- 1Department of Dermatology, Zhongshan Hospital (Xiamen), Fudan University, Xiamen, China
- 2Department of Dermatology, Zhongshan Hospital, Fudan University, Shanghai, China
Aims: To investigate levels of 25-hydroxyvitamin-D (25HVD), interleukin-17 (IL-17), IL-27, IL-35, and transforming growth factor β1 (TGF-β1) in chronic spontaneous urticaria (CSU) patients and their correlations with disease activity.
Methods: An observational, case-control study was conducted. Eighty-one CSU patients and fifty-eight healthy individuals were recruited into two groups. Serum levels of 25HVD, IL-17, IL-27, IL-35, TGF-β1 were determined via enzyme-linked immunosorbent assay (ELISA). Disease activity of CSU was assessed using urticaria activity score (UAS).
Results: Serum level of 25HVD was significantly lower in CSU group compared to control group (P < 0.001). Serum levels of IL-17 (P < 0.001), IL-27 (P < 0.001), TGF-β1 (P = 0.01) were significantly higher in CSU group than those in control group. In CSU group, the level of 25HVD was negatively associated with IgE (P < 0.01) and positively correlated with IL-35 (P < 0.01). In addition, serum level of IL-17 was negatively correlated with disease activity among female patients (P < 0.05).
Conclusion: Deficiency of 25HVD is associated with development of CSU, the underlying mechanism might be related with IL-35. Likewise, IL-17, IL-27, IL-35, and TGF-β1 might also contribute to development of CSU.
Introduction
Chronic spontaneous urticaria (CSU) is a prevalent dermatological disease characterized by recurrent symptoms and varying durations [1], which severely affects the quality of life of patients. The etiology and pathogenesis of CSU are still intricate. Recent studies have demonstrated that the occurrence and development of CSU may be related to 25-hydroxyvitamin-D (25HVD) deficiency. In addition, earlier studies have established that an imbalance in Th17/Treg plays a crucial role in pathogenesis of autoimmune diseases and is closely related to the occurrence of CSU [2, 3]. IL-17, a type of pro-inflammatory cytokine mainly secreted by Th17 cells, plays an immune surveillance role in relation to autoimmune and inflammatory responses. Some studies have announced the higher level of IL-17 in chronic urticaria [1, 3]. IL-27 is a cytokine that regulates Th17/Treg cells, exerting bidirectional regulatory effects on Treg cells and preventing differentiation of Th17 cells. Treg cells can secrete IL-10, IL-35, and TGF-β1, and IL-35 can promote Treg cells expansion. The effect of TGF-β1 is closely related to its concentration. At low concentrations, it promotes the differentiation of Th17 and induces the chemotaxis of inflammatory cells such as eosinophils, mast cells, et al. At high concentrations, it can promote Treg cells differentiation by inhibiting the proliferation and activity of inflammatory cells and reducing the secretion of some cytokines [1–3]. The regulatory mechanism of the Th17/Treg cells axis and its related cytokines in CSU is not fully understood. In addition, the relationship between these associated cytokines with vitamin D level in CSU remains scarce. In the present study, serum levels of vitamin D and cytokines associated with Th17/Treg in CSU patients were analyzed to establish possible correlations between these factors with CSU activity.
Materials and methods
Study population
CSU patients who admitted to the department of dermatology in Fudan University Zhongshan hospital (Xiamen) between January 2021 and December 2022 and fulfilled the follow criteria were recruited to the CSU group. Inclusion criteria for CSU group included: ① fulfilling all the diagnostic criteria outlined in the European Academy of Allergology and Clinical Immunology/the Global Allergy and Asthma European Network (EAACI/GA2LEN) guidelines; ② disease duration >6 weeks; ③ developed skin erythema and wind mass almost daily; ④ had not received vitamin D drugs. Exclusion criteria: ① patients with lesions lasting beyond 24 h; ② patients with urticaria arising from a definite cause; ③ patients with severe cardiac, hepatic, renal, and other organ dysfunction or autoimmune diseases; ④ those who refused to participate in serum test. Finally, eighty-one patients were included in CSU group. Fifty-eight individuals who underwent physical examination in the health examination center in the same hospital during the same period and dosen’t have any newly diagnosed or history of autoimmune diseases, infections, or malignancies were recruited to the control group. Informed consents were obtained from all patients for this study. An ethics approval letter was obtained for this study from ethics committee of our hospital (Approval No. B2021-041).
