocytes, responsible for the production of specific antibodies against TBEV, was proven by Czech scientists in a mouse model. They noted an increase in CD19 mRNA levels in the brain tissue of TBEV-infected mice, which correlated with high levels of TBEV-neutralizing antibodies and thus less susceptibility to TBEV. By demonstrating the strong production of cytokine/chemokine mRNA in the brain, they indicated that other immunopathological mechanisms are also involved in the outcome of the disease (Palus et al., 2013).
Cellular immunity against TBEV
Generally, T-lymphocytes are characterized by the expression of the cell surface marker CD3, which forms a complex with receptors specific for T-lymphocytes. The expression of other surface CD markers divides them into two groups with different immune functions: helper (CD4+) and cytotoxic (CD8+). Activated CD4+ T-lymphocytes by expressing various cytokines activate B lymphocytes, CD8+ T-lymphocytes, macrophages and other cells of the immune system. CD4+ T-lymphocytes have a central “helper” role. CD8+ T-lymphocytes kill infected host cells by releasing cytolytic proteins such as perforin and granzyme, most also act as cytokine producers. Effector CD8+ T cells contribute to clearance of infection and provide long-lasting immunity. In TBE, CSF cytosis is dominated by CD3+CD4+ and CD3+CD8+ T-lymphocyte, but their pathogenetic roles and mechanisms of migration into the CNS are unclear. The activation of immune responses in parallel with virus-induced damage was partially known between 1970 and 1980 (reviewed by Ruzek et al., 2010).
In the 1980s, in the context of the T-lymphocytes response to TBEV infection, the “transfer factor” was the subject of intensive research. The mechanism of cytotoxic T-lymphocyte-mediated specific immunity was studied by Gajdošová and her team. At that time, the results suggested that these should be immunologically specific mediator molecule-peptides that require interaction with the naïve cell to subsequently enable the naïve recipient to mount a secondary rather than a primary immunological response upon first encountering the antigen. It was a delayed-type hypersensitivity from sensitized donors to non-sensitized recipients using leukocyte blood lysates. Transfer factors “primed” recipients to express cell-mediated immunity. This effect is specific for the antigen to which they have bound in an immunologically specific way. Gaidosova, Oravec, Mayer and coworkers found that antigen-specific transfer factor (STF) from LGTV-immunized mice enhanced the induction activity in semi-purified splenocyte dialysates by inducing specific T-lymhocyte cytotoxic activity. This effect was group-reactive. Transfer factor molecules induced during attenuated TBEV infection in “donor” mice induced a state of high resistance to challenge with virulent tick-borne encephalitis virus in recipient mice. STF-induced T-lymphocytes killing activity showed cross-reactivity within the flavivirus genus. STF administered before live virus increased specific cytolytic T-lymphocytes response (Gajdošová et al., 1980; Gajdošová et al., 1981; Mayer et al., 1980; Mayer et al., 1982; Mayer et al., 1983; Mayer et al., 1985). The characterization of the spectrum of lymphocyte subpopulations in TBE patients has been addressed by Czech, Polish, Slovenian or German researchers. In 2002, Holub and his team analysed the numbers of lymphocyte subsets in the CSF of patients with tick-borne encephalitis (TBE) and acute neuroborreliosis; a lower accumulation of lymphocyte subsets in the CSF was identified in the case of TBE (Holub et al., 2002). Examination of postmortem human brains revealed only a weak topographical correlation between inflammatory changes (consisting primarily of T-lymphocytes and macrophages/microglia) and viral antigen distribution (Gelpi et al., 2005; Gelpi et al., 2006). Clinical studies by Gelpi’s team suggest an immunopathological rather than protective role for CD8+ T-lymphocytes during TBEV infection of the brain, as brain tissue biopsies from fatal TBE cases showed CD8+ T-lymphocyte infiltration in close proximity to TBEV-infected neurons (Gelpi et al., 2006). By analyzing the role of CD4+ and CD8+ T-lymhocytes in immunocompromised and immunocompetent mouse models, Ruzek et al. (2009a), Ruzek et al. (2009b) demonstrated that while CD4+ T-lymhocytes are more effective in limiting the virus, CD8+ T-lymhocytes contribute to immunopathological responses in the infected brain. However, their results also suggest that CD8+ T-lymhocytes may contribute to increased survival in the setting of infection with a low pathogenic strain of TBEV. By studying changes in blood-brain barrier (BBB) permeability in two susceptible animal models (BALB/c and C57Bl/6 mice) infected with TBE virus at different days after infection, Ruzek´team observed BBB breakdown in mice deficient in CD8+ T-lymphocytes. Their results suggest that CD8+ T-lymhocytes are not involved in the increase in BBB permeability that occurs during TBE (Ruzek et al., 2011).
The importance of CD4+ T-lymhocytes in the immune response to TBEV has been investigated by several European teams. Lepej et al. (2007) as well as Grygorczuk et al. (2016) showed that the CSF leukocyte population is dominated by lymphocytes, mainly CD4+ subsets, accompanied by CD8+ T cells. Gelpi et al. (2006) also found the same populations in inflammatory lesions of the brain parenchyma, where their proportions may differ, with CD8+ cells tending to equal or exceed CD4+ T-lymhocytes. Lepej et al. (2007), Palus et al. (2014a), Palus et al. (2014b), Bogovič et al. (2019) showed that the Th1 type dominates in the intrathecal response to TBEV as well as in the CSF. Bogovic’s team found that cytokines associated with innate and Th1 responses are expressed intrathecally in TBE patients and their high concentrations in CSF are associated with clinical severity and encephalitic picture. Detection lower Th17 mediators’ levels in the serum of patients with PES compared to patients with cured symptoms suggest a protective role of intense Th17 responses in TBE.
CD4+ T-lymphocytes role has been studied in particular in the context of vaccine development and monitoring of immune responses triggered by these vaccines. CD4+ T-lymphocytes responses are essential for neutralizing antibody production. In 2004, Gomez et al. (2004) analyzed the ability of CD4+ T-lymhocytes from young and older subjects to produce IL-2 and IFN-γ after in vitro stimulation with recombinant TBEV E protein using ELISPOT technology. In unfractionated blood mononuclear cells or purified CD4 (+) T-lymhocytes, they found no or minimal differences in response to neoantigen stimulation with autologous DCs. They demonstrated an intact ability of elderly CD4+ T-lymhocytes to respond to neoantigen stimulation, and even an increased ability of CD4+ T-lymhocytes to differentiate into effector cells. Schwaiger et al. (2014) compared the specificity and immunodominance of CD4+ T-lymphocyte responses induced by the viral structural proteins (C, prM/M and E) contained in the whole-virus TBE vaccine with those induced by the same proteins after natural infection. They revealed immunodominant regions in both proteins C and E that correlated with specific protein elements and domains in computer-predicted epitopes on CD4+ T-lymhocytes, providing evidence for a strong influence of structural properties on antigen processing and the MHC-II loading pathway. By comprehensive analysis of the cytokine patterns of CD4+ T-lymphocyte responses of TBE vaccinated human in comparison to patients with TBEV infection Aberle et al. (2015) showed the polyfunctional nature of TBEV-specific CD4+ T cell responses. Cytokine patterns after vaccination differed from those after infection. Stimulation with peptides covering TBEV structural proteins contained in the vaccine (C, prM/M and E) led to expresion ofTh1 specific cytokines (IFN-γ, IL-2, TNF-α), CD40 Tbet (ligand and Th1 lineage specifying transcription factor). CSF CD4+ T-lymphocyte enrichment likely due to elevated CXCL13 levels in TBE was recently confirmed by Toczylowski et al. (2020). The spectrum of CD3+CD4+ and CD3+CD8+ T-lymphocytes, B-lymphocytes (CD19+), as well as the NK (CD16+/56+) fraction, but also chemotactic axes in patients in correlation with various neurological manifestations of the disease such as meningitis, meningoencephalitis and meningoencephalomyelitis were monitored by Gygorczuk´s teams (Grygorczuk et al., 2020; Grygorczuk et al., 2023). TBEV infection caused an increase in the number of lymphocytes in the CSF, but did not affect the proportions of lymphocytes compared to uninfected patients. More severe forms of the disease and neurological disability were correlated with higher pleocytosis and expansion of CD4+ CD8+ and B-lymphocytes. Th lymphocytes were associated with encephalopathy, myelitis and mild cerebellar syndrome, B lymphocytes with myelitis and at least moderate encephalopathy, double positive T-lymhocytes with myelitis and reduced encephalopathy. In the CSF, the CD4+ Th1- and Th2-lymphocyte population was enriched in CCR5-positive cells, and the concentration of CCL5 was increased and associated with a milder presentation. NK cells were decreased in patients with neurological deficit. In children with TBE, the number of CD8+ T-lymphocytes and B cells increased at the expense of CD4+ lymphocytes compared to adults. It appears that specific populations including B, Th and Tc cells are likely to be specifically associated with TBE presenting as myelitis, encephalopathy and cerebellitis. No correlation of double-positive T and NK cells with severity suggested a likely protective function against TBEV. Upregulated CXCL10 mediated the intrathecal immune response but is probably not directly responsible for T cell migration.
Due to the low frequency of reported cases of gastrointestinal autonomic dysfunction in TBEV infection, which has been studied e.g., in German patients (Kaiser, 1999; Kleiter et al., 2006; Dobler et al., 2016), Boelke in their mouse model study investigated the presence of enteric intramural ganglion inflammation as a common feature in TBEV-infected mice. In animals with ganglioneuritis and macroscopic changes in the GI tract, they observed significantly high levels of infiltrating CD3+ T-lymphocytes and, to a lesser extent, macrophages (Boelke et al., 2021).
Conclusion
Tick-borne encephalitis (TBE) is the most important tick-borne disease in Europe. More than 90 years after the discovery of TBEV, there is still much to learn concerning the host and viral factors that govern the outcome of infection. In addition to studying its causative agent TBEV, its distribution in nature, reservoirs and pathogenesis, there is still an urgent need to develop safe and effective vaccines. This process requires a better understanding of the immunological mechanisms involved during infection. As showed within this review, decades of research indicate a paradoxical role of the immune response against TBEV: although the immune response is critical for controlling, clearing and preventing infection, poor clinical outcomes are often associated with virus-specific immunity and immunopathogenesis. Our work offers a comprehensive overview of the data obtained by scientists in Central Europe or in the framework of their collaboration with colleagues outside the monitored region in the field of host defense against TBEV. The obtained information showed a significant contribution of Central Europe in the research of the immune response of mammal host and humans against TBEV and the disease it causes. While at the beginning the studies were focused on the virus isolation and monitoring the basic parameters of the immune response such as the production of antibodies, later gradually more detailed information appeared on the synthesis of specific soluble factors of innate and acquired immunity, including immunity associated with IFNs, on cytokines/chemokines/growth factors as mediators of inflammation, T- or B-cell immune response. The information obtained was closely related to the development of diagnostic and research methods (some of them designed by researchers themselves), but also to the development of international cooperation. Some works confirmed theories, others represented the first step towards the creation of new ones. Clinical studies have verified in vitro research on cell lines or in vivo experiments on laboratory animals and vice versa. Although an effective vaccine is currently available, vaccination is not mandatory and may not be available everywhere. In addition, no specific chemotherapeutic agents have been developed against TBEV. Given the annual increase in incidence as well as the extreme severity of TBE, the identification of immune response biomarkers as candidates for rapid diagnosis, prognosis, monitoring or treatment of TBE is of paramount importance, to which the research conducted in this thesis has contributed. The most promising candidate seems to be MMP-9 in combination with monitoring the ratio of MMP-9 to tissue inhibitors of metalloproteinase – 1 (TIMP-1) (Barkhash et al., 2018). At the same time, specific antibodies against TBEV should be detected, in particular against the NS1 protein (Salat and Ruzek, 2020), or against domains I (DI) and II (DII) of the TBEV E protein or possibly against the C-terminal region of the RNA-dependent RNA polymerase NS5, respectively. We believe that the evaluation of the biomarkers described above may be useful in the diagnosis of TBE in clinical practice. However, further studies are needed to confirm this.
