Abstract
While traditionally considered a disorder of the basal ganglia, brainstem, and cerebellum, multiple reports have shown that spinal cord pathologies may lead to dystonia. In this article, we first discuss various spinal movement disorders and the differences between tonic spasms, spinal dystonia, spinal myoclonus, spinal tremors, and paroxysmal dyskinesia. We review potential pathogenesis of spinal dystonia. We then focus on reports of dystonia secondary to spinal cord demyelinating diseases such as multiple sclerosis and neuromyelitis optica spectrum disorders. We conclude by discussing the potential treatment options for spinal dystonia.
Introduction
Dystonia, the third most common movement disorder, was traditionally considered a disorder of basal ganglia; but recent literature has emphasized the role of the cerebellum, cerebral cortex, brainstem, and the network connecting these regions. There remains a question regarding the role of the spinal cord, a critical part of the network responsible for sensorimotor function. There have been increasing numbers of dystonia cases reported in patients with spinal cord pathology [1–3]. It is certain that dystonia can be seen in spinal cord disorders, but it is much under-recognized, just as other forms of spinal movement disorders. Spinal cord pathologies can present with diverse phenomenology of dystonia that is usually focal or segmental. Cervical lesions can produce a hemidystonia involving one-half of the body below the cranium. Spinal dystonia could be fixed or paroxysmal in nature. Failure to evaluate the spinal cord in patients with these dystonic phenotypes can delay appropriate treatment or result in permanent disability of what would have otherwise been correctable conditions. Although any spinal cord pathology can result in spinal dystonia, demyelinating diseases like multiple sclerosis (MS) and neuromyelitis optica spectrum disorders (NMOSD) [4] are among the leading causes of this relatively rare movement disorder. We begin this review with a brief overview of spinal generated movement disorders followed by a focus on spinal dystonia. As demyelination is the most important cause of spinal dystonia, we will highlight reports of demyelination-related spinal dystonia in patients with MS, NMOSD, and related disorders [5, 6]. We end by a discussion of management options.
Methodology
We searched PubMed database for keywords “dystonia” and “spinal cord,” “dystonia” and “multiple sclerosis,” “dystonia” and “neuromyelitis optica,” “dystonia” and “NMO” “dystonia” and “myelitis,” “dystonia” and “transverse myelitis,” “dyskinesia” and “spinal cord,” “dyskinesia and multiple sclerosis,” “dyskinesia” and “NMO,” “dyskinesia” and “myelitis,” “dyskinesia” and “transverse myelitis.” Reports of paroxysmal dyskinesia were reclassified to dystonia if there was complex posturing without a component of chorea.
An overview of spinal movement disorders
A subset of movement disorders originates in the spinal cord. These disorders typically tend to be hyperkinetic. Even ones that appear hypokinetic may instead be classified as sustained hyperkinetic spasms [3]. Table 1 lists these different subtypes of spinal movement disorders.
TABLE 1
| Spinal movement disorder | Definition | Comparison to spinal dystonia |
|---|---|---|
| Spinal Dystonia | Sustained muscle contraction of antagonistic muscles resulting in complex abnormal posture (other than simple flexion, extension, or adduction) that can be paroxysmal or non-paroxysmal secondary to spinal cord pathology | |
| Paroxysmal focal dystonia | Paroxysmal involuntary sustained muscle contraction of antagonistic muscle groups resulting in abnormal posture (other than simple flexion, extension, or adduction) secondary to spinal cord pathology. | |
| Non-paroxysmal (fixed) focal dystonia | Persistent (non-paroxysmal) sustained muscle contraction of antagonistic muscle groups resulting in fixed abnormal posture secondary to spinal cord pathology. | |
| Spinal hemi-dystonia | Fixed (or rarely paroxysmal) involuntary sustained muscle contraction of antagonistic muscle groups resulting in abnormal posture of the hemi-body (upper limb, lower limb, trunk) secondary to spinal cord pathology. | |
| Tonic Spasms | Sustained increase in muscle tone involving one set of muscle groups leading to isometric contraction or simple posturing in flexion, extension or adduction | Contraction is typically isometric or involves one set of muscle groups resulting in simple posturing. Complex posturing not observed |
| Flexor tonic spasm | Paroxysmal sustained increase in muscle tone resulting in visible tonic posturing of the affected body part (often the whole limb or part of the limb) in flexion secondary to spinal cord pathology. | |
| Extensor tonic spasm | Paroxysmal sustained increase in muscle tone resulting in visible tonic posturing of the affected body part (often the whole limb or part of the limb) in extension secondary to spinal cord pathology. | |
| Adductor tonic spasm | Paroxysmal sustained increase in muscle tone resulting in visible tonic posturing of the affected body part (often the whole limb or part of the limb) in adduction (or inversion) secondary to spinal cord pathology. | |
| Isometric tonic spasm | Paroxysmal sustained increase in muscle tone that can be felt by the patient and palpated by the examiner but does not result in visible change in posture (e.g., abdominal wall muscles) secondary to spinal cord pathology. | |
| Complex tonic spasm | A combination of two or more tonic spasms of combined phenomenology affecting two or more adjacent or distant body parts. | |
| Spinal Myoclonus | Sudden, brief (non-sustained) shock-like muscle contraction that can be focal, segmental or propriospinal. | Muscle contraction is not sustained and does not result in abnormal posturing |
| Focal/Segmental spinal myoclonus | Sudden, brief (non-sustained), shock-like focal muscle contraction of one or more adjacent body parts secondary to spinal cord pathology. | |
| Propriospinal myoclonus | Sudden, brief, shock-like, arrhythmic jerks of the trunk, hips, and knees (sparing the head) in flexion or extension secondary to spinal cord pathology. | |
| Spinal Movement Disorders Associated with Sensory Phenomenon | Abnormal movement triggered by sensory changes in the affected limb secondary to spinal cord pathology | No abnormal posturing. Prominent associated sensory symptoms |
| Secondary restless leg syndrome | Unpleasant or uncomfortable urge to move the legs during periods of inactivity that is transiently relieved by movement secondary to spinal cord pathology. | |
| Painful legs moving toes syndrome | Involuntary slow writhing movement of the toes in vertical or horizontal planes associated with painful sensation in the legs/feet secondary to spinal cord pathology. | |
| Pseudoathetosis | Athetotic-like movement of the distal limbs due to deep sensory deprivation impairing joint position sense, often aggravated by eye closure or visual distraction, secondary to spinal cord pathology involving the posterior column. | |
| Spinal Tremor | Postural/action or orthostatic tremors due to interruption of the spinocerebellar tracts secondary to spinal cord pathology | To and fro regular movement, no abnormal posturing or sustained increase in muscle tone. Tremor frequency and amplitude are stable and do not change with limb movement or in different postures |
| Kinetic spinal tremor | Postural and/or action tremor occurring after spinal cord pathological event involving the spinocerebellar tracts in absence of brainstem, cerebellar, or ganglionic lesions and without a personal or family history of essential tremor. | |
| Spinal orthostatic tremor | Fast (13–18 Hz) tremor of the legs while standing that is relieved by sitting or walking, and is often palpable rather than visible, secondary to spinal cord pathology. | |
| Spontaneous clonus | Spontaneous (non-induced) involuntary, rhythmic muscle contractions and relaxations associated with spasticity secondary to spinal cord pathology. It commonly involves the ankle or the wrist and appears in certain positions or with certain movements. | No abnormal posturing in isolated clonus but may accompany spinal focal dystonia of the foot or hand. |
Proposed classification and definitions of spinal movement disorders.
