Abstract
Treatments are challenging in generalized granuloma annulare. We reported two cases of generalized granuloma annulare without diabetes mellitus; one exhibited non-annular papules and the other did patch/annular lesions. Since they were refractory to multiple treatments, we treated them with JAK inhibitor baricitinib at 2 mg daily. After 1–2-month treatment, they responded well to baricitinib with good tolerance. Given increased JAK1 signaling, high interferon-α and -γ production and macrophage activation in granuloma annulare, JAK inhibitors are considered to be a reasonable choice for recalcitrant generalized granuloma annulare. In addition to the reported cases, our two cases further support the effectiveness of JAK inhibitors for generalized granuloma annulare.
Introduction
Granuloma annulare (GA) is an inflammatory skin disease and subdivided into localized GA and generalized or disseminated GA [1, 2]. Generalized GA (GGA) is a rare and recalcitrant condition with a chronic course [1–3]. The skin manifestations of GGA include annular lesions and non-annular papules [2], and large annular lesions evolve into patches [1]. Diabetes mellitus (DM) and dyslipidemia are well-known associated conditions, and the frequencies of DM are 21.0% and 10.3% in GGA and localized GA, respectively [2].
GGA are usually resistant or variably responds to various therapeutic modalities, including systemic corticosteroids, phototherapies, isotretinoin, dapsone, pentoxifylline, hydroxychloroquine, cyclosporine, interferon (IFN)-γ, potassium iodide, nicotinamide, niacinamide, salicylic acid, dipyridamole, photodynamic therapy, fumaric acid ester, etanercept, infliximab, and adalimumab [3]. Recently, preferable therapeutic effects of Janus kinase (JAK) inhibitors on GA have been accumulated [4, 5]. Here, we report two patients with GGA, who showed non-annular papules and patch/annular lesions, respectively, and responded well to baricitinib.
Report of cases
Case 1
A 51-year-old Japanese man was referred to us because of a 5-month history of a generalized papular eruption on the trunk and four extremities without pruritus. He consulted three dermatologic clinics and was treated individually with oral 10 mg-daily methylprednisone or clarithromycin, and additional topical corticosteroids without remarkable therapeutic effects. On examination, the patient had multiple, solid papules densely present with cobblestone-like appearance on the trunk and four limbs (Figure 1A), sparing the face, palms and soles. Individual papules were 2–3 mm in diameter and had a slightly depressed center (Figure 1B). Some papules were observed in a linear fashion, indicating Köbner phenomenon. Peripheral blood examination revealed that T-cell production of interferon-γ to tuberculosis peptides, angiotensin converting enzyme (ACE), anti-nuclear antibodies, infection tests of syphilis and hepatitis viruses, hemoglobin A1c, and serum lipids were all negative or within normal limits. Complete blood cell counts and differential leukocyte counts were normal, except for monocytosis (20.4%). The frequencies of CD3+, CD4+ and CD8+ cells were within normal ranges. Soluble IL-2 receptor level was slightly increased (522 U/mL; normal, ≤496). He had hypertension and was given amlodipine, which was estimated as a potential culprit drug and was discontinued upon our first examination. His family history was unremarkable.