Assessment of 25HVD, IL-17, IL-27, IL-35, TGF-β1, C reactive protein (CRP), immunoglobulin E (IgE), and erythrocyte sedimentation rate (ESR) levels
A 5 mL of fasting venous blood was collected and centrifuged at 3,000 r/min for 10 min. The supernatant was subsequently collected. Levels of 25HVD, IL-17, IL-27, IL-35, and TGF-β1 were determined using ELISA kits purchased from Shanghai Weiao Biotechnology Co., Ltd., following manufacturer’s instructions. Serum levels of IgE, CRP, and ESR of CSU patients were measured via ELISA in accordance with instructions of manufacturer’s kits of DiaSys Diagnostic Systems GmbH and Roche Diagnostics (Shanghai) Co., Ltd., respectively. Grouping criteria of level of 25HVD was as follows: 25HVD ≥ 30 ng/mL was considered sufficient, 20 ng/mL ≤ 25HVD<30 ng/mL was considered mild insufficiency, 10 ng/mL ≤ 25HVD < 20 ng/mL was considered significant insufficiency, and 25HVD < 10 ng/mL was considered 25HVD deficiency.
Assessment of urticaria activity
CSU disease activity was assessed using urticaria activity score (UAS) [4], encompassing number of wheals, diameter of largest wheal, and pruritus intensity. Pruritus intensity was scored as follow: absent = 0; mild = 1; moderate = 2; severe = 3. Diameter of largest wheal was graded as follow: diameter of wheal <1.5 cm = 1; diameter of wheal between 1.5 and 3.0 cm = 2; diameter of wheal >3.0 cm = 3. Number of wheals was classified as follow: no wheals = 0; <20 wheals/24 h = 1; 20–50 wheals/24 h = 2; >50 wheals/24 h = 3.
Statistical analysis
Statistical analyses were conducted using SPSS version 20.0 (IBM). Numerical variables were expressed as means and standard deviations, whereas categorical variables were presented as numbers and percentages. LSD-t test was used to compare intergroup differences between the two groups. Spearman’s Rank correlation was used for the comparison of non-normally distributed variables. Multiple logistic regression analysis was used to correlate different disease variables. A two-sided p-value <0.01 was considered highly statistically significant. A two-sided p-value <0.05 was considered statistically significant.
Results
Demographic data and laboratory results
The clinical and laboratory characteristics of both groups were presented in Table 1 and Figure 1. The CSU group included 39 males and 42 females, with a mean age of 39.65 ± 12.92 years, a mean disease duration of 12.77 ± 31.85 months, and a mean disease activity score of 4.20 ± 1.10. The control group included 22 males and 36 females, with a mean age of 37.78 ± 13.17 years. Serum levels of TGF-β1, IL-17, and IL-27 were significantly higher in patients in CSU group than those in control group (P = 0.01 for TGF-β1 and <0.001 for others). IL-35 was slightly lower in CSU group than control group, but without statistically difference. Serum level of 25HVD was significantly lower in CSU group compared to control group (P < 0.001). Among patients in CSU group, 70.1% had an insufficient level of 25HVD, with 33.9% manifesting significant insufficiency/deficiency, whereas in control group, 34.5% of patients had insufficient levels of 25HVD, with 21.9% suffering from significant insufficiency/deficiency.
