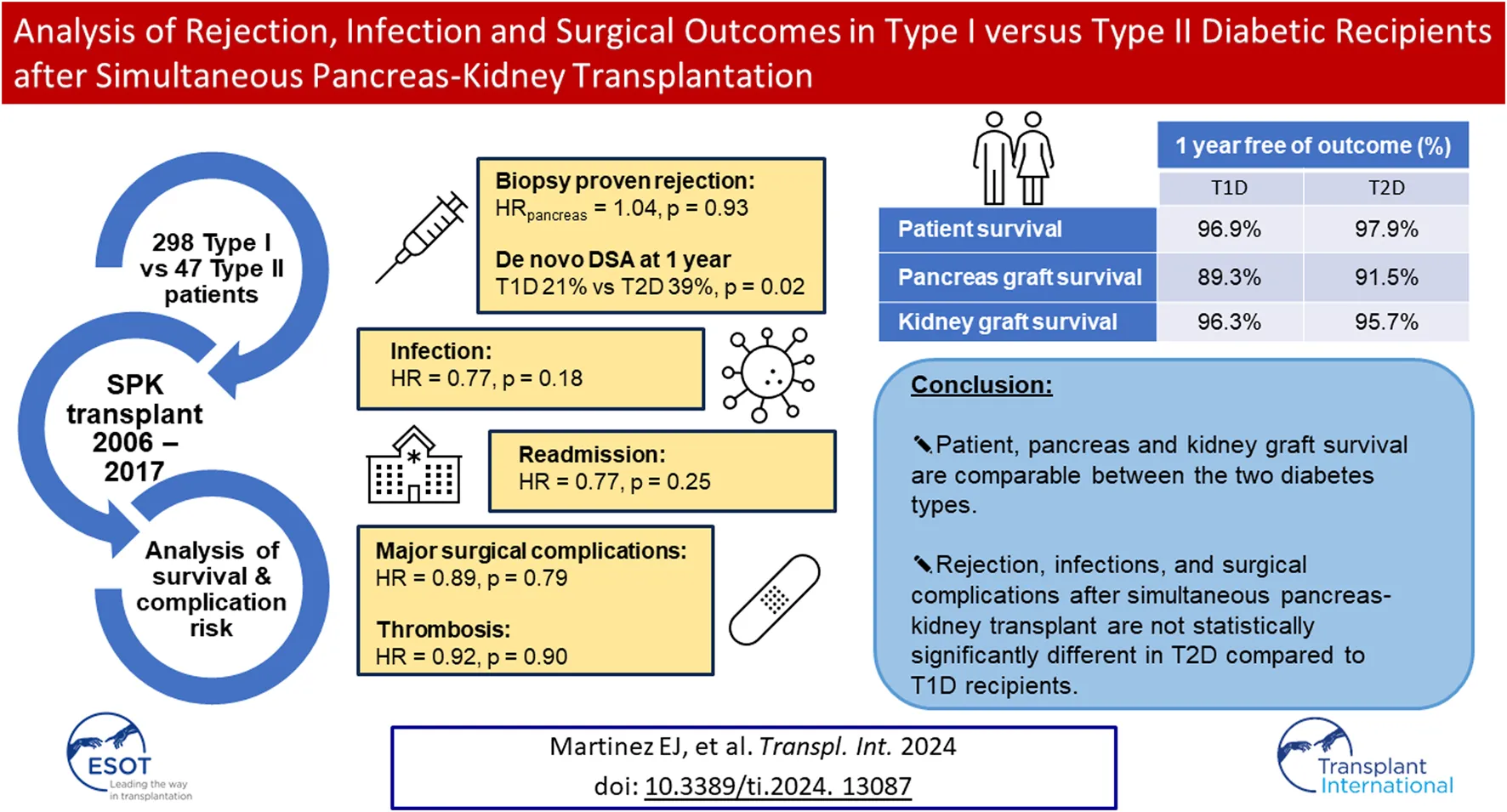
Abstract
Given the increasing frequency of simultaneous pancreas-kidney transplants performed in recipients with Type II diabetes and CKD, we sought to evaluate possible differences in the rates of allograft rejection, infection, and surgical complications in 298 Type I (T1D) versus 47 Type II (T2D) diabetic recipients of simultaneous pancreas-kidney transplants between 2006-2017. There were no significant differences in patient or graft survival. The risk of biopsy-proven rejection of both grafts was not significantly different between T2D and T1D recipients (HRpancreas = 1.04, p = 0.93; HRkidney = 0.96; p = 0.93). Rejection-free survival in both grafts were also not different between the two diabetes types (ppancreas = 0.57; pkidney = 0.41). T2D had a significantly lower incidence of de novo DSA at 1 year (21% vs. 39%, p = 0.02). There was no difference in T2D vs. T1D recipients regarding readmissions (HR = 0.77, p = 0.25), infections (HR = 0.77, p = 0.18), major surgical complications (HR = 0.89, p = 0.79) and thrombosis (HR = 0.92, p = 0.90). In conclusion, rejection, infections, and surgical complications after simultaneous pancreas-kidney transplant are not statistically significantly different in T2D compared to T1D recipients.
Introduction
Simultaneous pancreas-kidney transplantation (SPKT) in Type I diabetes (T1D)with end-stage renal disease (ESRD) has produced significant improvement in prolongation and quality of life. Patient survival approaches 97% and 92% at 1 year and 3 years, respectively [1]. The half-life of pancreas allografts has increased to 15.5 years [2] secondary to advances in immunosuppressive therapy, surgical techniques, and immune monitoring [1, 3–5]. SPKT is also associated with improved kidney graft survival [6, 7] and improved preservation of kidney graft ultrastructure and function [8] compared to deceased donor kidney transplant alone.
Concerning SPKT in Type II diabetes mellitus (T2D) patients with ESRD, many studies have addressed the outcomes of pancreas transplantation for such patients [9]. Such studies have found comparable results between the two types of recipients regarding various endpoints including insulin resistance and β-cell function [3], kidney and pancreas graft survival [9–16], post-transplant glycemic control, BMI control [9, 17], and patient survival [6, 11, 18].
However, the effect of diabetes type on graft rejection after pancreas transplantation is less well understood. Differing rates of allograft rejection are observed in other abdominal solid organ transplants based on the primary etiology of the organ failure, especially with autoimmune components [19–30]. Several studies have evaluated the effects of donor-specific anti-HLA antibodies (DSA) on graft outcomes [31–33] and noted significantly decreased kidney and pancreas allograft survival [33–36]. None of these studies, however, account for the type of diabetes as a distinguishing factor.
In addition, T2D patients may be obese and consequently may have an increased risk of surgical site infections [37, 38] and worse graft outcomes [39]. The inflammatory milieu of T2D may impact the risk of surgical infections, thrombosis, etc., [40–42]. While these theoretical risks may exist, the outcomes of T1D and T2D SPKT recipients with respect to important specific surgical and infection-related outcomes have not been thoroughly evaluated.
Thus, in this study, we sought to comprehensively examine whether the type of diabetes impacts the rates of acute biopsy-proven rejection and DSA development as well as other key surgical and infectious complications. Additionally, we globally analyze factors contributing to these outcomes in the T1D and T2D SPKT populations.
Material and Methods
A single center retrospective review of prospectively collected data from a comprehensive in-house Transplant Database, electronic medical records, and the UNOS/OPTN STAR file was approved by the local Institutional Review Board. Analysis included primary SPKT recipients from 2006–2017 with 1-year minimum post-transplant follow-up. Diabetes mellitus types were determined by a holistic assessment with a grading system that included factors of patients’ age at diabetes onset, need for immediate use of insulin, pre-transplant fasting C-peptide, family history of diabetes, and the presence of autoantibodies (GAD65, Insulin- and Islet-antibodies) [43]. Primary outcomes included patient and graft survival, incidence of biopsy proven pancreas and kidney rejection and dnDSA, readmissions, infections, and surgical complications, including bleeding, pancreatic graft thromboses and other surgical site complications (Figure 1).
FIGURE 1
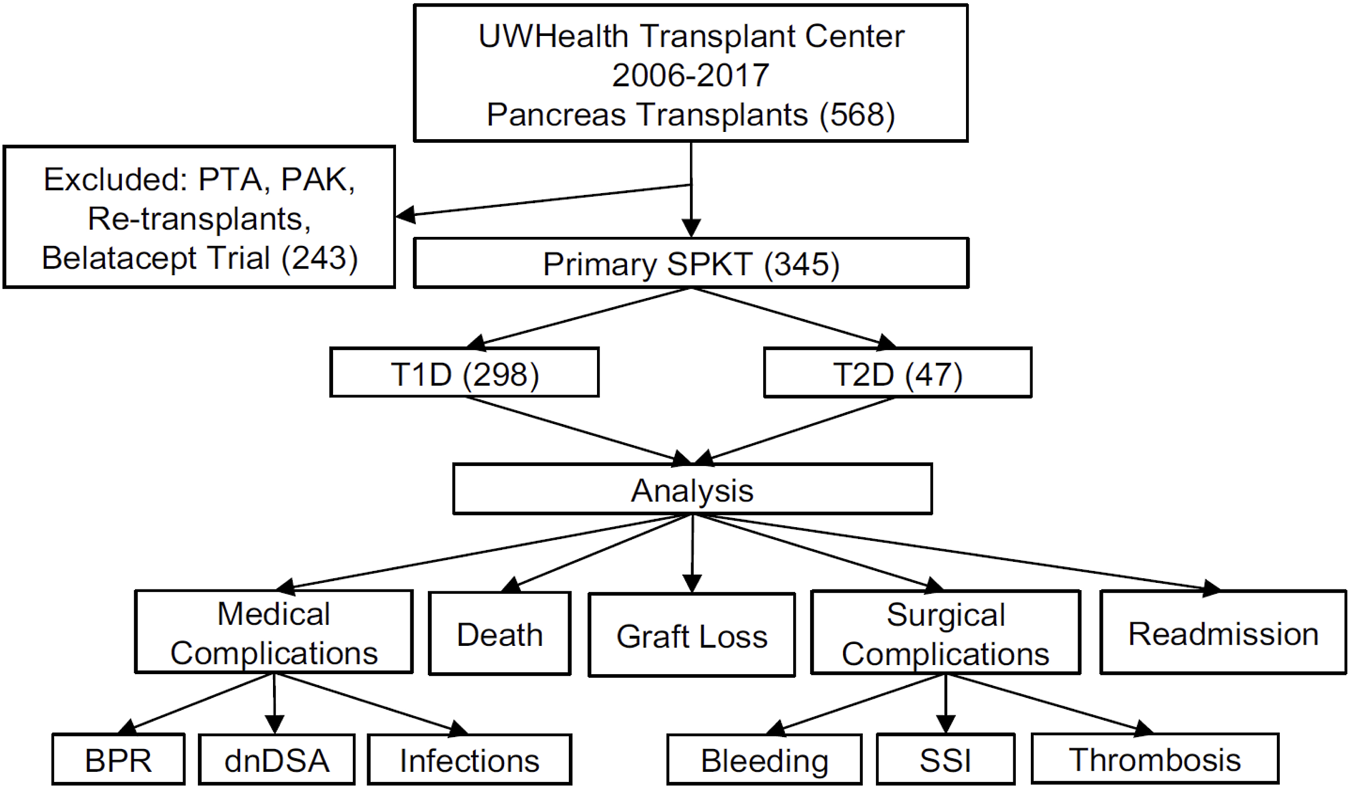
Schematic diagram of study design and data analysis. UWHC, University of Wisconsin Hospital and Clinics; PTA, Pancreas Transplant Alone; PAK, Pancreas After Kidney (transplant); SPKT, Simultaneous Pancreas Kidney Transplant; PTA, Pancreas Transplant Alone; PAK, Pancreas After Kidney (transplant); T1D, Type 1 Diabetes Mellitus; T2D, Type 2 Diabetes Mellitus; BPR, Biopsy Proven Rejection; dnDSA, de novo Donor Specific Antibody; SSI, Surgical Site Infection.
