Abstract
De novo thrombotic microangiopathy (TMA) is a rare and challenging condition in kidney transplant recipients, with limited research on its incidence and impact on graft survival. This study conducted a systematic review and meta-analysis of 28 cohorts/single-arm studies and 46 case series/reports from database inception to June 2022. In meta-analysis, among 14,410 kidney allograft recipients, de novo TMA occurred in 3.20% [95% confidence interval (CI): 1.93–4.77], with systemic and renal-limited TMA rates of 1.38% (95% CI: 06.5–2.39) and 2.80% (95% CI: 1.27–4.91), respectively. The overall graft loss rate of de novo TMA was 33.79% (95% CI: 26.14–41.88) in meta-analysis. This study provides valuable insights into the incidence and graft outcomes of de novo TMA in kidney transplant recipients.
Introduction
Thrombotic microangiopathy (TMA) is a rare complication of kidney transplantation that is often associated with poor graft and patient outcomes. TMA can be diagnosed based on clinical or histopathological features. Clinical recognition of TMA requires evidence of (a) microangiopathic hemolytic anemia: fragmented red blood cells on a peripheral blood smear, decreased haptoglobin levels, elevated lactate dehydrogenase and indirect bilirubin levels, and a decline in hemoglobin levels; (b) thrombocytopenia; and (c) evidence of organ damage. The common sites are the kidneys, central nervous system, and gastrointestinal tract [1]. Allograft biopsy is the gold standard method for establishing the diagnosis. Histologically, TMA is characterized by the patchy distribution of the vessel wall and detachment of edematous endothelial cells from the basement membrane. This causes intravascular platelet aggregation with subsequent formation of platelet-rich thrombi within the microcirculation and obstruction of vessel lumina [2].
Post-transplant TMA is classified into recurrent TMA and de novo TMA. Recurrent TMA is characterized by the same disease process that manifests as TMA involving the native kidney and recurs in the case of the allograft. In contrast, de novo TMA develops for the first time in kidney transplant recipients who had no evidence of the disease before transplantation. A study based on the United States Renal Data System indicated that the incidence of overall TMA in kidney allograft recipients was 5.6 episodes per 1,000 person-years, with approximately 50% patient mortality at 3 years [3]. As for de novo TMA, the incidence had been reported with a wide range and could be incorrectly estimated due to missed diagnosis of TMA before kidney transplantation. The incidence of de novo TMA has been reported to range from 3% to 14%, and the allograft loss rate ranges from 10% to 57%, both with a wide range [4]. De novo TMA not only causes acute decline of allograft function but also different degrees of sequelae. Graft loss in the case of de novo TMA is up to 40% within 2 years of diagnosis [3]. Outcomes range from transient renal dysfunction with mild clinical significance to acute renal failure requiring temporary dialysis therapy, potential allograft loss, and patient mortality. The outcome depends on the histopathological severity of the TMA, the promptness of the diagnosis, and the initiation of treatment [5].
The etiologies of kidney allograft de novo TMA include calcineurin inhibitors (CNIs), mammalian target of rapamycin inhibitors, ischemia-reperfusion injury, antibody-mediated rejection (AMR), viral infection, thrombotic thrombocytopenic purpura, and atypical hemolytic uremic syndrome (aHUS). CNIs, both cyclosporine and tacrolimus, are well-documented medications that cause de novo TMA [6–11]. Mammalian target of rapamycin inhibitors, such as sirolimus and everolimus, comprise much of the drug-related etiologies of TMA [12–15]. AMR is also a common and well-recognized cause of post-transplant TMA [16, 17]. Other less common causes, which can lead to TMA, include various viral infections such as infection of hepatitis C, cytomegalovirus, parvovirus, and BK virus [18–23]. Antiviral therapy [24], disseminated histoplasmosis [25], and thrombotic thrombocytopenic purpura are also among the reported etiologies [26–28]. aHUS is also an important cause. The presence of genetic mutations in complement systemic regulation can trigger an uncontrolled alternative complement pathway activity, resulting in endothelial injury, the pathogenetic basis of TMA [29].
Existing evidence about the incidence and outcome of de novo TMA is mainly based on case series and retrospective studies, comprising a wide range of data. Studies on the incidence and graft outcomes of de novo TMA are lacking. Therefore, this study aimed to present comprehensive data on the incidence, graft loss, and survival of kidney allografts in patients with de novo TMA.
Materials and Methods
Study Design
We conducted a systematic review and meta-analysis to evaluate the incidence and survival of kidney allografts in patients with de novo TMA. This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines and The Cochrane Collaboration form [30, 31].
Search Strategy and Eligibility Criteria
We systematically searched PubMed, Cochrane Library, and EMBASE (until April 2022). A manual search of the reference lists of relevant studies was performed to complement our search results. Search terms included kidney transplantation, de novo, and thrombotic microangiopathy, including all subheadings of the Medical Subject Headings and text searches for articles that were not indexed. No language restrictions were used to reduce funnel plot asymmetry. Automatic e-mail updates were built to periodically acquire new research results from the databases. Full details of the search strategy are presented in Supplementary Table S1. The reference lists of the relevant reports were manually searched to identify any missing relevant research articles or strategies.
Study Selection
All randomized controlled trials, observational studies, case reports, and case series were included in this systematic review and meta-analysis if they reported the following: 1) kidney allograft recipient; 2) de novo TMA; 3) incidence; or 4) graft survival. The exclusion criteria were as follows: 1) studies without retrievable endpoints; 2) studies with recurrent TMA; and 3) studies with posters or editorial comments only. The titles, abstracts, and contents were screened by three authors (C-YHs, S-HW, and C-YHu) to determine whether the studies met the inclusion criteria. The full texts of potentially relevant studies were retrieved and assessed in more detail.
Data Extraction
Three reviewers (C-YHu, S-HW, and C-YHs) independently assessed the studies for eligibility and extracted the data using a standardized data extraction form. Disagreements were resolved through a discussion with a fourth author (H-YC). The following parameters were extracted from each study: general characteristics (first author, year of publication, study terms, study design, and country), patient characteristics (number of patients in each treatment arm, patient age, sex, kidney donor types, genetic variants for complement dysregulation, cause of end-stage renal disease [ESRD], anti-rejection regimen, kidney pathological features, treatment of TMA, and follow-up duration), TMA incidence, and kidney allograft survival.
Quality Assessment
All cohort studies that met the inclusion criteria were subjected to quality appraisal using the Newcastle–Ottawa Scale, which contains 8 items within 3 domains and a total maximum score of 9 for cohort studies. Scores of 7–9 indicate high quality, 4–6 indicate high risk, and 0–3 indicate a very high risk of bias. All the case reports that met the inclusion criteria were subjected to quality appraisal using the CARE checklist and were recorded as “YES,” “PARTLY,” or “NO,” according to information reported by the included studies. The responses were assigned scores of 1, 0.5, and 0, respectively. The overall score was the sum of the 21 sub-items and was defined as “high” (more than 15), “medium” (10.5–14.5), and “low” (less than 10) [32]. These quality assessments were judged independently by two reviewers (S-HW and C-YHs), and any conflict was discussed with the third reviewer (C-YHu).