FIGURE 1
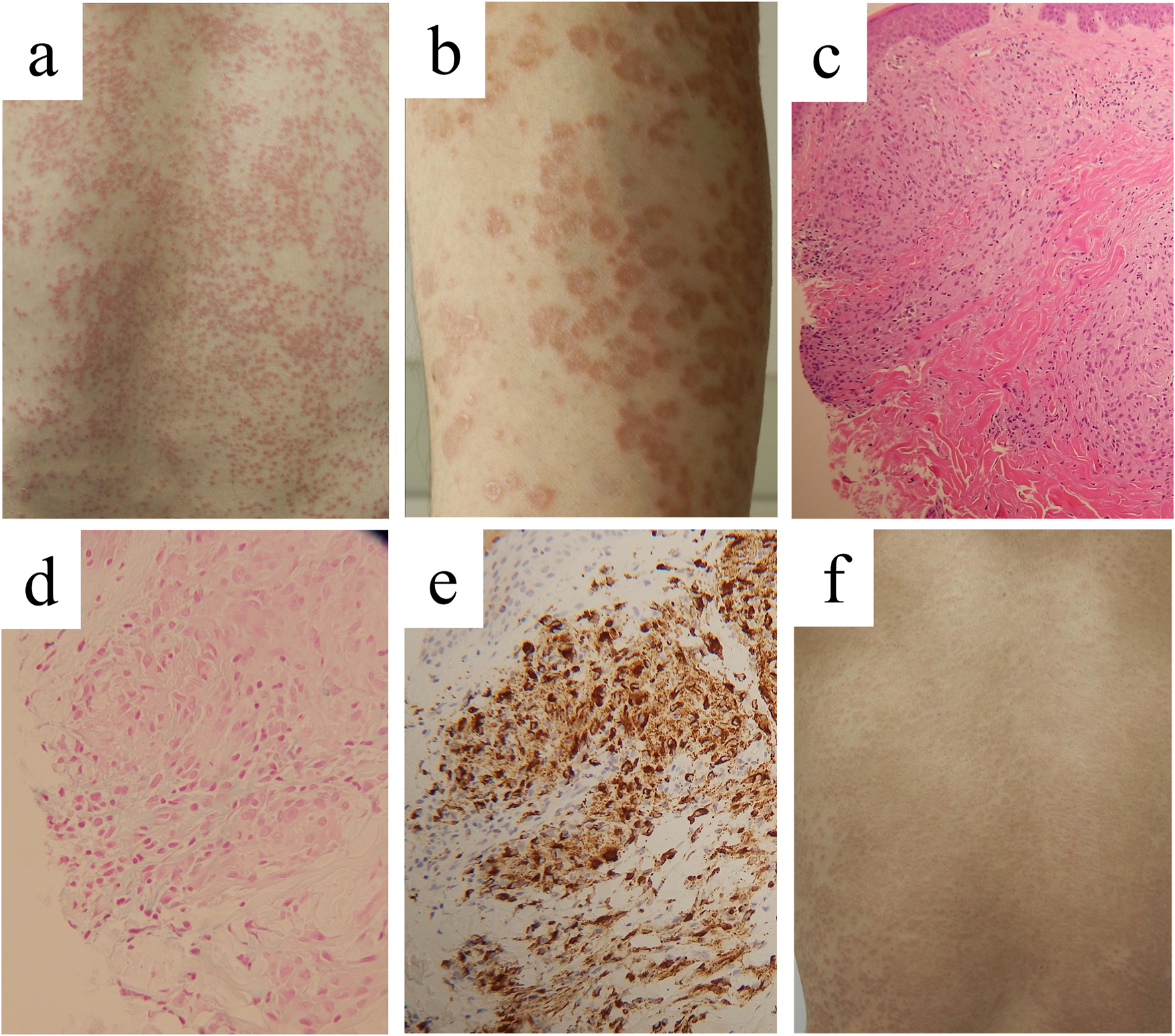
Clinical appearance and histopathological findings in case 1. Clinical pictures on our first examination, showing multiple solid papules on the back (A) and close inspection of papules on the lower leg (B). Infiltration of histiocytes in the dermis and degeneration of dermal collagen fibers lined by a palisading granulomatous reaction [(C), ×50], with Alcian-blue-positive mucin deposition [(D), ×200]. Immunostaining for CD68 [(E), ×200]. Improvement of the skin lesions on the back by 3-month administration with baricitinib at 2 mg daily (F).
A biopsy specimen taken from a papule on the abdomen disclosed focal or perivascular accumulation of histiocytes in the dermis and subcutaneous tissue. The histiocytes had round or oval nuclei without atypia. Multinucleated giant cells were slightly observed. There was degeneration of dermal collagen fibers lined by a palisading granulomatous reaction (Figure 1C) with Alcian-blue-positive mucin deposition (Figure 1D). No pathogen was found with Periodic acid-Schiff, Grocott, or Ziel-Neelsen staining. Immunohistochemically, the histiocytoid cells were positive for CD68 (Figure 1E), CD163, lysozyme and fascin, indicating histiocytes/macrophages, and negative for S-100 protein, CD1a and langerin, negating the possibilities of Langerhans cell histiocytosis and intermediate cell histiocytosis. We thus diagnosed the lesions as GGA. Sarcoidosis was excluded normal chest roentgenogram on annual health examination and normal serum ACE level.