Clinical Management
Systemic venous drainage and enteric exocrine drainage were performed in all SPKTs. Most patients were transferred to the transplant floor post-operatively with aspirin as the sole anticoagulation and without NG tube placement. Each patient’s immunosuppressive therapy was protocolized based on pre-transplant immunologic risk assessment. Either Alemtuzumab (ALEM) (30 mg, 1 dose), anti-thymocyte globulin (ATG) (1.5 mg/kg, 3-4 doses), or basiliximab (BAS) (20 mg, 2 doses) were used for induction therapy. Oral tacrolimus (initial target levels 8–10 ng/mL in the first year and 6–8 ng/mL thereafter) and oral mycophenolic acid (720 mg twice daily) were used as maintenance therapy in all patients. Dexamethasone 100 mg IV was administered intraoperatively and tapered to prednisone thereafter per protocol. Post-induction, selected patients underwent either early steroid withdrawal protocol or a rapid steroid taper to prednisone 5 mg daily by 1 month. All recipients receiving BAS induction received a more delayed steroid taper to prednisone 5–10 mg daily by 6 months. Nystatin and trimethoprim-sulfamethoxazole were given for 3 months and 1 year respectively. CMV prophylaxis with valganciclovir or acyclovir was given for 6 and 3 months depending on the recipients’ risk. Virtual crossmatching has been our standard minimal compatibility testing for the entire study period.
Outcome Definitions
Graft Failure
Per UNOS definitions, pancreas graft failure was defined by graft pancreatectomy, reregistration for pancreas transplant, registration for islet transplantation, use of insulin >0.5 unit/kg/day for 90 consecutive days, or recipient death. Kidney graft failure was defined by graft nephrectomy, return to maintenance dialysis, or recipient death [44].
Graft Rejection
Pancreas allograf biopsy indications included post-transplant elevation of amylase or lipase, DSA increase or dnDSA, and hyperglycemia. Pancreas and kidney biopsies were evaluated by light microscopy with assignment of a grade (indeterminate/borderline, I, II, and III) and degree of immunohistochemical staining for C4D (none, <5%, or >5%) according to the Banff grading schema [45]. Acute rejection outcome represents cellular rejection or antibody mediated rejection or both.
De Novo DSA
Donor-specific anti-HLA Class I and II antibodies were detected pre- and post-transplant using Luminex single antigen beads (One Lambda, Canoga Park, CA). Antibodies were identified using multiple criteria including patterns of epitope reactivity, mean fluorescence intensity (MFI) value, specific bead behaviors, and assay background [46]. Since 2014, routine post-transplant monitoring of DSA has been performed on all transplant recipients at 6 and 12 months, and annually thereafter. Patients with a pretransplant calculated panel reactive antibody greater than zero were tested at an additional 6-week time point, and patients with pre-transplant DSA were tested at additional 3-week, 6-week, and 3-month time points. All patients undergoing kidney or pancreas transplant biopsy for any reason had DSA testing as a part of the biopsy visit [35, 47]. The strength of de novo DSA (dnDSA) was represented as the sum of the MFI of all DSA. Patients were diagnosed with dnDSA if any one of the following occurred: i) no detectable pre-transplant DSA followed by the development of new antibodies post-transplant, ii) the sum MFI increased by at least 2 fold, or iii) new alleles were detected post-transplant.
Infections
Post-transplant infections were categorized as bacterial or opportunistic infections (including virus, fungus, listeria, nocardia, and CMV viremia) and surgical site related. Surgical site infections were defined as any wound or intraabdominal infection within 90 days post-transplantation. Urinary tract infections (UTI) within the first-year post-transplantation were also assessed.
Surgical Complications
Surgical complications were categorized as either bleeding, non-bleeding or thrombotic complications (see Table 2 footnote for specific complications). Pancreatic graft thrombotic events were defined as either partial thrombosis resulting in continued graft function or complete thrombosis requiring transplant pancreatectomy or causing early graft failure within 90 days post-transplantation.
Statistical Analysis
Differences in recipient and donor demographic factors between T1D and T2D recipients were analyzed using t-tests and Chi-square tests or Fisher’s exact tests. Multivariable Cox Proportional Hazards models, or multiple logistic regression, when appropriate, were used to investigate the association of all outcomes with diabetes types, while adjusting for recipient’s BMI, age at time of transplant, PDRI, KDPI, and induction immunosuppression. Death-censored-, rejection-free-, readmission free-, infection-free-, major surgical complication-free-, de novo DSA free-, thrombosis free-survival and thrombosis related to graft failure free-survival were compared between T1D and T2D using Kaplan Meier curves and log-rank tests. Post-transplant outcomes relating to the average number of episodes within the first year were analyzed using t-tests. Analyses were conducted using SAS software (version 9.4, SAS Institute Inc., Cary, NC) and p-values less than 0.05 were considered to be statistically significant.
Results
Study Population
A total of 345 SPKTs were categorized as 298 T1Ds and 47 T2Ds. The average post-transplant follow-up was 6.7 ± 3.6 years. Donor demographic factors were not significantly different between T1D and T2D recipients (Table 1). Several recipient demographic factors, not surprisingly, were significantly different between the cohorts. Besides the expected differences in several recipient factors such as age, BMI, ethnicity and duration of diabetes, T2D patients has lower positivity for GAD65 autoantibody and was more frequently treated with ATG and ALEM induction and early steroid withdrawal compared to T1D patients (p < .001). Lastly, there was no significant difference in the presence of pre-transplant DSA, or degree of pre-transplant DSA between the two groups.
TABLE 1
| T1D (n = 298) | T2D (n = 47) | P-value | |
|---|---|---|---|
| Donor – pre-transplant | |||
| Age, years (mean ± sd) | 29.1 ± 12.6 | 27 ± 12 | 0.28 |
| Males | 176 (59.1%) | 27 (57%) | 0.83 |
| BMI, kg/m2 (mean ± sd) | 24.0 ± 4.4 | 23.7 ± 4.2 | 0.61 |
| Type of transplant (%DBD) | 81.9% | 72.3% | 0.12 |
| PDRI (mean ± sd) | 1.31 ± 0.5 | 1.3 ± 0.4 | 0.59 |
| KDPI (mean ± sd) | 22.7% ± 18.7% | 23.1% ± 15.2% | 0.91 |
| Pancreas cold ischemic time, hours (mean ± sd) | 12.6 ± 4.1 | 12.8 ± 3.8 | 0.82 |
| Kidney cold ischemic time, hours (mean ± sd) | 13.9 ± 4.3 | 14.8 ± 3.8 | 0.23 |
| CMV (% positive) | 49% | 55% | 0.74 |
| EBV (% positive) | 88.4% | 80% | 0.27 |
| Donor HLA Mismatch | 0.67 | ||
| 0 | 2 (0.7%) | 0 (0%) | |
| 1 | 3 (1%) | 0 (0%) | |
| 2 | 12 (4%) | 3 (6.4%) | |
| 3 | 45 (15.1%) | 4 (8.5%) | |
| 4 | 86 (28.9%) | 13 (27.7%) | |
| 5 | 95 (31.9%) | 20 (42.6%) | |
| 6 | 55 (18.5%) | 7 (15%) | |
| Recipient – pre-transplant | |||
| Males (%) | 179 (60.1%) | 40(85.1%) | <.001 |
| Recipient Race | <.001 | ||
| American Indian or Alaska Native (%) | 2 (0.7%) | 2 (4.3%) | |
| Asian (%) | 3 (1.0%) | 4 (8.5%) | |
| Black or African American (%) | 23 (7.7%) | 10 (21%) | |
| White (%) | 270 (90.6%) | 31 (66%) | |
| Age at the time of diabetes mellitus diagnosis, years (mean ± sd) | 13.7 ± 7.6 | 28.3 ± 9.1 | <.001 |
| 25%–75% quartile range | 8.0–18.0 | 21.0–35.0 | |
| Median | 12.0 | 27.0 | |
| Age at the time of transplant, years (mean ± sd) | 42.5 ± 9.1 | 47.9 ± 9.1 | <.001 |
| 25%–75% quartile range | 35.3–49.4 | 39.5–55.4 | |
| Median | 42.3 | 51.8 | |
| Recipient Onset of Diabetes Greater than 30 Years | <.001 | ||
| No (%) | 290 (97.3%) | 25 (53.2%) | |
| Yes (%) | 8 (2.7%) | 22 (46.8%) | |
| BMI, kg/m2 (mean ± sd) | 25.6 ± 3.7 | 27.3 ± 3.4 | 0.004 |
| 25%–75% quartile range | 23.0–27.8 | 24.9–29.6 | |
| Median | 25.2 | 27.4 | |
| C-peptide, ng/mL (mean ± sd) | 0.19 ± 0.39 | 3.67 ± 3.24 | <.001 |
| 25%–75% quartile range | 0.10–0.10 | 1.33–4.90 | |
| Median | 0.10 | 3.20 | |
| HbA1c, % (mean ± sd) | 8.38 ± 1.62 | 7.71 ± 1.46 | 0.01 |
| 25%–75% quartile range | 7.20–9.30 | 6.65–8.80 | |
| Median | 8.30 | 7.70 | |
| Family history of diabetes (% yes) | 55% | 85.1% | <.001 |
| Insulin requirements pre-transplant, unit/day (mean ± sd) | 39.1 ± 16.1 | 44.5 ± 28.4 | 0.21 |
| 25%–75% quartile range | 27.0–50.0 | 20.0–60.0 | |
| Median | 37.0 | 40.5 | |
| CMV (% positive) | 39.4% | 55.3% | 0.04 |
| EBV (% positive) | 94.5% | 97.9% | 0.03 |
| PRA (% mean ± sd) | 7 ± 20.0 | 6.3 ± 16.3 | 0.83 |
| Pre-transplant DSA | 0.12 | ||
| Negative (%) | 283 (95.3%) | 42 (89.4%) | |
| <1000 MFI (%) | 8 (2.69%) | 4 (8.5%) | |
| >1000 MFI (%) | 6 (2.02%) | 1 (2.13%) | |
| NA (%) | 1 (0.003%) | 0 (0%) | |
| Auto antibody status | |||
| Number of tested patients | 42 | 28 | |
| Any auto-antibody (% positive) | 73.8% | 28.6% | <.001 |
| GAD65 (% positive) | 55.9% | 11.1% | <.001 |
| Insulin Ab (% positive) | 63.2% | 17.9% | <.001 |
| Islet IgG (% positive) | 0.0% | 8% | 0.14 |
| Steroid immunosuppression | 0.01 | ||
| Early steroid withdrawal (%) | 6 (2%) | 6 (12.8%) | |
| Induction and maintenance (%) | 266 (89.26%) | 41 (87.23%) | |
| Induction immunosuppression | <.001 | ||
| Anti-thymocyte globulin (ATG) (%) | 46 (15.4%) | 22 (46.8%) | |
| Alemtuzumab (ALEM) (%) | 79 (26.5%) | 10 (21.3%) | |
| Basiliximab (BAS) (%) | 173 (58%) | 15 (31.9%) | |
SPKT donor and recipient demographics.