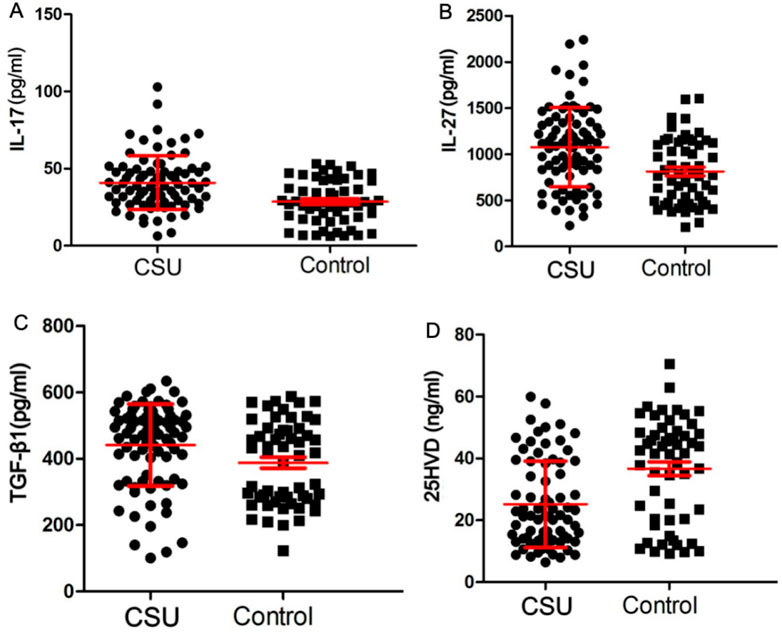
Figure 1. Serum levels of 25HVD and associated cytokines in two groups. (A) Serum level of IL-17 was significantly higher in patients in CSU group than those in control group (P < 0.001). (B) Serum level of IL-27 was significantly higher in patients in CSU group than those in control group (P < 0.001). (C) Serum level of TGF-β1 was significantly higher in patients in CSU group than those in control group (P = 0.01). (D) Serum level of 25HVD was significantly lower in CSU group compared to control group (P < 0.001). CSU, chronic spontaneous urticaria; IL-17, interleukin-17; IL-27, interleukin-27; TGF-β1, transforming growth factor β1; 25HVD, 25-hydroxyvitamin-D.
Correlation between serum levels of 25HVD, IL-17, IL27, IL-35, and TGF-β1 with different disease variables in CSU group
The correlation analysis results of serum levels of 25HVD, IL-17, IL27, IL-35, TGF-β1 and a variety of disease variables were shown in Supplementary Table 1. The level of 25HVD was negatively associated with IgE (spearman coefficient = −0.332) and positively correlated with IL-35 (spearman coefficient = 0.330) (P < 0.01, also shown in Figure 2). In addition, serum level of IL-27 was positively correlated with levels of IL-17, IL-35, and TGF-β1 (P < 0.05, Supplementary Table 1).
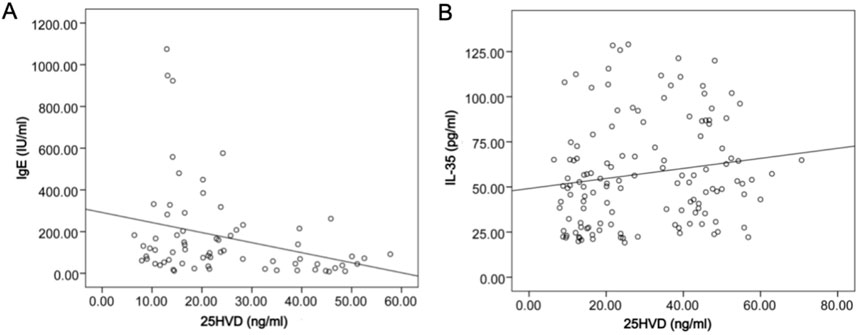
Figure 2. Correlation between serum levels of 25HVD with IgE and IL-35 in CSU group. (A) The level of 25HVD was negatively associated with IgE (spearman coefficient = −0.332, P < 0.01). (B) The level of 25HVD was positively correlated with IL-35 (spearman coefficient = 0.330, P < 0.01). 25HVD, 25-hydroxyvitamin-D; IgE, immunoglobulin E; IL-35, interleukin-35; CSU, chronic spontaneous urticaria.
Correlation between disease activity and disease variables in female CSU patients
Serum level of IL-17 was negatively correlated with disease activity among female patients in CSU group (p < 0.05, Table 2). In addition, positive correlation between disease activity with IL-35 and disease duration in female CSU patients were noted (p < 0.05). There were no correlation between disease activity with age, CRP, ESR, IgE, 25HVD, IL27, or TGF-β1.