Despite overlap in medical literature, spinal dystonia should be differentiated from tonic spasms. Produced in large by spinal pathology, tonic spasms refer to an activation of one set of muscle groups leading to isometric contraction or simple posturing in flexion, extension or adduction. Focal dystonia refers to tonic contraction of antagonistic muscles resulting in complex abnormal posture (other than simple flexion, extension, or adduction) that can be paroxysmal or non-paroxysmal [5, 7]. Although pain is more typically linked to tonic spasms, both tonic spasms and paroxysmal dystonia could be either painful or painless [4]. Demyelination, most commonly in the spinal cord and less so in the cerebral peduncles or internal capsules, may lead to ephaptic transmission and hyperexcitability of the motor neurons resulting in tonic spasms or paroxysmal dystonia [3]. Paroxysmal dystonias and tonic spasms may often be misclassified by clinicians as they can be confused with one another or at times referred to as paroxysmal dyskinesia or spinal seizures [8–11]. The term paroxysmal dyskinesia should be reserved for cases manifesting with a combination of different movement phenomenologies including dystonia, chorea, and other involuntary movements.
Spinal myoclonus refers to quick muscle jerks that can be focal, segmental or propriospinal. Spinal segmental myoclonus is due to loss of inhibition of interneurons leading to hyperexcitability in a myotomal distribution. Propriospinal myoclonus is hypothesized to be due a lesion in propriospinal pathways affecting axial muscles [3, 5, 12]. Tremors of spinal origin present as action (kinetic) or orthostatic tremors due to interruption of the spinocerebellar tracts [3]. Although spinal movement disorders can occur in the absence of spasticity, they commonly co-exist with spasticity and overlap with spasticity-related involuntary movements [5]. It is also important to differentiate spinal movement disorders from functional neurological disorders (FND). Fixed dystonia, focal, or propriospinal myoclonus are common presentation of FND but tend to be variable, distractible, and not associated with spinal pathology on imaging. Functional dystonia is the second most common form of functional movement disorders but can be a diagnostic challenge requiring the clinician to take a thorough history and physical exam to determine risk factors and key exam findings [13].
Spinal dystonia
Pathogenesis of spinal dystonia
Many of the positive symptoms seen in demyelinating disease, such as paresthesias, dystonia, and spasms, may occur due to a dysregulation in the excitability of demyelinated axons [14]. Ectopic bursts of impulses, ephaptic interactions, and mechanosensitivity of axons are all proposed mechanisms of the positive symptoms seen in demyelinating diseases [15]. Even the slightest changes in the natural cellular environment can induce axonal hyperexcitability. For example, hyperventilation can reduce extracellular free calcium and lead to hyperexcitability [16]. Many or all of these mechanisms may play a role in the paroxysmal symptoms experienced by patients with demyelinating diseases. In this section, we focus on ephaptic transmission.
Ephaptic transmission or coupling is a type of neuronal communication where neurons can influence surrounding neurons without synapsing to them but instead by changing the electric potential of the extracellular medium via action potentials and synaptic currents [17]. Mice studies show a physiologic use of this phenomenon in the cerebellum where climbing fibers non-synaptically influence neighboring Purkinje cells via ephaptic signaling [18]. On the flipside, a computational study involving rodent hippocampi showed that ephaptic coupling can pathologically generate small electrical fields to induce seizure-like activity [19]. Previous in vivo and in vitro animal studies have shown that ephaptic transmission is common in axonal tracts affected by demyelination [20–22].
Ephaptic transmission is one of the proposed mechanisms of altered excitability in MS and other demyelinating diseases [23]. Traugott and Lebon showed that MS plaques contain cells expressing many interferons. The pattern of interferon expression in active plaques versus inactive plaques or unaffected white matter is suggestive of how inflammatory factors may lead to axonal irritation [24]. Within one to 2 weeks of the initial attack, these inflamed axons may lead to hyperexcitability and ephaptic transmission causing recurrent symptoms like spasms or neuralgic pain [15, 25]. Osterman and Westerberg proposed that ephaptic activation of axons in demyelinated areas of fiber tracts lead to a transverse spread of activation. Figure 1 demonstrates the proposed mechanism of ephaptic transmission. In the original report, they describe these phenomena as similar in nature to seizure-like activity. For example, Lhermitte’s sign (a shock-like sensation traveling down the back triggered by neck bending) may be from ephaptic transmission down the posterior columns of the spinal cord [26].