Patient and Graft Survival
Patient survival (97.9% in T2D vs. 96.9% in T1D at 1 year) and pancreas graft survival (91.5% in T2D vs. 89.3% in T1D at 1 year) were not statistically significantly different between T1D and T2D SPKT recipients (Figures 2A, B; Table 2). Kidney graft survival was also not different between the two types of diabetes recipients (95.7% in T2D vs. 96.3% in T1D at 1 year) (Figure 2D; Table 2).
FIGURE 2
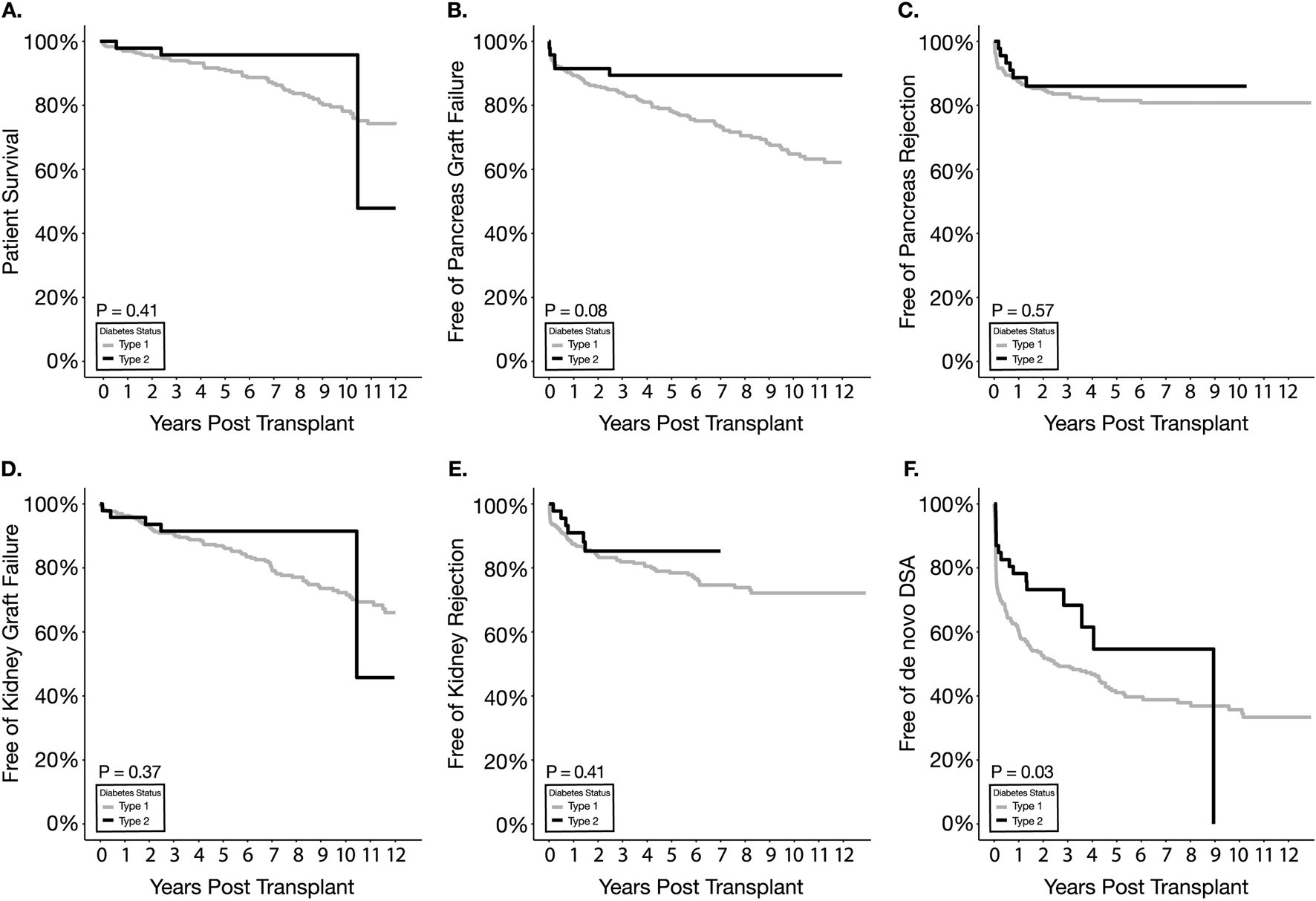
Kaplan Meier survival estimates for patient survival (A), pancreas graft failure (B), pancreas graft rejection (C), kidney graft failure (D), kidney graft rejection (E), and de novo DSA (F).
TABLE 2
| Outcomes | p-value (overall event-free survival) | 1 year free of outcome (%) | |
|---|---|---|---|
| T1D | T2D | ||
| Survival | |||
| Patient survival | 0.41 | 96.9% | 97.9% |
| Pancreas graft survival | 0.08 | 89.3% | 91.5% |
| Kidney graft survival | 0.37 | 96.3% | 95.7% |
| Rejection | |||
| Pancreas Rejection | 0.57 | 87.3% | 89.0% |
| Kidney Rejection | 0.41 | 87.6% | 91.2% |
| Post-transplant complications | |||
| De novo DSA | 0.03 | 60.6% | 78.5% |
| Readmission | 0.07 | 39.2% | 52.7% |
| Infectiona | 0.12 | 27.6% | 33.3% |
| Infection (UTI) | 0.27 | 63.9% | 75.8% |
| Major surgical complicationb | 0.84 | 84.5% | 84.6% |
| Thrombosisc | 0.46 | 90.7% | 93.5% |
Summary of major Rejection, Infection and Surgical Complication Endpoints. Kaplan-Meier Survival Estimates.
Infection, unless otherwise specified, includes both bacterial and opportunistic infections.
Major surgical complication includes both bleeding and non-bleeding complication but exclude thrombosis events. Bleeding complication is defined as any of the following: intraperitoneal (intra-abdominal) bleeding, bleeding from Jackson Pratt drain site, gastrointestinal or enteric anastomotic bleeding, pancreas arterial or venous anastomotic bleeding, renal arterial or venous anastomotic bleeding, and intravesicular hematoma. Non-bleeding complications include: chylous ascites, duodenojejunostomy leak, pancreatic enzyme leak without enteric leak (capsular or retrograde via common bile duct or pancreatic duct), pancreatic pseudocyst, ureteroneocystostomy leak, ureteral stricture, and lymphocele.
Included both partial and complete thrombosis events. Specific diagnoses included partial thrombosis of the pancreatic allograft arterial or venous systems (e.g., portions of iliac Y graft, superior mesenteric artery or vein, splenic artery or vein), or complete occlusive thrombus of the pancreatic arterial or venous systems leading to pancreatectomy and early graft loss.
Pancreas Rejection
Pancreas biopsy-proven rejection (BPR)-free survival and 1-year BPR-free survival were similar between the two types of diabetic recipients (89.0% for T2D and 87.3% for T1D) (Figure 2C; Table 2). Further stratification of rejection endpoints by grade of rejection, C4d positivity, and assessing average episodes per patient (Table 3) also failed to elucidate statistically significantly different rejection outcomes in the T1D vs. T2D recipients. Multivariable analysis (Table 4) showed that diabetes type has little association with overall pancreas BPR or other rejection subcategories. Interestingly, increasing BMI was a significant protective factor against pancreas BPR with and without Indeterminate/borderline pathology included, Grade 1 BPR, and C4d > 5% staining on biopsy (HR = 0.90, 0.89, 0.86, 0.88 respectively, all p < 0.05). Increasing PDRI was not significantly associated with any pancreas rejection endpoints. Meanwhile, increasing KDPI was significantly associated with a higher risk of pancreas BPR with Indeterminate/borderline pathology included (HR = 1.02, p = 0.03) but was not significant when excluding Indeterminate/borderline pathology. Increasing age at transplant was protective against C4d > 5% staining on biopsy (HR = 0.95, p = 0.01). Compared to BAS, both ALEM and ATG showed a trend, though not significant, to being protective toward overall pancreas BPR and BPR subcategories. Univariate analysis by induction type failed to demonstrate significant differences in index outcomes (Table 5).