Outcomes
The study outcomes were the de novo TMA incidence and graft survival rates. De novo TMA incidence was divided into systemic and renal-limited TMA. Some studies that were not classified as systemic or renal-limited TMA were classified as unknown type of TMA. Therefore, we reported the following four different TMA incidences: 1) systemic TMA, 2) renal-limited TMA, 3) total TMA, and 4) unknown type of TMA.
The graft outcomes included graft loss and graft survival. Some studies showed graft survival of 1, 2, 3, 4, 5, 8, and 10 years.
Measurements
De novo TMA incidence was reported as a percentage and event per person-year. The pooled estimated incidence of de novo TMA was reported with a 95% confidence interval (CI). The graft survival rate was reported as a percentage. The pooled estimated graft survival was also reported with a 95% CI.
Meta-Analyses
The effect of baseline characteristics on the incidence of de novo TMA was analyzed. These factors included C4d, acute AMR, acute cell-mediated rejection, and the use of tacrolimus or cyclosporine. A random effects model was used for the meta-analysis.
Statistical Analysis
We used the MedCalc statistical software version Medal 20.110 (Acacialaan 22 8400 Ostend Belgium) to conduct meta-analyses and SPSS version 23.0 (IBM Corp., Armonk, New York) for descriptive analyses. Statistical heterogeneity of studies was assessed using I2 (inconsistency) from the fixed-effects model. All results were analyzed using a random-effects model if I2 was greater than 50% to minimize the potential heterogeneity effect and between-study variance. For descriptive analyses, continuous data were reported as mean ± standard deviation. p < 0.05 was considered statistically significant.
Results
Study Selection
Overall, 229 potentially relevant articles were identified in the literature search. Based on the review of the titles and abstracts, 126 studies were excluded. Further, 103 full-text articles were assessed for their eligibility; 31 records were excluded for the reasons of posters, insufficient data, or an editorial protocol. Finally, 75 studies met the inclusion criteria. Supplementary Figure S1 summarizes the flowchart of the search. Of the 75 included studies, 46 were case reports and case series, 21 were single-arm studies, and 8 were cohort studies. Eculizumab was approved for aHUS treatment by the Food and Drug Administration of the United States in 2011. Most of the studies published in 2012 collected data before 2012. Therefore, we categorized studies as published before 2013 and after 2013 (Supplementary Figure S2).
Study Characteristics of Single-Arm and Cohort Studies
Supplementary Table S4 summarizes the characteristics of single-arm and cohort studies. The percentage of males in the included studies ranged from 17% to 77.8%. The recruitment years of the studies ranged from 1980 to 2019, and 13 studies were published before 2013. The mean age was not reported in 9 studies, while the mean age reported in the other 20 studies was >23 years. The proportion of sex was not reported in 15 studies, whereas the male sex percentage was ranged from 0% to 77.7% in 14 studies. The study population was divided into kidney allograft recipients and renal biopsy recipients. The causes of ESRD included presumed chronic glomerulonephritis, presumed chronic interstitial nephritis, IgA nephropathy, focal segmental glomerulosclerosis, diabetic nephropathy, nephrosclerosis, lupus nephropathy, polycystic kidney disease, and hypertensive nephrosclerosis; however, they were not reported in 22 studies. Regarding management, CNI adjustment was reported in seven studies, two studies reported the efficacy of plasma exchange (PE), and three studies reported eculizumab therapy. Moreover, the proportion of AMR was mentioned in five studies, whereas the proportion of ABO-incompatible cases was mentioned in one study. Finally, one study reported pregnancy outcomes.
Study Characteristics of Case Reports and Case Series
Supplementary Table S2 and Supplementary Figure S3 summarize the characteristics of the case reports and case series. A total of 46 case reports and case series of 62 kidney allograft de novo TMA recipients were identified. A total of 42 (68%) recipients were tacrolimus users, 15 (24%) were cyclosporine users, and 5 (8%) were sirolimus users. The gene mutation data were limited. Of the 42 tacrolimus users, 9 possessed a complement factor H mutation, 2 possessed a complement factor I mutation, 1 possessed a factor II mutation, and 1 had a factor V mutation. Further, 5 of the 62 patients had a history of kidney transplants, 20 were living donor recipients, and 38 were deceased donor recipients. The onset timings (mean ± SD) of TMA were 11.26 ± 37.38, 16.68 ± 32.99, and 1.71 ± 2.96 months among tacrolimus, cyclosporine, and sirolimus users, respectively.
Six patients had AMR, six had cell-mediated rejection, and six had C4d+ on kidney pathology. Five patients were ABO-incompatible. The management of TMA included tapering the CNI and sirolimus dose, and then shifting to other immunosuppressive agents, eculizumab therapy, PE or infusion therapy, or belatacept therapy. A total of 34 (55%) patients received PE or infusion, and 18 (29%) patients received eculizumab therapy. The follow-up periods, months (mean ± SD) were 19.10 ± 37.23, 14.81 ± 13.74, and 4.45 ± 4.68 among tacrolimus, cyclosporine, and sirolimus users, respectively. Finally, 8 of the 62 individuals showed graft loss, whereas 48 individuals showed improvement in serum creatinine levels.
Incidence of De Novo Thrombotic Microangiopathy
The detailed de novo TMA incidence in the individual studies is summarized in Table 1. Among the studies included in our analysis, 20 reported on the incidence of de novo TMA. Of them, 18 studies focused on de novo TMA in kidney allograft recipients, whereas the remaining 2 studies [16, 36] reported on de novo TMA detected in kidney allograft biopsies. Two studies reported only on the incidence of TMA, without specifying the number of kidney allograft recipients or biopsies involved [34, 36]. Therefore, these studies were excluded from the meta-analysis.