We treated the patients with oral tranilast, 100 mg daily, for 4 weeks, and subsequently with narrow-band ultraviolet-B, up to 0.8 J/cm2, at a 2-week interval for 8 weeks, with limited therapeutic effects. Since he was refractory to multiple treatments by the three precedent dermatologists and us, baricitinib, 2 mg daily, was initiated. Written informed consent was obtained from the patient. After 9-week treatment, the papules were markedly flattened (Figure 1F). The patient had good intervention adherence and tolerated the treatment. Currently, his eruption subsided without recurrence.
Case 2
A 62-year-old Japanese woman was referred to us because of multiple lesions on the lower limbs, abdomen, and chest without pruritus. She developed an erythematous eruption on the abdomen 1 year before our examination, and then, reddish papules began to spread at the peripheries of the abdominal plaques. Subsequently, the same papular or annular lesions occurred on the chest and thighs, forming multifocal crops of papules. She was treated by a dermatologist topically with a strongest corticosteroid, which did not improve the eruption. Her family history was unremarkable. She had lipidemia. Peripheral blood examination showed that the following tests were negative or within normal limits: complete blood cell counts, differential leukocyte counts, T-cell tuberculosis test, ACE, anti-nuclear antibodies, syphilis and hepatitis virus tests, sugar level, and hemoglobin A1c. Blood chemistries disclosed high levels of total cholesterol (269 mg/dL; normal, 130–220) and LDL (181 mg/dL; normal, 70–139). Liver and renal functions were normal. On our initial examination, the patient had large erythematous patches on the abdomen with papules scattered from the peripheries (Figure 2A). Papules, measured mostly 5 mm in diameter and exhibiting depressed center were spread to the chest and thighs near the knees (Figure 2B).
FIGURE 2
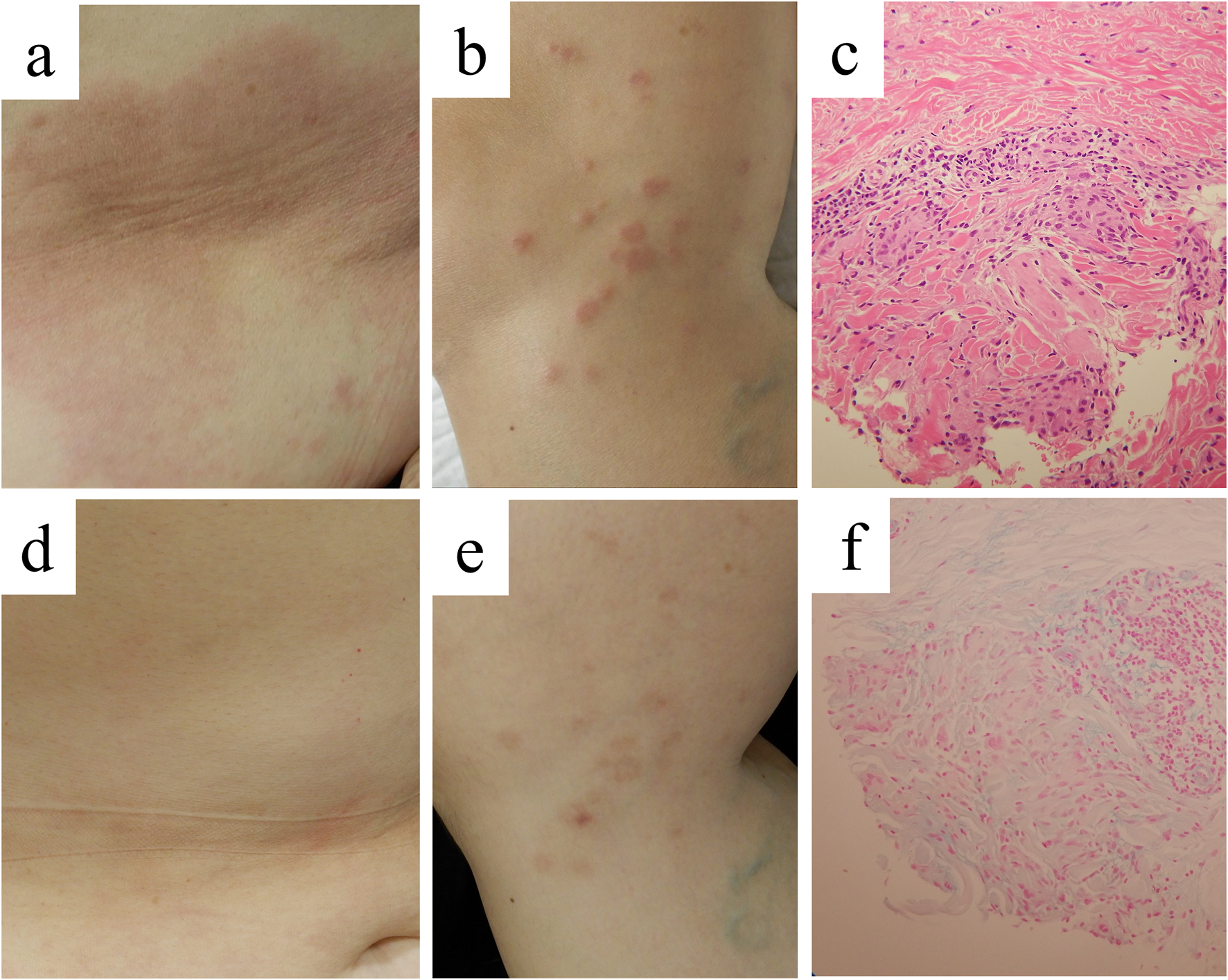