TABLE 3
| T1D (n = 298) | T2D (n = 47) | P-value | |
|---|---|---|---|
| Pancreas graft rejection (Biopsy proven) | |||
| Number patients with at least 1 rejection episode within 1st year | |||
| BPR without Indeterminate/borderline | 36 (12.6%) | 5 (11%) | 0.71 |
| BPR with Indeterminate/borderline | 39 (13.7%) | 5 (11%) | 0.57 |
| Grade 1 | 22 (7.77%) | 5 (11%) | 0.50 |
| Grade 2 | 11 (3.89%) | 0 (0%) | 0.18 |
| Grade 3 | 6 (2.12%) | 0 (0%) | 0.33 |
| Indeterminate/borderline | 7 (2.48%) | 0 (0%) | 0.29 |
| C4d > 5% on biopsy | 20 (7.07%) | 1 (2.2%) | 0.22 |
| Average episodes per patient within 1st year | |||
| BPR without Indeterminate/borderline (mean ± sd) | 0.13 ± 0.40 | 0.09 ± 0.29 | 0.41 |
| BPR with Indeterminate/borderline (mean ± sd) | 0.16 ± 0.46 | 0.09 ± 0.29 | 0.22 |
| Grade 1 (mean ± sd) | 0.08 ± 0.31 | 0.09 ± 0.29 | 0.84 |
| Grade 2 (mean ± sd) | 0.03 ± 0.18 | 0 ± 0 | 0.002 |
| Grade 3 (mean ± sd) | 0.02 ± 0.17 | 0 ± 0 | 0.03 |
| Indeterminate/borderline (mean ± sd) | 0.02 ± 0.18 | 0 ± 0 | 0.02 |
| C4d > 5% on biopsy (mean ± sd) | 0.07 ± 0.34 | 0 ± 0 | <0.001 |
| Kidney graft rejection (Biopsy proven) | |||
| Number of patients with at least 1 rejection episode at 1 year | 36 (12.4%) | 4 (8.8%) | 0.47 |
| Average episodes per patient within 1st year (mean ± sd) | 0.10 ± 0.30 | 0.07 ± 0.26 | 0.51 |
| De Novo DSA within 1st year(%) | 116 (39.4%) | 10 (21.3%) | 0.02 |
| Readmission | |||
| Number of patients with at least 1 readmission episode at 1 year | 179 (60.7%) | 22 (47%) | 0.11 |
| Average episodes per patient within 1st year (mean ± sd) | 1.11 ± 1.29 | 0.88 ± 1.18 | 0.28 |
| Number of patients at 90 days | |||
| Any readmission | 137 (46.8%) | 19 (41%) | 0.42 |
| Wound-related | 7 (2.43%) | 2 (4.4%) | 0.45 |
| Infection-related | 56 (19.2%) | 10 (22%) | 0.70 |
| Rejection-related | 22 (7.67%) | 1 (2.2%) | 0.17 |
| Other-related | 93 (31.8%) | 15 (32%) | 0.89 |
| Infection (number and % of patient who have at least 1 episode) | |||
| Bacterial infection within the 1st year | 139 (47.1%) | 21 (45%) | 0.64 |
| Opportunistic infection within the 1st year | 141 (47.9%) | 20 (43%) | 0.73 |
| Surgical site infection (within 90 days) | 47 (16.0%) | 6 (13%) | 0.55 |
| Non surgical site infection (within 90 days) | 143 (48.2%) | 18 (39%) | 0.27 |
| UTI within the 1st year | 88 (30.3%) | 11 (24%) | 0.36 |
| Major surgical complication (number and % of patient who have at least 1 episode) | |||
| Any complication within 1st year | 45 (15.5%) | 7 (15%) | 0.96 |
| Non-bleeding fluid collection within 1st year | 41 (14.2%) | 7 (15%) | 0.84 |
| Bleeding complications within 1st year | 7 (2.46%) | 0 (0%) | 0.29 |
| Pancreas graft thrombosis event (number and % of patient who have at least 1 episode) | |||
| Thrombosis (partial and complete) within 90 days | 25 (8.52%) | 3 (6.5%) | 0.62 |
| Graft failures due to thrombosis within 90 days | 9 (3.09%) | 1 (2.2%) | 0.73 |
Univariate analysis for post-transplant outcomes.
TABLE 4
| Outcomes | Type II vs. type I | BMI | PDRI | KDPI | Age at transplant | ALEM vs. BAS | ATG vs. BAS | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) or OR (95% CI) | P-value | HR (95% CI) or OR (95% CI) | P-value | HR (95% CI) or OR (95% CI) | P-value | HR (95% CI) or OR (95% CI) | P-value | HR (95% CI) or OR (95% CI) | P-value | HR (95% CI) or OR (95% CI) | P-value | HR (95% CI) or OR (95% CI) | P-value | |
| Biopsy proven rejection (BPR) of pancreas graft without Indeterminate/borderline | 1.04 (0.42–2.55) | 0.93 | 0.90 (0.83–0.98) | 0.01 | 0.65 (0.25–1.66) | 0.37 | 1.02 (0.99–1.04) | 0.08 | 0.98 (0.95–1.01) | 0.19 | 0.48 (0.22–1.03) | 0.06 | 0.81 (0.38–1.72) | 0.81 |
| BPR with Indeterminate/borderline | 0.88 (0.36–2.14) | 0.77 | 0.89 (0.83–0.96) | 0.003 | 0.57 (0.24–1.39) | 0.22 | 1.02 (1.00–1.05) | 0.03 | 0.98 (0.96–1.01) | 0.30 | 0.55 (0.27–1.11) | 0.09 | 0.89 (0.44–1.83) | 0.76 |
| Grade 1 BPR | 1.40 (0.51–3.85) | 0.52 | 0.86 (0.78–0.95) | 0.003 | 0.89 (0.28–2.78) | 0.84 | 1.01 (0.98–1.04) | 0.48 | 0.98 (0.94–1.01) | 0.21 | 0.69 (0.28–1.71) | 0.42 | 1.28 (0.55–2.96) | 0.56 |
| C4d > 5% on biopsy | 0.46 (0.06–3.52) | 0.45 | 0.88 (0.79–0.99) | 0.03 | 0.74 (0.21–2.65) | 0.65 | 1.01 (0.98–1.04) | 0.46 | 0.95 (0.91–0.99) | 0.01 | 0.69 (0.28–1.74) | 0.44 | 0.40 (0.09–1.71) | 0.21 |
| Kidney graft rejection (Biopsy proven) | 0.96 (0.40–2.30) | 0.93 | 1.02 (0.95–1.10) | 0.54 | 1.11 (0.54–2.24) | 0.78 | 1.02 (0.99–1.04) | 0.07 | 0.97 (0.94–1.00) | 0.05 | 0.72 (0.41–1.27) | 0.26 | 0.40 (0.16–0.97) | 0.04 |
| De Novo DSA | 0.70 (0.41–1.21) | 0.20 | 0.95 (0.91–0.99) | 0.02 | 1.38 (0.86–2.23) | 0.18 | 0.99 (0.98–1.01) | 0.80 | 0.99 (0.98–1.01) | 0.59 | 0.38 (0.26–0.57) | <.001 | 0.63 (0.40–1.00) | 0.05 |
| Readmission | 0.77 (0.50–1.20) | 0.25 | 0.97 (0.93–1.00) | 0.08 | 1.00 (0.68–1.47) | 0.99 | 1.01 (0.99–1.02) | 0.08 | 0.99 (0.98–1.01) | 0.20 | 1.02 (0.76–1.39) | 0.87 | 1.06 (0.74–1.53) | 0.74 |
| Infection (Any) | 0.77 (0.52–1.13) | 0.18 | 0.97 (0.94–1.00) | 0.08 | 0.97 (0.67–1.40) | 0.86 | 1.01 (0.99–1.02) | 0.11 | 0.99 (0.98–1.01) | 0.58 | 1.21 (0.92–1.61) | 0.17 | 1.28 (0.93–1.77) | 0.13 |
| Surgical site infectiona | 0.74 (0.30–1.84) | 0.52 | 1.02 (0.94–1.10) | 0.62 | 1.14 (0.52–2.52) | 0.73 | 1.01 (0.98–1.03) | 0.59 | 0.98 (0.95–1.02) | 0.31 | 1.44 (0.76–2.77) | 0.27 | 1.54 (0.74–3.23) | 0.24 |
| Non-surgical site infectiona | 0.87 (0.51–1.46) | 0.60 | 0.96 (0.92–1.01) | 0.09 | 1.03 (0.63–1.67) | 0.91 | 1.01 (0.99–71.02) | 0.28 | 0.99 (0.97–1.01) | 0.32 | 1.49 (1.03–2.14) | 0.03 | 1.08 (0.69–1.69) | 0.73 |
| UTI | 0.91 (0.51–1.62) | 0.74 | 0.95 (0.91–0.99) | 0.04 | 0.98 (0.57–1.67) | 0.94 | 1.01 (0.99–1.03) | 0.10 | 0.97 (0.95–0.99) | 0.05 | 0.76 (0.49–1.16) | 0.20 | 1.06 (0.66–1.71) | 0.79 |
| Major surgical complication | 0.89 (0.38–2.10) | 0.79 | 1.01 (0.94–1.08) | 0.80 | 1.93 (0.99–3.77) | 0.05 | 0.99 (0.97–1.02) | 0.85 | 1.00 (0.97–1.03) | 0.93 | 0.83 (0.43–1.60) | 0.57 | 1.09 (0.54–2.22) | 0.80 |
| Thrombosis | 0.92 (0.26–3.22) | 0.90 | 0.88 (0.79–0.98) | 0.02 | 2.13 (0.86–5.28) | 0.10 | 1.00 (0.97–1.03) | 0.96 | 1.02 (0.98–1.06) | 0.41 | 1.22 (0.54–2.76) | 0.64 | 0.47 (0.13–1.62) | 0.23 |
Multivariable analysis for post-transplant outcomes.
Logistic Regression was used instead of Cox Hazard Model.