Discussion
The etiology and pathogenesis of CSU are complex and have not been fully defined. Numerous studies have observed that CSU patients typically experience vitamin D insufficiency [5, 6]. Vitamin D plays a physiological role, predominantly in the form of 25HVD in vivo. Vitamin D can bind with the nuclear receptor, thereby regulating nuclear transcription at gene level [7, 8]. A study conducted by Woo [6] concluded that serum level of 25HVD is significantly lower in patients with chronic urticaria, and 25HVD levels are negatively correlated with urticaria activity and duration. The results of present study demonstrated that serum level of 25HVD was significantly lower in CSU group than in control group, which was consistent with previous studies [5, 6]. A substantial reduction in 25HVD serum level was noted in CSU patients. However, correlation analysis revealed that there was no significant correlation between 25HVD level and disease activity or duration, which may be attributed to the limited sample size of this study, the complex pathogenesis of CSU, and various factors that impact disease activity and duration. As is well documented, 25HVD plays a pivotal role in development of CSU. While several studies have pointed out that vitamin D exerts an immunomodulatory role in vivo, the specific mechanism underlying its influence on urticarial remains to be elucidated.
The relationship between T lymphocytes and chronic urticaria has been previously reported [9]. Littman et al. [10, 11] described that Treg cells that mediate immune tolerance and Th17 cells that mediate inflammatory responses are also closely related to pathogenesis of CSU. IL-17 is a type of pro-inflammatory cytokine mainly secreted by Th17 cells, plays an immune surveillance role in relation to autoimmune and inflammatory responses. A study undertaken by Atwa et al. [12, 13] exposed that IL-17 was higher in chronic urticaria patients. In present study, serum level of IL-17 was significantly higher in CSU patients than that in controls, in line with findings of prior investigations. Further on, the correlation analysis showed a positive correlation between IL-17 and disease duration (>30 months) in whole CSU patients (Supplementary Table 2). Although serum level of IL-17 had no significant correlation with disease activity in whole CSU group, it is worth mentioning that there was a negative correlation between disease activity and IL-17 in female CSU patients, in contrary to most previous studies. In other hand, serum level of IL-17 was significantly higher in male patients than those of females in CSU group in this study (Supplementary Table 3). This suggested that there might be gender difference of the role of IL17 in the pathogenesis that deserves further exploration. Taken together, our results uncovered that IL-17 may be a predictive factor for development of CSU.
Treg cells, a group of effector T cells that exert negative regulatory effects on the proliferation of CD4+ CD8+ T cells, has been implicated in the pathogenesis of chronic urticaria [14]. Besides, these cells synthesize cytokines such as IL-35 and TGF-β1, and IL-35 can promote Treg cells expansion. Chen et al. [15] reported that IL-35 was significantly lower in CSU patients. However, other studies observed increased levels of IL-35 in chronic urticaria patients [16]. In the current study, the level of IL-35 was slightly lower in CSU group compared to control group, but without statistical difference. However, serum level of IL-35 was positively correlated with disease activity among female patients in CSU group. These differences may be ascribed to the variability in subjects included in this study and highlight the multifaceted role of IL-35 in pathogenesis of CSU and at different stages of the disease.
Furthermore, the effect of TGF-β1 is closely related to its concentration. At low concentrations, it promotes the differentiation of Th17 and induces the chemotaxis of inflammatory cells such as eosinophils, macrophages, and mast cells. At high concentrations, it can promote Treg cells differentiation by inhibiting the proliferation and activity of inflammatory cells such as T, B lymphocytes and macrophages, and reducing the secretion of cytokines such as IL-1, IL-2, and IL-4 [2, 3]. Some studies have evinced that Treg cells and TGF-β1 are significantly lower during the active phase of chronic urticaria [17]. Paradoxically, in other studies TGF-β1was higher during the active phase of chronic urticaria [18, 19]. In this study, serum level of TGF-β1 was significantly higher in CSU group compared with that in control group, in agreement with findings of some earlier studies, possibly owing to its role in promoting the differentiation of Th17 cells and Treg cells. Nonetheless, the level of TGF-β1 was not correlated with disease activity. Thus, the effects of IL-35 and TGF-β1 on balance of Th17/Treg warrant further investigation.
IL-27 is a cytokine that modulates the Th17/Treg ratio, concomitantly preventing differentiation of Th17 cells and exerting bidirectional regulatory effects on Treg cells [20]. Herein, serum level of IL-27 was significantly higher in CSU group than in control group, and positively correlated with both IL-35 and TGF-β1 levels. We speculate that an increased level of IL-27 may lead to a disproportion of T cell subsets in CSU patients and exert a positive regulatory effect on Treg cells. Meanwhile, IL-27 was positively correlated with that of IL-17, in contrast to the physiological effect of IL-27 on Th17 cells. This observation might be attributed to impact of other factors affecting Th17 cells via different pathways in CSU patients. Therefore, further studies on relationship between dysregulated expression of Th17/Treg related cytokines in the pathogenesis of CSU are necessitated.