Clinical appearance and histopathological findings in case 2. Clinical pictures on our first examination, showing large erythematous patches on the abdomen (A) and small annular lesions on the thigh (B). Infiltration of histiocytes in the dermis [(C), ×200], with Alcian-blue-positive mucin deposition [(F), ×200]. Improvement of the skin lesions on the abdomen (D) and the thigh (E) by 2-month administration with baricitinib at 2 mg daily.
Histopathologically, a papular lesion showed that histiocytes and multinucleated giant cells infiltrated in the upper to middle dermis (Figure 2C). The infiltrate was mainly present between collogen fibers, as reported in the previous study that the predominant pattern in GGA is interstitial rather than palisading [6]. In some areas, collagen fibers were slightly degenerated and alcian blue-positive material was deposited in the same foci (Figure 2F). The eruption was diagnosed as GGA. Sarcoidosis was excluded normal chest roentgenogram on annual health examination and normal serum ACE level.
Since the patient’s eruption, consisting of large patches and scattered papules, did not respond well to the strongest topical corticosteroid or 100 mg-daily tranilast, we treated her with varicitinib, 2 mg daily. Written informed consent was obtained from the patient. The patient had good intervention adherence and tolerated the treatment. Twelve weeks after the initiation, the patches and annular or popular lesions were markedly improved, leaving hyperpigmented sequelae (Figures 1D, E).
Discussion
Various conventional treatments have been used for GGA [3], but their effectiveness is mostly limited, and the long-term use may result in adverse reactions. We chose baricitinib at 2 mg daily to treat our two cases with different GGA types [1, 2]: cases 1 and 2 exhibited non-annular papules [1, 2] and patch/annular lesions [1, 7], respectively. Both showed 1–2-month rapid effectiveness of this JAK inhibitor even at a low dose with good tolerance. The effectiveness of JAK inhibitors on GA has been documented in GGA patients treated with baricitinib (JAK1/2 inhibitor) [8–10], upadacitinib (mainly JAK1 inhibitor) [7, 11–13], abrocitinib (mainly JAK1 inhibitor) [14, 15], and tofacitinib (JAK1/3 inhibitor) [16]. Our findings support the previous notion and provide further evidence for its off-labeled use for GGA.