FIGURE 1
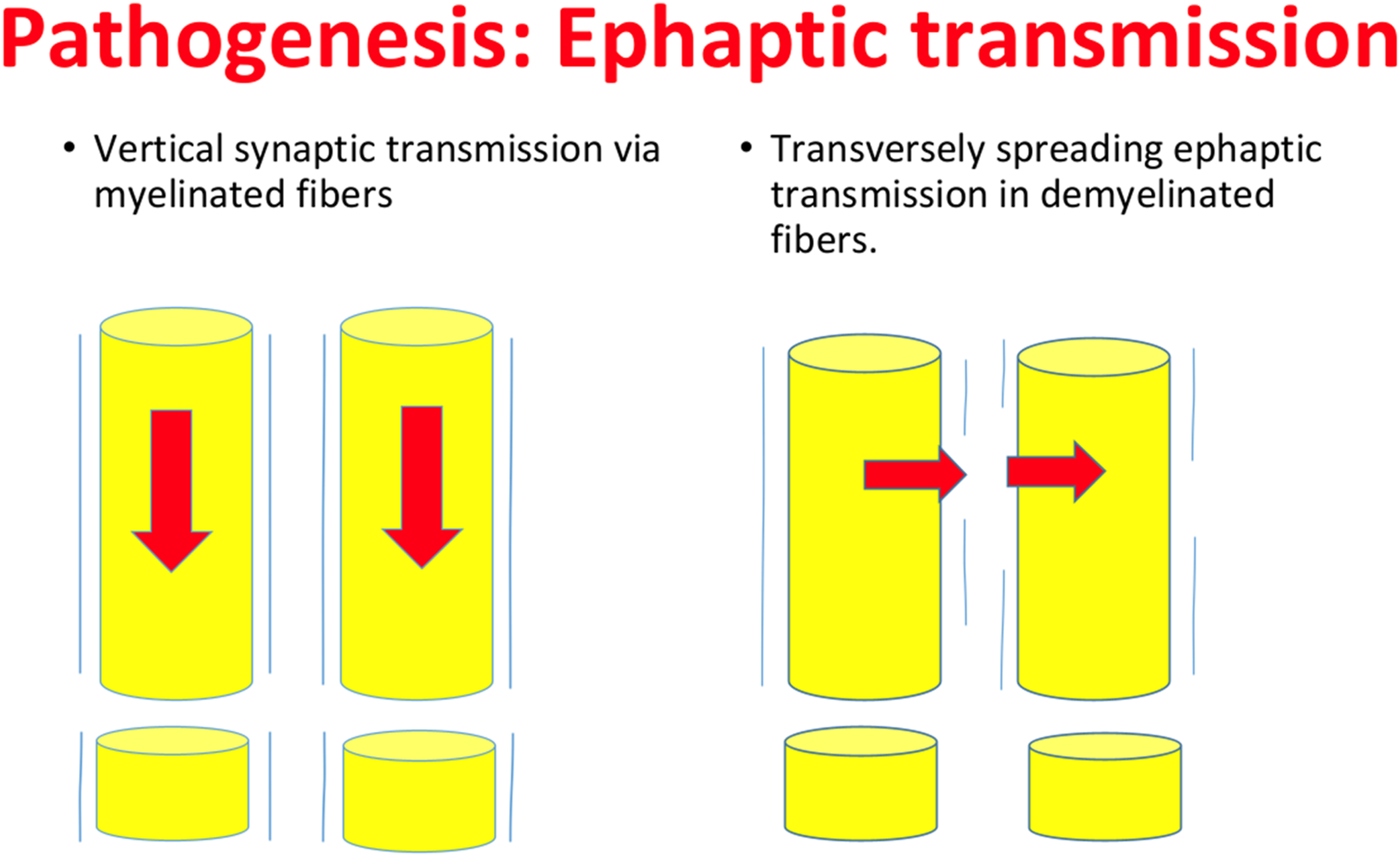
Ephaptic neural Transmission. Damage to white matter, from demyelination in MS or NMOSD, can lead to disruption in normal synaptic transmission. Altered neuronal field potentials may cause transverse as opposed to vertical activation of various neuronal tracts. This phenomenon occurs physiologically in the cerebellum, but has also been shown to lead to spasm and pain in demyelinated fibers especially in the spinal cord. Similar to tonic spasm, paroxysmal dystonia may also be caused by ephaptic transmission in demyelinated spinal cord tracts.
While the exact nature of paroxysmal phenomena have yet to be elucidated, it is plausible to hypothesize that ephaptic transmission in various white matter tracts in the spinal cord may lead to these events. Tonic spasms, which are described as brief episodes of simple posturing are one of the most well studied paroxysmal MS symptoms. They are often associated with lesions of the corticospinal tract in the spinal cord, cerebral peduncles, or internal capsules [14]. Otmani et al suggested that ephaptic transmission in the corticospinal tract at various levels might be responsible for paroxysmal dystonia in MS in a manner similar to tonic spasms [27]. It is unclear whether ephaptic transmission results in direct stimulation of antagonistic muscle groups leading to dystonic posturing versus an impairment of external feedback to motor centers resulting in dystonia.
Upper motor neuron lesions, whether it may be from demyelination or infarction, generally lead to a disruption in various spinal reflex arcs followed by hyperexcitability of certain muscles. The damage to inhibitory vestibulospinal or reticulospinal tracts is likely the source of the loss of inhibition. Spasticity is related to a dysregulation of the spinal cord reflexes. It presents as a velocity-dependent form of hypertonia that is caused by upper motor neuron lesions leading to a disinhibition of spinal reflexes. This change in balance between excitatory and inhibitory circuitry can lead to increased tone and spasticity or spastic dystonia [28]. Classically, this can lead to the clasp-knife phenomenon [29]. Failure of reciprocal inhibition between an agonist-antagonist muscle pair can lead to co-contraction of both muscles (for example, the biceps and triceps simultaneously) with spastic posturing. Spastic posturing/dystonia results from pyramidal tract damage and can be made analogous to the dystonia seen classically from extrapyramidal pathway damage [30].
Previous studies have shown that there is no specific location in the neural axis that, when lesioned, would cause dystonia. The basal ganglia was originally thought to be implicated due to improvements with levodopa intake as well as deep brain stimulation of the globus pallidus [31]. Earlier reports of dystonia suggest that lesions to the internal capsule, putamen, caudate, thalamus, or brainstem are responsible for the phenomenon [32–36]. One systematic review of 20 papers comparing primary dystonias specifically found that lesions of the globus pallidus, putamen, brainstem, and cerebellum were implicated in dystonia [37]. While the putamen and globus pallidus seem to be the most important locations for dystonia, functional imaging showed that secondary dystonia can happen due to lesions anywhere in the motor system [38].
The neuronal integrator has been described in literature as a circuit that operates to integrate various sensory inputs to create a type of motor output. This integrator requires precise, optimal tuning and is susceptible to “leaking” causing pathologic movement. Shaikh et al proposed a neural integrator for abnormal head movements and dystonia involving structures such as the cerebellum, basal ganglia, various proprioceptive tracts, peripheral sensory organs, and other nuclei in the brainstem. For example, in monkeys, inactivation of the interstitial nucleus of Cajal would lead to ipsilateral torticollis and activation causes contralateral torticollis. Recent transcranial magnetic stimulation experiments show that disruption of cerebellar function can lead to dystonic posturing [39, 40]. In addition to the structures already included, the model could be expanded to include the spinal cord. Damage anywhere along the motor pathway could alter the fine-tuning of the neural integrator. Insults to the corticospinal tracts or any of the various proprioceptive tracts in the spinal cord may alter integration causing dystonia. As we display here, many case reports have shown that mechanical or demyelinating lesions interrupting the senseriomotor and/or spinocerebellar tracts in the spinal cord can result in spinal dystonia highlighting the potential role of the spinal cord as a part of the neural integrator model.