TABLE 5
| T1D (fail/Total) (%) | T2D (fail/Total) (%) | P-value | |
|---|---|---|---|
| Induction | |||
| Anti-Thymoglobulin | |||
| Outcomes within 1 year post-transplant | |||
| Pancreas - BPR without Indeterminate/borderline | 2/46 (4.4%) | 3/22 (14%) | 0.20 |
| Pancreas - BPR with Indeterminate/borderline | 3/46 (7.5%) | 3/22 (14%) | 0.38 |
| Death-censored pancreas graft failure | 4/46 (8.7%) | 1/22 (4.5%) | 0.55 |
| Kidney rejection | 1/46 (2.2%) | 1/22 (4.6%) | 0.58 |
| Death-censored kidney graft failure | 1/46 (2.2%) | 1/22 (4.6%) | 0.58 |
| Basiliximab | |||
| Outcomes within 1 year post-transplant | |||
| Pancreas - BPR without Indeterminate/borderline | 29/173 (16.7%) | 0/15 (0.0%) | 0.10 |
| Pancreas - BPR with Indeterminate/borderline | 31/173 (17.9%) | 0/15 (0.0%) | 0.09 |
| Death-censored pancreas graft failure | 13/173 (7.51%) | 1/15 (6.7%) | 0.92 |
| Kidney rejection | 26/173 (15.1%) | 1/15 (6.7%) | 0.39 |
| Death-censored kidney graft failure | 5/173 (2.89%) | 0/15 (0.0%) | 0.51 |
| Alemtuzumab | |||
| Outcomes within 1 year post-transplant | |||
| Pancreas - BPR without Indeterminate/borderline | 5/79 (6.4%) | 2/10 (20%) | 0.14 |
| Pancreas - BPR with Indeterminate/borderline | 5/79 (6.4%) | 2/10 (20%) | 0.14 |
| Death-censored pancreas graft failure | 10/79 (13%) | 2/10 (20%) | 0.55 |
| Kidney rejection | 9/79 (11%) | 2/10 (20%) | 0.40 |
| Death-censored kidney graft failure | 0/79 (0%) | 1/10 (10%) | 0.005 |
Univariate analysis for post-transplant outcomes.
Kidney Rejection
Overall kidney rejection-free survival between T2D and T1D was not significantly different (p = 0.41) (Figure 2E; Table 2). In univariate analysis, the rate of kidney BPR within the first year was 8.8% in T2D recipients vs. 12.4% in T1D recipients (p = 0.47) (Table 3). The lack of association between diabetes type and kidney graft rejection was confirmed in multivariable analysis (Table 4). Unlike for pancreas graft rejection, neither BMI nor KDPI were significantly associated with an increased risk of kidney rejection. Older age at transplant was marginally protective against rejection (HR = 0.97, p = 0.05) (Table 4). Compared to BAS, ATG was significantly associated with decreased kidney BPR (HR = 0.40, p = 0.04).
De Novo DSA
Overall dnDSA-free survival between T2D and T1D was significantly different (p = 0.03) (Figure 2F; Table 2). A significantly lower incidence of dnDSA was observed in T2D (21%) compared to T1D (39%) within the first year (p = 0.02) (Table 3). Multivariable analysis, however, showed that type of diabetes has no association with developing de novo DSA while suggesting that increasing BMI was protective against such an outcome (HR = 0.95, p = 0.02) (Table 4). Regarding peri-operative induction agent use, compared to BAS, ALEM was significantly associated with decreased development of dnDSA (HR = 0.38, p < .001).
Readmission
Kaplan-Meier analysis of freedom from readmission showed no difference between the two types of diabetes (p = 0.07) (Figure 3A; Table 2). The percentage of readmissions within the first-year post-transplantation was not significantly different when comparing T2D with T1D recipients (47% vs. 60.7%, p = 0.11) though there was a trend to fewer readmissions in T2D recipients (Table 3). Positive trends in favor of T2D were also identified in the average number of readmission episodes per patient within the first year as well as overall readmissions within the first 90 days, though the results did not reach statistical significance. In the multivariable analysis (Table 4), neither type of diabetes nor other factors were associated with overall readmission risk.
FIGURE 3
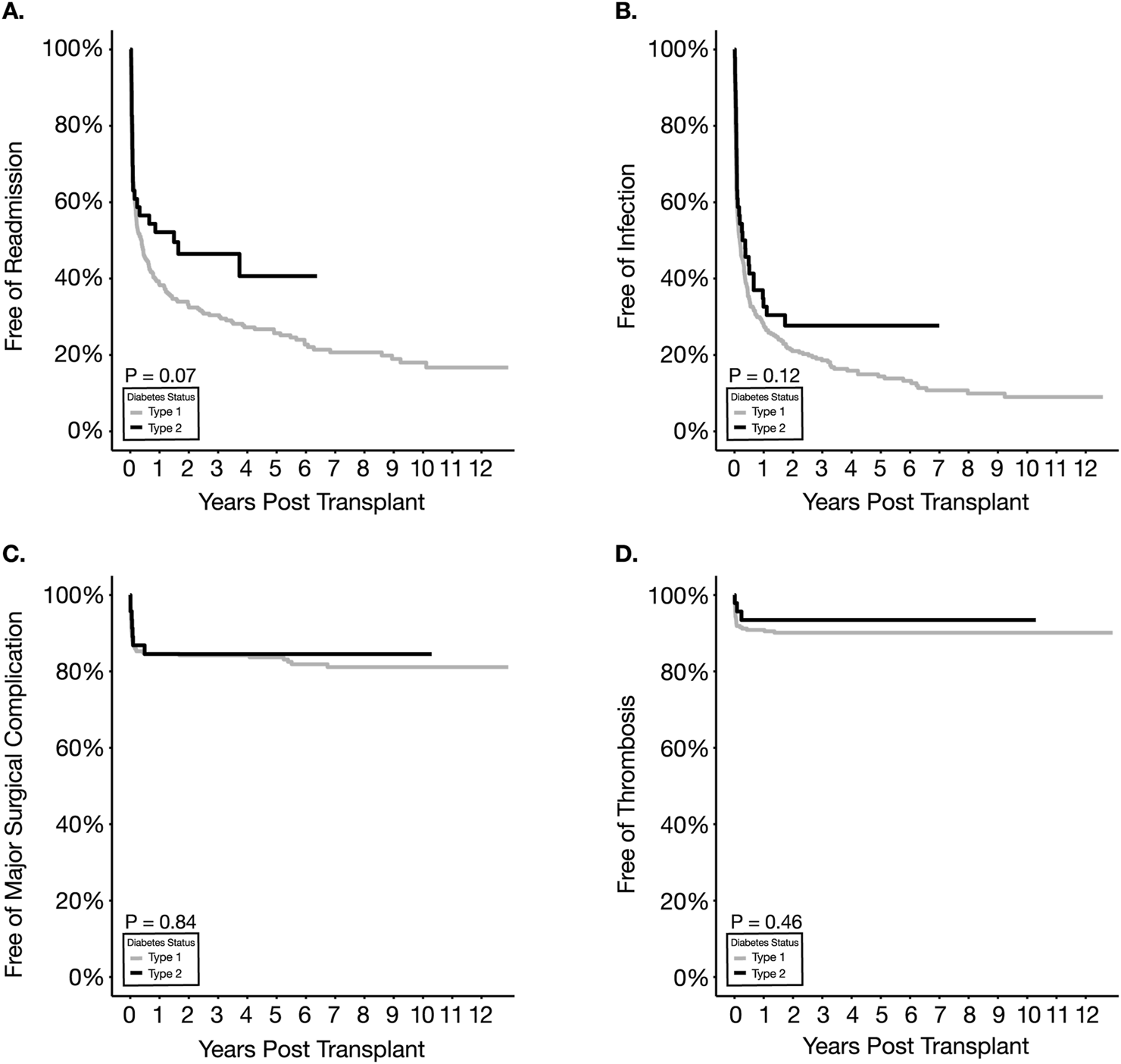
Kaplan Meier survival estimates for readmission (A), infection (B), major surgical complications (C) and thrombosis (D).
Post-Transplant Infections
No statistical difference was observed between T2D and T1D recipients with respect to overall infection-free survival (p = 0.12) and UTI-free survival (p = 0.27) (Figure 3B; Table 2). There was no significant difference between the two types of diabetic recipients regarding the sub-categories of infection (Table 3). Multivariable analysis also supported the similarity between the two types in overall infection, UTI, surgical- and non-surgical site infection (Table 4). Increasing BMI was significantly associated with decreased risk of UTI (HR = 0.95, p = 0.04), whereas using ALEM was significantly associated with an increased risk of non-surgical site infection (HR = 1.49, p = 0.03).
Major Surgical Complications
Overall surgical complication-free survival was not significantly different between the two groups (Figure 3C; Table 2). A significant difference was not observed in the frequency or distribution of major surgical complications or subtypes (i.e., bleeding and non-bleeding) within the first -year post-transplantation between T1D and T2D recipients (Table 3). Multivariable analysis also showed that none of the variables tested, including diabetes types, were significantly associated with an increased risk of major surgical complication (Table 4).
Thrombosis Events
No difference in thrombosis-free survival was detected between T1D and T2D recipients with 1-year survivals of 90.3% and 94.8% in T1D and T2D respectively (Figure 3D; Table 2). Within the first 90 days post-SPKT, partial pancreatic thrombotic events and pancreas graft failures secondary to thrombosis were also not different between T1D and T2D in both univariate and multivariable analyses (Table 3, 4). Interestingly, on multivariable analysis, increasing BMI was significantly associated with a lower risk of thrombosis (HR = 0.88, p = 0.02).
Discussion
Whereas the majority of studies focus on patient and graft survival outcomes between T1D and T2D recipients, few address key infectious, surgical, and immunological outcomes. The current study addresses this gap and demonstrates that similar post-transplant outcomes, such as the incidence of acute BPR, readmissions, infections, UTIs, thrombosis, and other major surgical complications can be achieved between T1D and T2D SPKT recipients. Also, consistent with findings from previous studies demonstrating improvement in patient survival with advancing eras [9, 10, 43], the present study demonstrates acceptable and comparable patient-, pancreas allograft- and kidney allograft-survival in T2D versus T1D SPKT recipients.