Author contributions
Conceptualization IS and PB, writing—original draft preparation, IS, PB, and JD, writing—review and editing, IS and PB. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Publication of this article was financially supported by Biomedical Research Center SAS, Slovakia and the Scientific Grant Agency of the Ministry of Education, Science, Research and Sport of the Slovak Republic and the Slovak Academy of Sciences, project VEGA 2/0108/22.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Aberle, J. H., Schwaiger, J., Aberle, S. W., Stiasny, K., Scheinost, O., Kundi, M., et al. (2015). Human CD4+ T helper cell responses after tick-borne encephalitis vaccination and infection. PLoS One 10 (10), e0140545. doi:10.1371/journal.pone.0140545
Achazi, K., Ruzek, D., Donoso-Mantke, D., Schlegel, M., Ali, H. S., Wenk, M., et al. (2011). Rodents as sentinels for the prevalence of tick-borne encephalitis virus. Vector-Borne Zoonotic Dis. 11, 641–647. doi:10.1089/vbz.2010.0236
Ackermann-Gäumann, R., Eyer, C., Leib, S. L., and Niederhauser, C. (2019). Comparison of four commercial IgG-enzyme-linked immunosorbent assays for the detection of tick-borne encephalitis virus antibodies. Vector Borne Zoonotic Dis. 19 (5), 358–364. doi:10.1089/vbz.2018.2359
Ackermann-Gäumann, R., Tritten, M. L., Hassan, M., and Lienhard, R. (2018). Comparison of three commercial IgG and IgM ELISA kits for the detection of tick-borne encephalitis virus antibodies. Ticks Tick. Borne Dis. 9 (4), 956–962. doi:10.1016/j.ttbdis.2018.03.031
Agudelo, M., Palus, M., Keeffe, J. R., Bianchini, F., Svoboda, P., Salát, J., et al. (2021). Broad and potent neutralizing human antibodies to tick-borne flaviviruses protect mice from disease. J. Exp. Med. 218 (5), e20210236. doi:10.1084/jem.20210236
Amicizia, D., Domnich, A., Panatto, D., Lai, P. L., Cristina, M. L., Avio, U., et al. (2013). Epidemiology of tick-borne encephalitis (TBE) in Europe and its prevention by available vaccines. Hum. Vaccines Immunother. 9, 1163–1171. doi:10.4161/hv.23802
Atrasheuskaya, A. V., Fredeking, T. M., and Ignatyev, G. M. (2003). Changes in immune parameters and their correction in human cases of tick-borne encephalitis. Clin. Exp. Immunol. 131 (1), 148–154. doi:10.1046/j.1365-2249.2003.02050.x
Bagó, Z., Bauder, B., Kolodziejek, J., Nowotny, N., and Weissenböck, H. (2002). Tick borne encephalitis in a mouflon (Ovis ammon musimon). Vet. Rec. 150, 218–220. doi:10.1136/vr.150.7.218
Baker, D. G., Woods, T. A., Butchi, N. B., Morgan, T. M., Taylor, R. T., Sunyakumthorn, P., et al. (2013). Toll-like receptor 7 suppresses virus replication in neurons but does not affect viral pathogenesis in a mouse model of Langat virus infection. J. Gen. Virol. 94 (Pt 2), 336–347. doi:10.1099/vir.0.043984-0
Balling, A., Beer, M., Gniel, D., and Pfeffer, M. (2015). Prevalence of antibodies against tick-borne encephalitis virus in dogs from saxony, Germany. Berl. Muench. Tieraerztl. Wochenschr. 128, 297–303.
Balling, A., Plessow, U., Beer, M., and Pfeffer, M. (2014). Prevalence of antibodies against tick-borne encephalitis virus in wild game from Saxony, Germany. Ticks Tick. Borne Dis. 5, 805–809. doi:10.1016/j.ttbdis.2014.06.007
Balogh, Z., Ferenczi, E., Szeles, K., Stefanoff, P., Gut, W., Szomor, K. N., et al. (2010). Tick-borne encephalitis outbreak in Hungary due to consumption of raw goat milk. J. Virol. Methods. 163, 481–485. doi:10.1016/j.jviromet.2009.10.003
Barkhash, A. V., Voevoda, M. I., and Romaschenko, A. G. (2013). Association of single nucleotide polymorphism rs3775291 in the coding region of the TLR3 gene with predisposition to tick-borne encephalitis in a Russian population. Antivir. Res. 99, 136–138. doi:10.1016/j.antiviral.2013.05.008
Barkhash, A. V., Yurchenko, A., Yudin, N. S., Ignatieva, E. V., Kozlova, I. V., Borishchuk, I. A., et al. (2018). Amatrix metalloproteinase 9 (MMP9) gene single nucleotide polymorphism is associated with predisposition to tick-borne encephalitis virus-induced severe central nervous system disease. Ticks Tick. Borne Dis. 9, 763–767. doi:10.1016/j.ttbdis.2018.02.010
Bauer, B. U., Runge, M., Schneider, M., Könenkamp, L., Steffen, I., Rubel, W., et al. (2023). Co-exposure to Anaplasma spp., Coxiella burnetii and tick-borne encephalitis virus in sheep in southern Germany.2023. Acta Vet. Scand. 65 (1), 6. doi:10.1186/s13028-022-00659-6
Beauté, J., Spiteri, G., Warns-Petit, E., and Zeller, H. (2018). Tick-borne encephalitis in Europe, 2012 to 2016. Euro Surveill. 23 (45), 1800201. doi:10.2807/1560-7917.ES.2018.23.45.1800201
Beicht, J., Kubinski, M., Zdora, I., Puff, C., Biermann, J., Gerlach, T., et al. (2023). Induction of humoral and cell-mediated immunity to the NS1 protein of TBEV with recombinant Influenza virus and MVA affords partial protection against lethal TBEV infection in mice. Front. Immunol. 14, 1177324. doi:10.3389/fimmu.2023.1177324
Beránková, Z., Khanna, R., Spěváková, M., Langhansová, H., Kopecký, J., and Lieskovská, J. (2024). Cellular stress is triggered by tick-borne encephalitis virus and limits the virus replication in PMJ2-R mouse macrophage cell line. Ticks Tick. Borne Dis. 15 (1), 102269. doi:10.1016/j.ttbdis.2023.102269
Beránková, Z., Kopecký, J., Kobayashi, S., and Lieskovská, J. (2022). Dual control of tick-borne encephalitis virus replication by autophagy in mouse macrophages. Virus Res. 315, 198778. doi:10.1016/j.virusres.2022.198778
Biernat, B., Karbowiak, G., Werszko, J., and Stańczak, J. (2014). Prevalence of tick-borne encephalitis virus (TBEV) RNA in Dermacentor reticulatus ticks from natural and urban environment, Poland. Pol. Exp. Appl. Acarol. 64, 543–551. doi:10.1007/s10493-014-9836-5
Bílý, T., Palus, M., Eyer, L., Elsterová, J., Vancová, M., and Ruzek, D. (2015). Electron tomography analysis of tick-borne encephalitis virus infection in human neurons. Sci. Rep. 5, 10745. doi:10.1038/srep10745
Blaškovič, D. (1961). Tick-borne encephalitis in Europe: Some aspects of epidemiology and control. Trans. N. Y. Acad. Sci. 23 (3 Ser II), 215–232. doi:10.1111/j.2164-0947.1961.tb03116.x
Blaškovič, D., and Nosek, J. (1972). The ecological approach to the study of tickborne encephalitis. Progr Med. Virol. 14, 275–320.
Blom, K., Braun, M., Pakalniene, J., Lunemann, S., Enqvist, M., Dailidyte, L., et al. (2016). NK cell responses to human tick-borne encephalitis virus infection. J. Immunol. 197 (7), 2762–2771. doi:10.4049/jimmunol.1600950
Bock, H. L., Klockmann, U., Jüngst, C., Schindel-Künzel, F., Theobald, K., and Zerban, R. (1990). A new vaccine against tick-borne encephalitis: Initial trial in man including a dose-response study. Vaccine 8 (1), 22–24. doi:10.1016/0264-410x(90)90172-i
Boelke, M., Puff, C., Becker, K., Hellhammer, F., Gusmag, F., Marks, H., et al. (2021). Enteric ganglioneuritis, a common feature in a subcutaneous TBEV murine infection model. Microorganisms 9 (4), 875. doi:10.3390/microorganisms9040875
Bogovič, P., Kastrin, A., Lotrič-Furlan, S., Ogrinc, K., Avšič Županc, T., Korva, M., et al. (2022). Comparison of laboratory and immune characteristics of the initial and second phase of tick-borne encephalitis. Emerg. Microbes Infect. 11 (1), 1647–1656. doi:10.1080/22221751.2022.2086070
Bogovič, P., Lotrič-Furlan, S., Avšič-Županc, T., Korva, M., Lusa, L., Strle, K., et al. (2021). Low virus-specific IgG antibodies in adverse clinical course and outcome of tick-borne encephalitis. Microorganisms 9 (2), 332. doi:10.3390/microorganisms9020332
Bogovič, P., Lusa, L., Korva, M., Pavletič, M., Resman Rus, K., Lotrič-Furlan, S., et al. (2019). Inflammatory immune responses in the pathogenesis of tick-borne encephalitis. J. Clin. Med. 8, 731. doi:10.3390/jcm8050731
Bogovič, P., Stupica, D., Rojko, T., Lotrič-Furlan, S., Avšič-Županc, T., Kastrin, A., et al. (2018). The long-term outcome of tick-borne encephalitis in Central Europe. Ticks Tick-Borne Dis. 9, 369–378. doi:10.1016/j.ttbdis.2017.12.001
Böhm, B., Schade, B., Bauer, B., Hoffmann, B., Hoffmann, D., Ziegler, U., et al. (2017). Tick-borne encephalitis in a naturally infected sheep. BMC Vet. Res. 13, 267. doi:10.1186/s12917-017-1192-3
Brandenburg, P. J., Obiegala, A., Schmuck, H. M., Dobler, G., Chitimia-Dobler, L., and Pfeffer, M. (2023). Seroprevalence of tick-borne encephalitis (TBE) virus antibodies in wild rodents from two natural TBE foci in bavaria, Germany. Pathogens 12 (2), 185. doi:10.3390/pathogens12020185
Brockmann, S. O., Oehme, R., Buckenmaier, T., Beer, M., Jeffery-Smith, A., Spannenkrebs, M., et al. (2018). A cluster of two human cases of tick-borne encephalitis (TBE) transmitted by unpasteurised goat milk and cheese in Germany, May 2016. Euro Surveill. 23 (15), 17-00336–00336. doi:10.2807/1560-7917.ES.2018.23.15.17-00336
Burri, C., Korva, M., Bastic, V., Knap, N., Avšič-Županc, T., and Gern, L. (2012). Serological Evidence of tick-borne encephalitis virus infection in rodents captured at four sites in Switzerland. J. Med. Entomol. 49, 436–439. doi:10.1603/ME11084
Caini, S., Szomor, K., Ferenczi, E., Szekelyne Gaspar, A., Csohan, A., Krisztalovics, K., et al. (2012). Tick-borne encephalitis transmitted by unpasteurised cow milk in western Hungary, September to October 2011. Euro Surveill. 17 (12), 20128. doi:10.2807/ese.17.12.20128-en
Carpio, K. L., Thompson, J. K., Widen, S. G., Smith, J. K., Juelich, T. L., Clements, D. E., et al. (2023). Differences in genetic diversity of mammalian tick-borne flaviviruses. Viruses 15 (2), 281. doi:10.3390/v15020281
Černý, V. (1975). The role of mammals in natural foci of tick-borne encephalitis in Central Europe. Folia Parasit. (Praha). 22, 271–273.
Chitimia-Dobler, L., Lemhöfer, G., Król, N., Bestehorn, M., Dobler, G., and Pfeffer, M. (2019). Repeated isolation of tick-borne encephalitis virus from adult Dermacentor reticulatus ticks in an endemic area in Germany. Parasites Vectors 12, 90. doi:10.1186/s13071-019-3346-6
Chmielewska, A. M., Gómez-Herranz, M., Gach, P., Nekulova, M., Bagnucka, M. A., Lipińska, A. D., et al. (2022). The role of IFITM proteins in tick-borne encephalitis virus infection. J. Virol. 96 (1), e0113021. doi:10.1128/JVI.01130-21
Cisak, E., Wójcik-Fatla, A., Zając, V., Sroka, J., Buczek, A., and Dutkiewicz, J. (2010). Prevalence of tick-borne encephalitis virus (TBEV) in samples of raw milk taken randomly from cows, goats and sheep in eastern Poland. Ann. Agric. Environ. Med. 17 (2), 283–286.
Csank, T., Drzewnioková, P., Korytár, Ľ., Major, P., Gyuranecz, M., Pistl, J., et al. (2018). A serosurvey of flavivirus infection in horses and birds in Slovakia. Vector Borne Zoonotic Dis. 18 (4), 206–213. doi:10.1089/vbz.2017.2216
Dai, X., Shang, G., Lu, S., Yang, J., and Xu, J. (2018). A new subtype of eastern tick-borne encephalitis virus discovered in Qinghai-Tibet Plateau, China. Emerg. Microbes Infect. 7, 1–9. doi:10.1038/s41426-018-0081-6
Daniel, M., Benes, C., Danielová, V., and Kríz, B. (2011). Sixty years of research of tick-borne encephalitis a basis of the current knowledge of the epidemiological situation in Central Europe. Epidemiol. Mikrobiol. Imun 60 (4), 135–155.