Although spinal dystonia may be related to abnormal descending extrapyramidal tracts, most cases of spinal dystonia associate pyramidal pathology and can be viewed as a “pyramidal movement disorder” as opposed to the typical extrapyramidal prototype.
Dystonia secondary to non-demyelinating spinal lesions
While the most important and prominent cause of spinal dystonia is demyelination, we will start by briefly highlighting case reports of spinal dystonia due to non-demyelinating causes [5, 6].
Multiple case reports highlight cervical spinal tumors with ensuing compression causing focal paroxysmal dystonia. In 1994, a report was published detailing a 14 year old boy presenting with torticollis secondary to a spinal astrocytoma [41]. In 1995, Madhusudanan reported a patient with dystonia and athetosis due to a spinal glioma [42]. One report from 2006 highlighted five pediatric cases of cervical spine tumors leading to torticollis. Interestingly, the torticollis was the presenting symptom of the tumor, which is rare. Of the five cases, three were astrocytomas located in either the medulla, C1-6 or posterior cranial fossa. One case was an ependymoma spanning the entire cervical spine. The final case was an eosinophilic granuloma from C3 to C7 [43]. In 2020, Styrcek et al reported a patient presenting with episodic right anterolaterocollis with shoulder elevation lasting 10–60 s with no alteration of consciousness. These episodes occurred 2–6 times a day. MRI revealed a heterogenous mass in segments C1-2 with edema extending caudally to segment C6 [44].
Syringomyelia has also been highlighted as a cause of spinal dystonia. In 1995, Madhusudanan et al reported two patients who developed dystonia and athetosis as a result of syringomyelia [42]. In 1999, Hill et al reported two additional patients suffering from movement disorders secondary to syringomyelia. One developed torticollis while the other developed choreoathetoid-dystonic movements of the hand and arm [45].
In 2011, Benz et al reported a patient with spinal cord infiltration by a B-cell non-Hodgkin lymphoma. Brain MRI was unremarkable but spinal MRI showed intramedullary lesions spanning the cord from C1 and below. Initial symptoms included singultus, nausea, and vomiting, but was quickly followed by progressing symmetrical tetraparesis. The patient was treated with corticosteroids, and 3 weeks after the onset of symptoms, the patient began having involuntary movements and posturing of the fingers in the left hand, which the authors noted was similar to paroxysmal non-kinesigenic dyskinesia. Both the tetraparesis and posturing movements slowly resolved with treatment [46].
Spinal dystonia secondary to multiple sclerosis
Historically, movement disorders with the exception of tremors, were thought to be uncommon in patients with MS. More recent reports suggest that dystonia and other movement disorders, are not uncommon in MS patients, [6, 47–52]. Dystonia can in fact be the first manifestation of MS [47, 53].
Earlier literature on spinal dystonia and MS was limited to case reports. Cervical dystonia due to demyelination in the spinal cord has been reported frequently in the literature. It has been hypothesized that spinal demyelination may interrupt proprioceptive afferents leading to abnormal posturing [54]. The first such report of spinal dystonia secondary to demyelination was in 1993 by Klostermann et al displaying a patient with upper cervical spinal cord lesions presenting with spasmodic torticollis [55]. In 1996, Consentino reported a patient with MS presenting with paroxysmal kinesigenic dystonia in the upper limbs. The patient had an MRI showing high intensity signals from C2 to C7 [56]. In 2004, Rüegg et al reported a patient who presented with cervical dystonia with an MRI showing lesions in the C3 and C4 spinal segments alongside some periventricular and juxtacortical lesions [53].
Some interesting reports of acute hand dystonia and writer’s cramp in MS patients suggested a link to demyelination of the cervical spinal cord. In a patient with acute hand dystonia, the MRI showed lesions in the posterolateral cervical spine without any involvement of the basal ganglia or thalamus [57]. In the case with writer’s cramp, the patient had demyelinating lesions in the periventricular white matter alongside spinal segments C6 and C7 [58].
Yucesan et al reported a patient who presented with left hemidystonia secondary to cervical demyelinating lesions. MRI showed no basal ganglia involvement but lesions in the left posterolateral spine at C2 and C3 levels with right posterolateral spine lesions at C4 [59].
A review conducted in 2013 by Mehanna and Jankovic suggested that painful tonic spasms or paroxysmal dystonias are the second most common movement disorder in multiple sclerosis [48]. Although the review, similar to most of the older reports, did not distinguish tonic spasms from paroxysmal dystonia, it did highlight the important but under-recognized connection between MS and dystonia beyond individual case reports.
In the only prospective study of movement disorders in MS patients conducted by our group in 2016, 18% of patients with early MS had focal dystonia, which was mostly paroxysmal in nature. After MRI analysis, the anatomical origin of the focal dystonia was deemed to be in the spinal cord in over 70% of the patients. Dystonia involved either the upper or the lower extremity depending on the level of the culprit demyelinating lesion in the spinal cord. In fact, other than tremor, most of the movement disorders in this study were spinal in origin [6].
Spinal dystonia secondary to neuromyelitis optica spectrum disorders
NMOSD is an autoimmune disease with a special predilection to the optic nerves and the spinal cord [11]. Like MS, the pathogenesis of dystonia in NMOSD may be from axonal irritability secondary to inflammation or ephaptic activation of axons due to secondary demyelination [23]. Spinal cord inflammation is often more extensive in NMOSD than MS and is more likely to involve the spinal grey matter [60]. Some of the data aimed at determining the prevalence of movement disorders in MS came before NMOSD was established as a distinct disease. More recent literature from Kim et al suggests that tonic spasms and paroxysmal dystonia are more common in NMOSD than MS. Kim et al conducted a retrospective study in 2012 and found that 25% of patients with NMOSD had paroxysmal dystonia compared to only 2.9% in MS [60]. A study by Candeias da Silva et al., showed that paroxysmal dystonia is more common and more closely associated with NMOSD when compared to MS; however, this study did not perform MRIs to determine if the dystonic posturing was spinal in origin [61].
Schmidt et al described some of the first cases of paroxysmal dystonia secondary to NMOSD. They presented four patients that developed paroxysmal dystonias during the recovery from NMOSD attacks. Of the four, one patient had NMOSD for 37 years, two had it for 8 years, and one had it for 1 year. MRIs of these patients showed lesions in the cervical and thoracic spinal cord [11].