TABLE 1
| Study | Study design | Study population | Total TMA incidence | Systemic TMA incidence | Renal-limited TMA incidence | Study follow-up time (month) | C4d+ | C4d− | Tacrolimus base regimen | Cyclosporine base regimen | Acute antibody-mediated rejection | Acute cell-mediated rejection | ABO incompatible |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baid, 1999 [33] | Single-arm | KAR | 3.2% (12/379) | 3.2% (12/379) | 84 | ||||||||
| Braet, 2016 [34] | Single-arm | KAR | 2.20% | N/A | 428 | ||||||||
| Caires, 2012 [35] | Single-arm | KAR | 1.1% (17/1,549) | 1.1% (17/1,549) | 132 | ||||||||
| Dessaix, 2019 [36] | Single-arm | KAB | 4.80% | 6.6 | |||||||||
| Doradla, 2020 [37] | Single-arm | KAR | 0.85% (17/2,000) | 0.2% (4/2,000) | 0.65% (13/2,000) | ||||||||
| Fortin, 2004 [38] | Cohort | KAR | 3.53% (13/368) | 3.53% (13/368) | 1.29% (3/233) | 3.70% (2/54) | |||||||
| Franco, 2003 [39] | Single-arm | KAR | 0.26% (10/3,862) | 0.26% (10/3,862) | 24 | ||||||||
| Futamura, 2020 [40] | Single-arm | KAR | 5.16% (69/1,336) | 211 | |||||||||
| Gumber, 2014 [41] | Single-arm | KAR | 2.89% (34/1,175) | 72 | |||||||||
| Kocak, 2015 [42] | Single-arm | KAR | 2.72% (13/477) | 2.72% (13/477) | 36 | 2.72% (13/477) | |||||||
| Langer, 2001 [43] | Single-arm | KAR | 1.5% (10/672) | 1.5% (10/672) | 212 | 1.5% (10/672) | |||||||
| Nava, 2014 [44] | Cohort | KAR | 7.3% (36/496) | 180 | N/A | ||||||||
| Oyen, 2006 [45] | Single-arm | KAR | 0.82% (7/850) | 48 | 0.82% (7/850) | ||||||||
| Ozedemir, 2018 [46] | Single-arm | KAR | 33.33% (30/90) | 17.6% (9/51) | |||||||||
| Reynolds, 2003 [3] | Cohort | KAR | 4.9/1,000 PY | ||||||||||
| Santos, 2003 [47] | Single-arm | KAR | 5% (6/115) | 5% (6/115) | |||||||||
| Satoskar, 2010 [16] | Single-arm | KAB | 13.6% (33/243) | 23.6% (6/715) | |||||||||
| Schwimmer, 2003 [4] | Single-arm | KAR | 3% (21/742) | 1.07% (8/742) | 1.75% (13/742) | 52% (11/21) | 48% (10/21) | ||||||
| Tasaki, 2019 [17] | Cohort | KAR | 7.5% (15/201) | 7.5% (15/201) | 214 | 17.2% (15/87) | |||||||
| Zarifian, 1999 [48] | Single-arm | KAR | 13.8% (26/188) | 1.06% (2/188) | 12.7% (24/188) |
Thrombotic microangiopathy incidence in included cohort and single-arm studies.
KAR, kidney allograft recipients; KAB, kidney allograft biopsies; PY, person-years; TMA, thrombotic microangiopathy.
Among kidney allograft recipients, the overall incidence of de novo TMA was 3.2% (95% CI: 1.93–4.77) (Figure 1A). The incidence of systemic and renal-limited de novo TMA was 1.38% (95% CI: 0.65–2.39) and 2.79% (95% CI: 1.27–4.91), respectively (Figures 1B, C). The unknown type of TMA incidence was 3.64% (95% CI: 1.50–6.67) (Figure 1D). All the outcomes showed significant heterogeneity (I2 > 89%). Stratifying the analysis based on distinct follow-up periods provided information on the overall incidence of thrombotic microangiopathy (TMA). Within 5 years follow up time, the TMA incidence was 1.04% (95% CI: 0.16–2.68) with significant heterogeneity (I2: 92.17%) (Supplementary Figure S4). As the follow-up duration extended to the 5–10 years, the TMA incidence was 3.02% (95% CI: 2.23–3.92) with low heterogeneity (I2: 0.00%) (Supplementary Figure S5). If follow-up was more than 10 years, the TMA incidence was 4.15% (95% CI: 1.64–7.75) with significant heterogeneity (I2: 95.54%) (Supplementary Figure S6). In a cohort study conducted in 2003, the incidence of de novo TMA in kidney allograft recipients was 4.9 episodes per 1,000 person-years [3].
FIGURE 1
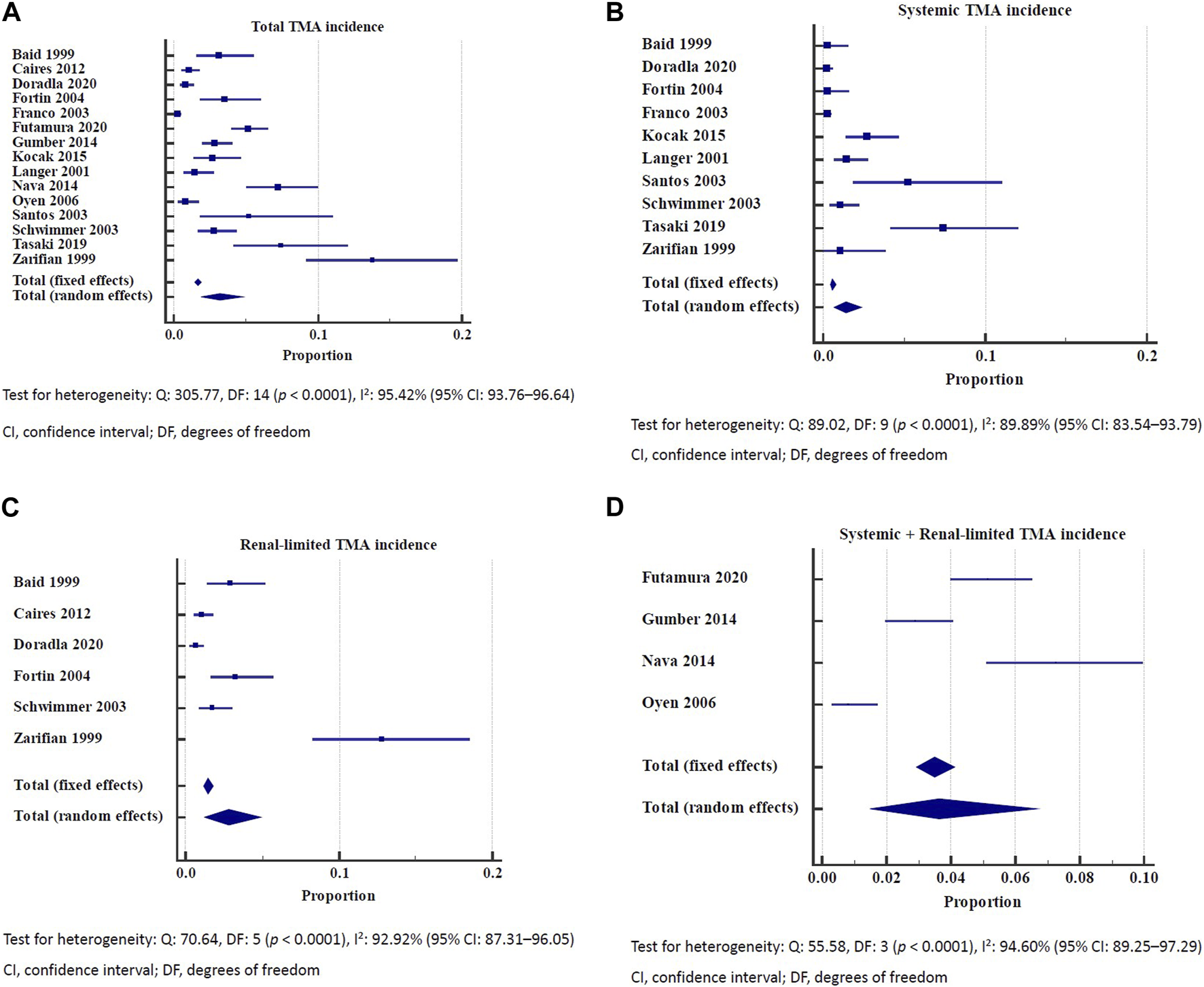
(A) Incidence of total de novo thrombotic microangiopathy. Test for heterogeneity: Q: 305.77, DF: 14 (p < 0.0001), I2: 95.42% (95% CI: 93.76–96.64). CI, confidence interval; DF, degrees of freedom. (B) Incidence of systemic thrombotic microangiopathy. Test for heterogeneity: Q: 89.02, DF: 9 (p < 0.0001), I2: 89.89% (95% CI: 83.54–93.79). CI, confidence interval; DF, degrees of freedom. (C) Incidence of renal-limited thrombotic microangiopathy. Test for heterogeneity: Q: 70.64, DF: 5 (p < 0.0001), I2: 92.92% (95% CI: 87.31–96.05). CI, confidence interval; DF, degrees of freedom. (D) Incidence of systemic and renal-limited thrombotic microangiopathy. Test for heterogeneity: Q: 55.58, DF: 3 (p < 0.0001), I2: 94.60% (95% CI: 89.25–97.29). CI, confidence interval; DF, degrees of freedom.