Daniel, M., Zitek, K., Danielová, V., Kríz, B., Valter, J., and Kott, I. (2006). Risk assessment and prediction of Ixodes ricinus tick questing activity and human tick-borne encephalitis infection in space and time in the Czech Republic. Int. J. Med. Microbiol. 296 (Suppl. 40), 41–47. doi:10.1016/j.ijmm.2006.02.008
de Heus, P., Bagó, Z., Weidinger, P., Lale, D., Trachsel, D. S., Revilla-Fernández, S., et al. (2023). Severe neurologic disease in a horse caused by tick-borne encephalitis virus, Austria, 2021. Viruses 15 (10), 2022. doi:10.3390/v15102022
de Heus, P., Kolodziejek, J., Hubálek, Z., Dimmel, K., Racher, V., Nowotny, N., et al. (2021). West nile virus and tick-borne encephalitis virus are endemic in equids in eastern Austria. Viruses 13 (9), 1873. doi:10.3390/v13091873
Deviatkin, A. A., Karganova, G. G., Vakulenko, Y. A., and Lukashev, A. N. (2020). TBEV subtyping in terms of genetic distance. Viruses 12, 1240–1251. doi:10.3390/v12111240
Dobler, G., Bestehorn, M., Antwerpen, M., and Overby-Wernstedt, A. (2016). Complete genome sequence of a low-virulence tick-borne encephalitis virus strain. Genome announc. 4, 1–2. doi:10.1128/genomeA.01145-16
Dobler, G., Kaier, K., Hehn, P., Böhmer, M. M., Kreusch, T. M., and Borde, J. (2020). Tick-borne encephalitis virus vaccination breakthrough infections in Germany: A retrospective analysis from 2001 to 2018. Clin. Microbiol. Infect. 26, 1090.e7–1090.e13. doi:10.1016/j.cmi.2019.12.001
Dorko, E., Hockicko, J., Rimárová, K., Bušová, A., Popaďák, P., Popaďáková, J., et al. (2018). Milk outbreaks of tick-borne encephalitis in Slovakia, 2012-2016. Cent. Eur. J. Public Health 26 (Suppl. l), S47–S50. doi:10.21101/cejph.a5272
Dörrbecker, B., Dobler, G., Spiegel, M., and Hufert, F. T. (2010). Tick-borne encephalitis virus and the immune response of the mammalian host. Travel Med. Infect. Dis. 8, 213–222. doi:10.1016/j.tmaid.2010.05.010
Du, Y., Mi, Z., Xie, Y., Lu, D., Zheng, H., Sun, H., et al. (2021). Insights into the molecular basis of tick-borne encephalitis from multiplatform metabolomics. PLoS Negl. Trop. Dis. 15 (3), e0009172. doi:10.1371/journal.pntd.0009172
Egyed, L., Zöldi, V., and Szeredi, L. (2015). Subclinical tick-borne encephalitis virus in experimentally infected Apodemus agrarius. Intervirology 58, 369–372. doi:10.1159/000443833
Elbaz, M., Gadoth, A., Shepshelovich, D., Shasha, D., Rudoler, N., and Paran, Y. (2022). Systematic review and meta-analysis of foodborne tick-borne encephalitis, Europe, 1980-2021. Emerg. Infect. Dis. 28 (10), 1945–1954. doi:10.3201/eid2810.220498
Elsterova, J., Palus, M., Sirmarova, J., Kopecky, J., Niller, H., and Ruzek, D. (2017). Tick-borne encephalitis virus neutralization by high dose intravenous immunoglobulin. Ticks Tick. Borne Dis. 8 (2), 253–258. doi:10.1016/j.ttbdis.2016.11.007
Ernek, E. (1962). Experimental pathogenicity of the tick-borne encephalitis virus for Domestic Ducks. in: Paper presented at the Biology of viruses of the tick-borne encephalitis complex: proceedings of a symposium, held at Smolenice.
Ernek, E., Kozuch, O., Hudec, K., and Urbán, S. (1967). Occurrence of tick-borne encephalitis virus neutralizing antibody in aquatic birds in Central Europe. Acta Virol. 11 (6), 562.
Ernek, E., Kozuch, O., Lichard, M., and Nosek, J. (1968). The role of birds in the circulation of tick-borne encephalitis virus in the Tribec region. Acta Virol. 12 (5), 468–570.
Ernek, E., Kožuch, O., and Nosek, J. (1969a). The relation between tick-borne encephalitis virus and the wild duck (Anas platyrhynchos) II. Chronic latent infection. Acta Virol. 13, 303–308.
Ernek, E., Kožuch, O., Nosek, J., and Hudec, J. (1969b). The relation between tick-borne encephalitis virus and the wild duck (Anas platyrhynchos) I. Acute infection. Acta Virol. 13, 296–302.
Ernek, E., Kozuch, O., Nosek, J., Hudec, K., and Folk, C. (1975). Virus neutralizing antibodies to arboviruses in birds of the order Anseriformes in Czechoslovakia. Acta Virol. 19 (4), 349–353.
Ernek, E., KožuchO, L. M., Nosek, J., and Albrecht, P. (1963). Experimental infection of Clethrionomys glareolus and Apodemus flavicollis with tick-borne encephalitis virus. Acta Virol. 7, 434–436.
Ernek, E., and Lichard, M. (1964). Role of the English sparrow Passer domesticus in the circulation of tick-borne encephalitis virus. J. Hyg. Epidemiol. Microbiol. Immunol. 8, 375–379.
Etna, M. P., Signorazzi, A., Ricci, D., Severa, M., Rizzo, F., Giacomini, E., et al. (2021). Human plasmacytoid dendritic cells at the crossroad of type I interferon-regulated B cell differentiation and antiviral response to tick-borne encephalitis virus. PLoS Pathog. 17 (4), e1009505. doi:10.1371/journal.ppat.1009505
European Centre for Disease Prevention and Control (2022). “Tick-borne encephalitis,” in ECDC. Annual epidemiological report for 2020 (Stockholm: ECDC). Available at: www.ecdc.europa.eu.
Feal, G. (1949). Laboratory infection caused by tick-borne encephaltis virus. Cas. Lék. Ces. 88, 224–229.
Fialova, A., Cimburek, Z., Iezzi, G., and Kopecky, J. (2010). Ixodes ricinus tick saliva modulates tick-borne encephalitis virus infection of dendritic cells. Microbes Infect./Inst. Pasteur 12 (7), 580–585. doi:10.1016/j.micinf.2010.03.015
Földvári, G., Široký, P., Szekeres, S., Majoros, G., and Sprong, H. (2016). Dermacentor reticulatus: a vector on the rise. Parasites Vectors 9, 314. doi:10.1186/s13071-016-1599-x
Fortova, A., Barkhash, A. V., Pychova, M., Krbkova, L., Palus, M., Salat, J., et al. (2023b). Genetic polymorphisms in innate immunity genes influence predisposition to tick-borne encephalitis. J. Neurovirol 29, 699–705. doi:10.1007/s13365-023-01182-8
Fortova, A., Hönig, V., Salat, J., Palus, M., Pychova, M., Krbkova, L., et al. (2023a). Serum matrix metalloproteinase-9 (MMP-9) as a biomarker in paediatric and adult tick-borne encephalitis patients. Virus Res. 324, 199020. doi:10.1016/j.virusres.2022.199020
Frańková, V., Chýle, M., Duniewicz, M., Marhoul, Z., Kolman, J. M., and Mancal, P. (1978). Rapid diagnosis of tick - borne encephalitis by immunofluorescence assay of specific serum IgM antibodies. J. Hyg. Epidemiol. Microbiol. Immunol. 22 (4), 502–510.
Füzik, T., Formanová, P., Ruzek, D., Yoshii, K., Niedrig, M., and Plevka, P. (2018). Structure of tick-borne encephalitis virus and its neutralization by a monoclonal antibody. Nat. Commun. 9 (1), 436. doi:10.1038/s41467-018-02882-0
Gajdosová, E., Mayer, V., and Oravec, C. (1980). Cross-reactive killer T lymphocytes in a flavivirus infection. Acta Virol. 24 (4), 291–293.
Gajdosová, E., Oravec, C., and Mayer, V. (1981). Cell-mediated immunity in flavivirus infections. I. Induction of cytotoxic T lymphocytes in mice by an attenuated virus from the tick-borne encephalitis complex and its group-reactive character. Acta Virol. 25 (1), 10–18.
Gaunt, M., Sall, A. A., de Lamballerie, X., Falconar, A. K. I., Dzhivanian, T. I., and Gould, E. A. (2001). Phylogenetic relationships of flaviviruses correlate with their epidemiology, disease association and biogeography. J. Gen. Virol. 82, 1867–1876. doi:10.1099/0022-1317-82-8-1867
Gelpi, E., Preusser, M., Garzuly, F., Holzmann, H., Heinz, F. X., and Budka, H. (2005). Visualization of Central European tick-borne encephalitis infection in fatal human cases. J. Neuropathol. Exp. Neurol. 64, 506–512. doi:10.1093/jnen/64.6.506
Gelpi, E., Preusser, M., Laggner, U., Garzuly, F., Holzmann, H., Heinz, F. X., et al. (2006). Inflammatory response in human tick-borne encephalitis: Analysis of postmortem brain tissue. J. Neurovirol. 12 (4), 322–327. doi:10.1080/13550280600848746
Ghita, L., Breitkopf, V., Mulenge, F., Pavlou, A., Gern, O. L., Durán, V., et al. (2021). Sequential MAVS and MyD88/TRIF signaling triggers anti-viral responses of tick-borne encephalitis virus-infected murine astrocytes. J. Neurosci. Res. 99 (10), 2478–2492. doi:10.1002/jnr.24923
Girl, P., Bestehorn-Willmann, M., Zange, S., Borde, J. P., Dobler, G., and von Buttlar, H. (2020). Tick-borne encephalitis virus nonstructural protein 1 IgG enzyme-linked immunosorbent assay for differentiating infection versus vaccination antibody responses. J. Clin. Microbiol. 58 (4), 1–19. doi:10.1128/JCM.01783-19
Gomez, I., Marx, F., Gould, E. A., and Grubeck-Loebenstein, B. (2004). T cells from elderly persons respond to neoantigenic stimulation with an unimpaired IL-2 production and an enhanced differentiation into effector cells. Exp. Gerontol. 39 (4), 597–605. doi:10.1016/j.exger.2003.11.018
Gothe, L. M. R., Ganzenberg, S., Ziegler, U., Obiegala, A., Lohmann, K. L., Sieg, M., et al. (2023). Horses as sentinels for the circulation of flaviviruses in eastern-Central Germany. Viruses 15 (5), 1108. doi:10.3390/v15051108
Grard, G., Moureau, G., Charrel, R. N., Lemasson, J. J., Gonzalez, J. P., Gallian, P., et al. (2007). Genetic characterization of tick-borne flaviviruses: new insights into evolution, pathogenetic determinants and taxonomy. Virology 361, 80–92. doi:10.1016/j.virol.2006.09.015
Gresikova, M. (1958a). Excretion of the Tick-borne encephalitis virus in the milk of subcutaneously infected cows. Acta virol. 2, 188–192.
Gresikova, M. (1958b). Recovery of the tick-borne encephalitis virus from the blood and milk of subcutaneously infected sheep. Acta virol. 2, 113–119.
Gresikova, M. (1960). “The transmission of the virus of the Czechoslovak tick encephalitis by goat milk. In: zeckenenzephalitis in Europa Tick-borne encephalitis in Europe,” in Abhandlungen der Deutschen Akademie der Wissenschaften zu Berlin. Akademieverlag Berlin. Editor H. Libikova, 1960, 121–122.
Grešíková, M. (1972). Studies on tick-borne arboviruses isolated in Central Europe. Biol. Práce 18 (2), 1–116. Vyd. SAV, Bratislava.
Grešíková, M. (1999). Kliešťová encefalitída-trvalý verejno-zdravotnícky problem. Bratislava: VEDA Vyd. SAV.
Gresikova, M., and Calisher, C. H. (1988). “Tick-borne encephalitis,” in The arboviruses: epidemiology and ecology. Editor T. P. Monath, IV, 177–202. (Chapter 45). doi:10.1201/9780429289170-10
Grešíková, M., and Ernek, E. (1965). “The role of some bird species in the ecology of tick-borne encephalitis,” in Theoretical questions of natural foci of diseases. Editors B. Rosicky, and K. Heyberger (Prague, Czech Republic: Publishing House of the Czechoslovak Academy of Sciences), 193–198.
Gresikova, M., and Kaluzova, M. (1997). Biology of tick-borne encephalitis virus. Acta Virol. 41 (2), 115–124.
Grešíková, M., Nosek, J., Řeháček, J., and Albrecht, P. (1962). The role of birds in a natural focus of tick-borne encephalitis. II. Experimental infection of great tits (Parus major L.) with tick-borne encephalitis virus. Journal of Hygiene, Epidemiology. Microbiol. Immunol. 6, 339–342.
Gresikova, M., and Rehacek, J. (1959). Isolation of the tick encephalitis virus from the blood and milk of domestic animals sheep and cow after infection by ticks of the family Ixodes ricinus L. Arch. Ges. Virusforsch. 9 (3), 360–364. doi:10.1007/bf01248828
Grešíková-Kohútová, M., and Albrecht, P. (1959). Experimental pathogenicity of the tick-borne encephalitis virus for the green lizard, Lacerta viridis (Laurenti 1768). J. Hyg. Epidemiol. Microbiol. Immunol. 3 (2), 58–263.
Gresíková, M., and Sekeyová, M. (1978). 2-mercapto-ethanol application in tick-borne encephalitis serologic diagnostics (author's transl). Imunologie 27 (1), 26–28.