A case review conducted by Usmani et al in 2012 examined 57 patients with NMOSD, eight of which had tonic spasms (this paper used the term tonic spasms and dystonia interchangeably). Two of these patients had the spasms in their initial episodes. The report also indicated that these spasms are more common in NMOSD when compared to MS. The lesions were widespread, however, and tonic spams were reported in patients with lesions in the spinal cord, cerebral peduncles, internal capsules, thalami, and basal ganglia [23]. In response to this paper, Fragoso et al reported a case of a woman with progressive dystonia and an MRI showing a longitudinally extensive lesion in the cervical spinal cord. She went without a proper diagnosis until she developed bilateral optic neuritis 9 months after her initial presentation highlighting the need to recognize spinal dystonia as a potential presenting symptom of spinal cord demyelination [62].
In 2013, Abaroa et al gave 19 NMOSD patients detailed questionnaires to determine the prevalence of tonic spasms/paroxysmal dystonia (again, used interchangeably) [63]. Eighteen of the 19 patients reported tonic spasms/paroxysmal dystonia suggesting that these movement disorders may be highly prevalent in NMOSD patients [64]. One case report by Balasa from 2014 reported a patient diagnosed with NMOSD who developed painful paroxysmal dystonia 9 months after the onset of her NMOSD. MRI showed a hyperintense lesion at the level of C1-4 suggesting acute myelitis with no associated brain lesions [65]. Abaroa et al responded to the Balasa report suggesting that longitudinally extensive transverse myelitis alongside tonic spasms may be a prominent presentation of NMOSD [63].
In a retrospective study conducted by our group, 43% of 37 NMOSD patients had spinal movement disorders. Those with spinal movement disorders were older and less likely to be African Americans. Focal dystonia was seen in 5.4% of the whole cohort and 12.5% of those with spinal movement disorders. MRIs showed lesions from the cervicomedullary junction to the level of T8 and no lesions in the basal ganglia or brainstem [7]. In a prospective study conducted by our group on 62 patients with myelitis due to idiopathic disease, NMOSD, or MOG-associated disease; focal dystonia was the second most common spinal movement disorder present in 25% of patients (presented at the 2022 Consortium of Multiple Sclerosis Centers Annual Meeting). Myelitis-related spinal movement disorders including dystonia were most common in patients with NMOSD with AQP4 IgG and often occurred during recovery from a spinal attack. A subset of spinal paroxysmal dystonia was painful. Spinal movement disorders especially tonic spasms and paroxysmal dystonia are among the most bothersome residual symptoms in patients with NMOSD and related disorders.
Treatment of spinal dystonia
One recent retrospective study by Cedeno et al found that 70% of patients with movement disorders from demyelination responded to first trial of movement disorder-directed symptomatic therapy [66]. A study conducted by our group in 2019 showed that in a cohort of 61 patients with spasticity, only 53% of patients with spasticity-associated involuntary movements responded to anti-spasticity treatment suggesting that etiology may be important when deciding on a therapeutic plan [5]. Distinguishing demyelination-related from non-demyelination related dystonia could inform treatment options since corticosteroid therapy may ameliorate symptoms of acute demyelination attacks including acute dystonia. The vast majority of dystonia treatment, however, is symptomatic due to the lack of understanding of the exact mechanisms and the fact that dystonia often occurs in the recovery phase of demyelinating attacks [67]. Here, we briefly cover the broad classes of symptomatic drugs that can be used to treat spinal dystonia.
Botulinum toxin
Botulinum toxin (BT) is the initial treatment of choice for idiopathic or symptomatic focal and segmental dystonia including blepharospasm, spasmodic dysphonia, cervical, oromandibular, and lingual dystonia and is the most commonly used treatment [67–69]. BT was used as an effective treatment in a few of the cases we reported [47, 54, 55]. These reports were cases of cervical dystonia. Azzimondi et al reported a patient whose dystonia showed only mild improvement to corticosteroid use, however, BT injections to the bilateral splenius capitus and sternocleidomastoids resulted in near resolution of symptoms [47]. Klostermann et al reported a patient with spasmodic torticollis secondary to a cervical spine lesion. While the cervical cord lesion remained unchanged for up to 2 years, BT injections markedly improved the torticollis [55].
Anti-cholinergics
Of all the oral medications for dystonia, anticholinergics may be the most effective, regardless of etiology [69]. These drugs are muscarinic antagonists with the commonly used drugs in this class being trihexyphenidyl and benztropine. The main uses for anticholinergics are in generalized and segmental dystonia rather than focal dystonia [67]. There is a wide therapeutic range, which can lead to significant improvement in symptoms with these agents, but unfortunately, they must also be titrated properly due to a significant side effect profile [67, 69]. Surprisingly, not many of the cases we reviewed used this class of medications. One reported use of trihexyphenidyl came from Pettigrew et al who reported three patients with hemidystonia who showed a slight improvement in response to this medication [33].
Anti-epileptics
Anti-convulsants work generally by reducing excitability of neurons. Carbamazepine, a sodium channel blocker, has also been shown to be effective in dystonia [70, 71]. It is also especially effective in paroxysmal tonic spasms or dystonia caused by MS and NMOSD [3]. Often times, it may be used in combination with acetazolamide for improved outcomes [48, 72]. Many patients in the case reports we discussed had a good response to carbamazepine and a select few are discussed here. Ciampi et al reported a favorable outcome in six patients that received both carbamazepine and acetazolamide and theorized that the resultant acidosis from acetazolamide could potentially be therapeutic [34]. Zafar et al reported a patient with dystonia secondary to MS who was originally diagnosed with seizures. Corticoteroids and carbamazepine therapy were initiated which reduced the frequency of dystonic posturing from thrice a day to once a day [8]. Dehailan also reported a similar case of a patient with dystonia who was started on concurrent corticosteroids and carbamazepine therapy resulting in a significant decrease in frequency of posturing [10]. Finally, Fragoso et al reported a patient with dystonia so severe that it rendered the patient unable to walk. However, the patient had a dramatic response to carbamazepine therapy and regained proper function of her limbs [62]. Schmidt et al reported improvement in four patients who had dystonia secondary to NMOSD with the use of carbamazepine [11].
Other antiepileptics like zonisamide works to block voltage gated sodium and calcium channels as well as inhibits monoamine oxidase. It was found to be useful in the treatment of dystonia [67, 73]. Gabapentin is a calcium channel blocker that has also been shown to be effective in treating dystonia, especially in pediatric patients [74].
Muscle relaxants
Muscle relaxants are a broad class of drugs that can be used to treat dystonia. Oral and/or intrathecal baclofen are used for patients with generalized dystonia [67]. Baclofen works as a GABAB agonist to inhibit synaptic transmission. Its main clinical use is for spasticity but it can also be used for dystonia [67, 69]. Studies indicate that intrathecal baclofen pump may be superior to the oral form of baclofen in controlling hyperkinetic involuntary movements [5, 67]. Benzodiazepines, such as clonazepam, may be used as treatment for dystonia. These drugs work similarly to baclofen except they are agonists at the GABAA receptor. While one study found that benzodiazepines were one of the most common drugs prescribed for all etiologies of dystonia second to BT, use may be limited due to their extensive side effect profile including drowsiness and addiction [13, 68].