Organ transplant recipients whose primary etiology of organ failure is autoimmune in nature may have higher rates of rejection and recurrence, especially in kidney transplantation [19–23] and liver transplantation [24–30]. However, a UNOS registry review did not find a significant association of rejection between T2D and T1D when combining kidney and pancreas rejection outcomes [10]. This study has the caveat however that kidney and pancreas rejection were not analyzed separately, and the majority of centers did not perform routine pancreas allograft biopsies in SPKT recipients, thereby potentially leading to underreporting of pancreas rejection. Thus, we posited that T1D SPKT recipients may experience higher rates of pancreas rejection than T2D SPKT recipients given the autoimmune nature of diabetes in the former. However, we did not observe a significantly different 1-year pancreas, or kidney, BPR rate in T2D vs. T1D patients. The overall rates of rejection in our population are consistent with those previously reported in the literature (4%–38%) [48–53]. The current study provides greater granularity particular to rejection type and severity compared to prior studies [6, 9, 10, 14, 15, 52]. These prior studies, additionally, did not meticulously categorize T1D and T2D recipients [14, 15, 54], assess pancreas BPR separately from kidney BPR [10, 14, 15, 55, 56] or specifically look at pancreas BPR [6, 9, 52, 57]. Thus, the current study adds a more comprehensive assessment of the rejection risk confronting T2D SPKT recipients. It also suggests a very low overall incidence of pancreas antibody-mediated rejection (ABMR) based on the ∼6% overall incidence of C4d>5% staining on biopsies in both T1D and T2D recipients, which is consistent with previously reported data [50].
While not definitive, our data does suggest a possible signal with regard to more rejection in T1D recipients. For example, we observed a greater number of episodes of Grade 2-3 ACR, indeterminate ACR, and C4d+ rejection, and numerically more patients with these rejection diagnoses within the first year in T1D patients. Moreover, we observed a higher incidence of dnDSA in T1D patients compared to T2D patients. Thus, this type of diabetes may be associated with an increased risk of pancreas rejection endpoints. Though we observed a higher rate of pancreas rejection signals by univariate analysis, we failed to detect a significant difference in multivariable analyses. Given the lack of major differences between T1D and T2D SPKT recipients, the current study suggests that a primary autoimmune pathology does not pose a substantially increased risk of BPR, nor does it suggest T2D confers higher rates of pancreas or kidney BPR. Thus, the type of diabetes thus should not affect candidacy for SPKT from the rejection perspective.
Similar patient and graft survival outcomes have been described with both T-cell depleting and non-depleting agents for SPKT [53, 58–60]. Overall, lower rates of early acute rejection have been described in SPKT with T-cell-depleting agents versus non-depleting agents [53]. Comparing types of T-cell-depleting therapies, ALEM versus ATG has been associated with comparable surgical complications, readmissions, thromboses, and bleeding [61]. These studies however involve very few T2D recipients. The results of our study are congruent with these findings and indicate that, compared to BAS, ALEM induction might be beneficial for pancreas graft rejection and was associated with lower risk of dnDSA development, while ATG induction was associated with reduced kidney graft rejection. We also did not find an association between ALEM and kidney graft rejection, consistent with Sampaio et al [10]. Induction trends in our cohort are also consistent with those reported in T2D recipients represented in registry data, with an increasing trend toward use of T-cell-depleting antibodies in more recent eras [9]. Larger cohorts of T2D SPKT recipients are needed to make definitive conclusions regarding any differences in the rejection rate between induction regimens based on diabetes type.
The development of dnDSA after pancreas and SPK transplantation has been identified as a significant risk factor for pancreas and kidney rejection, and for graft failure [33–35]. We demonstrated a significantly lower incidence of dnDSA within the first year in T2D versus T1D SPKT recipients. This result may be explained by differences in induction immunosuppression mentioned earlier (i.e., more BAS induction in T1D vs. T2D recipients), and therefore should not necessarily be construed as definitively indicating T2D SPKT recipients would require less intensive immunosuppression or less vigorous postoperative-immune monitoring, though these benefits remain a possibility.
Previous analysis of SPKT registry data from over a decade ago [10] and more recent UK registry data [55] has suggested that the type of diabetes did not significantly impact the rate of surgical complications including abscess formation, anastomotic leak, pancreatitis, and primary non-function. Obesity, frequently associated with T2D, on the other hand, has been associated with increased risk of postoperative infections, a need for postoperative invasive procedures [62, 63], increased risk of patient death, pancreas graft loss, and kidney graft loss [39]. Our findings demonstrate no difference in risks of major surgical complications (bleeding and non-bleeding), surgical site infections, incidental image-identified pancreatic graft thrombotic lesions, and pancreatic graft losses secondary to thrombosis in T2D vs. T1D recipients. In the absence of significantly worse infectious and surgical complications and similar rejection rates between T2D and T1D SPKT recipients, it seems very reasonable to continue to offer selected IDDM/CKD patients an SPKT regardless of their diabetes labels. Prospective trials would also be valuable to definitively compare efficacy and safety outcome endpoints, but await a significant multi-center effort to accrue a sufficient number of patients. In the meantime, we recommend a careful and systematic center-specific approach to offering SPKT to T2D/CKD patients.
Given the rising rates of T2D-associated CKD and obesity, safe criteria for SPKT in the T2D/CKD population should be established [64, 65]. Though we found some marginal protective effect associated with older age with regard to pancreas and kidney rejection, elderly patients tend to preform poorly due to having more comorbidities. UNOS/OPTN policy still requires patients to be insulin-dependent, though weight or BMI restrictions were recently eliminated [44]. Consequently, the indication for SPKT for T2D and CKD at most centers in the US is quite narrow and the majority of T2D/CKD patients presenting to centers are not considered candidates for SPKT but are generally offered a kidney transplant alone. Morbidly obese patients with CKD who do not require insulin most likely have residual beta cell mass, and their diabetes could be reversed by bariatric surgery [66–70]. However, if they have undetectable or minimal C-peptide, their diabetes is unlikely reversed by bariatric surgery alone. CKD patients whose diabetes is controlled by non-insulin oral or injectable agents, diet or exercise are not eligible for pancreas transplantation currently in the US based on allocation policy. However, it is well understood by the transplant community that once they receive a kidney transplant and the requisite immunosuppression, the patient’s diabetes will worsen and ultimately require long-term insulin for control. In this situation, they may benefit from a pancreas-after-kidney transplant, but would it be reasonable to offer a “preemptive” SPKT to this population, preempting their requirement for insulin, just as we offer kidneys preemptively in patients with CKD prior to dialysis? Understanding the relative mortality risk of T2D/CKD waiting list patients who are controlled without insulin to those who are on insulin may support future policy decisions.
We recognize potential limitations to the broader applicability of the results presented here given the non-randomized, single-center, and retrospective nature of our study. Despite using an objective multiparametric approach to classify diabetes type, mis-categorization is possible as not all patients fit neatly into the classically defined T1D and T2D categories, though we believe that this approach is more holistic and objective. We also acknowledge that dnDSA and rejection may still develop after our 1 year minimum follow-up period. Therefore, to ensure valid conclusions can be made, we limited our incidence analysis of immunological, surgical, and infectious complications to the first year or less so that every patient had an equal chance to realize these complications. Consequently, we cannot describe medium or longer-term outcomes relative to these complications. Lastly, our T2D population is relatively small compared to registry data, albeit one of the larger single-center experiences presented to date. However, we feel that the granularity of our data, the recent cohort, and the greater homogeneity of candidate selection, surgical technique, immunosuppression, and post-operative practices at a single center than exists in registry data are benefits to teasing out differences between these populations and to provide updated information. Nonetheless, we believe these data provide useful guidance by comprehensively examining immunological, infectious, and surgical complications after SPKT in T2D recipients.
In conclusion, with the increasing prevalence of T2D related ESRD and an increasing trend of SPKT performed in T2D(9) this study found similar outcomes regarding rejection, major surgical complications, infections, and readmissions between SPKT T1D and T2D recipients. It further demonstrates the success that SPKT can achieve in carefully selected T2D recipients, and provides valuable reassurance to the transplant community for continued careful protocolized application of SPKT to low cardiovascular risk T2D/CKD patients.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the UW School of Medicine and Public Health IRB. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because some patients have lost their grafts or died, if informed consent would be mandated then this would bias this study; therefore, consent was waived by our IRB. This is a retrospective study and because this is a retrospective study and for the above reasons our IRB consistently allows studies of this nature to have waived consent.
Author contributions
All authors participated in the design of the study, interpretation of the results and review of the manuscript. EM, PP, JW, and BW collected the data; EM, PP, LS, GL, and NM analyzed the data; EM, PP, and JO wrote the manuscript; TA-Q, DM, SP, HS, DK, RR, and JO reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number T35DK062709. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Conflict of interest
The authors of this manuscript have conflicts of interest to disclose as described by the Transplant International. JO is co-founder of, has equity interest in and serves as Chair of the Scientific Advisory Board of Regenerative Medical Solutions, Inc. He receives clinical trial support from Veloxis Pharmaceuticals, CareDx Transplant Management, Inc., Natera, Inc. and Vertex Pharmaceuticals, Inc. DK reports serving as a scientific advisor for, or member of, eGenesis, and receiving research funding from Medeor Pharma and the National Institutes of Health.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
ALEM, Alemtuzumab; ATG, Antithymocyte globulin; BAS, Basiliximab; BMI, body mass index; BPR, biopsy proven rejection; CIT, cold ischemia time; CMV, cytomegalovirus; dnDSA, de novo anti-HLA donor specific antibody; DCD, donation after circulatory death; ESRD, end stage renal disease; KDPI, kidney donor profile index; OPTN/UNOS, Organ Procurement and Transplant Network/United Network for Organ Sharing; PDRI, pancreas donor risk index; SPKT, simultaneous pancreas kidney transplant; STAR, Standard Transplant Analysis and Research; T1D, Type I diabetes mellitus; T2D, Type II diabetes mellitus; UW, University of Wisconsin.
References
1.
Redfield RR Rickels MR Naji A Odorico JS . Pancreas Transplantation in the Modern Era. Gastroenterol Clin North America (2016) 45:145–66. 10.1016/j.gtc.2015.10.008
2.
Kandaswamy R Stock PG Gustafson SK Skeans MA Urban R Fox A et al OPTN/SRTR 2018 Annual Data Report: Pancreas. Am J Transplant (2020) 20(s1):131–92. 10.1111/ajt.15673
3.
Shin S Jung CH Choi JY Kwon HW Jung JH Kim YH et al Long-Term Metabolic Outcomes of Functioning Pancreas Transplants in Type 2 Diabetic Recipients. Transplantation (2017) 101(6):1254–60. 10.1097/TP.0000000000001269
4.