Gresikova, M., Sekeyova, M., Stupalova, S., and Necas, S. (1975). Sheep milk-borne epidemic of tick-borne encephalitis in Slovakia. Intervirology 5, 57–61. doi:10.1159/000149880
Grubhoffer, L., Krivanec, K., Kopecký, J., and Tomková, E. (1988). Rapid serological diagnosis of tick-borne encephalitis by means of enzyme immunoassay. Folia Parasitol. (Praha). 35 (2), 181–187.
Grygorczuk, S., Czupryna, P., Pancewicz, S., Świerzbińska, R., Dunaj, J., Siemieniako, A., et al. (2021). The increased intrathecal expression of the monocyte-attracting chemokines CCL7 and CXCL12 in tick-borne encephalitis. J. Neurovirol 27 (3), 452–462. doi:10.1007/s13365-021-00975-z
Grygorczuk, S., Czupryna, P., Pancewicz, S., Świerzbińska, R., Kondrusik, M., Dunaj, J., et al. (2018a). Intrathecal expression of IL-5 and humoral response in patients with tick-borne encephalitis. Ticks Tick. Borne Dis. 9 (4), 896–911. doi:10.1016/j.ttbdis.2018.03.012
Grygorczuk, S., Osada, J., Parczewski, M., Moniuszko, A., Świerzbińska, R., Kondrusik, M., et al. (2016). The expression of the chemokine receptor CCR5 in tick-borne encephalitis. J. Neuroinflammation 13, 45. doi:10.1186/s12974-016-0511-0
Grygorczuk, S., Osada, J., Sulik, A., Toczyłowski, K., Dunaj-Małyszko, J., Czupryna, P., et al. (2023). Associations of the cerebrospinal fluid lymphocyte population with a clinical presentation of tick-borne encephalitis. Ticks Tick. Borne Dis. 14 (5), 102204. doi:10.1016/j.ttbdis.2023.102204
Grygorczuk, S., Osada, J., Toczyłowski, K., Sulik, A., Czupryna, P., Moniuszko-Malinowska, A., et al. (2020). The lymphocyte populations and their migration into the central nervous system in tick-borne encephalitis. Ticks Tick. Borne Dis. 11 (5), 101467. doi:10.1016/j.ttbdis.2020.101467
Grygorczuk, S., Parczewski, M., Moniuszko, A., Świerzbińska, R., Kondrusik, M., Zajkowska, J., et al. (2015). Increased concentration of interferon lambda-3, interferon beta and interleukin-10 in the cerebrospinal fluid of patients with tick-borne encephalitis. Cytokine 71, 125–131. doi:10.1016/j.cyto.2014.10.001
Grygorczuk, S., Parczewski, M., Świerzbińska, R., Czupryna, P., Moniuszko, A., Dunaj, J., et al. (2017). The increased concentration of macrophage migration inhibitory factor in serum and cerebrospinal fluid of patients with tick-borne encephalitis. J. Neuroinflammation 14 (1), 126. doi:10.1186/s12974-017-0898-2
Grygorczuk, S., Świerzbińska, R., Kondrusik, M., Dunaj, J., Czupryna, P., Moniuszko, A., et al. (2018b). The intrathecal expression and pathogenetic role of Th17 cytokines and CXCR2-binding chemokines in tick-borne encephalitis. J. Neuroinflammation 15 (1), 115. doi:10.1186/s12974-018-1138-0
Grygorczuk, S., Zajkowska, J., Swierzbinska, R., Pancewicz, S., Kondrusik, M., and Hermanowska-Szpakowicz, T. (2006a). Elevated concentration of the chemokine CCL3 (MIP-1alpha) in cerebrospinal fluid and serum of patients with tick-borne encephalitis. Adv. Med. Sci. 51, 340–344.
Grygorczuk, S., Zajkowska, J., Swierzbińska, R., Pancewicz, S., Kondrusik, M., and Hermanowska-Szpakowicz, T. (2006b). Concentration of the beta-chemokine CCL5 (RANTES) in cerebrospinal fluid in patients with tick-borne encephalitis. Neurol. Neurochir. Pol. 40 (2), 106–111. Polish.
Grzybek, M., Alsarraf, M., Tołkacz, K., Behnke-Borowczyk, J., Biernat, B., Stańczak, J., et al. (2018). Seroprevalence of TBEV in bank voles from Poland-a long-term approach. Emerg. Microbes Infect. 7 (1), 1–8. doi:10.1038/s41426-018-0149-3
Gudowska-Sawczuk, M., Czupryna, P., Moniuszko-Malinowska, A., Pancewicz, S., and Mroczko, B. (2021). Free immunoglobulin light chains in patients with tick-borne encephalitis: before and after treatment. J. Clin. Med. 10 (13), 2922. doi:10.3390/jcm10132922
Günther, G., Haglund, M., Lindquist, L., Sköldenberg, B., and Forsgren, M. (1997). Intrathecal IgM, IgA and IgG antibody response in tick-borne encephalitis. Long-term follow-up related to clinical course and outcome. Virol. 8 (1), 17–29. doi:10.1016/s0928-0197(97)00273-0
Harabacz, I., Bock, H., Jüngst, C., Klockmann, U., Praus, M., and Weber, R. (1992). A randomized phase II study of a new tick-borne encephalitis vaccine using three different doses and two immunization regimens. Vaccine 10 (3), 145–150. doi:10.1016/0264-410x(92)90003-3
Haslwanter, D., Blaas, D., Heinz, F. X., and Stiasny, K. (2017). A novel mechanism of antibodymediated enhancement of flavivirus infection. PLoS Pathog. 13 (9), e1006643. doi:10.1371/journal.ppat.1006643
Haut, M., Girl, P., Oswald, B., Romig, T., Obiegala, A., Dobler, G., et al. (2020). The red fox (Vulpes vulpes) as sentinel for tick-borne encephalitis virus in endemic and non-endemic areas. Microorganisms 8 (11), 1817. doi:10.3390/microorganisms8111817
Haviernik, J., Eyer, L., Yoshii, K., Kobayashi, S., Cerny, J., Nougairède, A., et al. (2021). Development and characterization of recombinant tick-borne encephalitis virus expressing mCherry reporter protein: a new tool for high-throughput screening of antiviral compounds, and neutralizing antibody assays. Antivir. Res. 185, 104968. doi:10.1016/j.antiviral.2020.104968
Heigl, Z., and Zeipel, G. V. (1966). Experimental infection with tick-borne encephalitis virus in Clethrionomys glareolus, Apodemus flavicollis, Apodemus sylvaticus and Mus musculus. Acta Pathol. Microbiol. Scand. 66, 489–509. doi:10.1111/apm.1966.66.4.489
Heinz, F. X., Holzmann, H., Essl, A., and Kundi, M. (2007). Field effectiveness of vaccination against tick-borne encephalitis. Vaccine 25, 7559–7567. doi:10.1016/j.vaccine.2007.08.024
Heinz, F. X., Stiasny, K., Holzmann, H., Grgic-Vitek, M., Kriz, B., Essl, A., et al. (2013). Vaccination and tick-borne encephalitis, central Europe. Emerg. Infect. Dis. 19 (1), 69–76. doi:10.3201/eid1901.120458
Hellenbrand, W., Kreusch, T., Böhmer, M. M., Wagner-Wiening, C., Dobler, G., Wichmann, O., et al. (2019). Epidemiology of tick-borne encephalitis (TBE) in Germany, 2001–2018. Pathogens 8 (2), 42. doi:10.3390/pathogens8020042
Hofmann, H., Frisch-Niggemeyer, W., and Kunz, C. (1978). Protection of mice against tick-borne encephalitis by different classes of immunoglobulins. Infection 6, 154–157. doi:10.1007/bf01641903
Hofmann, H., and Popow-Kraupp, T. (1982). Tick-borne encephalitis (TBE) infection: Antiviral antibodies in cerebrospinal fluid. Zentralbl Bakteriol. Mikrobiol. Hyg. A Med. Mikrobiol. Infekt. Parasitol. 253 (3), 305–311. doi:10.1016/s0174-3031(82)80065-6
Hofmann, H., Radda, A., and Pretzmann, G. (1971). Interferon production by 2 species of mice after infection with tick-borne encephalitis (TBE) virus. Brief report. Arch. Ges. Virusforsch. 34, 385–387. doi:10.1007/bf01242986
Holub, M., Klucková, Z., Beran, O., Aster, V., and Lobovská, A. (2002). Lymphocyte subset numbers in cerebrospinal fluid: Comparison of tick-borne encephalitis and neuroborreliosis. Acta Neurol. Scand. 106 (5), 302–308. doi:10.1034/j.1600-0404.2002.01314.x
Holzmann, H. (2003). Diagnosis of tick-borne encephalitis. Vaccine 21 (S), 36–40. doi:10.1016/s0264-410x(02)00819-8
Holzmann, H., Aberle, S. W., Stiasny, K., Werner, P., Mischak, A., Zainer, B., et al. (2009). Tick-borne encephalitis from eating goat cheese in a mountain region of Austria. Infect. Dis. 15 (10), 1671–1673. doi:10.3201/eid1510.090743
Hubálek, Z. (2021). History of arbovirus research in the Czech republic. Viruses 13, 2334–2368. doi:10.3390/v13112334
Hubálek, Z., Černý, V., Mittermayer, T., Kilik, J., Halouzka, J., Juricova, Z., et al. (1986). Arbovirological survey in silica plateau area, roznava district, Czechoslovakia. J. Hyg. Epidemiol. Microbiol. Immunol. 30, 87–98.
Hudopisk, N., Korva, M., Janet, E., Simetinger, M., Grgič-Vitek, M., Gubenšek, J., et al. (2013). Tick-borne encephalitis associated with consumption of raw goat milk, Slovenia, 2012. Emerg. Infect. Dis. 19 (5), 806–808. doi:10.3201/eid1905.121442
Im, J. H., Baek, J. H., Durey, A., Kwon, H. Y., Chung, M. H., and Lee, J. S. (2020). Geographic distribution of Tick-borne encephalitis virus complex. J. Vector Borne Dis. 57 (1), 14–22. doi:10.4103/0972-9062.308794
Janik, M., Płaczkowska, S., Woźniak, M., and Bil-Lula, I. (2020). Analysis of multiple risk factors for seronegative rate of anti-tick-borne encephalitis virus immunization in human serum. Medicina 56 (5), 244. doi:10.3390/medicina56050244
Jarmer, J., Zlatkovic, J., Tsouchnikas, G., Vratskikh, O., Strauß, J., Aberle, J. H., et al. (2014). Variation of the specificity of the human antibody responses after tick-borne encephalitis virus infection and vaccination. J. Virol. 88 (23), 13845–13857. doi:10.1128/JVI.02086-14
Jezyna, C. A. (1976). Milk-borne outbreak of tick-borne encephalitis in the olsztyn province. Prz. Epidemiol. 30, 479–490.
Kaiser, R. (1999). The clinical and epidemiological profile of tick-borne encephalitis in southern Germany 1994–1998: A prospective study of 656 patients. Brain 122 (Pt 11), 2067–2078. doi:10.1093/brain/122.11.2067
Kaiser, R. (2008). Tick-borne encephalitis. Infect. Dis. Clin. North Am. 22 (3), 561–575. doi:10.1016/j.idc.2008.03.013
Kaiser, R. (2012). Tick-borne encephalitis: clinical findings and prognosis in adults. Wien Med. Wochenschr 162 (11-12), 239–243. doi:10.1007/s10354-012-0105-0
Kaiser, R., and Holzmann, H. (2000). Laboratory findings in tick-borne encephalitis-correlation with clinical outcome. Infection 28, 78–84. doi:10.1007/s150100050051
Karbowiak, G. (2014). The occurrence of the Dermacentor reticulatus tick – its expansion to new areas and possible causes. Ann. Parasitol. 60, 37–47.
Karbowiak, G., Biernat, B., Szewczyk, T., and Sytykiewicz, H. (2015). The role of particular tick developmental stages in the circulation of tick-borne pathogens affecting humans in Central Europe. 1. The general pattern. Ann. Parasitol. 61, 221–228. doi:10.17420/ap6104.11
Karbowiak, G., and Kiewra, D. (2010). New locations of Dermacentor reticulatus ticks in western Poland: The first evidence of merge in D. reticulatus occurrence areas? Wiad. Parazytol. 54, 333–336.
Kawai, T., and Akira, S. (2006). Innate immune recognition of viral infection. Nat. Immunol. 7, 131–137. doi:10.1038/ni1303
Kazimírová, M. (2022). “Tick borne infections in central Europe,” in Climate, tick and disease. Editor P. Nuttall (Oxfordshire: CAB International), 430–436. OX10 8DE UK. doi:10.1079/9781789249637.0000
Kerlik, J., Avdičová, M., Štefkovičová, M., Tarkovská, V., Pántiková Valachová, M., Molčányi, T., et al. (2018). Slovakia reports highest occurrence of alimentary tick-borne encephalitis in Europe: analysis of tick-borne encephalitis outbreaks in Slovakia during 2007-2016. Travel Med. Infect. Dis. 26, 37–42. doi:10.1016/j.tmaid.2018.07.001
Khozinsky, V. V., Semenov, B. F., Gresíková, M., Chunikhin, S. P., Sekeyová, M., and Kožuch, O. (1985). Role of macrophages in the pathogenesis of experimental tick-borne encephalitis in mice. Acta Virol. 29 (3), 194–202.