T lymphocytes are considered chief target cells for immunomodulatory function of vitamin D. Vitamin D can induce the conversion of CD4+ T lymphocytes into Treg cells, thus inhibiting immune response [21]. In vitro experiments found that vitamin D inhibited the proliferation and differentiation of Th17 cells and attenuated their ability to synthesize IL-17. In this study, significant differences were noted in IL-35 and TGF-β1 levels at different serum levels of 25HVD in CSU group (Supplementary Table 4), while Spearman’s rank test validated that the level of 25HVD was positively correlated with that of IL-35. This partly corroborates that 25HVD can affect the differentiation of Treg cells by mediating the levels of IL-35 and TGF-β1. However, the relationship between vitamin D and Th17/Treg ratio remains underexplored and requires further investigation. It is worthwhile acknowledging that the small sample size in this study might have partially compromised the robustness of our findings.
In summary, this study demonstrated significantly lower serum level of 25HVD and significantly higher levels of IL-17, IL-27 and TGF-β1 in CSU patients. Additionally, negative correlation of IL-17 and disease activity in female CSU patients was noted, while significantly higher level of IL-17 was found in male patients, suggesting gender difference may plays a role in the pathogenesis of CSU. Importantly, the present study revealed positive correlation between 25HVD and IL-35, suggesting that the role of 25HVD in CSU might be associated with mediating the change of IL-35 level and affecting the differentiation of Treg cells. However, the complex relationship between 25HVD and Th17/Treg associated cytokines in CSU and their correlation with disease activity and course require further investigation.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by ethics committee of Zhongshan Hospital (Xiamen), Fudan University. Approval No. B2021-041. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YQ designed the study. YQ and XQ analysed the data and drafted the manuscript. YQ and LG drew the figures and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was supported by the grants from the Xiamen science and technology plan guiding project (3502Z20209058) and Fujian provincial health technology project (2023GGB10).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/jcia.2024.13636/full#supplementary-material
References
1. Gaig, P, Olona, M, Muñoz Lejarazu, D, Caballero, M, Domínguez, F, Echechipia, S, et al. Epidemiology of urticaria in Spain. J Investig Allergol Clin Immunol (2004) 14(3):214–20.
2. Wenzel, SE, Trudeau, JB, Barnes, S, Zhou, X, Cundall, M, Westcott, JY, et al. TGF-beta and IL-13 synergistically increase eotaxin-1 production in human airway fibroblasts. J Immunol (2002) 169(8):4613–9. doi:10.4049/jimmunol.169.8.4613
3. Zhou, L, Lopes, JE, Chong, MM, Ivanov, II, Min, R, Victora, GD, et al. TGF-β-induced Foxp3 inhibits Th17 cell differentiation by antagonizing RORγt function. Nature (2008) 8(453):7192. doi:10.1038/nature06878
4. Ye, YM, Park, JW, Kim, SH, Choi, JH, Hur, GY, Lee, HY, et al. Clinical evaluation of the computerized chronic urticaria-specific quality of life questionnaire in Korean patients with chronic urticaria. Clin Exp Dermatol (2012) 37(7):722–8. doi:10.1111/j.1365-2230.2012.04414.x
5. Rasool, R, Masoodi, KZ, Shera, IA, Yosuf, Q, Bhat, IA, Qasim, I, et al. Chronic urticaria merits serum vitamin D evaluation and supplementation; a randomized case control study. World Allergy Organ J (2015) 8(1):15. doi:10.1186/s40413-015-0066-z
6. Woo, YR, Jung, KE, Koo, DW, and Lee, JS. Vitamin D as a marker for disease severity in chronic urticaria and its possible role in pathogenesis. Ann Dermatol (2015) 27(4):423–30. doi:10.5021/ad.2015.27.4.423