The incidence of de novo TMA among kidney allograft biopsies ranged from 0.26% to 4.8% across the studies [34, 36, 39]. The incidence of systemic de novo TMA was 0.26% [39].
Graft Survival Rate in Patients With De Novo Thrombotic Microangiopathy
The detailed individual de novo TMA graft survival rate is summarized in Table 2. Our analysis included a total of 18 studies that reported on kidney allograft survival, of which, 8 were eligible for inclusion in the meta-analysis [16, 17, 37, 38, 41, 45, 47, 50]. The overall graft loss rate of de novo TMA was 33.79% (95% CI: 26.14–41.88). No significant heterogeneity (I2 = 18.04%) was observed (Figure 2). The meta-analysis of seven studies reporting 1-year graft survival outcomes revealed a rate of 55.39% (95% CI: 36.46–73.54). However, a substantial degree of heterogeneity (I2 = 88.12%) was observed (Supplementary Figure S7).
TABLE 2
| Graft loss | Graft survival | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Study design | Total graft loss | Plasma exchange | Either tacrolimus or cyclosporine, shift to sirolimus (%) | Acute antibody-mediated rejection (%) | Acute cell-mediated rejection (%) | C4d+ | C4d− (%) | 1-year survival (%) | 2-year survival (%) | 3-year survival (%) | 4-year survival (%) | 5-year survival (%) | 8-year survival (%) | 10-year survival (%) |
| Baid, 1999 [33] | Single-arm | 40 | |||||||||||||
| Braet, 2016 [34] | Single-arm | 32 | |||||||||||||
| Caires, 2012 [35] | Single-arm | 43 | |||||||||||||
| Costa, 2013 [49] | Single-arm | 73.30 | |||||||||||||
| Dessaix, 2019 [36] | Single-arm | 8 | |||||||||||||
| Doradla, 2020 [37] | Single-arm | 53% (9/17) | 100 | 100 | 47 | 35 | 35 | ||||||||
| Fortin, 2004 [38] | Single-arm | 30.77% (4/13) | |||||||||||||
| Gumber, 2014 [41] | Single-arm | 17.65% (6/34) | |||||||||||||
| Le Quintrec, 2008 [50] | Single-arm | 33.33% (8/24) | 67 | ||||||||||||
| Meehan, 2011 [51] | Single-arm | 57% | |||||||||||||
| Oyen, 2006 [45] | Single-arm | 28.57% (2/7) | 28.6 | ||||||||||||
| Ozedemir, 2018 [46] | Single-arm | 83 | 51 | 51 | |||||||||||
| Reynolds, 2003 [3] | Cohort | 47 | 35 | ||||||||||||
| Santos, 2003 [47] | Single-arm | 33.33% (2/6) | |||||||||||||
| Satoskar, 2010 [16] | Single-arm | 40.68% (24/59) | 35% (8/23) | 40% | 42 | ||||||||||
| Tasaki, 2019 [17] | Cohort | 26.67% (4/15) | |||||||||||||
| Wu, 2016 [52] | Cohort | 70.0 | 48.3 | 28.0 | |||||||||||
| Zarifian, 1999 [48] | Single-arm | 81 | 69 | ||||||||||||
Graft outcome in included cohort and single-arm studies.
FIGURE 2
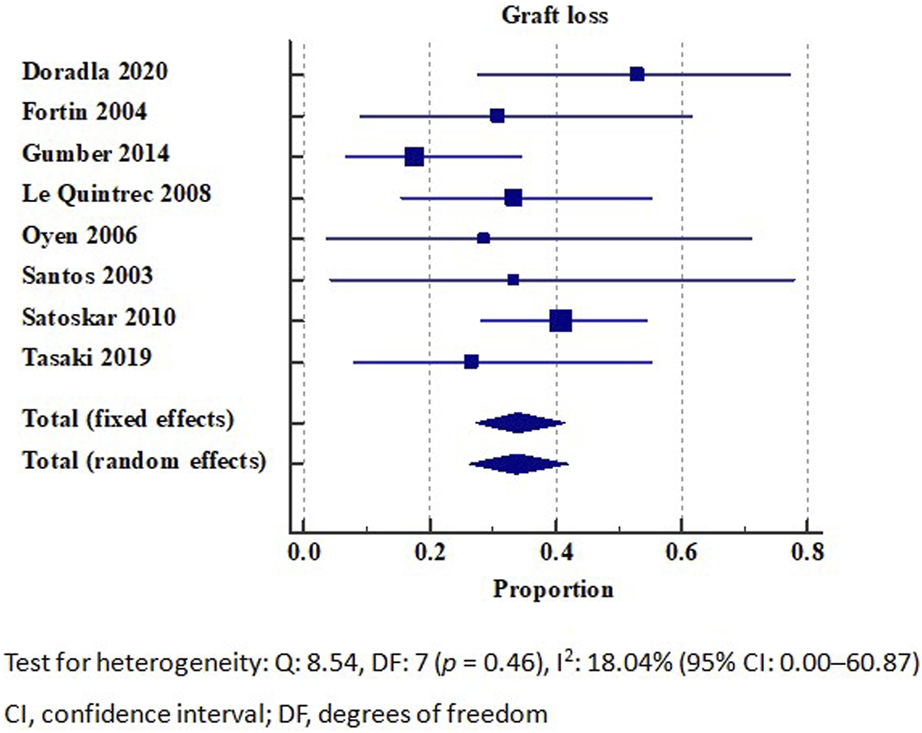
Graft loss in patients with de novo thrombotic microangiopathy. Test for heterogeneity: Q: 8.54, DF: 7 (p = 0.46), I2: 18.04% (95% CI: 0.00–60.87). CI, confidence interval; DF, degrees of freedom.