Conclusion
Spinal dystonia is an underrecognized phenomenon in spinal cord disorders especially demyelinating diseases. Historically it was thought that dystonia was solely a basal ganglia phenomenon. However, recent literature has suggested that there is not one such nucleus or region in the central nervous system that may cause dystonia but rather abnormal neural transmission along the pyramidal or extrapyramidal motor pathways.
In this review, we discussed case reports and series of dystonia that were specifically spinal in origin, over the past few decades. While there have been a few reports of spinal dystonia secondary to tumors and other disease states causing cord compression, by far the most common etiology of spinal dystonia is demyelination [5, 6]. Recent studies suggest that dystonia is not uncommon in demyelinating diseases and could be one of the presenting symptoms of the disease. In fact, it may even be one of the more important symptoms to look for in patients with NMOSD as there is a much stronger association of dystonia with NMOSD than MS [60].
Treatment of dystonia remains mostly symptomatic as the neurologic correlates and pathomechanisms of this movement disorder are poorly understood [67]. While we focused on the broad classes of medications that may be used clinically, it is important to note that when the dystonia manifests in the setting of an acute demyelinating attack, anti-inflammatory drugs such as corticosteroids may be the most effective route of treatment [47, 48, 53].
Recent literature trends towards recognizing dystonia as a spinal phenomenon, however, more data is needed to determine the true prevalence of spinal dystonia and the exact anatomical generators of dystonia within the spinal cord.
Statements
Author contributions
SS: conceptualization, literature review, writing original draft, writing: review and editing. TL: conceptualization, writing original draft, writing: review and editing. HA: conceptualization, supervision, writing original draft, writing: review and editing, final approval of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
HA is a consultant for Biogen, Genentech, BMS, Alexion, and Horizon. He receives research support from Novartis, BMS, Genentech, Sanofi-Genzyme, and the Guthy-Jackson Charitable Foundation.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
Albin RL Young AB Penney JB . The functional anatomy of basal ganglia disorders. Trends Neurosci (1989) 12(10):366–75. 10.1016/0166-2236(89)90074-x
2.
Dietrichs E . Movement disorders and basal ganglia function. Tidsskr Nor Laegeforen (2008) 128(17):1968–71.
3.
Termsarasab P Thammongkolchai T Frucht SJ . Spinal-generated movement disorders: A clinical review. J Clin Mov Disord (2015) 2:18. 10.1186/s40734-015-0028-1
4.
Lu A Zimmermann HG Specovius S Motamedi S Chien C Bereuter C et al Astrocytic outer retinal layer thinning is not a feature in AQP4-IgG seropositive neuromyelitis optica spectrum disorders. J Neurol Neurosurg Psychiatry (2022) 93(2):188–195. 10.1136/jnnp-2021-327412
5.
Abboud H Macaron G Yu XX Knusel K Fernandez HH Bethoux F . Defining the spectrum of spasticity-associated involuntary movements. Parkinsonism Relat Disord (2019) 65:79–85. 10.1016/j.parkreldis.2019.05.007
6.
Abboud H Yu XX Knusel K Fernandez HH Cohen JA . Movement disorders in early MS and related diseases: A prospective observational study. Neurol Clin Pract (2019) 9(1):24–31. 10.1212/CPJ.0000000000000560
7.
Abboud H Fernandez HH Mealy MA Levy M . Spinal movement disorders in neuromyelitis optica: An under-recognized phenomenon. Mov Disord Clin Pract (2016) 3(6):596–602. 10.1002/mdc3.12321
8.
Zafar AA Mohsin Almajid AM . Paroxysmal dystonia masquerading as focal onset seizure in patient with multiple sclerosis. Saudi J Health Sci (2016) 5(3):142–144.
9.
Spatt J Chaix R Mamoli B . Epileptic and non-epileptic seizures in multiple sclerosis. J Neurol (2001) 248(1):2–9. 10.1007/s004150170262
10.
Al Dehailan AS . Paroxysmal dystonia as an initial presentation of multiple sclerosis posing a diagnostic challenge. Neurosciences (Riyadh) (2019) 24(3):236–9. 10.17712/nsj.2018.3.20190025
11.
Schmidt FR Costa FHR Silva FMLC Maultasch H Rosso AL Nicaretta DH et al Paroxysmal dystonia and neuromyelitis optica. Arq Neuropsiquiatr (2012) 70(4):271–2. 10.1590/s0004-282x2012005000011
12.
van der Salm SM Erro R Cordivari C Edwards MJ Koelman JHTM van den Ende T et al Propriospinal myoclonus: Clinical reappraisal and review of literature. Neurology (2014) 83(20):1862–70. 10.1212/WNL.0000000000000982
13.
Batla A . Dystonia: A review. Neurol India (2018) 66:S48–S58. 10.4103/0028-3886.226439
14.
Rae-Grant AD . Unusual symptoms and syndromes in multiple sclerosis. Continuum (Minneap Minn) (2013) 19(4):992–1006. 10.1212/01.CON.0000433287.30715.07
15.
Smith KJ McDonald WI . The pathophysiology of multiple sclerosis: The mechanisms underlying the production of symptoms and the natural history of the disease. Philos Trans R Soc Lond B Biol Sci (1999) 354(1390):1649–73. 10.1098/rstb.1999.0510
16.
Burke D . Microneurography, impulse conduction, and paresthesias. Muscle Nerve (1993) 16(10):1025–32. 10.1002/mus.880161005
17.
Schmidt H Hahn G Deco G Knösche TR . Ephaptic coupling in white matter fibre bundles modulates axonal transmission delays. Plos Comput Biol (2021) 17(2):e1007858. 10.1371/journal.pcbi.1007858
18.
Han KS Chen CH Khan MM Guo C Regehr WG . Climbing fiber synapses rapidly and transiently inhibit neighboring Purkinje cells via ephaptic coupling. Nat Neurosci (2020) 23(11):1399–409. 10.1038/s41593-020-0701-z
19.
Shivacharan RS Chiang CC Wei X Subramanian M Couturier NH Pakalapati N et al Neural recruitment by ephaptic coupling in epilepsy. Epilepsia (2021) 62(7):1505–17. 10.1111/epi.16903
20.
Jefferys JG . Nonsynaptic modulation of neuronal activity in the brain: Electric currents and extracellular ions. Physiol Rev (1995) 75(4):689–723. 10.1152/physrev.1995.75.4.689
21.