In dermatology, JAK inhibitors, such as baricitinib, upadacitinib and abrocitinib, are used for the treatment of atopic dermatitis (AD) and alopecia areata, and currently under investigation for a variety of skin diseases [5]. The critical action of JAK inhibitors on allergy is to inhibit JAK1, a main signaling transducer for type 2 immune response, where interleukin (IL-4) and IL-13 play a crucial role [17, 18]. However, JAK1 inhibitors also suppress the expression of interferon (IFN)-γ, IFN-α/β, and IL-22, because JAK1 also participates in the signal transduction of these cytokines [18]. In GA, IFN-γ is over-expressed [19], and the production of IFN-γ by CD4+ T cells is upregulated and is associated with inflammatory polarization of macrophages and fibroblasts [16]. Moreover, JAK-signal transducer and activator of transcription (STAT) pathway is activated in the pathophysiology of GA [20]. IFN-α is known to induce GGA in patients administered with pegylated IFN-α [21]. Finally, IL-22 may promote granuloma as seen in sarcoidosis [22]. These findings suggest that JAK1 inhibitors depress type 1/2 IFNs and IL-22 and are reasonable choice for GGA. A recent observation indicates that topical JAK inhibitor is effective for GA [23].
DM is occasionally associated with GGA [1, 2], and treatment of DM might improve GGA in DM-bearing patients. When the patients, like the present cases, do not have such an associated disease, treatments would be challenging. A limitation of the present report is documentation of only two cases. Although there remains a paucity of randomized controlled studies, our cases underscore the potential of JAK inhibitors as promising therapeutic options for recalcitrant GGA. Further case series and prospective clinical trials are needed to determine whether JAK inhibitors are truly effective in GAA.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YT and MY contributed to conception and design of the study. YT wrote the first draft of the manuscript. MY, SK, and MO wrote parts of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
References
1.
Joshi TP Duvic M . Granuloma annulare: an updated review of epidemiology, pathogenesis, and treatment options. Am J Clin Dermatol (2022) 23(1):37–50. 10.1007/s40257-021-00636-1
2.
Dabski K Winkelmann RK . Generalized granuloma annulare: clinical and laboratory findings in 100 patients. J Am Acad Dermatol (1989) 20(1):39–47. 10.1016/s0190-9622(89)70005-0
3.
Lukács J Schliemann S Elsner P . Treatment of generalized granuloma annulare - a systematic review. J Eur Acad Dermatol Venereol (2015) 29(8):1467–80. 10.1111/jdv.12976
4.
Damsky W Thakral D McGeary MK Leventhal J Galan A King B . Janus kinase inhibition induces disease remission in cutaneous sarcoidosis and granuloma annulare. J Am Acad Dermatol (2020) 82(3):612–21. 10.1016/j.jaad.2019.05.098
5.
Chapman S Gold LS Lim HW . Janus kinase inhibitors in dermatology: Part II. A comprehensive review. J Am Acad Dermatol (2022) 86(2):414–22. 10.1016/j.jaad.2021.06.873
6.
Ehret M Lenormand C Scrivener JN Gusdorf L Lipsker D Cribier B . Generalized granuloma annulare: a clinicopathological study. Ann Dermatol Venereol (2020) 147(4):271–8. 10.1016/j.annder.2019.09.617
7.
Sondermann W Hadaschik E Specker C . Successful therapy of disseminated patch-type granuloma annulare with upadacitinib in a patient with rheumatoid arthritis. Dermatol Ther (2022) 35(1):e15211. 10.1111/dth.15211
8.
Yan TM Zhang H Wu XY Zhang ZY . Successful treatment of generalized granuloma annulare with baricitinib. J Eur Acad Dermatol Venereol (2022) 36(7):e500–2. 10.1111/jdv.18031
9.
Jadoul A Huygen L Leemans G Grosber M Kortekaas Krohn I Gutermuth J . JAK1/2 pathway-specific treatment of disseminated granuloma annulare with baricitinib. J Eur Acad Dermatol Venereol (2023) 28. 10.1111/jdv.19073
10.