33% of patients with CNI-related TMA (4 out of 12) developed ESRD, while all patients with rejection-associated TMA developed ESRD [37]. The overall 1-year graft survival rate was 47%, whereas the 5- and 10-year graft survival rates were 35%. Additionally, there was no significant difference in the graft survival rate between the renal-limited and systemic TMAs (p = 0.4) [37].
In one study, among 33 C4d-positive TMA patients, 23 (70%) underwent plasmapheresis, with a graft loss rate of 35% (8 out of 23). Conversely, the remaining 30% (10 patients) did not receive plasmapheresis, and among these, the graft loss rate was higher at 50% [16].
Study Quality of Included Cohort Studies
All observational studies scored from 6 to 9 on the Newcastle–Ottawa Scale criteria and were included in the quantitative analysis (Supplementary Table S3). Five cohort studies were considered to be of high quality (Newcastle–Ottawa score ≥ 7).
Discussion
Our systematic review and meta-analysis encompassed 75 studies, including 29 cohort or single-arm studies and 46 case series or case reports, to provide a comprehensive examination of the incidence and graft survival rate in kidney allograft recipients with de novo TMA. Among 14,410 kidney allograft recipients, 306 individuals developed de novo TMA, corresponding to an incidence of 3.20%, while the incidences of systemic TMA and renal-limited TMA were 1.38% and 2.80%, respectively. Among the 200 kidney allograft recipients who developed de novo TMA, 138 individuals remained dialysis-free 1 year after transplantation. However, among the 175 individuals with de novo TMA who were followed up for graft outcomes, 59 individuals eventually experienced graft loss, resulting in an overall graft loss rate of 33.79%.
Data on the incidence difference between kidney recipients with renal-limited TMA and systemic TMA are inconsistent. A study involving 21 individuals with pathology-proven kidney allograft TMA showed that 60% of the individuals had systemic TMA, and 40% had renal-limited TMA [4]. However, in contrast, only 5% of 43 individuals with pathology-proven lupus nephritis and concomitant TMA were found to have systemic TMA [53]. Moreover, comparative studies investigating differences in graft survival rates between patients with renal-limited and systemic TMA are scarce. In a case series involving 21 individuals with kidney allograft TMA, including 8 with renal-limited TMA and 13 with systemic TMA, Kaplan–Meier analysis revealed that those with renal-limited TMA had better graft survival than those with systemic TMA, with an average follow-up of 62 months [4]. Therefore, a well-designed future study is needed to examine the difference in incidence and outcomes between renal-limited and systemic TMA.
More than 90% of kidney recipients are treated with CNIs, and only a few develop de novo TMA. Therefore, caution should be exercised before attributing de novo TMA to CNIs until other predisposing factors have been ruled out [54]. The incidence of CNIs-related de novo TMA ranges from 1.29% to 3.7% [43, 55]. Several mechanisms explain the relationship between CNIs and de novo TMA. In CNI users, an imbalance of vasodilators (prostaglandin E2, prostacyclin I2, and nitric oxide) and vasoconstrictors (thromboxane A2 and endothelin) leads to glomerular arteriolar vasoconstriction and endothelial damage [56, 57]. The release of microparticles from CNIs-exposed endothelium is reported to activate the complement alternative pathway, causing endothelial cell damage [11]. Our analysis of case reports and series revealed that tapering down the CNIs-dose is the most common strategy, followed by shifting to other CNIs or sirolimus. However, the graft survival rate remains unfavorable and is reported to be 28.6% [45].
C4d is an indicator of an activated classical complement pathway, and linear C4d staining in the peritubular capillary is a key diagnostic feature of AMR [58]. A retrospective study involving 59 individuals with kidney allograft TMA revealed that those with peritubular capillaries linear C4d staining had a nearly 4-fold higher incidence of TMA than did those without C4d staining (C4d+ vs. C4d−: 13.6% vs. 3.6%) [16]. However, the 2-year graft loss rate was similar between the two groups, with nearly 40% in each group. In contrast, another study of 74 individuals with kidney allograft TMA found that those with C4d+ had a higher graft loss rate than those without C4d staining (55.6% vs. 30%) [46]. Nevertheless, C4d deposits are not uncommon in kidney allograft TMA, particularly in the glomeruli. In a study of 32 individuals with renal TMA, which included 12 kidney allograft sections and 30 native kidney sections, C4d deposits were detected in 88% of TMA cases, while C5b-9 deposits were detected in 76% of TMA cases [58]. Notably, of the 12 kidney allograft TMA sections, C4d deposits were present in 75% of glomeruli, and C5b-9 deposits were present in 50%. The study showed that C4d and C5b-9 are common denominators in kidney allografts in patients with TMA and suggested that anti-terminal complement therapy may be beneficial in these patients. The management strategy for de novo TMA includes identifying and removing triggers, PE, and eculizumab therapy. However, the efficacy of PE in de novo TMA has not been fully established, owing to the heterogeneity of its etiologies. Although PE plays an important role in managing thrombotic thrombocytopenic purpura, it is also used as a bridging therapy to eculizumab in patients with aHUS. In a single-arm retrospective cohort study conducted in the United States in 2003 (pre-eculizumab era) to examine the efficacy of PE in 29 kidney allograft recipients with TMA, 6 (20%) of them suffered from graft loss. Among the 10 individuals who had histological acute rejection, 6 (60%) suffered from graft loss within 1 year [59]. In a comparative study conducted in 2010 (pre-eculizumab era), which aimed to explore the efficacy of PE with concurrent intravenous immunoglobulin in 33 kidney allograft recipients with de novo TMA and concomitant AMR, the graft loss rate was not different between those with and without PE + intravenous immunoglobulin (35% vs. 50%) [16]. In a single-arm retrospective cohort study conducted in Spain in 2020, which comprised 16 kidney allograft recipients with de novo aHUS, only 2 of 13 individuals who underwent PE achieved complete hematological and renal recovery. Eight individuals received rescue eculizumab owing to no or partial renal response to PE, and six (75%) of them achieved complete hematological and renal recovery after receiving rescue eculizumab [60]. Finally, according to our analysis of case reports and series, 52% of 48 individuals with de novo TMA who underwent PE achieved a renal response, whereas 83% of 18 individuals with de novo TMA receiving eculizumab achieved a renal response.
This study has few limitations. First, owing to the low prevalence of de novo TMA, there was a lack of randomized controlled trials, and the meta-analysis results were based on single-arm or observational cohort studies. Additionally, there was a wide variance in the number of cases among the enrolled studies. Second, there was heterogeneity in our meta-analysis of TMA incidence, which may be mainly attributed to the influence of the following three studies: a study by Tasaki et al., which included ABO-incompatible kidney allograft recipients and had a high TMA incidence; a study by Nava et al., which included older kidney allograft recipients and had a high TMA incidence; and a study by Zarifian et al., which had a higher proportion of individuals with chronic transplant nephropathy and was conducted in 1999 when all recipients were receiving cyclosporine for immunosuppression [17, 44, 53]. Finally, 9 of the 15 studies included in our TMA incidence meta-analysis and 5 of 8 studies included in our graft survival meta-analysis were conducted before 2013 (pre-eculizumab era). This may have led to a bias in both TMA incidence and graft survival rates in the current eculizumab era.