Katz B Schmitt OH . Electric interaction between two adjacent nerve fibres. J Physiol (1940) 97(4):471–88. 10.1113/jphysiol.1940.sp003823
22.
Granit R Skoglund CR . Facilitation, inhibition and depression at the;artificial synapse' formed by the cut end of a mammalian nerve. J Physiol (1945) 103(4):435–48. 10.1113/jphysiol.1945.sp004089
23.
Usmani N Bedi G Lam BL Sheremata WA . Association between paroxysmal tonic spasms and neuromyelitis optica. Arch Neurol (2012) 69(1):121–4. 10.1001/archneurol.2011.832
24.
Traugott U Lebon P . Multiple sclerosis: Involvement of interferons in lesion pathogenesis. Ann Neurol (1988) 24(2):243–51. 10.1002/ana.410240211
25.
Bruno A Dolcetti E Centonze D . Theoretical and therapeutic implications of the spasticity-plus syndrome model in multiple sclerosis. Front Neurol (2021) 12:802918. 10.3389/fneur.2021.802918
26.
Ostermann PO Westerberg CE . Paroxysmal attacks in multiple sclerosis. Brain (1975) 98(2):189–202. 10.1093/brain/98.2.189
27.
El Otmani H Benmansour Y Araqi-Houssaini A Benkirane N Dany F Abdoh Rafai M et al Paroxysmal dystonia and multiple sclerosis. Rev Neurol (Paris) (2014) 170(2):119–23. 10.1016/j.neurol.2013.07.031
28.
Dressler D Bhidayasiri R Bohlega S Chana P Chien HF Chung TM et al Defining spasticity: A new approach considering current movement disorders terminology and botulinum toxin therapy. J Neurol (2018) 265(4):856–62. 10.1007/s00415-018-8759-1
29.
Sheean G . The pathophysiology of spasticity. Eur J Neurol (2002) 9(1):3–9. 10.1046/j.1468-1331.2002.0090s1003.x
30.
Sheean G McGuire JR . Spastic hypertonia and movement disorders: Pathophysiology, clinical presentation, and quantification. PM R (2009) 1(9):827–33. 10.1016/j.pmrj.2009.08.002
31.
Ganguly J Kulshreshtha D Almotiri M Jog M . Muscle tone physiology and abnormalities. Toxins (Basel) (2021) 13(4):282. 10.3390/toxins13040282
32.
Marsden CDOJZJ Obeso JA Zarranz JJ Lang AE . The anatomical basis of symptomatic hemidystonia. Brain (1985) 108:463–83. 10.1093/brain/108.2.463
33.
J PLJ Jankovic J . Hemidystonia: A report of 22 patients and a review of the literature. J Neurol Neurosurg Psychiatry (1985) 48:650–7. 10.1136/jnnp.48.7.650
34.
Ciampi E Uribe-San-Martín R Godoy-Santín J Cruz JP Cárcamo-Rodríguez C Juri C . Secondary paroxysmal dyskinesia in multiple sclerosis: Clinical-radiological features and treatment. Case report of seven patients. Mult Scler (2017) 23(13):1791–5. 10.1177/1352458517702968
35.
Tuzun E Akman-Demir G Eraksoy M . Paroxysmal attacks in multiple sclerosis. Mult Scler (2001) 7(6):402–4. 10.1177/135245850100700609
36.
Oakes PK Srivatsal SR Davis MY Samii A . Movement disorders in multiple sclerosis. Phys Med Rehabil Clin N Am (2013) 24(4):639–51. 10.1016/j.pmr.2013.06.003
37.
Sharma N . Neuropathology of dystonia. Tremor Other Hyperkinet Mov (N Y) (2019) 9:569. 10.7916/d8-j6sx-b156
38.
Tanabe LM Kim CE Alagem N Dauer WT . Primary dystonia: Molecules and mechanisms. Nat Rev Neurol (2009) 5(11):598–609. 10.1038/nrneurol.2009.160
39.
Sanchez K Rowe FJ . Role of neural integrators in oculomotor systems: A systematic narrative literature review. Acta Ophthalmol (2018) 96(2):e111–8. 10.1111/aos.13307
40.
Shaikh AG Zee DS Crawford JD Jinnah HA . Cervical dystonia: A neural integrator disorder. Brain (2016) 139(10):2590–9. 10.1093/brain/aww141
41.
Bussieres A Cassidy JD Dzus A . Spinal cord astrocytoma presenting as torticollis and scoliosis. J Manipulative Physiol Ther (1994) 17(2):113–8.
42.
Madhusudanan M Gracykutty M Cherian M . Athetosis-dystonia in intramedullary lesions of spinal cord. Acta Neurol Scand (1995) 92(4):308–12. 10.1111/j.1600-0404.1995.tb00134.x
43.
Kumandas S Per H Gümüş H Tucer B Yikilmaz A Kontaş O et al Torticollis secondary to posterior fossa and cervical spinal cord tumors: Report of five cases and literature review. Neurosurg Rev (2006) 29(4):333–8. 10.1007/s10143-006-0034-8
44.
Strycek O Fiedler J Rektor I . Paroxysmal dystonia due to cervical spinal cord tumor. Parkinsonism Relat Disord (2020) 79:1–2. 10.1016/j.parkreldis.2020.08.011
45.
Hill MD Kumar R Lozano A Tator CH Ashby P Lang AE . Syringomyelic dystonia and athetosis. Mov Disord (1999) 14(4):684–8. 10.1002/1531-8257(199907)14:4<684::aid-mds1021>3.0.co;2-g
46.
Benz R Viecelli A Taverna C Schelosky L . Paroxysmal non-kinesigenic dyskinesia due to spinal cord infiltration of low-grade B cell non-Hodgkin's lymphoma. Ann Hematol (2012) 91(3):463–5. 10.1007/s00277-011-1258-4
47.
Azzimondi G Rinaldi R Liguori R Tonon C Martini E D'Alessandro R . Dystonia as an isolated symptom of multiple sclerosis?Ital J Neurol Sci (1997) 18(5):301–2. 10.1007/BF02083309
48.
Mehanna R Jankovic J . Movement disorders in multiple sclerosis and other demyelinating diseases. J Neurol Sci (2013) 328(1-2):1–8. 10.1016/j.jns.2013.02.007
49.
Minagar A Sheremata WA Weiner WJ . Transient movement disorders and multiple sclerosis. Parkinsonism Relat Disord (2002) 9(2):111–3. 10.1016/s1353-8020(02)00009-3
50.