Kim D Kang HY . Rapid improvement of refractory generalized granuloma annulare with the Janus kinase inhibitor baricitinib in two patients. Clin Exp Dermatol (2023) 48(4):375–6. 10.1093/ced/llac110
11.
De Greef A Benjelloun G Harkemanne E Baeck M . Successful treatment of disseminated granuloma annulare with upadacitinib. Dermatol Ther (Heidelb) (2024) 14(3):813–7. 10.1007/s13555-024-01117-z
12.
Slater KN Valk B Kartono F . A case of generalized granuloma annulare treated with upadacitinib. JAAD Case Rep (2023) 34:12–4. 10.1016/j.jdcr.2023.01.027
13.
Coican A Meckley A Sagasser N Greenfield M Song EJ El-Bahri J . Successful treatment of refractory generalized granuloma annulare with upadacitinib. Case Rep Dermatol Med (2024) 2024:8859178. 10.1155/2024/8859178
14.
Liu W Chen W Tian X Yu Y Zhu J Liang J et al Oral abrocitinib in the treatment of granuloma annulare: a case report. J Dermatolog Treat (2024) 35:2313090. 10.1080/09546634.2024.2313090
15.
Michels A Heiland R Hammerschmidt S Farcas A Voigt TP Braun SA et al Successful treatment of recalcitrant generalized granuloma annulare with the JAK inhibitor abrocitinib. J Dtsch Dermatol Ges (2024) 22(6):841–3. 10.1111/ddg.15426
16.
Wang A Rahman NT McGeary MK Murphy M McHenry A Peterson D et al Treatment of granuloma annulare and suppression of proinflammatory cytokine activity with tofacitinib. J Allergy Clin Immunol (2021) 147:1795–809. 10.1016/j.jaci.2020.10.012
17.
Dengler HS Wu X Peng I Rinderknecht CH K won Y Suto E et al Lung-restricted inhibition of Janus kinase 1 is effective in rodent models of asthma. Sci Transl Med (2018) 10(468):eaao2151. 10.1126/scitranslmed.aao2151
18.
He H Guttman-Yassky E . JAK inhibitors for atopic dermatitis: an update. Am J Clin Dermatol (2019) 20(2):181–92. 10.1007/s40257-018-0413-2
19.
Hwang E Lee T Okifo K Murphy M Damsky W . Retrospective assessment of immunologic and histologic heterogeneity in granuloma annulare by cytokine staining. Int J Dermatol (2024) 63(5):655–9. 10.1111/ijd.16998
20.
Choi R Wang JX Damsky W Wang A Galan A Leventhal J . Janus kinase-signal transducers and activators of transcription (JAK-STAT) activation in anti-programmed death-1 (PD-1) therapy-associated granuloma annulare: a case series. Int J Dermatol (2023) 62(6):e323–5. 10.1111/ijd.16375
21.
Ahmad U Li X Sodeman T Daboul I . Hepatitis C virus treatment with pegylated interferon-alfa therapy leading to generalized interstitial granuloma annulare and review of the literature. Am J Ther (2013) 20(5):585–7. 10.1097/MJT.0b013e318209e049
22.
Sakthivel P Bruder D . Mechanism of granuloma formation in sarcoidosis. Curr Opin Hematol (2017) 24(1):59–65. 10.1097/MOH.0000000000000301
23.
Piontkowski AJ Wei N Mumtaz A Gulati N . Ruxolitinib cream for the treatment of granuloma annulare. JAAD Case Rep (2024) 50:62–4. 10.1016/j.jdcr.2024.05.030
Summary
Keywords
baricitinib, granuloma annulare, JAK inhibitor, upadacitinib, abrocitinib
Citation
Yunoki M, Kondo S, Otsuka M and Tokura Y (2025) Case report: Generalized granuloma annulare successfully treated with baricitinib in two cases. J. Cutan. Immunol. Allergy 7:13892. doi: 10.3389/jcia.2024.13892
Received
07 October 2024
Accepted
16 December 2024
Published
06 January 2025
Volume
7 - 2024
Updates
Copyright
© 2025 Yunoki, Kondo, Otsuka and Tokura.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marina Yunoki, m.yunoki.731@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.