To conclude, the incidence of de novo TMA in patients with kidney allografts was 3.20%, whereas the incidences of systemic TMA and renal-limited TMA were 1.38% and 2.80%, respectively. The overall graft loss rate was 33.79%. These findings highlight the rare and complex nature of de novo TMA in kidney allograft recipients, which is associated with poor graft outcomes. Our study provides valuable insights into the incidence and graft outcomes.
Statements
Author contributions
H-YC conceived of the presented idea. S-HW and C-YHu verified the analytical methods and C-YHu performed data analysis. C-YHu and C-YHs wrote the manuscript with support from H-YC. C-YHs and H-YC investigated and supervised the findings of this work. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/ti.2024.12168/full#supplementary-material
Abbreviations
aHUS, atypical hemolytic uremic syndrome; AMR, antibody-mediated rejection; CI, confidence interval; CNIs, calcineurin inhibitors; ESRD, end-stage renal disease; PE, plasma exchange; TMA, thrombotic microangiopathy.
References
1.
Laurence J Haller H Mannucci PM Nangaku M Praga M Rodriguez de Cordoba S . Atypical Hemolytic Uremic Syndrome (aHUS): Essential Aspects of an Accurate Diagnosis. Clin Adv Hematol Oncol (2016) 14(11):2–15.
2.
Moschcowitz E . An Acute Febrile Pleiochromic Anemia With Hyaline Thrombosis of the Terminal Arterioles and Capillaries: An Undescribed Disease. Mt Sinai J Med (1925) 70(5):352–5.
3.
Reynolds JC Agodoa LY Yuan CM Abbott KC . Thrombotic Microangiopathy After Renal Transplantation in the United States. Am J Kidney Dis (2003) 42(5):1058–68. 10.1016/j.ajkd.2003.07.008
4.
Schwimmer J Nadasdy TA Spitalnik PF Kaplan KL Zand MS . De Novo Thrombotic Microangiopathy in Renal Transplant Recipients: A Comparison of Hemolytic Uremic Syndrome With Localized Renal Thrombotic Microangiopathy. Am J Kidney Dis (2003) 41(2):471–9. 10.1053/ajkd.2003.50058
5.
Roberts D Siegman I Andeen N Woodland D Deloughery T Rueda J et al De Novo Thrombotic Microangiopathy in Two Kidney Transplant Recipients From the Same Deceased Donor: A Case Series. Clin Transpl (2020) 34(7):e13885. 10.1111/ctr.13885
6.
Remuzzi G Bertani T . Renal Vascular and Thrombotic Effects of Cyclosporine. Am J Kidney Dis (1989) 13(4):261–72. 10.1016/s0272-6386(89)80032-0
7.
Ramírez C Olmo A O'Valle F Masseroli M Aguilar M Gómez-Morales M et al Role of Intrarenal Endothelin 1, Endothelin 3, and Angiotensin II Expression in Chronic Cyclosporin A Nephrotoxicity in Rats. Exp Nephrol (2000) 8(3):161–72. 10.1159/000020664
8.
Sahin G Akay OM Bal C Yalcin AU Gulbas Z . The Effect of Calcineurin Inhibitors on Endothelial and Platelet Function in Renal Transplant Patients. Clin Nephrol (2011) 76(3):218–25. 10.5414/cn106931
9.
Tomasiak M Rusak T Gacko M Stelmach H . Cyclosporine Enhances Platelet Procoagulant Activity. Nephrol Dial Transpl (2007) 22(6):1750–6. 10.1093/ndt/gfl836
10.
Verpooten GA Cools FJ Van der Planken MG Bedert LC Claes R van Gaal LF et al Elevated Plasminogen Activator Inhibitor Levels in Cyclosporin-Treated Renal Allograft Recipients. Nephrol Dial Transpl (1996) 11(2):347–51. 10.1093/oxfordjournals.ndt.a027265
11.
Renner B Klawitter J Goldberg R McCullough JW Ferreira VP Cooper JE et al Cyclosporine Induces Endothelial Cell Release of Complement-Activating Microparticles. J Am Soc Nephrol (2013) 24(11):1849–62. 10.1681/ASN.2012111064
12.
Keller K Daniel C Schöcklmann H Endlich KH Kerjaschki D Johnson RJ et al Everolimus Inhibits Glomerular Endothelial Cell Proliferation and VEGF, But Not Long-Term Recovery in Experimental Thrombotic Microangiopathy. Nephrol Dial Transpl (2006) 21(10):2724–35. 10.1093/ndt/gfl340
13.
Baas MC Gerdes VE Ten Berge IJ Heutinck KM Florquin S Meijers JC et al Treatment With Everolimus Is Associated With a Procoagulant State. Thromb Res (2013) 132(2):307–11. 10.1016/j.thromres.2013.07.004
14.
Sartelet H Toupance O Lorenzato M Fadel F Noel LH Lagonotte E et al Sirolimus-Induced Thrombotic Microangiopathy Is Associated With Decreased Expression of Vascular Endothelial Growth Factor in Kidneys. Am J Transpl (2005) 5(10):2441–7. 10.1111/j.1600-6143.2005.01047.x
15.
Miriuka SG Rao V Peterson M Tumiati L Delgado DH Mohan R et al mTOR Inhibition Induces Endothelial Progenitor Cell Death. Am J Transpl (2006) 6(9):2069–79. 10.1111/j.1600-6143.2006.01433.x
16.
Satoskar AA Pelletier R Adams P Nadasdy GM Brodsky S Pesavento T et al De Novo Thrombotic Microangiopathy in Renal Allograft Biopsies-Role of Antibody-Mediated Rejection. Am J Transpl (2010) 10(8):1804–11. 10.1111/j.1600-6143.2010.03178.x
17.
Tasaki M Saito K Nakagawa Y Imai N Ito Y Yoshida Y et al Analysis of the Prevalence of Systemic De Novo Thrombotic Microangiopathy After ABO-Incompatible Kidney Transplantation and the Associated Risk Factors. Int J Urol (2019) 26(12):1128–37. 10.1111/iju.14118
18.
De Keyzer K van Laecke S Peeters P Vanholder R . De Novo Thrombotic Microangiopathy Induced by Cytomegalovirus Infection Leading to Renal Allograft Loss. Am J Nephrol (2010) 32(5):491–6. 10.1159/000321328
19.
Java A Edwards A Rossi A Pandey R Gaut J Delos Santos R et al Cytomegalovirus-Induced Thrombotic Microangiopathy After Renal Transplant Successfully Treated With Eculizumab: Case Report and Review of the Literature. Transpl Int (2015) 28(9):1121–5. 10.1111/tri.12582
20.
Yamazaki S Takayama T Inoue K Higaki T Makuuchi M . Transplantation-Related Thrombotic Microangiopathy Triggered by Preemptive Therapy for Hepatitis C Virus Infection. Transplantation (2008) 86(7):1010–1. 10.1097/TP.0b013e31818747d8
21.