Kindberg, E., Vene, S., Mickiene, A., Lundkvist, A., Lindquist, L., and Svensson, L. (2011). A functional toll-like receptor 3 gene (TLR3) may Be a risk factor for tick-borne encephalitis virus (TBEV) infection. J. Infect. Dis. 203 (4), 523–528. doi:10.1093/infdis/jiq082
King, A. M. Q., Adams, M. J., Carstens, E. B., and Lefkowitz, E. J. (2011). in Virus taxonomy: classification and nomenclature of viruses: ninth report of the international committee on taxonomy of viruses.Elsevier Inc.
Klaus, C., Beer, M., Saier, R., Schau, U., Moog, U., Hoffmann, B., et al. (2012). Goats and sheep as sentinels for tick-borne encephalitis (TBE) virus-epidemiological studies in areas endemic and non-endemic for TBE virus in Germany. Ticks Tickborne Dis. 3, 27–37. doi:10.1016/j.ttbdis.2011.09.011
Klaus, C., Beer, M., Saier, R., Schubert, H., Bischoff, S., and Süss, J. (2011). Evaluation of serological tests for detecting tick-borne encephalitis virus (TBEV) antibodies in animals. Berl. Munch Tierarztl Wochenschr 124 (11-12), 443–449.
Klaus, C., Hoffmann, B., Beer, M., Müller, W., Stark, B., Bader, W., et al. (2010). Seroprevalence of tick-borne encephalitis (TBE) in naturally exposed monkeys (Macaca sylvanus) and sheep and prevalence of TBE virus in ticks in a TBE endemic area in Germany. Dis 1, 141–144. doi:10.1016/j.ttbdis.2010.06.001
Klaus, C., Hörügel, U., Hoffmann, B., and Beer, M. (2013). Tick-borne encephalitis virus (TBEV) infection in horses: clinical and laboratory findings and epidemiological investigations. Vet. Microbiol. 163, 368–372. doi:10.1016/j.vetmic.2012.12.041
Klaus, C., Ziegler, U., Hoffmann, D., Press, F., Fast, C., and Beer, M. (2019). Tick-borne encephalitis virus (TBEV) antibodies in animal sera - occurrence in goat flocks in Germany, longevity and ability to recall immunological information after more than six years. BMC Vet. Res. 15 (1), 399. doi:10.1186/s12917-019-2157-5
Klaus, C., Ziegler, U., Kalthoff, D., Hoffmann, B., and Beer, M. (2014). Tick-borne encephalitis virus (TBEV) - findings on cross reactivity and longevity of TBEV antibodies in animal sera. BMC Vet. Res. 10, 78. doi:10.1186/1746-6148-10-78
Klaus, C., Beer, M., Saierb, R., Schauc, U., Moogc, U., Hoffmann, B., et al. (2012). Goats and sheep as sentinels for tick-borne encephalitis (TBE) virus – epidemiological studies in areas endemic and non-endemic for TBE virus in Germany. Ger. Ticks Tick-borne Dis. 3, 27–37. doi:10.1016/j.ttbdis.2011.09.011
Kleiter, I., Steinbrecher, A., Flugel, D., Bogdahn, U., and Schulte-Mattler, W. (2006). Autonomic involvement in tick-borne encephalitis (TBE): report of five cases. Eur. J. Med. Res. 11, 261–265.
Klimes, J., Juricova, Z., Literak, I., Schanilec, P., Trachta, e, and Silva, E. (2001). Prevalence of antibodies to tickborne encephalitis and West Nile flaviviruses and the clinical signs of tickborne encephalitis in dogs in the Czech Republic. Vet. Rec. 148, 17–20. doi:10.1136/vr.148.1.17
Klockmann, U., Krivanec, K., Stephenson, J. R., and Hilfenhaus, J. (1991). Protection against European isolates of tick-borne encephalitis virus after vaccination with a new tick-borne encephalitis vaccine. Vaccine 9, 210–212. doi:10.1016/0264-410x(91)90157-2
Knap, N., Korva, M., Dolinšek, V., Sekirnik, M., Trilar, T., and Avšič-Županc, T. (2012). Patterns of tick-borne encephalitis virus infection in rodents in Slovenia. Vector Borne Zoonotic Dis. 12 (3), 236–242. doi:10.1089/vbz.2011.0728
Kohl, I., Kožuch, O., Elecková, E., Labuda, M., and Zaludko, J. (1996). Family outbreak of alimentary tick-borne encephalitis in Slovakia associated with a natural focus of infection. Eur. J. Epidemiol. 12 (4), 373–375. doi:10.1007/bf00145300
Kondrusik, M., Hermanowska-Szpakowicz, T., and Jaroszewicz, E. (1998). Concentrations of tumor necrosis factor alpha and interleukin-1 beta in cerebrospinal fluid in the course of tick-borne encephalitis. Pol. Merkur Lek. 4 (21), 126–129.
Kondrusik, M., Pancewicz, S., Zajkowska, J., and Hermanowska-Szpakowicz, T. (2001). Tumor necrosis factor alpha and interleukin 1-beta in serum of patients with tick-borne encephalitis. Pol. Merkur Lek. 11 (61), 26–28.
Kondrusik, M., Zajkowska, J., Pancewicz, S., Swierzbińska, R., Grygorczuk, S., and Hermanowska-Szpakowicz, T. (2005). Interferon gamma concentration in the cerebrospinal fluid of patients with tick-borne encephalitis. Neurol. Neurochir. Pol. 39, 109–113.
Kopecký, J., Grubhoffer, L., and Tomková, E. (1991a). Interaction of tick/borne encephalitis virus with mouse peritoneal macrophages. The effect of antiviral antibody and lectin. Acta Virol. 35 (3), 218–225.
Kopecký, J., Tomková, E., and Vlcek, M. (1991b). Immune response of the long-tailed field mouse (Apodemus sylvaticus) to tick-borne encephalitis virus infection. Folia Parasitol. (Praha) 38 (3), 275–282.
Koper, O. M., Kaminska, J., Grygorczuk, S., Zajkowska, J., and Kemona, H. (2018). CXCL9 concentrations in cerebrospinal fluid and serum of patients with tick-borne encephalitis. Arch. Med. Sci. 14, 313–320. doi:10.5114/aoms.2016.58667
Kotál, J., Langhansová, H., Lieskovská, J., Andersen, J. F., Francischetti, I. M., Chavakis, T., et al. (2015). Modulation of host immunity by tick saliva. J. Proteomics 128, 58–68. doi:10.1016/j.jprot.2015.07.005
Kovalev, S. Y., and Mukhacheva, T. A. (2017). Reconsidering the classification of tick-borne encephalitis virus within the Siberian subtype gives new insights into its evolutionary history. Infect. Genet. Evol. 55, 159–165. doi:10.1016/j.meegid.2017.09.014
Kožuch, O., Chunikhin, S. P., Gresikova, M., Nosek, J., Kurenkov, V. B., and Lysý, J. (1981). Experimental characteristics of viraemia caused by two strains of tick-borne encephalitis virus in small rodents. Acta Virol. 25, 219–224.
Kožuch, O., Grešíková, M., Nosek, J., Lichard, M., and Sekeyová, M. (1967). The role of small rodents and hedgehogs in a natural focus of tick-borne encephalitis. Bull. WHO 36 (Suppl. 1), 61–66.
Kožuch, O., Guryčová, D., Lysý, J., and Labuda, M. (1995). Mixed natural focus of tick-borne encephalitis, tularemia and haemorrhagic fever with renal syndrome in west Slovakia. Acta Virol. 39, 95–98.
Kožuch, O., Labuda, M., Lysý, J., Weismann, P., and Krippel, E. (1990). Longitudinal study of natural foci of Central European encephalitis virus in West Slovakia. Acta Virol. 34, 537–544.
Kožuch, O., Nosek, J., Chmela, J., Ernek, E., Uvizl, M., and Bolek, S. (1976). The ecology if the virus of tick-borne encephalitis in the natural foci in the Olomouc district (author's transl). Cesk. Epidemiol. Mikrobiol. Imunol. 25 (2), 88–96.
Kožuch, O., Nosek, J., Ernek, E., Lichard, M., and Albreicht, P. (1963). Persistence of tick-borne encephalitis virus in hibernating hedgehogs and dormice. Acta Virol. 7, 430–433.
Kreil, T. R., Burger, I., Bachmann, M., Fraiss, S., and Eibl, M. M. (1997). Antibodies protect mice against challenge with tick-borne encephalitis virus (TBEV)-infected macrophages. Clin. Exp. Immunol. 110 (3), 358–361. doi:10.1046/j.1365-2249.1997.4311446.x
Kreil, T. R., and Eibl, M. M. (1995). Viral infection of macrophages profoundly alters requirements for induction of nitric oxide synthesis. Virology 212, 174–178. doi:10.1006/viro.1995.1465
Kreil, T. R., and Eibl, M. M. (1996). Nitric oxide and viral infection: NO antiviral activity against a flavivirus in vitro, and evidence for contribution to pathogenesis in experimental infection in vivo. Virology 219 (1), 304–306. doi:10.1006/viro.1996.0252
Kreil, T. R., and Eibl, M. M. (1997). pre-and postexposure protection by passive immunoglobulin but no enhancement of infection with a flavivirus in a mouse model. J. Virol. 71 (4), 2921–2927. doi:10.1128/jvi.71.4.2921-2927.1997
Kreil, T. R., Maier, E., Fraiss, S., Attakpah, E., Burger, I., Mannhalter, J. W., et al. (1998b). Vaccination against tick-borne encephalitis virus, a flavivirus, prevents disease but not infection, although viremia is undetectable. Vaccine 16 (11–12), 1083–1086. doi:10.1016/s0264-410x(98)80102-3
Kreil, T. R., Maier, E., Fraiss, S., and Eibl, M. M. (1998a). Neutralizing antibodies protect against lethal flavivirus challenge but allow for the development of active humoral immunity to a nonstructural virus protein. J. Virol. 72 (4), 3076–3081. doi:10.1128/jvi.72.4.3076-3081.1998
Krejcí, J. (1949). Isolement d'un virus noveau en course d'un epidémie de meningoencephalite dans la region de Vyškov (Moraviae). Presse méd. Paris. 74, 1084.
Kříž, B., Beneš, C., and Daniel, M. (2009). Alimentary transmission of tick-borne encephalitis in the Czech Republic (1997-2008). Epidemiol. Mikrobiol. Imunol. 58 (2), 98–103.
Kříž, B., Beneš, C., Daniel, M., and Malý, M. (2013). Incidence of tick-borne encephalitis in the Czech republic in 2001-2011 in different administrative regions and municipalities with extended power. Epidemiol. Mikrobiol. Imunol. 62 (1), 9–18.
Kříž, B., Beneš, C., Daniel, M., and Malý, M. (2014). The role of game (wild boar and roe deer) in the spread of tick-borne encephalitis in the Czech Republic. Vector Borne Zoonotic Dis. 14 (11), 801–807. doi:10.1089/vbz.2013.1569
Król, M. E., Borawski, B., Nowicka-Ciełuszecka, A., Tarasiuk, J., and Zajkowska, J. (2019). Outbreak of alimentary tick-borne encephalitis in Podlaskie voivodeship, Poland. Przegl Epidemiol. 73 (2), 239–248. doi:10.32394/pe.73.01
Krzysiak, M. K., Anusz, K., Konieczny, A., Rola, J., Salat, J., Strakova, P., et al. (2021). The European bison (Bison bonasus) as an indicatory species for the circulation of tick-borne encephalitis virus (TBEV) in natural foci in Poland. Ticks Tick. Borne Dis. 12 (6), 101799. doi:10.1016/j.ttbdis.2021.101799
Kubinski, M., Beicht, J., Gerlach, T., Aregay, A., Osterhaus, ADME, Tscherne, A., et al. (2024). Immunity to tick-borne encephalitis virus NS3 protein induced with a recombinant modified vaccinia virus ankara fails to afford mice protection against TBEV infection. Vaccines 12, 105. doi:10.3390/vaccines12010105
Kubinski, M., Beicht, J., Zdora, I., Saletti, G., Kircher, M., Petry-Gusmag, M., et al. (2023). Cross-reactive antibodies against Langat virus protect mice from lethal tick-borne encephalitis virus infection. Front. Immunol. 14, 1134371. doi:10.3389/fimmu.2023.1134371
Kunz, C. (2003). TBE vaccination and the Austrian experience. Vaccine 21, 50–55. doi:10.1016/s0264-410x(02)00813-7
Kunz, C., Hofmann, H., Heinz, F. X., and Dippe, H. (1980). Efficacy of vaccination against tick-borne encephalitis. Wien Klin. Wochenschr 92, 809–813.