7. Yu, C, Fedoric, B, Anderson, PH, Lopez, AF, and Grimbaldeston, MA. Vitamin D(3) signalling to mast cells: a new regulatory axis. Int J Biochem Cel Biol (2011) 43(1):41–6. doi:10.1016/j.biocel.2010.10.011
8. Benson, AA, Toh, JA, Vernon, N, and Jariwala, SP. The role of vitamin D in the immunopathogenesis of allergic skin diseases. Allergy (2012) 67(3):296–301. doi:10.1111/j.1398-9995.2011.02755.x
10. Littman, DR, and Rudensky, AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell (2010) 140(6):845–58. doi:10.1016/j.cell.2010.02.021
11. Zepp, J, Wu, L, and Li, X. IL-17 receptor signaling and T helper 17-mediated autoimmune demyelinating disease. Trends Immunol (2011) 32(5):232–9. doi:10.1016/j.it.2011.02.007
12. Yang, X, Chen, L, Wang, S, Wu, Y, Zhou, X, and Meng, Z. The correlation between Th17/Treg immune dysregulation and the disease severity in chronic spontaneous urticaria patients. Immun Inflamm Dis (2023) 11(7):e920. doi:10.1002/iid3.920
13. Atwa, MA, Emara, AS, Youssef, N, and Bayoumy, NM. Serum concentration of IL-17, IL-23 and TNF-alpha among patients with chronic spontaneous urticaria: association with disease activity and autologous serum skin test. J Eur Acad Dermatol Venereol (2014) 28(4):469–74. doi:10.1111/jdv.12124
14. Arshi, S, Babaie, D, Nabavi, M, Tebianian, M, Ghalehbaghi, B, Jalali, F, et al. Circulating level of CD4+ CD25+ FOXP3+ T cells in patients with chronic urticaria. Int J Dermatol (2014) 53(12):e561–6. doi:10.1111/ijd.12630
15. Chen, T, Fu, LX, Sun, QM, Zhou, PM, and Guo, ZP. Decreased interleukin-35 serum levels in patients with chronic spontaneous urticaria. Ann Allergy Asthma Immunol (2018) 121(4):503–4. doi:10.1016/j.anai.2018.06.002
16. Collison, LW, Workman, CJ, Kuo, TT, Boyd, K, Wang, Y, Vignali, KM, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature (2007) 450(7169):566–9. doi:10.1038/nature06306
17. Yu, Q, Lin, W, Zhang, J, Peng, L, Kong, Q, Zhong, R, et al. The association of Th17/Treg cells expression in peripheral blood and chronic spontaneous urticaria: a protocol of systematic review and meta-analysis. Medicine (Baltimore) (2020) 99(36):e22014. doi:10.1097/MD.0000000000022014
18. Chen, Q, Zhong, H, Chen, WC, Zhai, Z, Zhou, Z, Song, Z, et al. Different expression patterns of plasma Th1-Th2-Th17- and Th22-related cytokines correlate with serum autoreactivity and allergen sensitivity in chronic spontaneous urticaria. J Eur Acad Dermatol Venereol (2018) 32(3):441–8. doi:10.1111/jdv.14541
19. Chen, W, Chiang, B, Liu, H, Leu, S, and Lee, Y. Defective functions of circulating CD4+CD25+ and CD4+CD25- T cells in patients with chronic ordinary urticaria. J Dermatol Sci (2008) 51(2):121–30. doi:10.1016/j.jdermsci.2008.02.012
20. Yoshida, H, Nakaya, M, and Miyazaki, Y. Interleukin 27: a double-edged sword for offense and defense. J Leukoc Biol (2009) 86(6):1295–303. doi:10.1189/jlb.0609445
Keywords: chronic spontaneous urticaria, interleukin-17, interleukin-27, interleukin-35, 25-hydroxyvitamin-D
Citation: Qiu Y, Qiu X, Chen Y, Chen M, Huang L, Lin X, Wei Y, Yang J and Gao L (2024) Changes of vitamin D, Th17/Treg related cytokines and their correlations with disease activity in chronic spontaneous urticaria. J. Cutan. Immunol. Allergy 7:13636. doi: 10.3389/jcia.2024.13636
Received: 08 August 2024; Accepted: 22 October 2024;
Published: 31 October 2024.
Copyright © 2024 Qiu, Qiu, Chen, Chen, Huang, Lin, Wei, Yang and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lujuan Gao, Z2FvX2x1anVhbkBmdWRhbi5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Yangyang Qiu
Yangyang Qiu Xiaoyan Qiu1†
Xiaoyan Qiu1† Lujuan Gao
Lujuan Gao