Baid-Agrawal S Farris AB 3rd Pascual M Mauiyyedi S Farrell ML Tolkoff-Rubin N et al Overlapping Pathways to Transplant Glomerulopathy: Chronic Humoral Rejection, Hepatitis C Infection, and Thrombotic Microangiopathy. Kidney Int (2011) 80(8):879–85. 10.1038/ki.2011.194
22.
Ardalan MR Shoja MM Tubbs RS Jayne D . Parvovirus B19 Microepidemic in Renal Transplant Recipients With Thrombotic Microangiopathy and Allograft Vasculitis. Exp Clin Transpl (2008) 6(2):137–43.
23.
Sethi S . Acute Renal Failure in a Renal Allograft: An Unusual Infectious Cause of Thrombotic Microangiopathy. Am J Kidney Dis (2005) 46(1):159–62. 10.1053/j.ajkd.2004.11.026
24.
Leca N Muczynski KA Jefferson JA de Boer IH Kowalewska J Kendrick EA et al Higher Levels of Leflunomide Are Associated With Hemolysis and Are Not Superior to Lower Levels for BK Virus Clearance in Renal Transplant Patients. Clin J Am Soc Nephrol (2008) 3(3):829–35. 10.2215/CJN.03930907
25.
Dwyre DM Bell AM Siechen K Sethi S Raife TJ . Disseminated Histoplasmosis Presenting as Thrombotic Microangiopathy. Transfusion (2006) 46(7):1221–5. 10.1111/j.1537-2995.2006.00873.x
26.
de la Rubia J Contreras E Del Río-Garma J . Thrombotic Thrombocytopenic Purpura [in Spanish]. Med Clin (Barc) (2011) 136(12):534–40. 10.1016/j.medcli.2010.02.011
27.
Pham PT Danovitch GM Wilkinson AH Gritsch HA Pham PC Eric TM et al Inhibitors of ADAMTS13: A Potential Factor in the Cause of Thrombotic Microangiopathy in a Renal Allograft Recipient. Transplantation (2002) 74(8):1077–80. 10.1097/00007890-200210270-00003
28.
Ulinski T Charpentier A Colombat M Desconclois C Deschênes G Bensman A et al From Humoral Rejection to Generalized Thrombotic Microangiopathy--Role of Acquired ADAMTS13 Deficiency in a Renal Allograft Recipient. Am J Transpl (2006) 6(12):3030–6. 10.1111/j.1600-6143.2006.01574.x
29.
Garg N Rennke HG Pavlakis M Zandi-Nejad K . De Novo Thrombotic Microangiopathy After Kidney Transplantation. Transpl Rev (Orlando) (2018) 32(1):58–68. 10.1016/j.trre.2017.10.001
30.
Shamseer L Moher D Clarke M Ghersi D Liberati A Petticrew M et al Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015: Elaboration and Explanation. BMJ (2015) 350:g7647. 10.1136/bmj.g7647
31.
Higgins JPT Green S . Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. London, United Kingdom: The Cochrane Collaboration (2011).
32.
Yang KL Lu CC Sun Y Cai YT Wang B Shang Y et al How About the Reporting Quality of Case Reports in Nursing Field? World J Clin Cases (2019) 7(21):3505–16. 10.12998/wjcc.v7.i21.3505
33.
Baid S Pascual M Williams WW Jr Tolkoff-Rubin N Johnson SM Collins B et al Renal Thrombotic Microangiopathy Associated With Anticardiolipin Antibodies in Hepatitis C-Positive Renal Allograft Recipients. J Am Soc Nephrol (1999) 10(1):146–53. 10.1681/ASN.V101146
34.
Braet P Sprangers B Dierickx D . Diagnosis of De Novo Thrombotic Microangiopathy After Kidney Transplantation. Acta Clinica Belgica: Int J Clin Lab Med (2016) 71:31.
35.
Caires RA Marques ID Repizo LP Sato VAH Carmo LPF Machado DJB et al De Novo Thrombotic Microangiopathy After Kidney Transplantation: Clinical Features, Treatment, and Long-Term Patient and Graft Survival. Transpl Proc (2012) 44(8):2388–90. 10.1016/j.transproceed.2012.07.039
36.
Dessaix K Bontoux C Aubert O Zuber J Sberro Soussan R Fremeaux Bacchi V et al Thrombotic Microangiopathy After Renal Transplantation: A Clinicopathological Study and Identification of Prognostic Factors. Transpl Int (2019) 32:11.
37.
Saikumar Doradla LP Lal H Kaul A Bhaduaria D Jain M Prasad N et al Clinical Profile and Outcomes of De Novo Posttransplant Thrombotic Microangiopathy. Saudi J Kidney Dis Transpl (2020) 31(1):160–8. 10.4103/1319-2442.279936
38.
Fortin MC Raymond MA Madore F Fugère JA Pâquet M St-Louis G et al Increased Risk of Thrombotic Microangiopathy in Patients Receiving a Cyclosporin-Sirolimus Combination. Am J Transpl (2004) 4(6):946–52. 10.1111/j.1600-6143.2004.00428.x
39.
Franco A Hernandez D Capdevilla L Errasti P Gonzalez M Ruiz JC et al De Novo Hemolytic-Uremic Syndrome/Thrombotic Microangiopathy in Renal Transplant Patients Receiving Calcineurin Inhibitors: Role of Sirolimus. Transpl Proc (2003) 35(5):1764–6. 10.1016/s0041-1345(03)00614-6
40.
Futamura K Norihiko G Fukuhara H Nawano T Kanda A Tomosugi T et al Incidence and Risk Factors of Thrombotic Microangiopathy After Kidney Transplantation. Nephrol Dial Transplant (2020) 35(3):iii1970. 10.1093/ndt/gfaa142.p1654
41.
Gumber M Vanikar A Kute V Shah P Patel H Engineer D et al De Novo Hemolytic Uremic Syndrome/Thrombotic Microangiopathy After Renal Transplantation: A Single Centre Experience. Transplantation (2014) 98:259. 10.1097/00007890-201407151-00790
42.
Kocak B Akyollu B Karatas C AkiNci S Arpali E Yelken B et al Eculizumab for De Novo HUS After Kidney Transplantation. Transpl Int (2015) 28:247.
43.
Langer RM Van Buren CT Katz SM Kahan BD . De Novo Hemolytic Uremic Syndrome After Kidney Transplantation in Patients Treated With Cyclosporine a Sirolimus Combination. Transpl Proc (2001) 33(7-8):3236–7. 10.1016/s0041-1345(01)02376-4
44.
Nava F Cappelli G Mori G Granito M Magnoni G Botta C et al Everolimus, Cyclosporine, and Thrombotic Microangiopathy: Clinical Role and Preventive Tools in Renal Transplantation. Transpl Proc (2014) 46(7):2263–8. 10.1016/j.transproceed.2014.07.062
45.