Kunze, U.ISW-TBE (2016). Tick-borne encephalitis—still on the map: report of the 18th annual meeting of the international scientific working group on tick-borne encephalitis. Ticks Tick-borne Dis. 7(5) 911–914. doi:10.1016/j.ttbdis.2016.04.009
Kurhade, C., Zegenhagen, L., Weber, E., Nair, S., Michaelsen-Preusse, K., Spanier, J., et al. (2016). Type I Interferon response in olfactory bulb, the site of tick-borne flavivirus accumulation, is primarily regulated by IPS-1. J. Neuroinflammation 13, 22. doi:10.1186/s12974-016-0487-9
Kvapil, P., Račnik, J., Kastelic, M., Pittermannová, P., Avšič-Zupanc, T., Bártová, E., et al. (2021). Detection of antibodies against tick-borne encephalitis virus and other flaviviruses in a zoological collection in Slovenia. Front. Vet. Sci. 8, 688904. doi:10.3389/fvets.2021.688904
Kwasnik, M., Rola, J., and Rozek, W. (2023). Tick-borne encephalitis-review of the current status. J. Clin. Med. 12 (20), 6603. doi:10.3390/jcm12206603
Labuda, M., Austyn, J. M., Zufova, E., Kožuch, O., Fuchsberger, N., Lysy, J., et al. (1996). Importance of localized skin infection in tick-borne encephalitis virus transmission. Virology 219, 357–366. doi:10.1006/viro.1996.0261
Labuda, M., Elecková, E., Licková, M., and Sabó, A. (2002). Tick-borne encephalitis virus foci in Slovakia. Int. J. Med. Microbiol. 291 (Suppl. 33), 43–47. doi:10.1016/s1438-4221(02)80008-x
Labuda, M., Kozuch, O., Zuffová, E., Elecková, E., Hails, R. S., and Nuttall, P. A. (1997). Tick-borne encephalitis virus transmission between ticks cofeeding on specific immune natural rodent hosts. Virology 235 (1), 138–143. doi:10.1006/viro.1997.8622
Labuda, M., Jones, L. D., Williams, T., Danielova, V., and Nuttall, P. A. (1993a). Efficient transmission of tick-borne encephalitis virus between cofeeding ticks. J. Med. Entomol. 30, 295–299. doi:10.1093/jmedent/30.1.295
Labuda, M., and Nuttall, P. A. (2008). “Viruses transmitted by ticks,” in Ticks: biology, disease and control. Editors A. S. Bowman, and P. A. Nuttall (Cambridge University Press), 253–280.
Labuda, M., Nuttall, P. A., Kožuch, O., Eleckova, E., Zuffova, E., Williams, T., et al. (1993b). Non-viraemic transmission of tick-borne encephalitis virus: A mechanism for arbovirus survival in nature. Experientia 49, 802–805. doi:10.1007/BF01923553
Labuda, M., and Randolph, S. E. (1999). Survival strategy of tick-borne encephalitis virus: cellular basis and environmental determinants. Zentralblatt Bakteriol. 289, 513–524. doi:10.1016/S0934-8840(99)80005-X
Lepej, S. Z., Misić-Majerus, L., Jeren, T., Rode, O. D., Remenar, A., Sporec, V., et al. (2007). Chemokines CXCL10 and CXCL11 in the cerebrospinal fluid of patients with tick-borne encephalitis. Acta Neurol. Scand. 115 (2), 109–114. doi:10.1111/j.1600-0404.2006.00726.x
Lester, S. N., and Li, K. (2014). Toll-like receptors in antiviral innate immunity. J. Mol. Biol. 426 (6), 1246–1264. doi:10.1016/j.jmb.2013.11.024
Ličková, M., Fumačová Havlíková, S., Sláviková, M., and Klempa, B. (2022). Alimentary infections by tick-borne encephalitis virus. Viruses 14, 56. doi:10.3390/v14010056
Ličková, M., Havlíková Fumačová, S., Sláviková, M., Slovák, M., Drexler, J. F., and Klempa, B. (2020). Dermacentor reticulatus is a vector of tick-borne encephalitis virus. Ticks Tick. Borne Dis. 11, 101414. doi:10.1016/j.ttbdis.2020.101414
Lieskovska, J., and Kopecky, J. (2012). Tick saliva suppresses IFN signalling in dendritic cells upon Borrelia afzelii infection. Parasite Immunol. 34 (1), 32–39. doi:10.1111/j.1365-3024.2011.01345.x
Lieskovská, J., Páleníková, J., Langhansová, H., Chmelař, J., and Kopecký, J. (2018). Saliva of Ixodes ricinus enhances TBE virus replication in dendritic cells by modulation of pro-survival Akt pathway. Virology 514, 98–105. doi:10.1016/j.virol.2017.11.008
Lindquist, L., and Vapalahti, O. (2008). Tick-borne encephalitis. Lancet 371 (9627), 1861–1871. doi:10.1016/s0140-6736(08)60800-4
Lindquist, R., Mundt, F., Gilthorpe, J. D., Wölfel, S., Gekara, N. O., Kröger, A., et al. (2016). Fast type I interferon response protects astrocytes from flavivirus infection and virus-induced cytopathic effects. J. Neuroinflammation 13, 277. doi:10.1186/s12974-016-0748-7
Lipowski, D., Popiel, M., Perlejewski, K., Nakamura, S., Bukowska-Osko, I., Rzadkiewicz, E., et al. (2017). A cluster of fatal tick-borne encephalitis virus infection in organ transplant setting. J. Infect. Dis. 215 (6), 896–901. doi:10.1093/infdis/jix040
Lommano, E., Dvořák, C., Vallotton, L., Jenni, L., and Gern, L. (2014). Tick-borne pathogens in ticks collected from breeding and migratory birds in Switzerland. Ticks Tick. Borne Dis. 5 (2014), 871–882. doi:10.1016/j.ttbdis.2014.07.001
Lotrič-Furlan, S., Bogovič, P., Avšič-Županc, T., Jelovšek, M., Lusa, L., and Strle, F. (2017). Tick-borne encephalitis in patients vaccinated against this disease. J. Intern. Med. 282, 142–155. doi:10.1111/joim.12625
Lotrič-Furlan, S., and Strle, F. (1995). Thrombocytopenia—a common finding in the initial phase of tick-borne encephalitis. Infection 23, 203–206. doi:10.1007/BF01781197
MacMicking, J. D. (2012). Interferon-inducible effector mechanisms in cell-autonomous immunity. Nat. Rev. Immunol. 12 (5), 367–382. doi:10.1038/nri3210
Markovinović, L., Kosanović Ličina, M. L., Tešić, V., Vojvodić, D., Vladušić Lucić, I., Kniewald, T., et al. (2016). An outbreak of tick-borne encephalitis associated with raw goat milk and cheese consumption, Croatia, 2015. Infection 44 (5), 661–665. doi:10.1007/s15010-016-0917-8
Marušić, M., Kopitar, A. N., Korva, M., Knap, N., Bogovič, P., Strle, F., et al. (2023). Dendritic cell activation and cytokine response in vaccine breakthrough TBE patients after in vitro stimulation with TBEV. Front. Immunol. 14, 1190803. doi:10.3389/fimmu.2023.1190803
Matuszczyk, I., Tarnowska, H., Zabicka, J., and Gut, W. (1997). The outbreak of an epidemic of tick-borne encephalitis in kielec province induced by milk ingestion. Prz. Epidemiol. 51, 381–388.
Mayer, V., Gajdosová, E., and Oravec, C. (1980). Transfer with dialysable transfer factor of T-lymphocyte cytolytic response to tick-bone encephalitis virus antigen in naive mice. Acta Virol. 24 (6), 459–463.
Mayer, V., Gajdosová, E., Valásková, M., Gombosová, A., and Oravec, C. (1982). Dialysable specific transfer factor in mice immunized with attenuated Langat virus from the tick-borne encephalitis complex: Generation, action and quantitative assay. Acta Virol. 26 (6), 453–465.
Mayer, V., Gajdosová, E., Valásková, M., and Oravec, C. (1985). Antigen-specific transfer factor from mice immunized with an attenuated flavivirus: Augmentation of inducing activity in semipurified splenocytic dialyzates. Acta Virol. 29 (1), 25–34.
Mayer, V., Valásková, M., Gajdosová, E., and Oravec, C. (1983). Concentration of murine antigen-specific transfer factor of defined potency. Acta Virol. 27 (3), 228–237.
Michel, F., Ziegler, U., Fast, C., Eiden, M., Klaus, C., Dobler, G., et al. (2021). Role of ducks in the transmission cycle of tick-borne encephalitis virus? Transbound. Emerg. Dis. 68 (2), 499–508. doi:10.1111/tbed.13704
Michelitsch, A., Wernike, K., Klaus, C., Dobler, G., and Beer, M. (2019). Exploring the reservoir hosts of tick-borne encephalitis virus. Viruses 11 (7), 669. doi:10.3390/v11070669
Mickiene, A., Pakalniene, J., Nordgren, J., Carlsson, B., Hagbom, M., Svensson, L., et al. (2014). Polymorphisms in chemokine receptor 5 and toll-like receptor 3 genes are risk factors for clinical tick-borne encephalitis in the Lithuanian population. PLoS ONE 9 (9), e106798. doi:10.1371/journal.pone.0106798
Minár, J. (1995). Natural foci of tick-borne encephalitis in Central Europe and the relationship of the incidence of Ixodes ricinus to original ecosystems. Cent. Eur. J. Public Health 3 (1), 33–37.
Moniuszko-Malinowska, A., Penza, P., Czupryna, P., Zajkowska, O., Pancewicz, S., Król, M., et al. (2019). Assessment of TLR-2 concentration in tick-borne encephalitis and neuroborreliosis. Scand. J. Clin. Lab. Invest 79 (7), 502–506. doi:10.1080/00365513.2019.1661510
Moniuszko-Malinowska, A., Penza, P., Czupryna, P., Zajkowska, O., Pancewicz, S., Świerzbińska, R., et al. (2018). Assessment of HMGB-1 concentration in tick-borne encephalitis and neuroborreliosis. Int. J. Infect. Dis. 70, 131–136. doi:10.1016/j.ijid.2018.03.013
Niedrig, M., Avsic, T., Aberle, S. W., Ferenczi, E., Labuda, M., Rozentale, B., et al. (2007). Quality control assessment for the serological diagnosis of tick-borne encephalitis virus infections. J. Clin. Virol. 38 (3), 260–264. doi:10.1016/j.jcv.2006.12.013
Niedrig, M., Vaisviliene, D., Teichmann, A., Klockmann, U., and Biel, S. S. (2001). Comparison of six different commercial IgG-ELISA kits for the detection of TBEV-antibodies. J. Clin. Virol. 20 (3), 179–182. doi:10.1016/s1386-6532(00)00178-5
Nosek, J. (1971). The ecology, bionomics and behaviour of Haemaphysalis (Aboimisalis) punctata tick. Ztschr Parasitenk 37, 198–210. doi:10.1007/BF00259499
Nosek, J. (1972). The ecology and public health importance of Dermacentor marginatus and D. reticulatus ticks in Central Europe. Folia Parasit. (Praha) 19, 93–102.
Nosek, J., Grešíková, M., ŘeháčekJ, K. O., and Albrecht, P. (1962). The role of birds in natural focus of tick-borne encephalitis, experimental infection of pheasants (Phasanius colchicus) with tick-borne encephalitis virus. J. Hyg. Epidemiol. Microbiol. Immunol. 6, 478–482.
Nosek, J., Korolev, M. B., Chunikhin, S. P., and KožuchO, C. F. (1984). The replication and eclipse-phase of the tick-borne encephalitis virus in Dermacentor reticulatus. Folia Parasitol. (Praha) 31, 187–189.
Nosek, J., and Kožuch, O. (1985). Replication of tick-borne encephalitis (TBE) virus in ticks Dermacentor marginatus. Angew. Parasitol. 26, 97–101.
Nosek, J., Kožuch, O., Grešíková, M., Lysý, J., Labuda, M., Teplan, J., et al. (1982). Detection of a natural focus of tick-borne encephalitis in Central Slovakia. II. Synecology of the tick-borne encephalitis virus in middle Povazie (author's transl). Bratisl. Lek. Listy 77, 264–269.
Nosek, J., Kožuch, O., and Grulich, I. (1970). The structure of tick-borne encephalitis (TBE) foci in Central Europe. Oecologia 5, 61–73. doi:10.1007/bf00345976
Nosek, J., Sixl, W., Kvíčala, P., and Waltinger, H. (1972). Central Europaean ticks (Ixodoidea), keys for determination. Mitteilungen der Abteilung fűr Zoologie am Landesmuseum. Joanneum Graz 1 (2), 61–92.