Tranchant C Bhatia KP Marsden CD . Movement disorders in multiple sclerosis. Mov Disord (1995) 10(4):418–23. 10.1002/mds.870100403
51.
Bachman DS Lao-Velez C Estanol B . Letter: Dystonia and choreoathetosis in multiple sclerosis. Arch Neurol (1976) 33(8):590. 10.1001/archneur.1976.00500080068016
52.
Coleman RJ Quinn NP Marsden CD . Multiple sclerosis presenting as adult onset dystonia. Mov Disord (1988) 3(4):329–32. 10.1002/mds.870030408
53.
Ruegg SJ Bühlmann M Renaud S Steck AJ Kappos L Fuhr P . Cervical dystonia as first manifestation of multiple sclerosis. J Neurol (2004) 251(11):1408–10. 10.1007/s00415-004-0544-7
54.
Cavallieri F Godeiro C Lino JC Moro E . Cervical dystonia in a case of longstanding secondary progressive multiple sclerosis. Rev Neurol (Paris) (2019) 175(4):269–71. 10.1016/j.neurol.2018.05.005
55.
Klostermann W Vieregge P Kompf D . Spasmodic torticollis in multiple sclerosis: Significance of an upper cervical spinal cord lesion. Mov Disord (1993) 8(2):234–6. 10.1002/mds.870080227
56.
Cosentino C Torres L Flores M Cuba JM . Paroxysmal kinesigenic dystonia and spinal cord lesion. Mov Disord (1996) 11(4):453–5. 10.1002/mds.870110422
57.
Uncini A Di Muzio A Thomas A Lugaresi A Gambi D . Hand dystonia secondary to cervical demyelinating lesion. Acta Neurol Scand (1994) 90(1):51–5. 10.1111/j.1600-0404.1994.tb02679.x
58.
Kim JS Guak TH Ahn JY Kim YI Kim TW Lee KS . Writer's cramp as a manifestation of cervical demyelinating lesions. Eur Neurol (2007) 58(1):54–6. 10.1159/000102169
59.
Yucesan C Tuncel D Akbostanci MC Yücemen N Mutluer N . Hemidystonia secondary to cervical demyelinating lesions. Eur J Neurol (2000) 7(5):563–6. 10.1046/j.1468-1331.2000.t01-1-00120.x
60.
Kim SM Go MJ Sung JJ Park KS Lee KW . Painful tonic spasm in neuromyelitis optica: Incidence, diagnostic utility, and clinical characteristics. Arch Neurol (2012) 69(8):1026–31. 10.1001/archneurol.2012.112
61.
Candeias da Silva C Bichuetti DB Azevedo Silva SMC Ferraz HB Oliveira EML Borges V . Movement disorders in multiple sclerosis and neuromyelitis optica: A clinical marker of neurological disability. Parkinsonism Relat Disord (2018) 51:73–8. 10.1016/j.parkreldis.2018.03.001
62.
Fragoso YD Brooks JB Oliveira CL . Severe dystonia as the first manifestation of neuromyelitis optica. Arq Neuropsiquiatr (2012) 70(9):749–50. 10.1590/s0004-282x2012000900022
63.
Rodriguez-Quiroga SA Abaroa L Arakaki T Garretto NS Villa AM . Commentary on neuromyelitis optica associated with painful paroxysmal dystonia: Case report and literature review. Acta Neurol Belg (2015) 115(3):523–4. 10.1007/s13760-014-0389-5
64.
Abaroa L Rodríguez-Quiroga SA Melamud L Arakaki T Garretto NS Villa AM . Tonic spasms are a common clinical manifestation in patients with neuromyelitis optica. Arq Neuropsiquiatr (2013) 71(5):280–3. 10.1590/0004-282X20130021
65.
Balasa R Bajkó Z Moţăţăianu A Maier A Maier S . Neuromyelitis optica associated with painful paroxysmal dystonia: Case report and literature review. Acta Neurol Belg (2015) 115(2):169–71. 10.1007/s13760-014-0325-8
66.
Suarez-Cedeno G Mehanna R . Movement disorders in multiple sclerosis and other demyelinating diseases: A retrospective review from a tertiary academic center. Neurologist (2021) 26(5):161–6. 10.1097/NRL.0000000000000333
67.
Balint B Mencacci NE Valente EM Pisani A Rothwell J Jankovic J et al Dystonia. Nat Rev Dis Primers (2018) 4(1):25. 10.1038/s41572-018-0023-6
68.
Pirio Richardson S Wegele AR Skipper B Deligtisch A Jinnah HA Dystonia Coalition Investigators. Dystonia treatment: Patterns of medication use in an international cohort. Neurology (2017) 88(6):543–550. 10.1212/WNL.0000000000003596
69.
Cloud LJ Jinnah HA . Treatment strategies for dystonia. Expert Opin Pharmacother (2010) 11(1):5–15. 10.1517/14656560903426171
70.
Isgreen WP Fahn S Barrett RE Snider SR Chutorian AM . Carbamazepine in torsion in dystonia. Adv Neurol (1976) 14:411–6.
71.
Termsarasab P Thammongkolchai T Frucht SJ . Medical treatment of dystonia. Med Treat dystonia J Clin Mov Disord (2016) 3:19. 10.1186/s40734-016-0047-6
72.
Sethi KD Hess DC Huffnagle VH Adams RJ . Acetazolamide treatment of paroxysmal dystonia in central demyelinating disease. Neurology (1992) 42(4):919–21. 10.1212/wnl.42.4.919
73.
Hainque E Vidailhet M Cozic N Charbonnier-Beaupel F Thobois S Tranchant C et al A randomized, controlled, double-blind, crossover trial of zonisamide in myoclonus-dystonia. Neurology (2016) 86(18):1729–35. 10.1212/WNL.0000000000002631
74.
Liow NY Gimeno H Lumsden DE Marianczak J Kaminska M Tomlin S et al Gabapentin can significantly improve dystonia severity and quality of life in children. Eur J Paediatr Neurol (2016) 20(1):100–7. 10.1016/j.ejpn.2015.09.007
Summary
Keywords
dystonia, multiple sclerosis, spinal dystonia, demyelination, neuromyelitis optica
Citation
Sarin S, Lawal T and Abboud H (2023) Spinal dystonia and other spinal movement disorders. Dystonia 2:11303. doi: 10.3389/dyst.2023.11303
Received
23 February 2023
Accepted
14 June 2023
Published
16 August 2023
Volume
2 - 2023
Edited by
Aasef Shaikh, Case Western Reserve University, United States
Updates
Copyright
© 2023 Sarin, Lawal and Abboud.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hesham Abboud, hesham.abboud@uhhospitals.org
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.