Oyen O Strøm EH Midtvedt K Bentdal Ø Hartmann A Bergan S et al Calcineurin Inhibitor-Free Immunosuppression in Renal Allograft Recipients With Thrombotic Microangiopathy/Hemolytic Uremic Syndrome. Am J Transpl (2006) 6(2):412–8. 10.1111/j.1600-6143.2005.01184.x
46.
Özdemir BH Atılgan A Akcay EY Özdemir G Soy EA Akdur A et al De Novo Thrombotic Microangiopathy in Renal Transplant Patients. Exp Clin Transpl (2018) 16(1):131–5. 10.6002/ect.TOND-TDTD2017.P27
47.
Santos ES Raez LE Kharfan-Dabaja MA Angulo J Restrepo A Byrnes JJ . Survival of Renal Allograft Following De Novo Hemolytic Uremic Syndrome After Kidney Transplantation. Transpl Proc (2003) 35(4):1370–4. 10.1016/s0041-1345(03)00441-x
48.
Zarifian A Meleg-Smith S O'donovan R Tesi RJ Batuman V . Cyclosporine-Associated Thrombotic Microangiopathy in Renal Allografts. Kidney Int (1999) 55(6):2457–66. 10.1046/j.1523-1755.1999.00492.x
49.
Costa RM Martul EV Reboredo J Rivera C . Transplant Glomerulopathy and De Novo Thrombotic Microangiopathy: Different Manifestations of Humoral Rejection. Nephrol Dial Transplant (2013) 28:i277–i8.
50.
Le Quintrec M Lionet A Kamar N Karras A Barbier S Buchler M et al Complement Mutation-Associated De Novo Thrombotic Microangiopathy Following Kidney Transplantation. Am J Transpl (2008) 8(8):1694–701. 10.1111/j.1600-6143.2008.02297.x
51.
Meehan SM Kremer J Ali FN Curley J Marino S Chang A et al Thrombotic Microangiopathy and Peritubular Capillary C4d Expression in Renal Allograft Biopsies. Clin J Am Soc Nephrol (2011) 6(2):395–403. 10.2215/CJN.05870710
52.
Wu K Budde K Schmidt D Neumayer HH Lehner L Bamoulid J et al The Inferior Impact of Antibody-Mediated Rejection on the Clinical Outcome of Kidney Allografts That Develop De Novo Thrombotic Microangiopathy. Clin Transpl (2016) 30(2):105–17. 10.1111/ctr.12645
53.
Strufaldi FL Menezes Neves PDMM Dias CB Yu L Woronik V Cavalcante LB et al Renal Thrombotic Microangiopathy Associated to Worse Renal Prognosis in Lupus Nephritis. J Nephrol (2021) 34(4):1147–56. 10.1007/s40620-020-00938-3
54.
Mulgaonkar S Kaufman DB . Conversion From Calcineurin Inhibitor-Based Immunosuppression to Mammalian Target of Rapamycin Inhibitors or Belatacept in Renal Transplant Recipients. Clin Transpl (2014) 28(11):1209–24. 10.1111/ctr.12453
55.
Forin MC Raymond MA Madore F Fugère JA Pâquet M St-Louis G et al Increased Risk of Thrombotic Microangiopathy in Patients Receiving a Cyclosporin–Sirolimus Combination. Am J Transpl (2004) 4(6):946–52. 10.1111/j.1600-6143.2004.00428.x
56.
Manzoor K Ahmed E Akhtar F Kazi JI Naqvi SA Rizvi SA . Cyclosporine Withdrawal in Post-Renal Transplant Thrombotic Microangiopathy. Clin Transpl (2006) 20(1):43–7. 10.1111/j.1399-0012.2005.00438.x
57.
Young BA Marsh CL Alpers CE Davis CL . Cyclosporine-Associated Thrombotic Microangiopathy/Hemolytic Uremic Syndrome Following Kidney and Kidney-Pancreas Transplantation. Am J Kidney Dis (1996) 28(4):561–71. 10.1016/s0272-6386(96)90468-0
58.
Chua JS Baelde HJ Zandbergen M Wilhelmus S van Es LA de Fijter JW et al Complement Factor C4d Is a Common Denominator in Thrombotic Microangiopathy. J Am Soc Nephrol (2015) 26(9):2239–47. 10.1681/ASN.2014050429
59.
Karthikeyan V Parasuraman R Shah V Vera E Venkat KK . Outcome of Plasma Exchange Therapy in Thrombotic Microangiopathy After Renal Transplantation. Am J Transpl (2003) 3(10):1289–94. 10.1046/j.1600-6143.2003.00222.x
60.
Portoles J Huerta A Arjona E Gavela E Agüera M Jimenez C et al Characteristics, Management and Outcomes of Atypical Haemolytic Uraemic Syndrome in Kidney Transplant Patients: A Retrospective National Study. Clin Kidney J (2020) 14(4):1173–80. 10.1093/ckj/sfaa096
61.
Elharrif K Lal Y Amin A Alhosainat N Gamilla-Crudo AK Hussain S et al Transplant Kidney Outcomes in Patients With Thrombotic Microangiopathy, Switched to Co-Stimulation Blocking Agent (Belatacept). Am J Kidney Dis (2019) 73(5):693.
62.
Kirsanova T Vinogradova M Kolyvanova A Kravchenko N Fedorova T . Preeclampsia, Hellp-Syndrome and Thrombotic Microangiopathy(tma)-Signs During Pregnancy After Kidney Transplantation. Nephrol Dial Transplant (2018) 33:i294.
63.
Mallett A Hughes P Szer J Tuckfield A Van Eps C Cambell SB et al Atypical Haemolytic Uraemic Syndrome Treated With the Complement Inhibitor Eculizumab: The Experience of the Australian Compassionate Access Cohort. Intern Med J (2015) 45(10):1054–65. 10.1111/imj.12864
64.
Portoles J Huerta A Arjona E Gavela E Aguera M Cavero T et al Clinical Patterns and Outcomes of Thrombotic Microangiopathy Related to Kidney Transplantation: A Nation-Wide Multicentre Study. Transpl Int (2019) 32:14.
65.
Rabant M Dessaix K Aubert O Bontoux C Zuber J Soussan RS et al P204 Thrombotic Microangiopathy After Renal Transplantation: A Clinicopathological Study and Identification of Prognostic Factors. Hum Immunol (2019) 80:215. 10.1016/j.humimm.2019.07.257
Summary
Keywords
thrombotic microangiopathy, kidney allograft, renal function, graft survival rate, graft loss rate
Citation
Hsiung C-Y, Chen H-Y, Wang S-H and Huang C-Y (2024) Unveiling the Incidence and Graft Survival Rate in Kidney Transplant Recipients With De Novo Thrombotic Microangiopathy: A Systematic Review and Meta-Analysis. Transpl Int 37:12168. doi: 10.3389/ti.2024.12168
Received
27 October 2023
Accepted
09 January 2024
Published
23 January 2024
Volume
37 - 2024
Updates
Copyright
© 2024 Hsiung, Chen, Wang and Huang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ching-Ying Huang, gingying0510@gmail.com
†These authors share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.