Nuttall, P. A. (2009). Molecular characterization of tick-virus interactions. Front. Biosci. 14, 2466–2483. doi:10.2741/3390
Orlinger, K. K., Hofmeister, Y., Fritz, R., Holzer, G. W., Falkner, F. G., Unger, B., et al. (2011). A tick-borne encephalitis virus vaccine based on the European prototype strain induces broadly reactive cross-neutralizing antibodies in humans. J. Infect. Dis. 203 (11), 1556–1564. doi:10.1093/infdis/jir122
Overby, A. K., Popov, V. L., Niedrig, M., and Weber, F. (2010). Tick-borne encephalitis virus delays interferon induction and hides its double-stranded RNA in intracellular membrane vesicles. J. Virol. 84 (17), 8470–8483. doi:10.1128/JVI.00176-10
Overby, A. K., and Weber, F. (2011). Hiding from intracellular pattern recognition receptors, a passive strategy of flavivirus immune evasion. Virulence 2 (3), 238–240. doi:10.4161/viru.2.3.16162
Palanova, A., Gresikova, M., Sekeyova, M., Pötheová, A., Galvánek, I., and Januska, J. (1992). Vaccination against tick-borne encephalitis in the Central Slovakia Region using a Soviet vaccine. Imunologie 41 (2), 69–77.
Palus, M., Bílý, T., Elsterová, J., Langhansová, H., Salát, J., Vancová, M., et al. (2014a). Infection and injury of human astrocytes by tick-borne encephalitis virus. J. Gen. Virol. 95 (Pt 11), 2411–2426. doi:10.1099/vir.0.068411-0
Palus, M., Formanova, P., Salat, J., Zampachova, E., Elsterova, J., and Ruzek, D. (2015). Analysis of serum levels of cytokines, chemokines, growth factors, and monoamine neurotransmitters in patients with tick-borne encephalitis: Identification of novel inflammatory markers with implications for pathogenesis. J. Med. Virol. 87, 885–892. doi:10.1002/jmv.24140
Palus, M., Sohrabi, Y., Broman, K. W., Strnad, H., Šíma, M., Ruzek, D., et al. (2018). A novel locus on mouse chromosome 7 that influences survival after infection with tick-borne encephalitis virus. BMC Neurosci. 19, 39. doi:10.1186/s12868-018-0438-8
Palus, M., Vancova, M., Sirmarova, J., Elsterova, J., Perner, J., and Ruzek, D. (2017). Tick borne encephalitis virus infects human brain microvascular endothelial cells without compromising blood-brain barrier integrity. Virology 507, 110–122. doi:10.1016/j.virol.2017.04.012
Palus, M., Vojtíšková, J., Salát, J., Kopecký, J., Grubhoffer, L., Lipoldová, M., et al. (2013). Mice with different susceptibility to tick-borne encephalitis virus infection show selective neutralizing antibody response and inflammatory reaction in the central nervous system. J. Neuroinflammation 10, 847. doi:10.1186/1742-2094-10-77
Palus, M., Zampachova, E., Elsterova, J., and Ruzek, D. (2014b). Serum matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 levels in patients with tick-borne encephalitis. J. Infect. 68, 165–169. doi:10.1016/j.jinf.2013.09.028
Paraličová, Z., Sekula, J., Jarčuška, P., Novotný, M., Rovňáková, A., Hockicko, J., et al. (2022). Outbreak of alimentary tick-borne encephalitis in eastern Slovakia: an analysis of affected patients and long-term outcomes. Pathogens 11 (4), 433. doi:10.3390/pathogens11040433
Pazdiora, P., Benesová, J., Brejcha, O., Holecek, J., Hrubá, P., Kubátová, A., et al. (2000). Incidence of tick-borne encephalitis in the west bohemia region 1960-1999--evaluation of vaccination. Epidemiol. Mikrobiol. Imunol. 49 (4), 148–152.
Peňazziová, K., Korytár, Ľ., Cingeľová Maruščáková, I., Schusterová, P., Loziak, A., Pivka, S., et al. (2022). Serologic investigation on tick-borne encephalitis virus, kemerovo virus and tribeč virus infections in wild birds. Microorganisms 10 (12), 2397. doi:10.3390/microorganisms10122397
Pfeffer, M., and Dobler, G. (2011). Tick-borne encephalitis virus in dogs—is this an issue? Parasites Vectors 4, 59. doi:10.1186/1756-3305-4-59
Pietruczuk, M., Pietruczuk, A., Pancewicz, S., Zajkowska, J., Swierzbińska, R., and Hermanowska-Szpakowicz, T. (2006). Czasteczki adhezji miedzykomórkowej sICAM-1, sICAM-2, sICAM-3 oraz IFNgamma w neuroboreliozie i kleszczowym zapaleniu mózgu [Intercellular adhesion molecules sICAM-1, sICAM-2, sICAM-3 and IFNgamma in neuroborreliosis and tick-borne encephalitis]. Przegl Epidemiol. 60 (Suppl. 1), 109–117.
Pikelj, F., Tomažič, J., Matičič, M., Sočan, M., and Muzlovič, I. (1995). Severe forms of tick-borne meningoencephalitis in Slovenia. J. Infect. 31, 83–85. doi:10.1016/s0163-4453(95)91860-4
Pintér, R., Madai, M., Horváth, G., Németh, V., Oldal, M., Kemenesi, G., et al. (2014): Molecular detection and phylogenetic analysis of tick-borne encephalitis virus in rodents captured in the transdanubian region of Hungary. Vector Borne Zoonotic Dis. 14 (8), 621–624. doi:10.1089/vbz.2013.1479
Pokorna Formanova, P., Palus, M., Salat, J., Hönig, V., Stefanik, M., Svoboda, P., et al. (2019). Changes in cytokine and chemokine profiles in mouse serum and brain, and in human neural cells, upon tick-borne encephalitis virus infection. J. Neuroinflammation 16 (1), 205. doi:10.1186/s12974-019-1596-z
Pospíšil, L., Jandasek, L., and Pesek, J. (1954). Isolation of new strains of meningoencephalitis virus in the Brno region during the summer of 1953. Lek. List. 9, 3–5.
Potokar, M., Korva, M., Jorgačevski, J., Avšič-Županc, T., and Zorec, R. (2014). Tick-borne encephalitis virus infects rat astrocytes but does not affect their viability. PLoS One 9 (1), e86219. doi:10.1371/journal.pone.0086219
Prokopowicz, D., Bobrowska, E., Bobrowski, M., and Grzeszczuk, A. (1995). Prevalence of antibodies against tick-borne encephalitis among residents of north-eastern Poland. Scand. J. Infect. Dis. 27 (1), 15–16. doi:10.3109/00365549509018965
Radda, A., Hofmann, H., and Kunz, Ch (1968a). Experimental infection with tick-borne encephalitis (TBE) virus of some European mammals. Zbl. Bacteriol. I. Orig. 208, 100–104.
Radda, A., Kunz, C., and Hofmann, H. (1968b). Demonstration of antibodies in sera of wild animals for the detection of foci of tick-borne encephalitis (TBE) virus in Lower Austria. Zentralbl Bakteriol. Orig. 208, 88–93. [In German].
Rampa, J. E., Askling, H. H., Lang, P., Zens, K. D., Gültekin, N., Stanga, Z., et al. (2020). Immunogenicity and safety of the tick-borne encephalitis vaccination (2009–2019): a systematic review. Travel Med. Infect. Dis. 37, 101876. doi:10.1016/j.tmaid.2020.101876
Rampas, J., and Gallia, F. (1949). Isolation of tick-borne encephalitis virus from ticks Ixodes ricinus. èas. lék. èes. 88, 1179–1180.
Řeháček, J. (1960). Experimental hibernation of the tick-borne encephalitis virus in engorged larvae of the tick Ixodes ricinus L. Acta Virol. 4, 106–109.
Řeháček, J. (1965). Development of animal viruses and rickettsiae in ticks and mites. Annu. Rev. Entomol. 10, 1–24. doi:10.1146/annurev.en.10.010165.000245
Řeháček, J., Grešíková, M., Nosek, J., and Albrecht, P. (1963). Experimental infection of the buzzard (Buteo buteo L.) and the kestrel (Falco tinnunculus L.) with tick-borne encephalitis virus. J Hyg Epidemiol. Microbiol. Immunol. 7, 145–150.
Rieger, M. A., Nübling, M., Kaiser, R., Tiller, F. W., and Hofmann, F. (1998). FSME-Infektionen durch Rohmilch--welche Rolle spielt dieser Infektionsweg? Untersuchungen aus dem südwestdeutschen FSME-Endemiegebiet [Tick-borne encephalitis transmitted by raw milk--what is the significance of this route of infection? Studies in the epidemic region of South-West Germany]. Gesundheitswesen 60 (6), 348–356.
Rieger, M. A., Nubling, M., Muller, W., Hasselhorn, H. M., and Hofmann, F. (1999). Foxes as indicators for TBE endemicity — a comparative serological investigation. Zentralbl. Bakteriol. 289, 610–618. doi:10.1016/s0934-8840(99)80017-6
Rieille, N., Klaus, C., Hoffmann, D., Péter, O., and Voordouw, M. J. (2017). Goats as sentinel hosts for the detection of tick-borne encephalitis risk areas in the Canton of Valais, Switzerland. BMC Vet. Res. 13 (1), 217. doi:10.1186/s12917-017-1136-y
Rockstroh, A., Moges, B., Berneck, B. S., Sattler, T., Revilla-Fernández, S., Schmoll, F., et al. (2019). Specific detection and differentiation of tick-borne encephalitis and West Nile virus induced IgG antibodies in humans and horses. Transbound. Emerg. Dis. 66 (4), 1701–1708. doi:10.1111/tbed.13205
Rónai, Z., and Egyed, L. (2020). Survival of tick-borne encephalitis virus in goat cheese and milk. Food Environ. Virol. 12 (3), 264–268. doi:10.1007/s12560-020-09427-z
Rosický, B. (1953). Bionomicko-faunistický nástin klíšťat (Ixodidae) z území ČSR. Zool. Entomol. Listy 2, 120–130.
Ruzek, D., Avšič Županc, T., Borde, J., Chrdle, A., Eyer, L., Karganova, G., et al. (2019). Tick-borne encephalitis in Europe and Russia: review of pathogenesis, clinical features, therapy, and vaccines. Antivir. Res. 164, 23–51. doi:10.1016/j.antiviral.2019.01.014
Ruzek, D., Dobler, G., and Donoso Mantke, O. (2010). Tick-borne encephalitis: Pathogenesis and clinical implications. Travel Med. Infect. Dis. 8, 223–232. doi:10.1016/j.tmaid.2010.06.004
Ruzek, D., Salát, J., Palus, M., Gritsun, T. S., Gould, E. A., Dyková, I., et al. (2009a). CD8+ T-cells mediate immunopathology in tick-borne encephalitis. Virology 384 (1), 1–6. doi:10.1016/j.virol.2008.11.023
Ruzek, D., Salát, J., Singh, S. K., and Kopecký, J. (2011). Breakdown of the blood-brain barrier during tick-borne encephalitis in mice is not dependent on CD8+ T-cells. PLoS One 6 (5), e20472. doi:10.1371/journal.pone.0020472
Ruzek, D., Vancova, M., Tesarova, M., Ahantarig, A., Kopecky, J., and Grubhoffer, L. (2009b). Morphological changes in human neural cells following tickborne encephalitis virus infection. J. Gen. Virol. 90, 1649–1658. doi:10.1099/vir.0.010058-0
Saksida, A., Duh, D., Lotrič-Furlan, S., Strle, F., Petrovec, M., and Avšič-Županc, T. (2005). The importance of tick-borne encephalitis virus RNA detection for early differential diagnosisof tick-borne encephalitis. J. Clin. Virol. 33, 331–335. doi:10.1016/j.jcv.2004.07.014
Saksida, A., Jakopin, N., Jelovšek, M., Knap, N., Fajs, L., Lusa, L., et al. (2018). Virus RNA load in patients with tick-borne encephalitis, Slovenia. Emerg. Infect. Dis. 24 (7), 1315–1323. doi:10.3201/eid2407.180059
Salat, J., Hunady, M., Schanilec, P., Strakova, P., Stefanik, M., Svoboda, P., et al. (2021). Experimental and natural infections of tick-borne encephalitis virus in dogs. Viruses 13 (10), 2039. doi:10.3390/v13102039
Salat, J., Hunady, M., Svoboda, P., Strelcova, L., Strakova, P., Fortova, A., et al. (2023). Efficacy and immunogenicity of a veterinary vaccine candidate against tick-borne encephalitis in dogs. Vaccine 41 (42), 6150–6155. doi:10.1016/j.vaccine.2023.09.019
Salat, J., and Ruzek, D. (2020). Tick-borne encephalitis in domestic animals. Acta Virol. 64, 226–232. doi:10.4149/av_2020_212
Salat, J., Strakova, P., Stefanik, M., Slosarkova, S., and Ruzek, D. (2022). Sero-epidemiology of tick-borne encephalitis in small ruminants in the Czech Republic. Ticks Tick. Borne Dis. 13 (5), 101996. doi:10.1016/j.ttbdis.2022.101996
Satapathy, P., Kumar, P., Chand, K., Gahtori, P., Rustagi, S., Sah, R., et al. (2023). The rising tide of tick-borne encephalitis across European nations. QJM An Int. J. Med. 116 (12), 973–975. doi:10.1093/qjmed/hcad226
Schneider, W. M., Chevillotte, M. D., and Rice, C. M. (2014). Interferon-stimulated genes: A complex web of host defenses. Annu. Rev. Immunol. 32, 513–545. doi:10.1146/annurev-immunol-032713-120231
Schwaiger, J., Aberle, J. H., Stiasny, K., Knapp, B., Schreiner, W., Fae, I., et al. (2014). Specificities of human CD4+ T cell responses to an inactivated flavivirus vaccine and infection: Correlation with structure and epitope prediction. J. Virol. 88 (14), 7828–7842. doi:10.1128/JVI.00196-14
Sekeyová, M., Gresíková, M., and Lesko, J. (1970). Formation of antibody to tick-borne encephalitis virus in Lacerta viridis and L. agilis lizards. Acta Virol. 14 (1), 87.