Rodríguez LM Knight RJ Heptulla RA . Continuous Glucose Monitoring in Subjects After Simultaneous Pancreas-Kidney and Kidney-Alone Transplantation. Diabetes Technol Ther (2010) 12(5):347–51. 10.1089/dia.2009.0157
5.
Gruessner AC Gruessner RWG . Pancreas Transplantation of US and Non-US Cases From 2005 to 2014 as Reported to the United Network for Organ Sharing (UNOS) and the International Pancreas Transplant Registry (IPTR). Rev Diabetic Stud (2016) 13(1):35–58. 10.1900/RDS.2016.13.35
6.
Margreiter C Resch T Oberhuber R Aigner F Maier H Sucher R et al Combined Pancreas-Kidney Transplantation for Patients With End-Stage Nephropathy Caused by Type-2 Diabetes Mellitus. Transplantation (2013) 95(8):1030–6. 10.1097/TP.0b013e3182861945
7.
Sung RS Zhang M Schaubel DE Shu X Magee JC . A Reassessment of the Survival Advantage of Simultaneous Kidney-Pancreas Versus Kidney-Alone Transplantation. Transplantation (2015) 99(9):1900–6. 10.1097/TP.0000000000000663
8.
Lindahl JP Reinholt FP Eide IA Hartmann A Midtvedt K Holdaas H et al In Patients With Type 1 Diabetes Simultaneous Pancreas and Kidney Transplantation Preserves Long-Term Kidney Graft Ultrastructure and Function Better Than Transplantation of Kidney Alone. Diabetologia (2014) 57(11):2357–65. 10.1007/s00125-014-3353-2
9.
Gruessner AC Laftavi MR Pankewycz O Gruessner RWG . Simultaneous Pancreas and Kidney Transplantation—Is It a Treatment Option for Patients With Type 2 Diabetes Mellitus? An Analysis of the International Pancreas Transplant Registry. Curr Diab Rep (2017) 17(6):44. 10.1007/s11892-017-0864-5
10.
Sampaio MS Kuo HT Bunnapradist S . Outcomes of Simultaneous Pancreas-Kidney Transplantation in Type 2 Diabetic Recipients. Clin J Am Soc Nephrol (2011) 6(5):1198–206. 10.2215/CJN.06860810
11.
Hau HM Jahn N Brunotte M Lederer AA Sucher E Rasche FM et al Short and Long-Term Metabolic Outcomes in Patients With Type 1 and Type 2 Diabetes Receiving a Simultaneous Pancreas Kidney Allograft. BMC Endocr Disord (2020) 20(1):30. 10.1186/s12902-020-0506-9
12.
Lo DJ Sayed BA Turgeon NA . Pancreas Transplantation in Unconventional Recipients. Curr Opin Organ Transpl (2016) 21(4):393–8. 10.1097/MOT.0000000000000334
13.
Wiseman AC Gralla J . Simultaneous Pancreas Kidney Transplant Versus Other Kidney Transplant Options in Patients With Type 2 Diabetes. Clin J Am Soc Nephrol (2012) 7(4):656–64. 10.2215/CJN.08310811
14.
Stratta RJ Rogers J Farney AC Orlando G El-Hennawy H Gautreaux MD et al Pancreas Transplantation in C-Peptide Positive Patients: Does “Type” of Diabetes Really Matter? J Am Coll Surg (2015) 220(4):716–27. 10.1016/j.jamcollsurg.2014.12.020
15.
Singh RP Rogers J Farney AC Hartmann EL Reeves-Daniel A Doares W et al Do Pretransplant C-Peptide Levels Influence Outcomes in Simultaneous Kidney-Pancreas Transplantation? Transpl Proc (2008) 40(2):510–2. 10.1016/j.transproceed.2008.01.048
16.
Light J Tucker M . Simultaneous Pancreas Kidney Transplants in Diabetic Patients with End-Stage Renal Disease: The 20-yr Experience. Clin Transpl (2013) 27(3):256–63. 10.1111/ctr.12100
17.
Andacoglu OM Himmler A Geng X Ahn J Ghasemian S Cooper M et al Comparison of Glycemic Control After Pancreas Transplantation for Type 1 and Type 2 Diabetic Recipients at a High Volume Center. Clin Transpl (2019) 33(8):e13656. 10.1111/ctr.13656
18.
Alhamad T Kunjal R Wellen J Brennan DC Wiseman A Ruano K et al Three-month Pancreas Graft Function Significantly Influences Survival Following Simultaneous Pancreas-Kidney Transplantation in Type 2 Diabetes Patients. Am J Transplant (2019) 20(3):788–96. 10.1111/ajt.15615
19.
Singh T Astor B Zhong W Mandelbrot D Djamali A Panzer S . Kidney Transplant Recipients With Primary Membranous Glomerulonephritis Have a Higher Risk of Acute Rejection Compared With Other Primary Glomerulonephritides. Transpl Direct (2017) 3(11):e223. 10.1097/TXD.0000000000000736
20.
Hariharan S Adams MB Brennan DC Davis CL First MR Johnson CP et al Recurrent and De Novo Glomerular Disease After Renal Transplantation: A Report from Renal Allograft Disease Registry (RADR). Transplantation (1999) 68(5):635–41. 10.1097/00007890-199909150-00007
21.
Dabade TS Grande JP Norby SM Fervenza FC Cosio FG . Recurrent Idiopathic Membranous Nephropathy After Kidney Transplantation: A Surveillance Biopsy Study. Am J Transplant (2008) 8(6):1318–22. 10.1111/j.1600-6143.2008.02237.x
22.
Cosio FG Cattran DC . Recent Advances in Our Understanding of Recurrent Primary Glomerulonephritis After Kidney Transplantation. Kidney Int (2017) 91(2):304–14. 10.1016/j.kint.2016.08.030
23.
Pruthi R McClure M Casula A Roderick PJ Fogarty D Harber M et al Long-term Graft Outcomes and Patient Survival Are Lower Posttransplant in Patients With a Primary Renal Diagnosis of Glomerulonephritis. Kidney Int (2016) 89(4):918–26. 10.1016/j.kint.2015.11.022
24.
Graziadei IW Wiesner RH Batts KP Marotta PJ Larusso NF Porayko MK et al Recurrence of Primary Sclerosing Cholangitis Following Liver Transplantation. Hepatology (1999) 29(4):1050–6. 10.1002/hep.510290427
25.
Neuberger J . Incidence, Timing, and Risk Factors for Acute and Chronic Rejection. Liver Transplant Surg (1999) 5(4 Suppl. 1):S30–6. 10.1053/JTLS005s00030
26.
Seiler CA Dufour JF Renner EL Schilling M Büchler MW Bischoff P et al Primary Liver Disease as a Determinant for Acute Rejection After Liver Transplantation. Langenbecks Arch Surg (1999) 384(3):259–63. 10.1007/s004230050201
27.
Farges O Saliba F Farhamant H Samuel D Bismuth A Reynes M et al Incidence of Rejection and Infection After Liver Transplantation as a Function of the Primary Disease: Possible Influence of Alcohol and Polyclonal Immunoglobulins. Hepatology (1996) 23(2):240–8. 10.1053/jhep.1996.v23.pm0008591847
28.
Narumi S Roberts JP Emond JC Lake J Ascher NL . Liver Transplantation for Sclerosing Cholangitis. Hepatology (1995) 22(2):451–7. 10.1002/hep.1840220213
29.
Jeyarajah DR Netto GJ Lee SP Testa G Abbasoglu O Husberg BS et al Recurrent Primary Sclerosing Cholangitis After Orthotopic Liver Transplantation: Is Chronic Rejection Part of the Disease Process? Transplantation (1998) 66(10):1300–6. 10.1097/00007890-199811270-00006
30.
Graziadei IW Wiesner RH Marotta PJ Porayko MK Eileen Hay J Charlton MR et al Long-term Results of Patients Undergoing Liver Transplantation for Primary Sclerosing Cholangitis. Hepatology (1999) 30(5):1121–7. 10.1002/hep.510300501
31.
Chaigne B Geneugelijk K Bédat B Ahmed MA Hönger G de Seigneux S et al Immunogenicity of Anti-HLA Antibodies in Pancreas and Islet Transplantation. Cell Transpl (2016) 25(11):2041–50. 10.3727/096368916X691673
32.
Becker LE Hallscheidt P Schaefer SM Klein K Grenacher L Waldherr R et al A Single-Center Experience on the Value of Pancreas Graft Biopsies and HLA Antibody Monitoring After Simultaneous Pancreas-Kidney Transplantation. Transpl Proc (2015) 47(8):2504–12. 10.1016/j.transproceed.2015.09.013
33.
Malheiro J Martins LS Tafulo S Dias L Fonseca I Beirão I et al Impact of De Novo Donor-specific Anti-HLA Antibodies on Grafts Outcomes in Simultaneous Pancreas-Kidney Transplantation. Transpl Int (2016) 29(2):173–83. 10.1111/tri.12687
34.
Mittal S Page SL Friend PJ Sharples EJ Fuggle SV . De Novo Donor-Specific HLA Antibodies: Biomarkers of Pancreas Transplant Failure. Am J Transplant (2014) 14(7):1664–71. 10.1111/ajt.12750
35.
Parajuli S Alagusundaramoorthy S Aziz F Garg N Redfield RR Sollinger H et al Outcomes of Pancreas Transplant Recipients With De Novo Donor-Specific Antibodies. Transplantation (2019) 103(2):435–40. 10.1097/TP.0000000000002339
36.
Uva PD Quevedo A Roses J Toniolo MF Pilotti R Chuluyan E et al Anti‐Hla Donor‐Specific Antibody Monitoring in Pancreas Transplantation: Role of Protocol Biopsies. Clin Transpl (2020) 34(8):e13998. 10.1111/ctr.13998
37.
Winfield RD Reese S Bochicchio K Mazuski JE Bochicchio GV . Obesity and the Risk for Surgical Site Infection in Abdominal Surgery. Am Surg (2016) 82(4):331–6. 10.1177/000313481608200418
38.
Lynch RJ Ranney DN Shijie C Lee DS Samala N Englesbe MJ . Obesity, Surgical Site Infection, and Outcome Following Renal Transplantation. Ann Surg (2009) 250(6):1014–20. 10.1097/SLA.0b013e3181b4ee9a
39.
Sampaio MS Reddy PN Kuo HT Poommipanit N Cho YW Shah T et al Obesity Was Associated With Inferior Outcomes in Simultaneous Pancreas Kidney Transplant. Transplantation (2010) 89(9):1117–25. 10.1097/TP.0b013e3181d2bfb2
40.
Mori DN Kreisel D Fullerton JN Gilroy DW Goldstein DR . Inflammatory Triggers of Acute Rejection of Organ Allografts. Immunol Rev (2014) 258(1):132–44. 10.1111/imr.12146
41.
Randeria SN Thomson GJA Nell TA Roberts T Pretorius E . Inflammatory Cytokines in Type 2 Diabetes Mellitus as Facilitators of Hypercoagulation and Abnormal Clot Formation. Cardiovasc Diabetol (2019) 18(1):72. 10.1186/s12933-019-0870-9
42.
Daryabor G Atashzar MR Kabelitz D Meri S Kalantar K . The Effects of Type 2 Diabetes Mellitus on Organ Metabolism and the Immune System. Front Immunol (2020) 11:1582. 10.3389/fimmu.2020.01582
43.
Pham PH Stalter LN Martinez EJ Wang JW Welch BW Leverson G et al Single Center Results of Simultaneous Pancreas-Kidney Transplantation in Patients with Type 2 Diabetes. Am J Transpl (2021) 21(8):2810–23. 10.1111/ajt.16462
44.
Removing BMI and C-Peptide from Kidney/pancreas Waiting Time Criteria - UNOS. 2010; Available from: https://unos.org/news/removing-bmi-and-cpeptide-from-kidney-pancreas-waiting-time-criteria/(Accessed August 14, 2010).
45.
Drachenberg CB Torrealba JR Nankivell BJ Rangel EB Bajema IM Kim DU et al Guidelines for the Diagnosis of Antibody-Mediated Rejection in Pancreas Allografts-Updated Banff Grading Schema. Am J Transplant (2011) 11(9):1792–802. 10.1111/j.1600-6143.2011.03670.x
46.
Ellis TM . Interpretation of HLA Single Antigen Bead Assays. Transpl Rev (2013) 27(4):108–11. 10.1016/j.trre.2013.07.001
47.
Parajuli S Reville PK Ellis TM Djamali A Mandelbrot DA . Utility of Protocol Kidney Biopsies for De Novo Donor-Specific Antibodies. Am J Transplant (2017) 17(12):3210–8. 10.1111/ajt.14466
48.
Dean PG Kudva YC Larson TS Kremers WK Stegall MD . Posttransplant Diabetes Mellitus After Pancreas Transplantation. Am J Transplant (2008) 8(1):175–82. 10.1111/j.1600-6143.2007.02018.x
49.
Stratta RJ Rogers J Orlando G Farooq U Al-Shraideh Y Farney AC . 5-Year Results of a Prospective, Randomized, Single-Center Study of Alemtuzumab Compared with Rabbit Antithymocyte Globulin Induction in Simultaneous Kidney-Pancreas Transplantation. Transpl Proc (2014) 46(6):1928–31. 10.1016/j.transproceed.2014.05.080
50.
Niederhaus SV Leverson GE Lorentzen DF Robillard DJ Sollinger HW Pirsch JD et al Acute Cellular and Antibody-Mediated Rejection of the Pancreas Allograft: Incidence, Risk Factors and Outcomes. Am J Transplant (2013) 13(11):2945–55. 10.1111/ajt.12443
51.
Neidlinger N Singh N Klein C Odorico J Munoz Del Rio A Becker Y et al Incidence of and Risk Factors for Posttransplant Diabetes Mellitus After Pancreas Transplantation. Am J Transplant (2010) 10(2):398–406. 10.1111/j.1600-6143.2009.02935.x
52.
Chakkera HA Bodner JK Heilman RL Mulligan DC Moss AA Mekeel KL et al Outcomes After Simultaneous Pancreas and Kidney Transplantation and the Discriminative Ability of the C-Peptide Measurement Pretransplant Among Type 1 and Type 2 Diabetes Mellitus. Transpl Proc (2010) 42(7):2650–2. 10.1016/j.transproceed.2010.04.065
53.
Niederhaus SV Kaufman DB Odorico JS . Induction Therapy in Pancreas Transplantation. Transpl Int (2013) 26(7):704–14. 10.1111/tri.12122
54.
Liu L Xiong Y Zhang T Fang J Zhang L Li G et al Effect of Simultaneous Pancreas-Kidney Transplantation on Blood Glucose Level for Patients With End-Stage Renal Disease With Type 1 and Type 2 Diabetes. Ann Transl Med (2019) 7(22):631. 10.21037/atm.2019.10.106
55.
Owen RV Carr HJ Counter C Tingle SJ Thompson ER Manas DM et al Multi-Centre UK Analysis of Simultaneous Pancreas and Kidney (SPK) Transplant in Recipients With Type 2 Diabetes Mellitus. Transpl Int (2024) 36:11792. 10.3389/ti.2023.11792
56.
Jeon HJ Koo TY Han M Kim HJ Jeong JC Park H et al Outcomes of Dialysis and the Transplantation Options for Patients With Diabetic End-Stage Renal Disease in Korea. Clin Transpl (2016) 30(5):534–44. 10.1111/ctr.12719
57.
Cao Y Liu X Lan X Ni K Li L Fu Y . Simultaneous Pancreas and Kidney Transplantation for End-Stage Kidney Disease Patients With Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Langenbecks Arch Surg (2022) 407(3):909–25. 10.1007/s00423-021-02249-y
58.
Fernández-Burgos I Montiel Casado MC Pérez-Daga JA Aranda-Narváez JM Sánchez-Pérez B León-Díaz FJ et al Induction Therapy in Simultaneous Pancreas-Kidney Transplantation: Thymoglobulin Versus Basiliximab. Transpl Proc (2015) 47(1):120–2. 10.1016/j.transproceed.2014.12.003
59.
Magliocca JF Odorico JS Pirsch JD Becker YT Knechtle SJ Leverson GE et al A Comparison of Alemtuzumab With Basiliximab Induction in Simultaneous Pancreas-Kidney Transplantation. Am J Transplant (2008) 8(8):1702–10. 10.1111/j.1600-6143.2008.02299.x
60.
Bazerbachi F Selzner M Boehnert MU Marquez MA Norgate A McGilvray ID et al Thymoglobulin Versus Basiliximab Induction Therapy for Simultaneous Kidney-Pancreas Transplantation: Impact on Rejection, Graft Function, and Long-Term Outcome. Transplantation (2011) 92(9):1039–43. 10.1097/TP.0b013e3182313e4f
61.
Stratta RJ Rogers J Orlando G Farooq U Al-Shraideh Y Doares W et al Depleting Antibody Induction in Simultaneous Pancreas-Kidney Transplantation: A Prospective Single-Center Comparison of Alemtuzumab Versus Rabbit Anti-thymocyte Globulin. Expert Opin Biol Ther (2014) 14(12):1723–30. 10.1517/14712598.2014.953049
62.
Afaneh C Rich B Aull MJ Hartono C Kapur S Leeser DB . Pancreas Transplantation Considering the Spectrum of Body Mass Indices. Clin Transpl (2011) 25(5):520–9. 10.1111/j.1399-0012.2011.01475.x
63.
Hanish SI Petersen RP Collins BH Tuttle-Newhall J Marroquin CE Kuo PC et al Obesity Predicts Increased Overall Complications Following Pancreas Transplantation. Transpl Proc (2005) 37(8):3564–6. 10.1016/j.transproceed.2005.09.068
64.
Orlando G Stratta RJ Light J . Pancreas Transplantation for Type 2 Diabetes Mellitus. Curr Opin Organ Transpl (2011) 16(1):110–5. 10.1097/MOT.0b013e3283424d1f
65.
Amara D Hansen KS Kupiec-Weglinski SA Braun HJ Hirose R Hilton JF et al Pancreas Transplantation for Type 2 Diabetes: A Systematic Review, Critical Gaps in the Literature, and a Path Forward. Transplantation (2022) 106(10):1916–34. 10.1097/TP.0000000000004113
66.
Chan G Garneau P Hajjar R . The Impact and Treatment of Obesity in Kidney Transplant Candidates and Recipients. Can J Kidney Health Dis (2015) 2(1):26. 10.1186/s40697-015-0059-4
67.
Modanlou KA Muthyala U Xiao H Schnitzler MA Salvalaggio PR Brennan DC et al Bariatric Surgery Among Kidney Transplant Candidates and Recipients: Analysis of the United States Renal Data System and Literature Review. Transplantation (2009) 87(8):1167–73. 10.1097/TP.0b013e31819e3f14
68.
Gheith O Al-Otaibi T Halim MA Mahmoud T Mosaad A Yagan J et al Bariatric Surgery in Renal Transplant Patients. Exp Clin Transplant (2017) 15(Suppl. 1):164–9. 10.6002/ect.mesot2016.P35
69.
Al-Bahri S Fakhry TK Gonzalvo JP Murr MM . Bariatric Surgery as a Bridge to Renal Transplantation in Patients With End-Stage Renal Disease. Obes Surg (2017) 27(11):2951–5. 10.1007/s11695-017-2722-6
70.
Yemini R Nesher E Carmeli I Winkler J Rahamimov R Mor E et al Bariatric Surgery Is Efficacious and Improves Access to Transplantation for Morbidly Obese Renal Transplant Candidates. Obes Surg (2019) 29(8):2373–80. 10.1007/s11695-019-03925-1
Summary
Keywords
infection, rejection, complication, pancreas-kidney transplantation, type 2 diabetes
Citation
Martinez EJ, Pham PH, Wang JF, Stalter LN, Welch BM, Leverson G, Marka N, Al-Qaoud T, Mandelbrot D, Parajuli S, Sollinger HW, Kaufman DB, Redfield RR III and Odorico JS (2024) Analysis of Rejection, Infection and Surgical Outcomes in Type I Versus Type II Diabetic Recipients After Simultaneous Pancreas-Kidney Transplantation. Transpl Int 37:13087. doi: 10.3389/ti.2024.13087
Received
03 April 2024
Accepted
10 September 2024
Published
19 September 2024
Volume
37 - 2024
Updates
Copyright
© 2024 Martinez, Pham, Wang, Stalter, Welch, Leverson, Marka, Al-Qaoud, Mandelbrot, Parajuli, Sollinger, Kaufman, Redfield and Odorico.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jon Scott Odorico, jon@surgery.wisc.edu
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.