Selinger, M., Wilkie, G. S., Tong, L., Gu, Q., Schnettler, E., Grubhoffer, L., et al. (2017). Analysis of tick-borne encephalitis virus-induced host responses in human cells of neuronal origin and interferon-mediated protection. J. Gen. Virol. 98, 2043–2060. doi:10.1099/jgv.0.000853
Shah, T., Li, Q., Wang, B., Baloch, Z., and Xia, X. (2023). Geographical distribution and pathogenesis of ticks and tick-borne viral diseases. Front. Microbiol. 14, 1185829. doi:10.3389/fmicb.2023.1185829
Sikutova, S., Hornok, S., Hubalek, Z., Dolezalkova, I., Juricová, Z., and Rudolf, I. (2009). Serological survey of domestic animals for tick-borne encephalitis and Bhanja viruses in northeastern Hungary. Vet. Microbiol. 135, 267–271. doi:10.1016/j.vetmic.2008.09.082
Simmonds, P., Becher, P., Bukh, J., Gould, E. A., Meyers, G., Monath, T., et al. (2017). ICTV virus taxonomy profile: Flaviviridae. J. Gen. Virol. 98, 2–3. doi:10.1099/jgv.0.000672
Skallova, A., Iezzi, G., Ampenberger, F., Kopf, M., and Kopecky, J. (2008). Tick saliva inhibits dendritic cell migration, maturation, and function while promoting development of Th2 responses. J. Immunol. 180 (9), 6186–6192. doi:10.4049/jimmunol.180.9.6186
Sláviková, M., Víchová, B., Blaňarová, L., et al. (2019). “Seroprevalence study of tick-borne encephalitis vírus in small ruminants from selected farms in Slovakia,” in Joint Czechoslovak virology conference 2019 and 1st SK-at structural virology meeting. Book of abstracts. Biologické centrum AV čr, České Budějovice. Editors B. Klempa, I. Nemčovičová, J. Černý, J. Tomášková, and P. Stolt-Bergner, 75.
Slovák, M., Kazimírová, M., Siebenstichová, M., Ustaníková, K., Klempa, B., Gritsun, T., et al. (2014). Survival dynamics of tick-borne encephalitis virus in Ixodes ricinus ticks. Ticks Tick-borne Dis. 5 (6), 962–969. doi:10.1016/j.ttbdis.2014.07.019
Smetana, A., and Malkova, D. (1966). Relation of Clethrionomys glareolus Schr. and Apodemus flavicollis Melch. to the virus of tick-borne encephalitis in 2 different biotopes. Cesk Epidemiol. Mikrobiol. Imunol. 15, 229–233. [In Czech].
Stancek, D., and Vilcek, J. (1965). The role of interferon in tick-borne encephalitis virus-infected L cells. I. acute infection. Acta Virol. 9, 1–8.
Stanko, M., Derdáková, M., Špitalská, E., and Kazimírová, M. (2022). Ticks and their epidemiological role in Slovakia: From the past till present. Biologia 77, 1575–1610. doi:10.1007/s11756-021-00845-3
Stiasny, K., Holzmann, H., and Heinz, F. X. (2009). Characteristics of antibody responses in tick-borne encephalitis vaccination breakthroughs. Vaccine 27 (50), 7021–7026. doi:10.1016/j.vaccine.2009.09.069
Stiasny, K., Leitner, A., Holzmann, H., and Heinz, F. X. (2021). Dynamics and extent of non-structural protein 1-antibody responses in tick-borne encephalitis vaccination breakthroughs and unvaccinated patients. Viruses 13 (6), 1007. doi:10.3390/v13061007
Süss, J. (2003). Epidemiology and ecology of TBE relevant to the production of effective vaccines. Vaccine 21 (Suppl. 1), S19–S35. doi:10.1016/s0264-410x(02)00812-5
Süss, J. (2011). Tick-borne encephalitis 2010: epidemiology, risk areas, and virus strains in Europe and Asia—an overview. Ticks, Tick-Borne Dis. 2, 2–15. doi:10.1016/j.ttbdis.2010.10.007
Süss, J., Beziat, P., Rohr, H. P., Treib, J., and Haass, A. (1996). Detection of the tick-borne encephalitis virus (TBEV) in ticks in several federal “Lander” of Germany by means of the polymerase chain reaction (PCR)—characterization of the virus. Infection 24, 403–404. doi:10.1007/bf01716096
Süss, J., Dobler, G., Zöller, G., Essbauer, S., Pfeffer, M., Klaus, C., et al. (2008). Genetic characterisation of a tick-borne encephalitis virus isolated from the brain of a naturally exposed monkey (Macaca sylvanus). Int. J. Med. Microbiol. 298, 295–300. doi:10.1016/j.ijmm.2008.02.001
Süss, J., Klaus, C., Diller, R., Schrader, C., Wohanka, N., and Abel, U. (2006). TBE incidence versus virus prevalence and increased prevalence of the TBE virus in Ixodes ricinus removed from humans. Int. J. Med. Microbiol. 296 (Suppl. 40), 63–68. doi:10.1016/j.ijmm.2005.12.005
Süss, J., Schrader, C., Falk, U., and Wohanka, N. (2004). Tick-borne encephalitis (TBE) in Germany--epidemiological data, development of risk areas and virus prevalence in field-collected ticks and in ticks removed from humans. Int. J. Med. Microbiol. 293 (Suppl. 37), 69–79. doi:10.1016/s1433-1128(04)80011-1
Svoboda, P., Haviernik, J., Bednar, P., Matkovic, M., Cervantes Rincón, T., Keeffe, J., et al. (2023). A combination of two resistance mechanisms is critical for tick-borne encephalitis virus escape from a broadly neutralizing human antibody. Cell Rep. 42 (9), 113149. doi:10.1016/j.celrep.2023.113149
Svobodová, M., Knízek, P., Kracmarová, R., Stepánová, V., and Förstl, M. (2010). Tick-borne encephalitis in the East Bohemia Region and its microbiological diagnostic pitfalls. Epidemiol. Mikrobiol. Imunol. 59 (3), 112–118.
Tkachev, S. E., Chicherina, G. S., Golovljova, I., Belokopytova, P. S., Tikunov, A. Y., Zadora, O. V., et al. (2017). New genetic lineage within the Siberian subtype of tick-borne encephalitis virus found in Western Siberia, Russia. Infect. Genet. Evol. 56, 36–43. doi:10.1016/j.meegid.2017.10.020
Toczylowski, K., Grygorczuk, S., Osada, J., Wojtkowska, M., Bojkiewicz, E., Wozinska-Klepadlo, M., et al. (2020). Evaluation of cerebrospinal fluid CXCL13 concentrations and lymphocyte subsets in tick-borne encephalitis. Int. J. Infect. Dis. 93, 40–47. doi:10.1016/j.ijid.2020.01.023
Tomazic, J., and Ihan, A. (1997). Flow cytometric analysis of lymphocytes in cerebrospinal fluid in patients with tick-borne encephalitis. Acta Neurol. Scand. 95, 29–33. doi:10.1111/j.1600-0404.1997.tb00064.x
Tonteri, E., Kipar, A., Voutilainen, L., Vene, S., Vaheri, A., Vapalahti, O., et al. (2013). The three subtypes of tick-borne encephalitis virus induce encephalitis in a natural host, the Bank Vole (Myodes glareolus). PLoS ONE 8 (12), e81214. doi:10.1371/journal.pone.0081214
Topp, A. K., Springer, A., Mischke, R., Rieder, J., Feige, K., Ganter, M., et al. (2023). Seroprevalence of tick-borne encephalitis virus in wild and domestic animals in northern Germany. Ticks Tick. Borne Dis. 14 (6), 102220. doi:10.1016/j.ttbdis.2023.102220
Trávniček, M., Guryčová, D., Halanová, M., Kožuch, O., Nadzamová, D., and Miško, J. (1999). Presence of antibodies to some zoonoses in mouflons (Ovis musimon Pall.) and fallow deer (Dama dama L.) in Eastern Slovakia. Vet. Med. 44:215–219.
Vilcek, J. (1960). An interferon-like substance released from tickborne encephalitis virus-infected chick embryo fibroblast cells. Nature 187, 73–74. doi:10.1038/187073a0
Weber, E., Finsterbusch, K., Lindquist, R., Nair, S., Lienenklaus, S., Gekara, N. O., et al. (2014). Type I interferon protects mice from fatal neurotropic infection with Langat virus by systemic and local antiviral responses. J. Virol. 88 (21), 12202–12212. doi:10.1128/JVI.01215-14
Weidmann, M., Schmidt, P., Hufert, F. T., Krivanec, K., and Meyer, H. (2006). Tick-borne encephalitis virus in Clethrionomys glareolus in the Czech Republic. Vector Borne Zoonotic Dis. 6, 379–381. doi:10.1089/vbz.2006.6.379
Weissbach, F. H., and Hirsch, H. H. (2015). Comparison of two commercial tick-borne encephalitis virus IgG enzyme-linked immunosorbent assays. Clin. Vaccine Immunol. 22 (7), 754–760. doi:10.1128/CVI.00096-15
Wójcik-Fatla, A., Cisak, E., Zając, V., Zwoliński, J., and Dutkiewicz, J. (2011). Prevalence of tick-borne encephalitis virus in Ixodes ricinus and Dermacentor reticulatus ticks collected from the Lublin region (eastern Poland). Ticks Tick-borne Dis. 2, 16–19. doi:10.1016/j.ttbdis.2010.10.001
Wurm, R., Dobler, G., Peters, M., and Kiessig, S. T. (2000). Serological investigations of red foxes (Vulpes vulpes L.) for determination of the spread of tick-borne encephalitis in Northrhine-Westphalia. J. Vet. Med. B 47, 503–509. doi:10.1046/j.1439-0450.2000.00373.x
Zajkowska, J., Moniuszko-Malinowska, A., Pancewicz, S. A., Muszyńska-Mazur, A., Kondrusik, M., Grygorczuk, S., et al. (2011). Evaluation of CXCL10, CXCL11, CXCL12 and CXCL13 chemokines in serum and cerebrospinal fluid in patients with tick borne encephalitis (TBE). Adv. Med. Sci. 56 (2), 311–317. doi:10.2478/v10039-011-0033-z
Zajkowska, J. M., Izycka, A., Jabłońska, E., Hermanowska-Szpakowicz, T., Kondrusik, M., Pancewicz, S., et al. (2005). Serum and cerebrospinal concentrations of sICAM-1 sICAM-2, sICAM-3 in neuroborrellosis and tick borne encephalitis--preliminary report. Pol. Merkur Lek. 19 (110), 152–157.
Zajkowska, J. M., Kondrusik, M., Pancewicz, S., Swierzbińska, R., Grygorczuk, S., and Hermanowska-Szpakowicz, T. (2006). Soluble CD40 and soluble CD40L concentrations in the serum and the cerebrospinal fluid of patients with tick borne encephalitis and neuroborreliosis. Neurol. Neurochir. Pol. 40 (1), 22–27.
Zidovec-Lepej, S., Vilibic-Cavlek, T., Ilic, M., Gorenec, L., Grgic, I., Bogdanic, M., et al. (2022). Quantification of antiviral cytokines in serum, cerebrospinal fluid and urine of patients with tick-borne encephalitis in Croatia. Vaccines (Basel) 10 (11), 1825. doi:10.3390/vaccines10111825
Zöldi, V., Ferenczi, E., and Egyed, L. (2013). Milk transmitted tick-borne encephalitis epidemics in Hungary. Magy. Állatorvosok Lapja 135, 48–56.
Keywords: tick-borne encephalis, innate immunity, adaptive immunity, central Europe, inflammation
Citation: Stibraniova I, Bartikova P and Dzubara J (2025) Response of host immune system to tick borne encephalitis virus. Acta Virol. 68:12936. doi: 10.3389/av.2024.12936
Received: 01 March 2024; Accepted: 24 October 2024;
Published: 03 January 2025.
Edited by:
Katarina Polcicova, Slovak Academy of Sciences, SlovakiaReviewed by:
Natasa Knap, University of Ljubljana, SloveniaJaroslava Lieskovska, University of South Bohemia in České Budějovice, Czechia
Copyright © 2025 Stibraniova, Bartikova and Dzubara. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Iveta Stibraniova, dmlydXZhbkBzYXZiYS5zaw==
†ORCID: Jozef Dzubara, orcid.org/0009-0003-8752-1748
 Iveta Stibraniova
Iveta Stibraniova Pavlina Bartikova
Pavlina Bartikova Jozef Dzubara†
Jozef Dzubara†